Abstract
The lipid raft hypothesis proposed that these microdomains are small (10–200 nM), highly dynamic and enriched in cholesterol, glycosphingolipids and signalling phospholipids, which compartmentalize cellular processes. These membrane regions play crucial roles in signal transduction, phagocytosis and secretion, as well as pathogen adhesion/interaction. Throughout evolution, many pathogens have developed mechanisms to escape from the host immune system, some of which are based on the host membrane microdomain machinery. Thus lipid rafts might be exploited by pathogens as signalling and entry platforms. In this review, we summarize the role of lipid rafts as players in the overall invasion process used by different pathogens to escape from the host immune system.
Keywords: cell signalling, lipid raft, membrane microdomain, parasite—host cell interaction, pathogen
Abbreviations used:
- AFM
atomic force microscopy
- APC
antigen‐presenting cell
- DAMP
damage‐associated molecular pattern
- DRM
detergent‐resistant membrane
- ER
endoplasmic reticulum
- EV‐1
echovirus type 1
- FRET
fluorescence resonance energy transfer
- gp
glycoprotein
- GPI
glycosylphosphatidylinositol
- HCV
hepatitis C virus
- HGF‐R
hepatocyte growth factor receptor
- Hsp
heat‐shock protein
- LPG
lipophosphoglycan
- LPS
lipopolysaccharide
- MLV
murine leukaemia virus
- PAMP
pathogen‐associated molecular pattern
- PIP2
phosphatidylinositol 4,5‐bisphosphate
- PrP(C)
(cellular) prion protein
- SARS‐CoV
severe acute respiratory syndrome coronavirus
- SV40
simian virus 40
- T3SS
type 3 secretion system
- TCR
T‐cell receptor
- TLR
Toll‐like receptor
Introduction
Lipid rafts: a heterogeneous membrane microdomain
Since it was first described as a simple barrier between extra and intracellular compartments, different plasma membrane structural and composition models have been proposed (Pike, 2009). Among these different models, the ‘fluid mosaic’ model proposed by Singer and Nicolson (1972) helps to provide answers about the different roles of the plasma membrane in cell biology, including transport processes, cell protection, cell—cell contact and, principally, cell signalling. The plasma membrane harbours many molecules involved in different cell signalling cascades, such as glycero‐ and sphingo‐lipids, proteins (receptord, kinases, etc.), which interacts upon different stimuli triggering different cell responses.
Over the last decade, several works have provided evidence that the plasma membrane is really more mosaic than fluid (Pike, 2003; Engelman, 2005). It was shown that lipids are not randomly distributed, leading to the proposal of a new model for understanding the biological membrane architecture (Pike, 2003; García‐Marcos et al., 2006). The new model suggests that the plasma membrane is patchy, with segregated portions that are distinct in structure and function and that can also vary in thickness and composition. From the first reports in the 1950s provided by Palade (1953) and Yamada (1955) showing stable flask‐like invaginations of the plasma membrane, to the current view of multiple membrane domains allowing different protein—lipid and protein—protein interactions that compartmentalize and temporarily order the membrane moiety, many different reports still bring some controversy to the theme.
The concept of lipid microdomains arose in the 1980s in the reports of Van Meer and Simons (1983) and Klausner and colleagues (1980). It is now widely accepted that lipid rafts are not pre‐existing domains in which proteins dynamically partition, but rather that the formation and disassembly of raft domains is a dynamic process (see, for example, Plowman et al., 2005). Later, rafts were visually observed by Simons and Ikonen (1997), who described them as lipid rafts, imagining these microdomains as floating islands in the membrane (Triantafilou et al., 2002; Luo et al., 2008). Further studies provided evidence that lipid rafts are mainly composed of cholesterol, (glyco)sphingolipids and (glycero)phospholipids, with a high degree of saturation in their fatty‐acid chains (Figure 1a). The tight interaction between these components provides the basis of their packing and rigidity, which leads to a phase separation (Simons and Ikonen, 1997).
Figure 1.
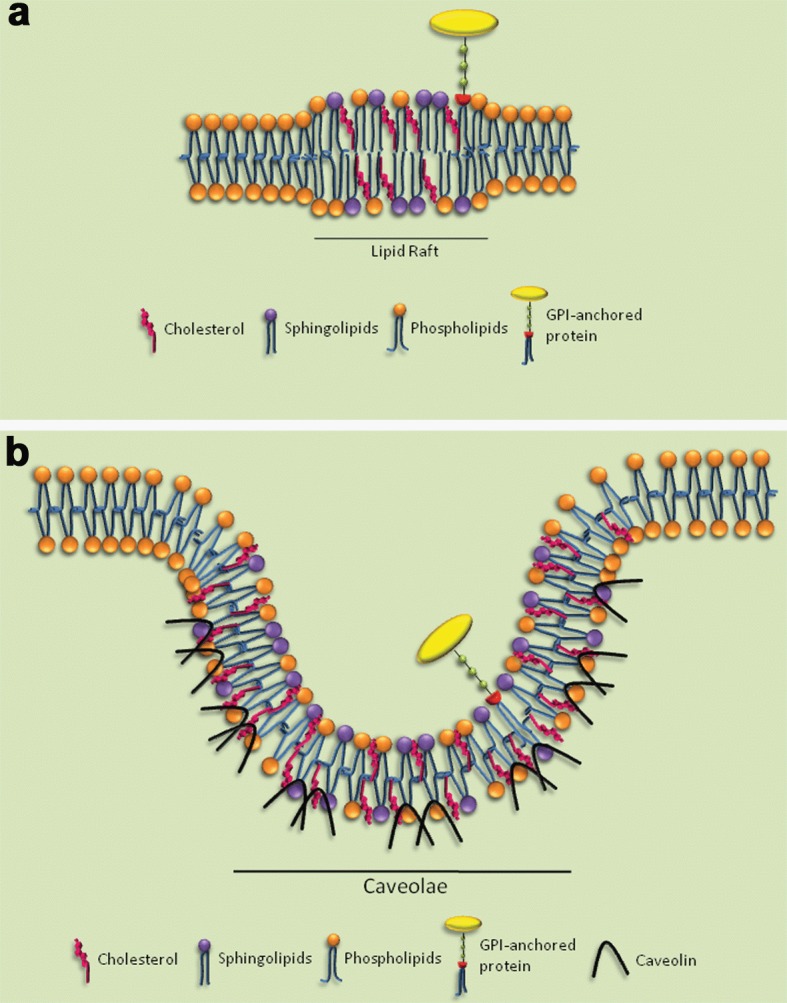
Membrane microdomains
(a) A classical lipid‐raft scheme. Membrane microdomains are enriched with cholesterol and sphingolipids and also possess associated proteins such as GPI‐anchored protein. (b) A caveolin‐rich lipid‐raft scheme. The membrane microdomain can form invaginations called caveolae. Caveolin is a marker protein of caveolae structure.
This tight‐packing organization of lipid rafts confers their resistance to solubilization by non‐ionic detergents, which allows their separation and isolation from the rest of the plasma membrane using sucrose‐density gradients (Brown and Rose, 1992). The development of this method is widely used for the isolation and analysis of lipid rafts and their associated proteins in different cell types (Triantafilou et al., 2002; Bouillon et al., 2003; Chazal and Gerlier, 2003; Laughlin et al., 2004; Tortelote et al., 2004; Vacca et al., 2004; Olsson and Sundler, 2006). However, new considerations about the term ‘detergent‐resistant membranes’ (DRMs) had already been reviewed. DRM no longer defines the bona fide rafts (Lai, 2003), because this technique can create several artifacts, such as the loss of membrane components during extraction (Edidin, 2003). However, many proteins functionally involved with microdomains had already been described via this method, as discussed by Lai (2003). Another efficient tool used to study lipid rafts is cholesterol depletion, by the use of cholesterol‐removing and ‐binding agents, such as cyclodextrins and filipin (Schnitzer et al., 1994; Luker et al., 2000). This method is based on the theory that cholesterol acts as a dynamic ‘glue’, holding lipid rafts together, and its removal from the membrane results in the dispersion of raft‐associated lipids and proteins (Mañes et al., 2003; Hawkes and Mak, 2006). Considering that cholesterol is a relatively rigid molecule, it was postulated that cholesterol‐rich microdomains have a slower mobility in the membrane than non‐raft regions (Shaw, 2006).
Nowadays, more accurate methods are employed in order to improve the research into lipid rafts. Fluorescent biosensors that are capable of tracking signalling events in live cells are powerful tools to aid the understanding of dynamic cellular signalling (Gao and Zhang, 2008). The proposals regarding microdomains postulated in the late 1990s can be now supported by, for example, FRET (fluorescence resonance energy transfer) microscopy and AFM (atomic force microscopy). Given the importance of the Akt pathway in cell proliferation and survival, and thus its involvement in cancer, this pathway has been increasingly studied. Gao and Zhang (2008) developed a genetically encodable Akt activity reporter and analysed the spatiotemporal dynamics of Akt activity within plasma membrane microdomains by FRET. Using this tool, it was observed that Akt activity is differentially regulated between raft and non‐raft regions of the plasma membrane (Gao and Zhang, 2008). In addition, AFM has been described as an essential tool to follow the motion of lipid‐raft microdomains and proteins that might be interacting with them (Henderson et al., 2004). Several works have demonstrated the importance of AFM in experimental approaches to study lipid rafts. Poole et al. (2004), studying MDCK (Madin—Darby canine kidney) cell microvilli, suggested that lipid rafts might be involved in the maintenance of these structures. These authors used a combination of AFM and laser‐scanning confocal microscopy in order to attest their hypothesis. Cecchi et al. (2009) interestingly observed an increase in specific amyloid ligands when raft components, such as cholesterol, are depleted. This group suggested that cholesterol can reduce membrane modifications triggered by amyloid residues at the lipid‐raft level, possibly involving physicochemical features. The techniques of AFM and confocal microscopy in combination with inhibitors of cholesterol synthesis and an agent that chelates cholesterol were allied in the study of lipid rafts in this context, as well as the pursuit of flotillin‐1 and the ganglioside GM1, which are known markers of microdomains.
The lipid raft size in vivo has been estimated to be 25–700 nm by using FRET and single‐molecule‐tracking microscopy. The size, arrangement and movement of lipid rafts are dynamic and can be found in localized cellular structures, such as filipodia and cell adhesion points (Chazal and Gerlier, 2003; Hawkes and Mak, 2006). It is accepted that membrane rafts in basal conditions are small regions of the lipid membranes, which tend to cluster after certain stimuli to form larger structures that are also called platforms (Liu and Anderson, 1995; Holopainen et al., 1998; Kusumi et al., 2004; Bollinger et al., 2005; Rao and Mayor, 2005). It is important to mention that the assembly of those small regions (pre‐rafts) seems to be dependent on the presence of ceramide (Liu and Anderson, 1995; Bollinger et al., 2005). In addition to the lipid components, a variety of cell receptors and signalling proteins are known to be associated with membrane rafts. They include the GPI (glycosylphosphatidylinositol)‐anchored proteins, kinases and adaptor molecules that act as intermediate transducers for many receptors, including TCRs (T‐cell receptors) and BCRs (B‐cell receptors) (Montixi et al., 1998; Cheng et al., 1999; Chazal and Gerlier, 2003; Hawkes and Mak, 2006).
The role of lipid rafts in different cell types has been the subject of numerous studies, and their physiological significance for cell biology has recently become clear. These membrane regions play an important role in a variety of cellular functions, including polarization, signal transduction, endocytosis, secretion, cell—cell and cell—pathogen adhesion (Mañes et al., 1999; Martin‐Belmonte et al., 2000; Harris et al., 2001; Grimmer et al., 2002; Ha et al., 2003; Pierini et al., 2003; Jacobson et al., 2007). One of the most widely appreciated roles of lipid rafts is the recruitment and concentration of molecules involved in cellular signalling. The formation of a molecular cluster and their signal transduction machinery in membrane rafts leads to enhanced signalling efficiency (Triantafilou et al., 2002).
