Summary
A joint working group established by the Haemato‐oncology subgroup of the British Committee for Standards in Haematology, the British Society for Bone Marrow Transplantation and the UK Clinical Virology Network has reviewed the available literature and made recommendations for the diagnosis and management of respiratory viral infections in patients with haematological malignancies or those undergoing haematopoietic stem cell transplantation. This guideline includes recommendations for the diagnosis, prevention and treatment of respiratory viral infections in adults and children. The suggestions and recommendations are primarily intended for physicians practising in the United Kingdom.
Keywords: influenza, respiratory syncytial virus, metapneumovirus, parainfluenza, stem cell transplant
A joint working group established by the Haemato‐oncology subgroup of the British Committee for Standards in Haematology (BCSH), the British Society of Blood and Marrow Transplantation (BSBMT) and the UK Clinical Virology Network has reviewed the available literature and made recommendations for the diagnosis, prevention and management of common respiratory viral infections in adults and children undergoing treatment for haematological malignancies or haematopoietic stem cell transplantation (HSCT). This guideline will focus on the following respiratory viral infections: influenza, respiratory syncytial virus (RSV), human metapneumovirus (HMPV), human parainfluenza virus (HPIV) and human rhinovirus (HRV). The management of adenovirus and opportunistic infections (notably cytomegalovirus, herpes simplex virus and varicella zoster virus), which may involve the respiratory tract, is beyond the scope of this manuscript and will not be discussed. Human coronavirus and other community acquired respiratory viruses, including bocavirus, will not be discussed due to the lack of available data in this area.
The key areas covered in this guideline are:
Diagnosis
Prevention
Treatment
Methodology
The production of these guidelines involved the following steps:
Establishment of a working group comprising experts in the field of virology, haemato‐oncology and HSCT followed by literature review to 6 February 2015. Medline was searched systematically for publications in English using the following key words: respiratory viral infection, stem cell transplant, chemotherapy, human rhinovirus (HRV), human metapneumovirus (HMPV), respiratory syncytial virus (RSV), human parainfluenza (HPIV), influenza, diagnosis, prevention, treatment. The reports of major conferences, including the American Society of Haematology and European Bone Marrow Transplant annual meetings, were also reviewed to 6 February 2015 using the same keywords.
Development of key recommendations based on randomized, controlled trial evidence. Due to the paucity of randomized studies some recommendations are based on literature review and a consensus of expert opinion.
The GRADE nomenclature was used to evaluate levels of evidence and to assess the strength of recommendations. The GRADE criteria are specified in the BCSH guideline pack and the GRADE working group website. Further information is available below and from the following websites:
Review by the BCSH committees, BSBMT executive committee, the UK Clinical Virology Network and the UK Paediatric Bone Marrow Transplant group.
Review by sounding board of the British Society for Haematology (BSH) and allogeneic transplant centres in the UK.
GRADE nomenclature for assessing levels of evidence and providing recommendations in guidelines
Strength of recommendations
Strong (grade 1): Strong recommendations (grade 1) are made when there is confidence that the benefits do or do not outweigh harm and burden. Grade 1 recommendations can be applied uniformly to most patients. Regard as ‘recommend’.
Weak (grade 2): Where the magnitude of benefit or not is less certain, a weaker grade 2 recommendation is made. Grade 2 recommendations require judicious application to individual patients. Regard as ‘suggest’.
Quality of evidence
The quality of evidence is graded as high (A), moderate (B) or low (C). To put this in context it is useful to consider the uncertainty of knowledge and whether further research could change what we know or our certainty.
(A) High: Further research is very unlikely to change confidence in the estimate of effect. Current evidence derived from randomized clinical trials without important limitations.
(B) Moderate: Further research may well have an important impact on confidence in the estimate of effect and may change the estimate. Current evidence derived from randomized clinical trials with important limitations (e.g. inconsistent results, imprecision – wide confidence intervals or methodological flaws – e.g. lack of blinding, large losses to follow‐up, failure to adhere to intention to treat analysis), or very strong evidence from observational studies or case series (e.g. large or very large and consistent estimates of the magnitude of a treatment effect or demonstration of a dose‐response gradient).
(C) Low: Further research is likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. Current evidence from observational studies, case series or just opinion.