Anderson et al. (2000) reported that MHC class II molecules are located in lipid rafts of murine and human B‐cell lines. Such a localization seems to be critical for T‐cell activation. The binding of TCRs to MHC class II molecules of APCs (antigen‐presenting cells) occurs in lipid rafts. The raft aggregation promotes tyrosine phosphorylation and recruitment of signalling proteins, but excludes certain proteins, such as the tyrosine phosphatases CD45 and CD43, which leads to the formation of a supramolecular activation cluster (Monks et al., 1998). However, in vivo, TCR does not constitutively reside in membrane lipid rafts. After T‐cell activation, the TCR moves into the rafts (Monks et al., 1998; Anderson et al., 2000; Luo et al., 2008). This characteristic was also observed for other molecules that migrate to the lipid rafts after specific stimulation in several physiological events.
There is a specific subtype of microdomain called caveolae. These structures are small membrane‐surface invaginations, which were initially described as cave‐like invaginations of the plasma membrane, 50–100 nm in size and found in many cell types (Figure 1b). Although they were identified by electron microscopy more than 50 years ago as an invagination in the plasma membrane with a flask‐shape morphology that can be singular or found in detached grape‐like clusters, caveolin caveolae have remained enigmatic structures (Palade, 1953; Yamada, 1955; Razani et al., 2002; Lajoie et al., 2009). Forty years after the description of caveolae, their structure could be more closely studied upon the discovery of caveolin, the signature protein present in calveolae (Rothberg et al., 1992). It has been suggested that caveolae can be stabilized by caveolin and, additionally, may further become immobilized by filamin, which binds to caveolin, as well as to the actin cytoskeleton (Stahlhut and van Deurs, 2000; Hommelgaard et al., 2005). Since that time, caveolae have been implicated and demonstrated to be important in a variety of cellular functions, including endocytic processes, cholesterol and lipid homoeostasis, signal transduction and tumour suppression (Razani et al., 2002; Duncan et al., 2002; Mañes et al., 2003; Cohen et al., 2004; Tortelote et al., 2004). The protein caveolin‐1 has itself been implicated in signal transduction, because of its direct interaction with a multitude of signalling molecules through the caveolin‐scaffolding domain. In addition, caveolin‐1 has been shown to be phosphorylated on tyrosine residues during some signalling events (Mastick and Saltiel, 1997; Okamoto et al., 1998; Ushio‐Fukai et al., 2001; Duncan et al., 2002).
Vesicular transport is one of the most important roles of caveolae, including endocytosis when caveolae are, indeed, functional endocytic vesicles (Lajoie and Nabi, 2007), and promotes transcytosis of specific macromolecules in endothelial cells (Minshall et al., 2003). Previous studies proposed that, as well as caveolae, caveolins also have an important involvement in signal transduction, principally due to its scaffold domain, which acts as a harbour for different cytosolic proteins involved in different signalling cascades (Sargiacomo et al., 1993; Lisanti et al., 1994; Razani et al., 2002; Cohen et al., 2004).
Pathogens and rafts: interacting to survive
It is well known that intracellular parasites have many mechanisms to avoid the host defence response. The inhibition of lysosomal fusion, a classical escape mechanism, was observed after infection by Mycobacterium, Chlamydia, Toxoplasma (Coutinho‐Silva et al., 2009) and Trypanosoma cruzi (Hall and Joiner, 1993), for example. Another way that pathogens can prolong their survival inside the host is by prevention of host‐cell apoptosis and by the modulation of reactive oxygen and nitrogen species generation (Coutinho‐Silva et al., 2009).
An interesting manner that allows pathogens to evade the immune system is through membrane microdomains. As signalling for the innate and adaptative immune responses is initiated in rafts, some pathogens have evolved mechanisms to subvert this signalling by co‐opting raft‐associated pathways (Mañes et al., 2003). Different pathogens, such as viruses, bacteria and protozoa, can use the host‐cell lipid rafts to secure their entrance and maintenance inside target cells (Figure 2). The benefit provided by interaction with lipid rafts can vary from one pathogen to another. The list of pathogens that hijack rafts also includes the non‐classical infectious agent, the scrapie PrP (prion protein). Here, we provide an update of how different pathogens modulate host immune response by using their lipid rafts.
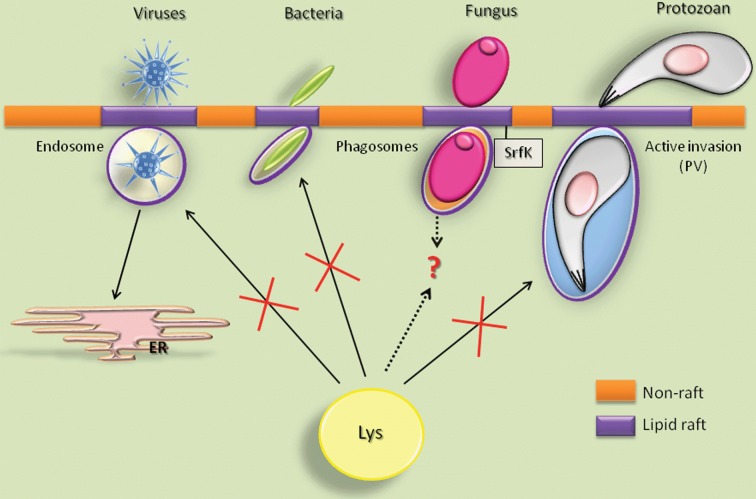
Figure 2.
Pathogen—raft interaction
Several pathogens can directly interact with different target cells through membrane microdomains in different manners. It is known that the entry via lipid rafts can avoid lysosomal fusion and therefore allow pathogen survival. In addition, parasites might modulate signalling pathways, including lipid‐raft‐associated protein kinases [Srfk (Src family kinases)]. Especially viruses, which do not have their own protein synthesis machinery, target the ER after subverting the lysosomal pathway. Lys, lysosome; PV, parasitophorous vacuole.
How do different pathogens interact with the host via lipid rafts?
Viruses: exploiting lipid rafts
Different viruses have evolved strategies to subvert raft‐associated signalling, enabling their efficient replication in immune cells, and at the same time blocking the immune response that is elicited by the target cells (Hawkes and Mak, 2006).
(a) Entry
Virus entry into a host cell involves the binding of the virus to one or more cell‐surface receptors, followed by entry into the cell. Many animal viruses exploit the endocytic machinery of their host cell for infection, and lipid rafts are often a site for entry, assembly and budding of microbial pathogens, as confirmed by biochemical approaches and microscopy evidence (Kovbasnjuk et al., 2001; Suomalainen, 2002; Lu et al., 2008).
For non‐enveloped viruses, after the attachment to cell‐surface receptors, the bound capsids are internalized, mostly by invagination of the plasma membrane and intracytoplasmic vesiculation. The involvement of lipid rafts in mediating this process has been described for several viruses, as reviewed by Chazal and Gerlier (2003). The most thoroughly studied of these is SV40 (simian virus 40). SV40 initiates infection by binding to the MHC class I molecules (Stang et al., 1997; Anderson et al., 1998; Duncan et al., 2002; Chazal and Gerlier, 2003). SV40 directly associates with caveolae, leading to a loss of actin stress fibres and the appearance of actin tails emanating from the virus containing caveolae (Duncan et al., 2002; Pelkmans et al., 2002). Moreover, caveolae transport SV40 particles to the ER (endoplasmic reticulum), where the virus is disassembled (Norkin et al., 2002; Chazal and Gerlier, 2003).
Both the polyoma virus and EV‐1 (echovirus type 1) also directly associate with caveolae (Richterová et al., 2001; Marjomaki et al., 2002). The polyoma virus associates with caveolin‐1 after entry, a possible association with ‘caveosomes’ and trafficking to the ER (Richterová et al., 2001). EV‐1 is internalized into caveolae using the integrin α2β1 as cellular receptor. Studies have shown that EV‐1, α2β1 integrin and caveolin‐1 were internalized together in vesicular structures and accumulated in a perinuclear compartment (Marjomaki et al., 2002). Interestingly, it was shown (Richterová et al., 2001; Marjomaki et al., 2002) that the entry of the virus does not occur by endocytosis through the classic clathrin‐coated vesicles. However, these authors observed that virus particles had merged with caveolin‐1, and incubation with methyl‐β‐cyclodextrin inhibited the virus entry.
Enveloped viruses also use rafts during the internalization and fusion process. The entry of enveloped virus involves virus attachment, followed by close apposition of the virus and plasma membranes. Then the two membranes fuse to deliver the virus' genomic RNA into the host cells, which requires conversion of the virus‐encoded envelope glycoprotein (Env) from its native state to its fusion‐activated form (Fantini et al., 2002; Chazal and Gerlier, 2003; Mañes et al., 2003). The glycoproteins of several viruses, including influenza virus, HIV, MLV (murine leukaemia virus), measles virus and Ebola virus, are associated with host‐cell membrane rafts (Scheiffele et al., 1997; Manie et al., 2000; Vincent et al., 2000; Pickl et al., 2001; Bavari et al., 2002). Additionally, there is biochemical evidence showing that cholesterol and possibly cholesterol‐rich lipid rafts are required for efficient porcine pseudorabies virus entry (Desplanques et al., 2008). Another recent study reported that the SARS‐CoV (severe acute respiratory syndrome coronavirus) receptor is located in lipid rafts and the productive entry of the SARS‐CoV pseudovirus into the host cell requires the presence of intact and functional lipid rafts (Lu et al., 2008). Fusion of SFV (Semliki Forest virus) and SIN (Sindbis virus), as well as other alphaviruses, depends on the presence of cholesterol and sphingolipid in the target membrane (Bron et al., 1993; Lu et al., 1999; Smit et al., 1999; Chazal and Gerlier, 2003), which are known to be abundant in lipid rafts (Pike, 2003). Other enveloped viruses enter the host cell using a pH‐independent fusion process, as found for HIV. The HIV‐1 Env is composed of two associated glycoprotein subunits, gp120 and gp41. The external gp120 is responsible for the attachment to the cellular receptors and co‐receptors (chemokine receptor family member CCR5 and/or CXCR4), whereas the transmembrane protein gp41 is responsible for the fusion of viral envelope with the plasma membrane of the target CD4+ T‐cells (Popik et al., 2002). Rafts are proposed to be the specific cell membrane regions in which these clustering events occur. It is important to point out that the entry of HIV‐1 through rafts may direct the virus complex into a favourable compartment for a productive infection (Fantini et al., 2002; Chazal and Gerlier, 2003; Mañes et al., 2003; Luo et al., 2008). A recent study showed that HIV entry into macrophages is sensitive to membrane cholesterol depletion, which favours the hypothesis for a role of macrophage lipid rafts in the HIV‐1 entry process (Carter et al., 2009).
The cellular receptor for the MLV, CAT1 (cationic amino acid transporter 1), is physically associated with caveolin in membrane rafts, and the disruption of rafts inhibits the early step of MLV infection, suggesting that the localization of the receptor within rafts is crucial for the virus entry (Lu and Silver, 2000).
It was already demonstrated that the penetration of filoviruses, such as Ebola virus and Marburg virus, is inhibited after cholesterol depletion of the host cell, and, after internalization, viral proteins co‐localized with caveolin (Bavari et al., 2002; Empig and Goldsmith, 2002; Chazal and Gerlier, 2003).
(b) Assembly
The late stages of the viral life cycle are the assembly of viral components into virions, maturation into infectious particles, and, in the case of enveloped viruses, release from the cell via a budding process (Ivanchenko et al., 2009). Assembly and budding are the last, but critical, steps in the virus life cycle for the survival of the virus and its disease‐producing ability in the host (Chen et al., 2008; Wang et al., 2009). An explanation as to why viruses use lipid rafts is that these structures offer an efficient system for concentrating all the virus proteins that are required for the assembly of new virions, as reviewed by Nayak et al. (2004).
HIV‐1 is enclosed in a lipid envelope enriched in cholesterol and sphingolipids, suggesting specific membrane localization for assembly (Aloia et al., 1993; Campbell et al., 2001; Raulin, 2002). Recent studies have reported that rafts represent a necessary step during HIV‐1 assembly. With similar methods of both assembly and budding within membrane rafts, many other viruses, including influenza virus, measles virus, Ebola virus and possibly Sendai virus, also use lipid rafts as assembly platforms (Luo et al., 2008). In this regard, it was also suggested that the RSV (respiratory syncytial virus) assembled within lipid rafts where viral proteins co‐localize with caveolin‐1 (Brown et al., 2002a, 2002b).
Although rafts are involved in virus assembly, we have to keep in mind that only a fraction of viral proteins are found associated with rafts; this could be due to the poor biochemical characterization of raft subsets or to the transient nature of the association.
(c) Budding
Whereas non‐enveloped viruses are released from the infected cell by disruption of the plasma membrane, enveloped viruses contain a host‐cell‐derived lipid bilayer, which is acquired during budding (Garoff et al., 1998; Chazal and Gerlier, 2003). Membrane lipids are not randomly incorporated into the viral envelope. In addition, some authors suggest that viral glycoproteins determine the site of virus assembly and budding (Garoff and Simons 1974; Allison et al., 1995; Vennema et al., 1996; Bruss, 2004). On the other hand, in polarized epithelial cells, the viral glycoproteins contain sorting signals or motifs and are directed to the specific site where assembly and budding will occur (Nayak et al., 2004). Lipid rafts function as microdomains for concentrating viral glycoproteins and may serve as a platform for virus budding. The structures have the ability to regulate budding; however, the mechanism by which the lipid raft can favour the budding and/or fission process is as yet unknown (Nayak et al., 2004; Luo et al., 2008). The budding of new virions from the raft allows the exclusion or inclusion of specific host‐cell membrane proteins in the virus particle, which could disrupt cellular and/or humoral immune responses to the virus (Vanderplasschen et al., 1998; Peterlin and Trono, 2003).
The lipid composition of the influenza virus family is due to affinity of the haemagglutinin and neuraminidase glycoproteins for these lipids, and some authors suggest that the influenza virus buds from raft domains (Chazal and Gerlier, 2003; Nayak et al., 2004).
Several reports suggest that HIV‐1 buds from lipid rafts (Campbell et al., 2001; Fantini et al., 2002). HIV incorporates raft‐associated complement regulatory proteins, which remain functionally active on the surface of the virus and down‐regulate the complement cascade (Mañes et al., 2003; Peterlin and Trono, 2003).
After budding from the host cell, viruses are released into the surrounding medium to infect other cells. The mechanism of this bud completion is as yet unclear and a number of both viral and host factors may affect this process (Nayak et al., 2004; Luo et al., 2008).
Bacteria: taking advantage of host‐cell membrane microdomains
Studies have been suggested that several bacteria interact with host lipid rafts to enter and survive inside the cell (Mañes et al., 2003; Hawkes and Mak, 2006). The mechanisms that underlie this interaction are starting to be unravelled. Activation of secretion, binding, perforation of the host‐cell membrane and signalling to trigger bacterial phagocytosis are involved with components of membrane microdomains (Lafont and van der Goot, 2005). It was found that the polarity of epithelial cells and the involvement of CD55 are important in the interaction of bacteria with lipid rafts (Peiffer et al., 1998). Two advantages in bacteria invasion were postulated: (1) avoidance of the intracellular degradative pathway and (2) triggering of the cell signalling cascades that lead to membrane ruffling and cytoskeleton rearrangement (Mañes et al., 2003).
The avoidance of the host immune pathway after phagocytosis was developed by several micro‐organisms, mainly bacterial cells. Subversion of phagosome fusion with lysosome and presentation to immune system was observed in pathogens such as Mycobacterium and Chlamydia. Gatfield and Pieters (2000) showed for the first time the relevant role of cholesterol for mycobacteria entry into macrophages. In addition, some bacteria hidden inside the cell took advantage of host lipids to generate phagosomes and survive inside them, such as Brucella spp. and Legionella pneumophila (Naroeni and Porte, 2002; Watarai et al., 2001, 2002). Besides, these parasites can hijack rafts, altering host‐cell signalling, for example Shigella flexneri (Lafont et al., 2002). The interaction between pathogens and the host cell can also modulate other host features, such as cytoskeletal dynamics. In the case of S. flexneri, cholesterol removal decreased the binding of an effector protein, called IpaB, with host CD44, which is known to be involved in cytoskeleton‐dependent signalling events (Hirao et al., 1996).
Seveau et al. (2004) demonstrated for the first time that the cell adhesion molecule, E‐cadherin, and HGF‐R (hepatocyte growth factor receptor) require host lipid rafts to mediate Listeria monocytogenes entry. It had already been reported by the same group that, in L. monocytogenes, two major proteins, internalin and InIB, mediate bacterial invasion into host and bind to E‐cadherin and HGF‐R respectively (Cossart et al., 2003).
Salmonella, Shigella and the entheropathogenic Escherichia coli have a common requirement for a T3SS (type III secretion system), which is a multicomponent molecular syringe that allows the translocation of so‐called effector proteins from bacterial cytoplasm, through the inner and outer bacterial membrane, as well as the host plasma membrane, directly into cytoplasm (van der Goot et al., 2004; Lafont and van der Goot, 2005). Activation of this system requires contact with the host cell, and has effector proteins, named SipB and SipC for Salmonella, IpaB and IpaC for Shigella and PopB and PopC for Pseudomonas. Hayward et al. (2005) showed a new requirement for cholesterol, for which the main binding determinant was SipB/IpaB to host cells, and Lafont and van der Goot (2005) suggest that this must occur downstream of the T3SS activation.
The pathogenic bacterium Brucella, which causes brucellosis, can avoid bactericidal activity of macrophages triggering the cAMP/PKA (protein kinase A) pathway. This process occurs immediately after the first contact with the target cell (Jimenez de Bagues et al., 2005). Lipid‐raft‐associated molecules, such as GPI‐anchored proteins, GM1 gangliosides and cholesterol, were found selectively incorporated into macropinosomes containing Brucella. In contrast, the lysosomal glycoprotein LAMP‐1 (lysosome‐associated membrane protein 1) and the host‐cell transmembrane protein CD44 were excluded from these macropinosomes (Watarai et al., 2002). Interestingly, it had already been demonstrated that Brucella abortus infection is related with PrPC (cellular PrP), one of the lipid raft‐associated molecules on the plasma membrane of different cell types. In addition, Watarai et al. (2002, 2004) postulated that the signal transduction induced by the interaction between bacterial Hsp60 (heat‐shock protein 60) and PrPC on macrophages contributes to the establishment of B. abortus infection. Coxiella burnetti, the causative agent of human acute and chronic Q fever, can be found in cholesterol‐rich vacuoles with lipid‐raft proteins, and also can modulate the cholesterol metabolism from the host cell (Howe and Heinzen, 2006). The importance of cholesterol in C. burnetti infection can be directly associated with its pathophysiology (Howe and Heinzen, 2006).
It was also suggested that pathogens and particles that bind to lipid‐raft components may trigger the macrophage autophagic machinery (Amer et al., 2005). L. pneumophila and a uropathogenic E. coli can stimulate autophagosome formation, which contains both lipid rafts and autophagy‐involved cell molecules. In addition, it was observed that internalization of pathogen and the autophagy stimulation are cholesterol sensitive and the pathogens harbouring in autophagosomes could avoid immediate killing (Amer et al., 2005). Components of lipid rafts do not appear to be essential for assembly of autophagosomes, but instead may affect a signal transduction pathway dedicated to host recognition of microbes, as suggested by Amer et al. (2005).
The specific subtype of microdomain, caveolae, also appeared to be directly involved in the interaction with bacteria (Duncan et al., 2002), such as Chlamydia trachomatis (Norkin et al., 2001), E. coli (Shin et al., 2000) and Campylobacter jejuni (Wooldridge et al., 1996). Among the Chlamydiae, depending on the serovar, or the species, one or both of the caveolin proteins (1 or 2) may play important roles in the developmental cycles (Stuart et al., 2003; Webley et al., 2004).
Porphyromonas gingivalis capitalizes on the lipid‐raft structure to down‐modulate innate defence mechanisms. Remarkably, this novel mechanism employs host‐cell signalling pathways through cross‐talk between TLR2 (Toll‐like receptor‐2)/chemokine receptor 4 to attenuate the protective and bactericidal response to P. gingivalis infection (Darveau, 2009).
Campylobacter enteritis, regardless of its own invasiveness, promotes the translocation of the non‐invasive bacteria E. coli across the intestinal epithelium via a lipid‐raft‐mediated transcellular process (Kalischuk et al., 2009).
Furthermore, bacterial toxins, such as cholera toxin, listeriolysin O and anthrax toxin, also target lipid domains (Orlandi and Fishman, 1998; Coconnier et al., 2000; Abrami et al., 2003). More recently, several pathogenic bacteria have been associated with lipid rafts, such as Francisella tularensis (Tamilselvam and Daefler, 2008), Helicobacter pylori (Lai et al., 2008), P.s gingivalis (Hajishengallis et al., 2006; Wang and Hajishengallis, 2008) and M. tuberculosis (Shin et al., 2008), which are members of an ever increasing list as the years progress. Recently, Caserta et al. (2008) described evidence for the first time that Clostridium perfringens enterotoxin acts independently of lipid microdomains.
Fungi: signalling modulation through host rafts
The involvement of fungal infection with lipid rafts is not yet well explored; however, for mycopathogens, a modulation in host‐cell signalling pathways has been reported, as described below. The invasion process of Candida albicans or Paracoccidioides brasiliensis had already been associated with activation of host‐cell tyrosine kinases (Belanger et al., 2002; Monteiro da Silva et al., 2007). The manipulation of signalling pathways, which involve the host‐cell kinases, can lead to an efficient way to enter, proliferate and exit the host cell during the infectious cycle (Münter et al., 2006). Recently, Maza et al. (2008) investigated yeast forms of P. brasiliensis in the context of kinase signalling. It was observed that this pathogen promotes the aggregation of lipid rafts in epithelial cells, which is an important step to fungal adhesion and Src kinase family activation. Thereby, for the first time, it was shown that a pathogenic fungus can interact with host‐cell membrane rafts to establish infection. Encephalitozoon cuniculi, a microsporidiam parasite that affects the nervous system, as well as the respiratory and digestive tracts, resides in a parasitophorous vacuole surrounded by host‐cell lipids, was labelled with DilC16 (1,1′‐dihexadecyl‐3,3,3′,3′‐tetramethylindocarbocyanine), a marker for lipid rafts, and DiO (3,3′‐dilinoleyloxacarbocyanine), a marker for non‐raft membrane domains, which suggests that both contribute to the formation of the vacuole membrane (Rönnebäumer et al., 2008). This report points to an alternative method of fungal infection in host cells that is raft‐independent.
Protozoa: hiding with lipid rafts
The protozoan invasion in the host cell occurs during specific stages of the pathogen life cycle. Intracellular entrance of these parasites does not depend on the endocytic machinery of the host cell, as in the case of bacteria and viruses. This can be explained due to the protozoan's larger size (5–10 μm). As shown for other pathogens, the exploitation of host membrane microdomains by protozoa constitutes a crucial step for its maintenance, survival and modulation of host immune response (Mañes et al., 2003).
Members of the Apicomplexa group, such as Toxoplasma gondii and Plasmodium falciparum, the aetiological agents of toxoplasmosis and malaria disease respectively, are obligatory intracellular parasites and actively enter their target cells (Aikawa et al., 1978; Suss‐Toby et al., 1996). Previous studies related that these parasites can interact with lipid rafts during the infection process, because parasitophorous vacuole membranes contain host raft lipids and proteins (Aikawa et al., 1978; Mordue et al., 1999; Lauer et al., 2000). This event shows that parasites might hijack or recruit these microdomains during infection. Furthermore, GPI‐anchored proteins, such as CD55 and CD59, that are major inhibitors of membrane complement, are progressively depleted from the infected cell surface (Haldar et al., 2002). It was further demonstrated that host raft cholesterol is important to vacuolar parasites because, when cholesterol was depleted from Plasmodium‐infected erythrocytes, the expulsion of non‐infective parasites occurred (Lauer et al., 2000). In addition, cholesterol depletion from red blood cells prevents P. falciparum infection (Samuel et al., 2001). Theileria parva, another member of Apicomplexa, also interacts with host‐cell rafts, with further regulation of host protein kinases (Dobbelaere et al., 2000; Baumgartner et al., 2003).
Studies indicated that raft association might not be sufficient to shuttle membrane molecules past the moving junctions (tight constrictions formed between parasite and host cell), for example, caveolin‐1 is excluded from the T. gondii parasitophorous vacuole (Mordue et al., 1999; Coppens and Joiner, 2003). Flotillin‐2, a raft protein anchored in plasma membrane by acylation, was also discarded from its parasitophorous vacuole (Charron and Sibley, 2004). It was demonstrated during T. gondii infection that selective portioning at the host—parasite interface is a highly complex process and that the raft interaction can benefit the parasite inclusion into parasitophorous vacuoles (Charron and Sibley, 2004). On the other hand, it was observed that the association with membrane microdomains is not necessary to direct insertion of host‐cell membrane molecules into T. gondii parasitophorous vacuole (Charron and Sibley, 2004). Murphy et al. (2007) related that different remodelling and sorting may occur in distinct endo‐vacuoles. In this case, primaquine was used to disturb red blood cell membranes and induce detergent‐free vesicles, which are enriched in cholesterol, raft proteins (flotillin and stomatin) and PIP2 (phosphatidylinositol 4,5‐bisphosphate). However, PIP2 was abrogated of Plasmodium parasitophorous vacuoles and another lipid was found, PS (phosphatidylserine). So, interestingly, erythrocyte raft lipid recruited to the site of invasion can be remodelled by malaria parasites to establish blood‐stage infection. Protein and lipid distribution in the erythrocyte membrane may be more ordered than previously expected (Murphy et al., 2007).
It is known that there is a unique relationship between cholesterol and caveolae (caveolins), which is involved in cholesterol homoeostasis. Therefore, caveolae become sensitive to cholesterol depletion and a cross‐link between cholesterol and caveolin has been demonstrated (Murata et al., 1995; Razani et al., 2002). Cholesterol also appeared to be important in the infection by the trypanosomatide, Leishmania (Pucadyil et al., 2004). This parasite can enter, survive and proliferate inside macrophages (Alexander and Russell, 1992). Leishmania donovani is responsible for the visceral leishmaniasis (Parson et al., 1983), which is characterized by defective cell‐mediated immunity (Basak et al., 1992; Saha et al., 1995; Sen et al., 2001). L. donovani LPG (lipophosphoglycan) requires intact membrane rafts to control host‐cell functions. It was reported that LPG associates with membrane rafts in the host cell and exerts its actions on host‐cell actin and phagosomal maturation through subversion of raft function (Winberg et al., 2009). Macrophages infected with Leishmania are unable to present, even processing‐independent peptide sequences, to T‐cells, and this event is not due to MHC expression (Prina et al., 1993). In this context, lipid rafts are also involved in the interaction between MHC and APC (Poloso and Roche, 2004). L. donovani can affect antigen presentation of macrophages due to the increase in membrane fluidity, which leads to a lipid‐raft disruption. Although the number of MHC complexes in infected cell surfaces was sufficient, there was no possibility of forming an aggregate and stimulating T‐cells (Chakraborty et al., 2005).
Host evolution: the other side of lipid rafts
Over millions of years, hosts and pathogens co‐evolved to improve their mechanisms of parasite elimination and maintenance of the infection respectively. As described above, pathogens can take advantage of lipid rafts for their own benefit. In the same way, the host membrane microdomains can trigger and enhance the immune response against micro‐organisms.
Studies of lipid rafts of mammals have described the interaction between TLRs and rafts. This family of receptors, which might be activated by PAMPs (pathogen‐associated molecular patterns), such as gram‐negative bacteria LPS (lipopolysaccharide), is important to pathogen recognition. However, only a few members constitutively co‐localize with membrane microdomains (Triantafilou et al., 2002). Besides, other TLRs can migrate to this specific region after activation. In the macrophage‐like cell line RAW 264.7, for example, LPS stimulation induces translocation of CD14, ERK‐2 (extracellular‐signal‐regulated kinase 2) and p38 to lipid rafts, but other proteins also involved in the LPS signalling response do not migrate within these microdomains (Triantafilou et al., 2007; Olsson and Sundler, 2006). In addition, Cuschieri et al. (2006) observed that when the human monocytic cell line THP‐1 was stimulated with LPS, there was a mobilization of TLR4 and HSP70 into the lipid raft. Taken together, these examples show the importance of the aggregation of specific receptor molecules within lipid rafts facilitating the LPS signalling to favour the clearance of intracellular pathogens (Triantafilou et al., 2002). Other molecules are able to induce receptor migration into lipid rafts, for example, DAMPs (damage‐associated molecular patterns). Extracellular ATP is a nucleotide which works as an important DAMP (Di Virgilio, 2005). P2 purinergic receptors, a family of nucleotide receptors, are involved with inflammatory responses (Burnstock and Knight, 2004; Bours et al., 2006; Burnstock, 2009) and the clearance of intracellular pathogens (Coutinho‐Silva et al., 2007, 2009). Several motifs in P2X7 receptors have been identified that are homologous with those known to be involved in protein—protein interactions and LPS binding. It was suggested that the C‐terminal region of the P2X7 receptor may directly associate with proteins and/or lipids that are important for regulating macrophage function (Denlinger et al., 2001). In addition, the activation of P2X7 receptors can induce ceramide generation and accumulation in macrophages (Raymond and Le Strunff, 2006), which is a sphingolipid implicated to be involved with the formation of larger rafts, so called signalling platforms (Gulbins et al., 2004).
Previous studies have already related that specific P2X and P2Y receptors can also be recruited towards membrane microdomain regions (Vacca et al., 2004; Bannas et al., 2005; Vial and Evans, 2005; García‐Marcos et al., 2006; Barth et al., 2007; Norambuena et al., 2008). Recently, we observed that the disruption of lipid rafts can reduce P2X7‐activated pore formation on dendritic cells and macrophages from humans and mice (F.S. Vieira and R. Coutinho‐Silva, unpublished data). Thus it is possible to suggest that the integrated signals from P2 receptors and TLRs located on rafts might explain the synergic effects of these sensors on stimulation of the immune response (Hu et al., 1998; Perregaux et al., 2000; García‐Marcos et al., 2009) (Figure 3).
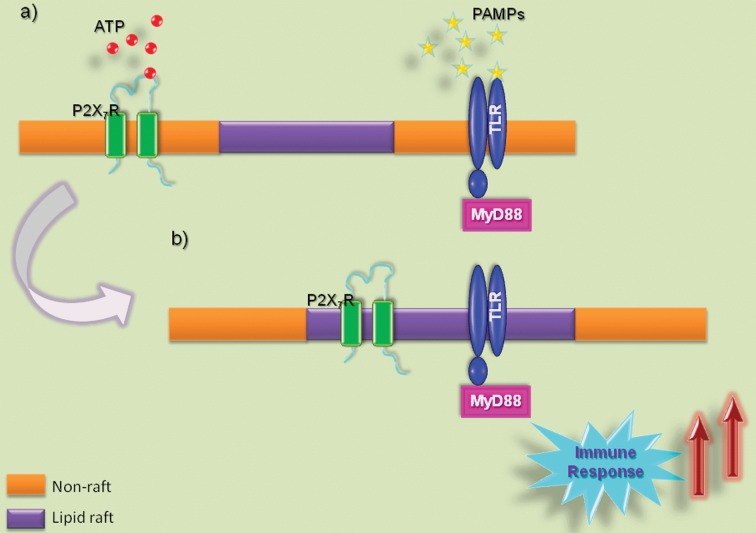
Figure 3.
P2X7 receptor and TLR interaction with lipid rafts
(a) P2X7 receptor (P2X7R) and/or TLR activation, by ATP or PAMPs respectively, in non‐raft membrane regions. (b) These receptors, when stimulated, migrate to lipid‐raft domains. In addition, the P2X7R is involved with inflammatory immune response, as well as TLRs which are related to the initial signal to the immune response. Thus it is suggested that the action of both receptors, together within lipid rafts, can lead to a more intense immune response. MyD88, myeloid differentiation primary response gene 88.
Lipid rafts as targets for chemotherapy: two sides of the coin
Nowadays, several groups are studying and developing new treatment strategies for less harmful chemotherapeutic agents, especially those against viral infections. One of these strategies could be to block HIV‐1 entry and its replication using natural dietary and plant‐derived compounds that target lipid rafts, principally due to its affinity for cholesterol (Verma, 2009). Sakamoto et al. (2005) showed that a secondary fungal metabolite (NA255) acts as a new anti‐HCV (hepatitis C virus) replication inhibitor that targets host lipid rafts, suggesting that the inhibition of sphingolipid metabolism may provide a new therapeutic strategy for treatment of HCV infection. In addition, a novel therapeutic strategy, considering the biochemistry of raft—pathogen interaction, called glycolipidomimetics, was proposed by Taïeb et al. (2004).
New concepts in the chemotherapy field interestingly reported the ability to specifically deliver therapeutic agents or drugs to selected cell types, thus minimizing systemic toxicity. This is the principal goal of nanoparticle‐based drug‐delivery approaches. It was reported by Partlow et al. (2008) that the predominant mechanism of direct delivery of lipophilic substances to the target cell plasma membrane acts via lipid mixing and subsequent intracellular trafficking through lipid‐raft‐dependent processes.
Final considerations
Initially, the special regions of plasma membrane, so‐called lipid rafts, were identified by their relative resistance to detergent extraction, namely DRM. However, several articles still refer to membrane microdomains as a DRM; this denomination should be used carefully, considering that this separation method is not completely reliable in unveiling these structures. There is an ongoing controversy regarding the nature and function of lipid rafts, because different experimental approaches have yielded different results. Meanwhile, these substantial experimental data in the literature have provided not only biochemical but also microscopical evidence for the close relationship between different pathogens and lipid rafts. Another important consideration is the now widely accepted view that lipid rafts may not be pre‐existing domains, but dynamic membrane regions that can fuse to each other to form larger rafts or signalling platforms enriched in ceramide (Liu and Anderson, 1995; Holopainen et al., 1998; Bollinger et al., 2005; Plowman et al., 2005). This fusion of small pre‐rafts may be induced in different cell types upon infection by different pathogens. Thus the association of a protein with lipid rafts during cell infection may be a unique event concerning this protein and rafts, which may mean that this association never occurs naturally. So upon the parasite adhesion, this protein is directed to the raft. Similarly, cholesterol is known to be involved in many different cellular functions, and not only the assembly and maintenance of lipid rafts, which leads us to propose that other cellular processes, rather than the disruption or disturbance of lipid rafts, may explain how some pathogens enter and survive within their hosts.
Taken together, the explanations above show us that each pathogen has developed its own strategies to maintain virulence and disseminate the disease. At present, lipid rafts have emerged as a safe entrance door to pathogens. Furthermore, host‐cell raft lipids were seen to be recruited to the invasion loci which may be remodelled by parasites to help in the establishment of the infection. There are at least two major mechanisms involving the host lipid raft by which parasites gain entry to the host cytoplasm and are able to survive: (i) the avoidance of lysosomal fusion and posterior degradation; and (ii) the modulation of host‐cell signalling pathways to its own benefits.
Each year the number of reports implicating new pathogens and their interactions with lipid rafts rapidly increases. New methods and techniques are also helping the researchers to identify, in a more precise way, the interaction of pathogens and lipid rafts. In this regard, biophysical approaches have been increasingly employed to better understand the aspects that are not yet fully elucidated regarding microdomains and pathogen association. In parallel, development of new drus targeting host lipid rafts has been extensively studied, especially for viruses. However, many questions about the immune evasion and infection control yet remain to be answered and further studies are necessary to minimize the historical controversy about the nature and cell biology of the membrane lipid rafts.
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil (CNPq); the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro‐Programa de Apoio a Núcleos de Excelência (FAPERJ/PRONEX); and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Footnotes
Fluid mosaic: A term used by Singer and Nicolson since 1972 to describe the structural features of biological membranes. The plasma membrane was considered fluid because its components, such as lipids and membrane proteins, move laterally or sideways throughout the membrane.
Lipid raft: A cholesterol‐ and sphingolipid‐enriched microdomain or platform found in cell membranes.
Sphingolipids: A class of lipids derived from the aliphatic amino alcohol sphingosine. These compounds play important roles in signal transmission and cell recognition.
Sucrose‐gradient centrifugation: A type of centrifugation often used to purify enveloped viruses and also to separate cell organelles. This method is also used to purify lipid rafts.
Cyclodextrins: Cyclodextrins (cycloamyloses) belong to a family of cyclic oligosaccharides. Methyl‐β‐cyclodextrin is employed for the cholesterol removal of cell membrane.
Filipin: Filipin was isolated from the fungus Streptomyces filipinensis. Often used in cellular biology as an inhibitor of the raft/caveolae endocytosis pathway in mammalian cells.
DRM: Detergent‐resistant membrane was a denomination used for an important early indication that rafts may exist in cells through the observation that cell membranes are not fully solubilized by non‐ionic detergents, such as Triton X‐100, at low temperatures.
FRET: Fluorescence (or Förster) resonance energy transfer is amechanism involving energy transfer between two fluorophores.
AFM: Atomic force microscopy is a very high‐resolution type of scanning probe microscopy, with a nanometre resolution.
Ceramides: A family of lipid molecules. A ceramide is composed of sphingosine and a fatty acid. Ceramides are found in high concentrations within the cell membrane.
GPI anchor: Glycosylphosphatidylinositol is a glycolipid that can be attached to the C‐terminus of a protein during post‐translational modification.
Caveola: A special type of lipid raft, comprising small (50–100 nanometre) invaginations of the plasma membrane, found in many vertebrate cell types.
Caveolins: A family of proteins involved in receptor‐independent endocytosis. The caveolin gene family has three members in vertebrates, caveolin‐1, −2 and −3.
Pathogens: The term pathogen is most commonly used to refer to infectious organisms. These include bacteria, viruses, protozoa and fungi.
Budding: In virology, budding is a form of viral shedding by which enveloped viruses acquire their external envelope from the host‐cell membrane, which bulges outwards and encloses the virion.
Flotillin: Flotillins belong to a family of lipid‐raft‐associated integral membrane proteins. Flotillin members are ubiquitously expressed and located to non‐caveolar microdomains on the cell plasma membrane. Two flotillin members have been described, flotillin‐1 and flotillin‐2.
Stomatin: Stomatin is a 32 kDa integral and lipid‐raft‐associated membrane protein that was first characterized in human red blood cells. Stomatin might play a fundamental role in the control of the surface expression of membrane proteins.
TLRs: Toll‐like receptors are a class of proteins that play a key role in the innate immune system. They are single membrane‐spanning non‐catalytic receptors that recognize structurally conserved molecules derived from microbes.
PAMPs: Pathogen‐associated molecular patterns are the molecules associated with groups of pathogens, which are recognized by cells of the innate immune system. PAMPs are recognized by TLRs.
DAMPs: Damage‐associated molecular pattern molecules can initiate and perpetuate the immune response in the non‐infectious inflammatory response. They serve as a start signal.
P2X receptors: A family of cation‐permeable ligand‐gated ion channels that open in response to the binding of ATP.
P2Y receptors: P2Y receptors are a family of purinergic receptors, and are G‐protein‐coupled receptors stimulated by nucleotides, such as ATP, ADP, UTP, UDP and UDP‐glucose.
Signalling platforms: Lipid rafts have been demonstrated to be aggregated in response to different stimuli. In addition, they play an important role in transmembrane signalling.
References
- Abrami L. Liu S. Cosson P. Leppla S.H. van der Goot F.G. Anthrax toxin triggers endocytosis of its receptor via a lipid raft‐mediated clathrin‐dependent process J. Cell Biol.. 2003. 160 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa M. Miller L.H. Johnson J. Rabbege J. Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite J. Cell Biol.. 1978. 77 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. Russell D.G. The interaction of Leishmania species with macrophages Adv. Parasitol. 1992. 31 175–254. [DOI] [PubMed] [Google Scholar]
- Allison S.L. Stadler K. Mandl C.W. Kunz C. Heinz F.X. Synthesis and secretion of recombinant tick‐borne encephalitis virus protein E in soluble and particulate form J. Virol. 1995. 69 5816–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloia R.C. Tian H. Jensen F.C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes Proc. Natl. Acad. Sci. U.S.A. 1993. 90 5181–5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amer A.O. Byrne B.G. Swanson M.S. Macrophages rapidly transfer pathogens from lipid raft vacuoles to autophagosomes Autophagy 2005. 1 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H.A. Chen Y. Norkin L.C. MHC class I molecules are enriched in caveolae but do not enter with simian virus 40 J. Gen. Virol. 1998. 79 1469–1477. [DOI] [PubMed] [Google Scholar]
- Anderson H.A. Hiltbold E.M. Roche P.A. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation Nat. Immunol. 2000. 1 156–162. [DOI] [PubMed] [Google Scholar]
- Bannas P. Adriouch S. Kahl S. Braasch F. Haag F. Koch‐Nolte F. Activity and specificity of toxin‐related mouse T cell ecto‐ADP‐ribosyltransferase ART2.2 depends on its association with lipid rafts Blood 2005. 105 3663–3670. [DOI] [PubMed] [Google Scholar]
- Barth K. Weinhold K. Guenther A. Young M.T. Schnittler H. Kasper M. Caveolin‐1 influences P2X7 receptor expression and localization in mouse lung alveolar epithelial cells FEBS J. 2007. 274 3021–3033. [DOI] [PubMed] [Google Scholar]
- Basak S.K. Saha B. Bhattacharya A. Roy S. Immunobiological studies on experimental visceral leishmaniasis. II. Adherent cell‐mediated down‐regulation of delayed‐type hypersensitivity response and up‐regulation of B cell activation Eur. J. Immunol. 1992. 22 2041–2045. [DOI] [PubMed] [Google Scholar]
- Baumgartner M. Angelisová P. Setterblad N. Mooney N. Werling D. Horejsí V. Langsley G. Constitutive exclusion of Csk from Hck‐positive membrane microdomains permits Src kinase‐dependent proliferation of Theileria‐transformed B lymphocytes Blood 2003. 101 1874–1881. [DOI] [PubMed] [Google Scholar]
- Bavari S. Bosio C.M. Wiegand E. Ruthel G. Will A.B. Geisbert T.W. Hevey M. Schmaljohn C. Schmaljohn A. Aman M.J. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses J. Exp. Med.. 2002. 195 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger P.H. Johnston D.A. Fratti R.A. Zhang M. Filler S.G. Endocytosis of Candida albicans by vascular endothelial cells is associated with tyrosine phosphorylation of specific host cell proteins Cell. Microbiol. 2002. 4 805–812. [DOI] [PubMed] [Google Scholar]
- Bollinger C.R. Teichgräber V. Gulbins E. Ceramide‐enriched membrane domains Biochim. Biophys. Acta 2005. 30 284–294. [DOI] [PubMed] [Google Scholar]
- Bouillon M. El Fakhry Y. Girouard J. Khalil H. Thibodeau J. Mourad W. Lipid raft‐dependent and ‐independent signaling through HLA‐DR molecules J. Biol. Chem.. 2003. 278 7099–7107. [DOI] [PubMed] [Google Scholar]
- Bours M.J. Swennena E.L.R. Di Virgilio F. Cronsteind B.N. Dagneliea P.C. Adenosine 5‐triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation Pharmacol. Ther.. 2006. 112 358–404. [DOI] [PubMed] [Google Scholar]
- Bron R. Wahlberg J.M. Garoff H. Wilschut J. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein EMBO J. 1993. 12 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.A. Rose J.K. Sorting of GPI‐anchored proteins to glycolipid‐enriched membrane subdomains during transport to the apical cell surface Cell 1992. 68 533–544. [DOI] [PubMed] [Google Scholar]
- Brown G. Aitken J. Rixon H.W. Sugrue R.J. Caveolin‐1 is incorporated into mature respiratory syncytial virus particles during virus assembly on the surface of virus‐infected cells J. Gen. Virol. 2002a. 83 611–621. [DOI] [PubMed] [Google Scholar]
- Brown G. Rixon H.W. Sugrue R.J. Respiratory syncytial virus assembly occurs in GM1‐rich regions of the host‐cell membrane and alters the cellular distribution of tyrosine phosphorylated caveolin‐1 J. Gen. Virol. 2002b. 83 1841–1850. [DOI] [PubMed] [Google Scholar]
- Bruss V. Envelopment of the hepatitis B virus nucleocapsid Virus Res.. 2004. 106 199–209. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic receptors and pain Curr. Pharm. Des.. 2009. 15 1717–1735. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Knight G.E. Cellular distribution and functions of P2 receptor subtypes in different systems Int. Rev. Cytol. 2004. 240 31–304. [DOI] [PubMed] [Google Scholar]
- Campbell S.M. Crowe S.M. Mak J. Lipid rafts and HIV‐1: from viral entry to assembly of progeny virions J. Clin. Virol. 2001. 22 217–227. [DOI] [PubMed] [Google Scholar]
- Carter G.C. Bernstone L. Sangani D. Bee J.W. Harder T. James W. HIV entry in macrophages is dependent on intact lipid rafts Virology 2009. 386 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta J.A. Hale M.L. Popoff M.R. Stiles B.G. McClane B.A. Evidence that membrane rafts are not required for the action of Clostridium perfringens enterotoxin Infect. Immun. 2008. 76 5677–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi C. Nichino D. Zampagni M. Bernacchioni C. Evangelisti E. Pensalfini A. Liguri G. Gliozzi A. Stefani M. Relini A. A protective role for lipid raft cholesterol against amyloid‐induced membrane damage in human neuroblastoma cells Biochim. Biophys. Acta 2009. 1788 2204–2216. [DOI] [PubMed] [Google Scholar]
- Chakraborty D. Banerjee S. Sen A. Banerjee K.K. Das P. Roy S. Leishmania donovani affects antigen presentation of macrophage by disrupting lipid rafts J. Immunol. 2005. 175 3214–3224. [DOI] [PubMed] [Google Scholar]
- Charron A.J. Sibley L.D. Molecular partitioning during host cell penetration by Toxoplasma gondii Traffic 2004. 5 855–867. [DOI] [PubMed] [Google Scholar]
- Chazal N. Gerlier D. Virus entry, assembly, budding, and membrane rafts Microbiol. Mol. Biol. Rev.. 2003. 67 226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B.J. Leser G.P. Jackson D. Lamb R.A. The influenza virus M2 protein cytoplasmic tail interacts with the M1 protein and influences virus assembly at the site of virus budding J. Virol. 2008. 82 10059–10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P.C. Dykstra M.L. Mitchell R.N. Pierce S.K. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting J. Exp. Med.. 1999. 190 1549–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coconnier M.H. Lorrot M. Barbat A. Laboisse C. Servin A.L. Listeriolysin O‐induced stimulation of mucin exocytosis in polarized intestinal mucin‐secreting cells: evidence for toxin recognition of membrane‐associated lipids and subsequent toxin internalization through caveolae Cell. Microbiol. 2000. 2 487–504. [DOI] [PubMed] [Google Scholar]
- Cohen A.W. Hnasko R. Schubert W. Lisanti M.P. Role of caveolae and caveolins in health and disease Physiol. Rev.. 2004. 84 1341–1379. [DOI] [PubMed] [Google Scholar]
- Coppens I. Joiner K.A. Host but not parasite cholesterol controls Toxoplasma cell entry by modulating organelle discharge Mol. Biol. Cell 2003. 14 3804–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart P. Pizarro‐Cerdá J. Lecuit M. Invasion of mammalian cells by Listeria monocytogenes: functional mimicry to subvert cellular functions Trends Cell Biol.. 2003. 13 23–31. [DOI] [PubMed] [Google Scholar]
- Coutinho‐Silva R. Corrêa G. Sater A.A. Ojcius D.M. The P2X7 receptor and intracellular pathogens: a continuing struggle Purinergic Signal. 2009. 5 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho‐Silva R. da Cruz C. Monteiro Persechini P.M. Ojcius D.M. The role of P2 receptors in controlling infections by intracellular pathogens Purinergic Signal. 2007. 3 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri J. Billigren J. Maier R. Endotoxin tolerance attenuates LPS‐induced TLR4 mobilization to lipid rafts: a condition reversed by PKC activation J. Leukoc. Biol.. 2006. 80 1289–1297. [DOI] [PubMed] [Google Scholar]
- Darveau R.P. Bacteria modulate host‐cell responses by capitalizing on the lipid raft structure Future Microbiol. 2009. 4 155–157. [DOI] [PubMed] [Google Scholar]
- Denlinger L.C. Fisette P.L. Sommer J.A. Watters J.J. Prabhu U. Dubyak G.R. Proctor R.A. Bertics P.J. Cutting edge: the nucleotide receptor P2X7 contains multiple protein‐ and lipid‐interaction motifs including a potential binding site for bacterial lipopolysaccharide J. Immunol. 2001. 167 1871–1876. [DOI] [PubMed] [Google Scholar]
- Desplanques A.S. Nauwynck H.J. Vercauteren D. Geens T. Favoreel H.W. Plasma membrane cholesterol is required for efficient pseudorabies virus entry Virology 2008. 376 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F. Purinergic mechanism in the immune system: a signal of danger for dendritic cells Purinergic Signal. 2005. 1 205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbelaere D.A. Fernandez P.C. Heussler V.T. Theileria parva: taking control of host cell proliferation and survival mechanisms Cell. Microbiol. 2000. 2 91–99. [DOI] [PubMed] [Google Scholar]
- Duncan M.J. Shin J.S. Abraham S.N. Microbial entry through caveolae: variations on a theme Cell. Microbiol. 2002. 4 783–791. [DOI] [PubMed] [Google Scholar]
- Edidin M. The state of lipid rafts: from model membranes to cells Annu. Rev. Biophys. Biomol. Struct. 2003. 32 257–283. [DOI] [PubMed] [Google Scholar]
- Empig C.J. Goldsmith M.A. Association of the caveola vesicular system with cellular entry by filoviruses J. Virol. 2002. 76 5266–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D.M. Membranes are more mosaic than fluid Nature 2005. 438 578–580. [DOI] [PubMed] [Google Scholar]
- Fantini J. Garmy N. Mahfoud R. Yahi N. Lipid rafts: structure, function and role in HIV, Alzheimer's and prion diseases Expert Rev. Mol. Med.. 2002. 4 1–22. [DOI] [PubMed] [Google Scholar]
- Gao X. Zhang J. Spatio temporal analysis of differential Akt regulation in plasma membrane microdomains Mol. Biol. Cell 2008. 10 4366–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Marcos M. Dehaye J.P. Marino A. Studying the role of plasma membrana microdomains in the modulation of P2XR‐mediated signalling FEBS J. 2009. 276 330–340. [DOI] [PubMed] [Google Scholar]
- García‐Marcos M. Pochet S. Tandel S. Fontanils U. Astigarraga E. Fernández‐González J.A. Kumps A. Marino A. Dehaye J.P. Characterization and comparision of raft‐like membranes isolated by two different methods from rat submandibular gland cells Biochim. Biophys. Acta 2006. 1758 796–806. [DOI] [PubMed] [Google Scholar]
- Garoff H. Simons K. Location of the spike glycoproteins in the Semliki Forest virus membrane Proc. Natl. Acad. Sci. U.S.A. 1974. 71 3988–3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H. Hewson R. Opstelten D.J. Virus maturation by budding Microbiol. Mol. Biol. Rev.. 1998. 62 1171–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatfield J. Pieters J. Essential role for cholesterol in entry of mycobacteria into macrophages Science 2000. 288 1647–1650. [DOI] [PubMed] [Google Scholar]
- Grimmer S. van Deurs B. Sandvig K. Membrane ruffling and macropinocytosis in A431 cells require cholesterol J. Cell Sci.. 2002. 4 772–784. [DOI] [PubMed] [Google Scholar]
- Gulbins E. Dreschers S. Wilker B. Grassmé H. Ceramide, membrane rafts and infections J. Mol. Med.. 2004. 82 357–363. [DOI] [PubMed] [Google Scholar]
- Ha H. Kwak H.B. Lee S.K. Na D.S. Rudd C.E. Lee Z.H. Kim H.H. Membrane rafts play a crucial role in receptor activator of nuclear factor κB signaling and osteoclast function J. Biol. Chem.. 2003. 278 18573–18580. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. Wang M. Harokopakis E. Triantafilou M. Triantafilou K. Porphyromonas gingivalis fimbriae proactively modulate β2 integrin adhesive activity and promote binding to and internalization by macrophages Infect. Immun. 2006. 74 5658–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar K. Mohandas N. Samuel B.U. Harrison T. Hiller N.L. Akompong T. Cheresh P. Protein and lipid trafficking induced in erythrocytes infected by malaria parasites Cell. Microbiol. 2002. 4 383–395. [DOI] [PubMed] [Google Scholar]
- Hall B.F. Joiner K.A. Developmentally‐regulated virulence factors of Trypanosoma cruzi and their relationship to evasion of host defences J. Eukaryot. Microbiol. 1993. 40 207–213. [DOI] [PubMed] [Google Scholar]
- Harris T.J. Awrey D.E. Cox B.J. Ravandi A. Tsang A. Siu C.H. Involvement of a Triton‐insoluble floating fraction in Dictyostelium cell—cell adhesion J. Biol. Chem.. 2001. 276 18640–18648. [DOI] [PubMed] [Google Scholar]
- Hawkes D.J. Mak J. Lipid membrane, a novel target for viral and bacterial pathogens Curr. Drug Targets 2006. 7 1615–1621. [DOI] [PubMed] [Google Scholar]
- Hayward R.D. Cain R.J. McGhie E.J. Phillips N. Garner M.J. Koronakis V. Cholesterol binding by the bacterial type III translocon is essential for virulence effector delivery into mammalian cells Mol. Microbiol. 2005. 56 590–603. [DOI] [PubMed] [Google Scholar]
- Henderson R.M. Edwardson J.M. Geisse N.A. Saslowsky D.E. Lipid rafts: feeling is believing News Physiol. Sci.. 2004. 19 39–43. [DOI] [PubMed] [Google Scholar]
- Hirao M. Sato N. Kondo T. Yonemura S. Monden M. Sasaki T. Takai Y. Tsukita S. Tsukita S. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho‐dependent signaling pathway J. Cell Biol.. 1996. 135 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen J.M. Subramanian M. Kinnunen P.K. Sphingomyelinase induces lipid microdomain formation in a fluid phosphatidylcholine/sphingomyelin membrane Biochemistry 1998. 15 17562–17570. [DOI] [PubMed] [Google Scholar]
- Hommelgaard A.M. Roepstorff K. Vilhardt F. Torgersen M.L. Sandvig K. van Deurs B. Caveolae: stable membrane domains with a potential for internalization Traffic 2005. 6 720–724. [DOI] [PubMed] [Google Scholar]
- Howe D. Heinzen R.A. Coxiella burnetii inhabits a cholesterol‐rich vacuole and influences cellular cholesterol metabolism Cell. Microbiol. 2006. 8 496–507. [DOI] [PubMed] [Google Scholar]
- Hu Y. Fisette P.L. Denlinger L.C. Guadarrama A.G. Sommer J.A. Proctor R.A. Bertics P.J. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric‐oxide synthase expression in RAW 264.7 macrophages J. Biol. Chem.. 1998. 273 27170–27175. [DOI] [PubMed] [Google Scholar]
- Ivanchenko S. Godinez W.J. Lampe M. Kräusslich H.G. Eils R. Rohr K. Bräuchle C. Müller B. Lamb D.C. Dynamics of HIV‐1 assembly and release PLoS Pathog. 2009. 5 e1000652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. Mouritsen O.G. Anderson R.G. Lipid rafts: at a crossroad between cell biology and physics Nat. Cell Biol.. 2007. 9 7–14. [DOI] [PubMed] [Google Scholar]
- de Bagues M.P. Jimenez Dudal S. Dornand J. Gross A. Cellular bioterrorism: how Brucella corrupts macrophage physiology to promote invasion and proliferation Clin. Immunol. 2005. 114 227–238. [DOI] [PubMed] [Google Scholar]
- Kalischuk L.D. Inglis G.D. Buret A.G. Campylobacter jejuni induces transcellular translocation of commensal bacteria via lipid rafts Gut Pathog. 2009. 1 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R.D. Kleinfeld A.M. Hoover R.L. Karnovsky M.J. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis J. Biol. Chem.. 1980. 255 1286–1295. [PubMed] [Google Scholar]
- Kovbasnjuk O. Edidin M. Donowitz M. Role of lipid rafts in Shiga toxin 1 interaction with the apical surface of Caco‐2 cells J. Cell Sci.. 2001. 114 4025–4031. [DOI] [PubMed] [Google Scholar]
- Kusumi A. Koyama‐Honda I. Suzuki K. Molecular dynamics and interactions for creation of stimulation‐induced stabilized rafts from small unstable steady‐state rafts Traffic 2004. 5 213–230. [DOI] [PubMed] [Google Scholar]
- Lafont F. van der Goot F.G. Oiling the key hole Mol. Microbiol. 2005. 56 575–577. [DOI] [PubMed] [Google Scholar]
- Lafont F. van Nhieu G. Tran Hanada K. Sansonetti P. van der Goot F.G. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft‐mediated CD44‐IpaB interaction EMBO J. 2002. 21 4449–4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai E.C. Lipid rafts make for slippery platforms J. Cell Biol.. 2003. 162 365–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C.H. Chang Y.C. Du S.Y. Wang H.J. Kuo C.H. Fang S.H. Fu H.W. Lin H.H. Chiang A.S. Wang W.C. Cholesterol depletion reduces Helicobacter pylori CagA translocation and CagA‐induced responses in AGS cells Infect. Immun. 2008. 76 3293–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie P. Nabi I.R. Regulation of raft‐dependent endocytosis J. Cell Mol. Med.. 2007. 11 644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie P. Goetz J.G. Dennis J.W. Nabi I.R. Lattices, rafts, and scaffolds: domain regulation of receptor signaling at the plasma membrane J. Cell Biol.. 2009. 185 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauer S. VanWye J. Harrison T. McManus H. Samuel B.U. Hiller N.L. Mohandas N. Haldar K. Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection EMBO J. 2000. 19 3556–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin R.C. McGugan G.C. Powell R.R. Welter B.H. Temesvari L.A. Involvement of raft‐like plasma membrane domains of Entamoeba histolytica in pinocytosis and adhesion Infect. Immun. 2004. 72 5349–5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M.P. Scherer P.E. Vidugiriene J. Tang Z.L. Hermanoski‐Vosatka A. Tu Y.H. Cook R.F. Sargiacomo M. Characterization of caveolin‐rich membrane domains isolated from an endothelial‐rich source: implications for human disease J. Cell Biol.. 1994. 126 111–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. Anderson R.G. Compartmentalized production of ceramide at the cell surface J. Biol. Chem.. 1995. 270 27179–27185. [DOI] [PubMed] [Google Scholar]
- Lu X. Silver J. Ecotropic murine leukemia virus receptor is physically associated with caveolin and membrane rafts Virology 2000. 276 251–258. [DOI] [PubMed] [Google Scholar]
- Lu Y.E. Cassese T. Kielian M. The cholesterol requirement for sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence J. Virol. 1999. 73 4272–4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. Liu D.X. Tama J.P. Lipid rafts are involved in SARS‐CoV entry into Vero E6 cells Biochem. Biophys. Res. Commun. 2008. 369 344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luker G.D. Pica C.M. Kumar A.S. Covey D.F. Piwnica‐Worms D. Effects of cholesterol and enantiomeric cholesterol on P‐glycoprotein localization and function in low‐density membrane domains Biochemistry 2000. 39 7651–7661. [DOI] [PubMed] [Google Scholar]
- Luo C. Wang K. de Liu Q. Li Y. Zhao Q.S. The functional roles of lipid rafts in T cell activation, immune diseases and HIV infection and prevention Cell Mol. Immunol. 2008. 5 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mañes S. Mira E. Gómez‐Moutón C. Lacalle R.A. Keller P. Labrador J.P. Martínez‐A C. Membrane raft microdomains mediate front‐rear polarity in migrating cells EMBO J. 1999. 18 6211–6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mañes S. del Real G. Martínez‐A C. Pathogens: raft hijackers Nat. Rev. Immunol. 2003. 3 557–568. [DOI] [PubMed] [Google Scholar]
- Manie S.N. Debreyne S. Vincent S. Gerlier D. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly J. Virol. 2000. 74 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjomaki V. Pietiainen V. Matilainen H. Upla P. Ivaska J. Nissinen L. Reunanen H. Huttunen P. Hyypia T. Heino J. Internalization of echovirus 1 in caveolae J. Virol. 2002. 76 1856–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Belmonte F. Alonso M.A. Zhang X. Arvan P. Thyroglobulin is selected as luminal protein cargo for apical transport via detergent‐resistant membranes in epithelial cells J. Biol. Chem.. 2000. 275 41074–41081. [DOI] [PubMed] [Google Scholar]
- Mastick C.C. Saltiel A.R. Insulin‐stimulated tyrosine phosphorylation of caveolin is specific for the differentiated adipocyte phenotype in 3T3‐L1 cells J. Biol. Chem.. 1997. 272 20706–20714. [DOI] [PubMed] [Google Scholar]
- Maza P.K. Straus A.H. Toledo M.S. Takahashi H.K. Suzuki E. Interaction of epithelial cell membrane rafts with Paracoccidioides brasiliensis leads to fungal adhesion and Src‐family kinase activation Microbes Infect. 2008. 10 540–547. [DOI] [PubMed] [Google Scholar]
- Minshall R.D. Sessa W.C. Stan R.V. Anderson R.G. Malik A.B. Caveolin regulation of endothelial function Am. J. Physiol. Lung Cell. Mol. Physiol. 2003. 285 L1179–L1183. [DOI] [PubMed] [Google Scholar]
- Monks C.R. Freiberg B.A. Kupfer H. Sciaky N. Kupfer A. Three‐dimensional segregation of supramolecular activation clusters in T cells Nature 1998. 166 4773–4779. [DOI] [PubMed] [Google Scholar]
- da Silva J.L. Monteiro Andreotti P.F. Benard G. Soares C.P. Miranda E.T. Mendes‐Giannini M.J. Epithelial cells treated with genistein inhibit adhesion and endocytosis of Paracoccidioides brasiliensis Antonie Van Leeuwenhoek 2007. 92 129–135. [DOI] [PubMed] [Google Scholar]
- Montixi C. Langlet C. Bernard A.M. Thimonier J. Dubois C. Wurbel M.A. Chauvin J.P. Pierres M. He H.T. Engagement of T cell receptor triggers its recruitment to low‐density detergent‐insoluble membrane domains EMBO J. 1998. 17 5334–5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordue D.G. Desai N. Dustin M. Sibley L.D. Invasion by Toxoplasma gondii establishes a moving junction that selectively excludes host cell plasma membrane proteins on the basis of their membrane anchoring J. Exp. Med.. 1999. 190 1783–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münter S. Way M. Frischknecht F. Signaling during pathogen infection Sci. STKE. 2006. 335 re5. [DOI] [PubMed] [Google Scholar]
- Murata M. Peranen J. Schreiner R. Weiland F. Kurzchalia T. Simons K. VIP21/caveolin is a cholesterol‐binding protein Proc. Natl. Acad. Sci. U.S.A. 1995. 92 10339–10343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S.C. Fernandez‐Pol S. Chung P.H. Murthy S.N. Prasanna Milne S.B. Salomao M. Brown H.A. Lomasney J.W. Mohandas N. Haldar K. Cytoplasmic remodeling of erythrocyte raft lipids during infection by the human malaria parasite Plasmodium falciparum Blood 2007. 110 2132–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naroeni A. Porte F. Role of cholesterol and the ganglioside GM1 in entry and short‐term survival of Brucella suis in murine macrophage Infect. Immun. 2002. 70 640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D.P. Hui E.K. Barman S. Assembly and budding of influenza virus Virus Res.. 2004. 106 147–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norambuena A. Poblete M.I. Donoso M.V. Espinoza C.S. González A. Huidobro‐Toro J.P. P2Y1 receptor activation elicits its partition out of membrane rafts and its rapid internalization from human blood vessels: implications for receptor signaling Mol. Pharmacol. 2008. 74 1666–1677. [DOI] [PubMed] [Google Scholar]
- Norkin L.C. Wolfrom S.A. Stuart E.S. Association of caveolin with Chlamydia trachomatis inclusions at early and late stages of infection Exp. Cell Res.. 2001. 266 229–238. [DOI] [PubMed] [Google Scholar]
- Norkin L.C. Anderson H.A. Wolfrom S.A. Oppenheim A. Caveolar endocytosis of simian virus 40 is followed by brefeldin A‐sensitive transport to the endoplasmic reticulum, where the virus disassembles J. Virol. 2002. 76 5156–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T. Schlegel A. Scherer P.E. Lisanti M.P. Caveolins, a family of scaffolding proteins for organizing ‘preassembled signaling complexes’ at the plasma membrane J. Biol. Chem.. 1998. 273 5419–5422. [DOI] [PubMed] [Google Scholar]
- Olsson S. Sundler R. The role of lipid rafts in LPS‐induced signaling in a macrophage cell line Mol. Immunol. 2006. 43 607–612. [DOI] [PubMed] [Google Scholar]
- Orlandi P.A. Fishman P.H. Filipin‐dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae‐like domains J. Cell Biol.. 1998. 141 905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G.E. Fine structure of blood capillaries J. Appl. Physiol 1953. 24 1424–1436. [Google Scholar]
- Parson R.D. Wheeler D.A. Harrison L.H. Kay H.D. The immunobiology of leishmaniasis Rev. Infect. Dis.. 1983. 5 907–927. [DOI] [PubMed] [Google Scholar]
- Partlow K.C. Lanza G.M. Wickline S.A. Exploiting lipid raft transport with membrane targeted nanoparticles: a strategy for cytosolic drug delivery Biomaterials 2008. 29 3367–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer I. Servin A.L. Bernet‐Camard M.F. Piracy of decay‐accelerating factor (CD55) signal transduction by the diffusely adhering strain Escherichia coli C1845 promotes cytoskeletal F‐actin rearrangements in cultured human intestinal INT407 cells Infect. Immun. 1998. 66 4036–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkmans L. Puntener D. Helenius A. Local actin polymerization and dynamin recruitment in SV40‐induced internalization of caveolae Science 2002. 296 535–539. [DOI] [PubMed] [Google Scholar]
- Perregaux D.G. McNiff P. Laliberte R. Conklyn M. Gabel C.A. ATP acts as an agonist to promote stimulus‐induced secretion of IL‐1b and IL‐18 in human blood J. Immunol. 2000. 165 4615–4623. [DOI] [PubMed] [Google Scholar]
- Peterlin B. Trono D. Hide, shield and strike back: how HIV‐infected cells avoid immune eradication Nat. Rev. Immunol. 2003. 3 97–107. [DOI] [PubMed] [Google Scholar]
- Pickl W.F. Pimentel‐Muinos F.X. Seed B. Lipid rafts and pseudotyping J. Virol. 2001. 75 7175–7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierini L.M. Eddy R.J. Fuortes M. Seveau S. Casulo C. Maxfield F.R. Membrane lipid organization is critical for human neutrophil polarization J. Biol. Chem.. 2003. 278 10831–10841. [DOI] [PubMed] [Google Scholar]
- Pike L.J. Lipid rafts: bringing order to chaos J. Lipid Res.. 2003. 44 655–667. [DOI] [PubMed] [Google Scholar]
- Pike L.J. The challenge of lipid rafts J. Lipid Res.. 2009. 50 S323–S328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plowman S.J. Muncke C. Parton R.G. Hancock J.F. H‐ras, K‐ras, and inner plasma membrane raft proteins operate in nanoclusters with differential dependence on the actin cytoskeleton Proc. Natl. Acad. Sci. U.S.A. 2005. 102 15500–15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloso N.J. Roche P.A. Association of MHC class II‐peptide complexes with plasma membrane lipid microdomains Curr. Opin. Immunol. 2004. 16 103–107. [DOI] [PubMed] [Google Scholar]
- Poole K. Meder D. Simons K. Müller D. The effect of raft lipid depletion on microvilli formation in MDCK cells, visualized by atomic force microscopy FEBS Lett.. 2004. 565 53–58. [DOI] [PubMed] [Google Scholar]
- Popik W. Alce T.M. Au W.C. Human immunodeficiency virus type 1 uses lipid raft‐colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells J. Virol. 2002. 76 4709–4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prina E. Jouanne C. de Lão S. Souza Szabo A. Guillet J.G. Antoine J.C. Antigen presentation capacity of murine macrophages infected with Leishmania amazonensis amastigotes J. Immunol. 1993. 151 2050–2061. [PubMed] [Google Scholar]
- Pucadyil T.J. Tewary P. Madhubala R. Chattopadhyay A. Cholesterol is required for Leishmania donovani infection: implications in leishmaniasis Mol. Biochem. Parasitol. 2004. 133 145–152. [DOI] [PubMed] [Google Scholar]
- Rao M. Mayor S. Use of Forster's resonance energy transfer microscopy to study lipid rafts Biochim. Biophys. Acta 2005. 1746 221–233. [DOI] [PubMed] [Google Scholar]
- Raulin J. Human immunodeficiency virus and host cell lipids. Interesting pathways in research for a new HIV therapy Prog. Lipid Res.. 2002. 41 27–65. [DOI] [PubMed] [Google Scholar]
- Raymond M.N. Le Stunff H. Involvement of de novo ceramide biosynthesis in macrophage death induced by activation of ATP‐sensitive P2X7 receptor FEBS Lett.. 2006. 580 131–136. [DOI] [PubMed] [Google Scholar]
- Razani B. Woodman S.E. Lisanti M.P. Caveolae: from cell biology to animal physiology Pharmacol. Rev.. 2002. 54 431–467. [DOI] [PubMed] [Google Scholar]
- Richterová Z. Liebl D. Horák M. Palková Z. Stokrová J. Hozák P. Korb J. Forstová J. Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VP1 pseudocapsids toward cell nuclei J. Virol. 2001. 75 10880–10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnebäumer K. Gross U. Bohne W. The nascent parasitophorous vacuole membrane of Encephalitozoon cuniculi is formed by host cell lipids and contains pores which allow nutrient uptake Eukaryot. Cell 2008. 7 1001–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K.G. Heuser J.E. Donzell W.C. Ying Y.S. Glenney J.R. Anderson R.G. Caveolin, a protein component of caveolae membrane coats Cell 1992. 68 673–682. [DOI] [PubMed] [Google Scholar]
- Saha B. Das G. Vohra H. Ganguly N.K. Mishra G.C. Macrophage‐T cell interaction in experimental visceral leishmaniasis: failure to express costimulatory molecules on Leishmania‐infected macrophages and its implication in the suppression of cell‐mediated immunity Eur. J. Immunol. 1995. 25 2492–2498. [DOI] [PubMed] [Google Scholar]
- Sakamoto H. Okamoto K. Aoki M. Kato H. Katsume A. Ohta A. Tsukuda T. Shimma N. Aoki Y. Arisawa M. Kohara M. Sudoh M. Host sphingolipid biosynthesis as a target for hepatitis C virus therapy Nat. Chem. Biol.. 2005. 1 333–337. [DOI] [PubMed] [Google Scholar]
- Samuel B.U. Mohandas N. Harrison T. McManus H. Rosse W. Reid M. Haldar K. The role of cholesterol and glycosylphosphatidylinositol‐anchored proteins of erythrocyte rafts in regulating raft protein content and malarial infection J. Biol. Chem.. 2001. 276 29319–29329. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M. Sudol M. Tang Z.L. Lisanti M.P. Signal transducing molecules and GPI‐linked proteins form a caveolin‐rich insoluble complex in MDCK cells J. Cell Biol.. 1993. 122 789–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P. Roth M.G. Simons K. Interaction of influenza virus haemagglutinin with sphingolipid‐cholesterol membrane domains via its transmembrane domain EMBO J. 1997. 16 5501–5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J.E. Oh P. Pinney E. Allard J. Filipin‐sensitive caveolae‐mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules J. Cell Biol.. 1994. 127 1217–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen E. Chattopadhyay S. Bandopadhyay S. De T. Roy S. Macrophage heterogeneity, antigen presentation, and membrane fluidity: implications in visceral Leishmaniasis Scand. J. Immunol. 2001. 53 111–120. [DOI] [PubMed] [Google Scholar]
- Seveau S. Bierne H. Giroux S. Prévost M.C. Cossart P. Role of lipid rafts in E‐cadherin‐ and HGF‐R/Met‐mediated entry of Listeria monocytogenes into host cells J. Cell Biol.. 2004. 166 743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A.S. Lipid rafts: now you see them, now you don't Nat. Immunol. 2006. 7 1139–1142. [DOI] [PubMed] [Google Scholar]
- Shin J.S. Gao Z. Abraham S.N. Involvement of cellular caveolae in bacterial entry into mast cells Science 2000. 289 785–788. [DOI] [PubMed] [Google Scholar]
- Shin D.M. Yang C.S. Lee J.Y. Lee S.J. Choi H.H. Lee H.M. Yuk J.M. Harding C.V. Jo E.K. Mycobacterium tuberculosis lipoprotein‐induced association of TLR2 with protein kinase C zeta in lipid rafts contributes to reactive oxygen species‐dependent inflammatory signalling in macrophages Cell. Microbiol. 2008. 10 1893–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K. Ikonen E. Functional rafts in cell membranes Nature 1997. 387 569–572. [DOI] [PubMed] [Google Scholar]
- Singer S.J. Nicolson G.L. The fluid mosaic model of the structure of cell membranes Science 1972. 175 720–731. [DOI] [PubMed] [Google Scholar]
- Smit J.M. Bittman R. Wilschut J. Low‐pH‐dependent fusion of Sindbis virus with receptor‐free cholesterol‐ and sphingolipid‐containing liposomes J. Virol. 1999. 73 8476–8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut M. van Deurs B. Identification of filamin as a novel ligand for caveolin‐1: evidence for the organization of caveolin‐1‐associated membrane domains by the actin cytoskeleton Mol. Biol. Cell 2000. 11 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang E. Kartenbeck J. Parton R.G. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae Mol. Biol. Cell 1997. 8 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart E.S. Webley W.C. Norkin L.C. Lipid rafts, caveolae, caveolin‐1, and entry by Chlamydiae into host cells Exp. Cell Res.. 2003. 287 67–78. [DOI] [PubMed] [Google Scholar]
- Suomalainen M. Lipid rafts and assembly of enveloped viruses Traffic 2002. 3 705–709. [DOI] [PubMed] [Google Scholar]
- Suss‐Toby E. Zimmerberg J. Ward G.E. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore Proc. Natl. Acad. Sci. U.S.A. 1996. 93 8413–8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taïeb N. Yahi N. Fantini J. Rafts and related glycosphingolipid‐enriched microdomains in the intestinal epithelium: bacterial targets linked to nutrient absorption Adv. Drug Deliv. Rev.. 2004. 56 779–794. [DOI] [PubMed] [Google Scholar]
- Tamilselvam B. Daefler S. Francisella targets cholesterol‐rich host cell membrane domains for entry into macrophages J. Immunol. 2008. 180 8262–8271. [DOI] [PubMed] [Google Scholar]
- Tortelote G.G. Valverde R.H. Lemos T. Guilherme A. Einicker‐Lamas M. Vieyra A. The plasma membrane Ca2+ pump from proximal kidney tubules is exclusively localized and active in caveolae FEBS Lett.. 2004. 576 31–35. [DOI] [PubMed] [Google Scholar]
- Triantafilou M. Miyake K. Golenbock D.T.K. Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide‐induced cell activation J. Cell Sci.. 2002. 115 2603–2611. [DOI] [PubMed] [Google Scholar]
- Triantafilou M. Gamper F.G. Lepper P.M. Mouratis M.A. Schumann C. Harokopakis E. Schifferle R.E. Hajishengallis G. Triantafilou K. Lipopolysaccharides from atherosclerosis‐associated bacteria antagonize TLR4, induce formation of TLR2/1/CD36 complexes in lipid rafts and trigger TLR2‐induced inflammatory responses in human vascular endothelial cells Cell. Microbiol. 2007. 9 2030–2039. [DOI] [PubMed] [Google Scholar]
- Ushio‐Fukai M. Hilenski L. Santanam N. Becker P.L. Ma Y. Griendling K.K. Alexander R.W. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol‐rich microdomains and focal adhesions in angiotensin II signaling J. Biol. Chem.. 2001. 276 48269–48275. [DOI] [PubMed] [Google Scholar]
- Vacca F. Amadio S. Sancesario G. Bernardi G. Volonté C. P2X3 receptor localizes into lipid rafts in neuronal cells J. Neurosci. Res.. 2004. 76 653–661. [DOI] [PubMed] [Google Scholar]
- van der Goot F.G. van Nhieu G. Tran Allaoui A. Sansonetti P. Lafont F. Rafts can trigger contact‐mediated secretion of bacterial effectors via a lipid‐based mechanism J. Biol. Chem.. 2004. 279 47792–47798. [DOI] [PubMed] [Google Scholar]
- Vanderplasschen A. Mathew E. Hollinshead M. Sim R. Smith G. Extracellular enveloped vaccinia virus is resistant to complement control proteins into its envelope Proc. Natl. Acad. Sci. U.S.A. 1998. 95 7544–7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G. Simons K. An efficient method for introducing defined lipids into the plasma membrane of mammalian cells J. Cell Biol.. 1983. 97 1365–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H. Godeke G.J. Rossen J.W. Voorhout W.F. Horzinek M.C. Opstelten D.J. Rottier P.J. Nucleocapsidindependent assembly of coronavirus‐like particles by coexpression of viral envelope protein genes EMBO J. 1996. 15 2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S.P. HIV: a raft‐targeting approach for prevention and therapy using plant‐derived compounds Curr. Drug Targets 2009. 10 51–59. [DOI] [PubMed] [Google Scholar]
- Vial C. Evans R.J. Disruption of lipid rafts inhibits P2X1 receptor‐mediated currents and arterial vasoconstriction J. Biol. Chem.. 2005. 280 30705–30711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S. Gerlier D. Manie S.N. Measles virus assembly within membrane rafts J. Virol. 2000. 74 9911–9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Fang S. Xiao H. Chen B. Tam J.P. Liu D.X. Interaction of the coronavirus infectious bronchitis virus membrane protein with β‐actin and its implication in virion assembly and budding PLoS One 2009. 4 e4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. Hajishengallis G. Lipid raft‐dependent uptake, signalling and intracellular fate of Porphyromonas gingivalis in mouse macrophages Cell. Microbiol. 2008. 10 2029–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai M. Derre I. Kirby J. Growney J.D. Dietrich W.F. Isberg R.R. Legionella pneumophila is internalized by a macropinocytotic uptake pathway controlled by the Dot/Icm system and the mouse Lgn1 locus J. Exp. Med.. 2001. 194 1081–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai M. Makino S. Michikawa M. Yanagisawa K. Murakami S. Shirahata T. Macrophage plasma membrane cholesterol contributes to Brucella abortus infection of mice Infect. Immun. 2002. 70 4818–4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watarai M. Interaction between Brucella abortus and cellular prion protein in lipid raft microdomains Microbes Infect. 2004. 6 93–100. [DOI] [PubMed] [Google Scholar]
- Webley W.C. Norkin L.C. Stuart E.S. Caveolin‐2 associates with intracellular chlamydial inclusions independently of caveolin‐1 BMC Infect. Dis.. 2004. 4 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg M.E. Holm A. Särndahl E. Vinet A.F. Descoteaux A. Magnusson K.E. Rasmusson B. Lerm M. Leishmania donovani lipophosphoglycan inhibits phagosomal maturation via action on membrane rafts Microbes Infect. 2009. 11 215–222. [DOI] [PubMed] [Google Scholar]
- Wooldridge K.G. Williams P.H. Ketley J.M. Host signal transduction and endocytosis of Campylobacter jejuni Microb. Pathog. 1996. 21 299–305. [DOI] [PubMed] [Google Scholar]
- Yamada E. The fine structure of the gall bladder epithelium of the mouse J. Biophys. Biochem. Cytol. 1955. 1 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]