Epidemiology
Respiratory viral infections are common in the general population and can pose a particular threat to patients with haematological malignancies and those undergoing HSCT. The incidence of infection with respiratory viruses in patients undergoing haematological malignancies or HSCT is likely to reflect the incidence in the community (Whimbey et al, 1997). The common respiratory viruses and their respective serotypes, seasonal variation, incubation period and mode of transmission are detailed in Table 1 and by the Public Health England National Infection Service (https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/471887/six_pathogens_-_Jan2004-Oct2015.pdf).
Table 1.
Common respiratory virus characteristics
| Virus | Type | Classification | Seasonal variation (USA, Western Europe) | Median incubation perioda | Mode of transmission |
|---|---|---|---|---|---|
| RSV |
Paramyxoviridae RNA virus – negative sense Genus Pneumovirus |
2 types: A and B (Moore et al, 2013) |
Autumn, winter, spring (Shah & Chemaly, 2011) |
4·4 d (Lessler et al, 2009) |
Direct or indirect contact, possibly droplet spray (Pica & Bouvier, 2012) |
| HPIV |
Paramyxoviridae RNA virus – negative sense HPIV 1 and 3: Genus Respirovirus; HPIV 2 and 4: Genus Rubulavirus (Schomacker et al, 2012) |
Serotypes: 1–4 (HPIV3 most likely to cause LRTI) (Schomacker et al, 2012) |
HPIV3: spring/summer HPIV1: autumn/winter HPIV2: autumn |
2·6 d (Lessler et al, 2009) |
Direct or close contact with droplets or large particle aerosols (limited evidence) (Henrickson, 2003) |
| Influenza |
Orthomyxoviridae RNA virus – negative sense Genus Influenzavirus |
3 subtypes: A, B, C further subtyped based on surface haemagglutinins (HA, H1‐16) and neuroaminidases (NA, N 1‐9) (Reviewed in Ison, 2011) |
Winter, novel influenza A can lead to pandemic outbreaks (Chretien et al, 2014) |
Influenza A –1·4 d Influenza B –0·6 d (Lessler et al, 2009 |
Contact, droplet spray and/or aerosol (Pica & Bouvier, 2012) |
| HMPV |
Paramyxoviridae, RNA virus – negative sense Genus Metapneumovirus (Haas et al, 2013) |
2 subtypes: HMPVA and HMPVB (Haas et al, 2013) |
Late winter, early spring (Haas et al, 2013) |
Range: 4–6 d (Haas et al, 2013) |
Direct or close contact with droplets, large particle aerosols or saliva (Haas et al, 2013) |
| Rhinovirus |
Picornaviridae RNA virus – positive sense Genus Rhinovirus (Moore et al, 2013) |
3 species: A, B, C, numerous subtypes (>100) (Moore et al, 2013) |
All seasons |
1·9 d (Lessler et al, 2009) |
Contact (direct or via fomite) or aerosol (large or small particle). Conjunctival/intranasal inoculation (not via oral route) (Jacobs et al, 2013b) |
RSV, respiratory syncytial virus; HPIV, human parainfluenza; HMPV, human metapneumovirus; LRTI, lower respiratory tract infection.
Not specific for immunocompromised patients.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Diagnosis
In many patients with haematological malignancies, particularly those undergoing HSCT, there is a risk of progression to lower respiratory tract infection (LRTI). Full clinical history and examination are essential in making the diagnosis of viral respiratory tract disease and a chest X‐Ray or computerized tomography (CT) scan may be helpful to confirm or exclude the presence of lower respiratory tract disease. The definitions of upper and lower respiratory tract infection differ between reports, but, for the purposes of these guidelines they have been defined as follows, based on the European Conference on Infections in Leukaemia (ECIL) recommendations (Hirsch et al, 2013).
Upper respiratory tract infection (URTI): detection of respiratory virus in samples taken from above and including the larynx.
Upper respiratory tract disease: URTI plus symptoms and/or signs of infection.
Lower respiratory tract infection (LRTI): detection of respiratory virus in samples taken from below the larynx.
Lower respiratory tract disease: detection of virus in respiratory secretion plus hypoxia, pulmonary infiltrates or pathological sputum production.
Respiratory viral infections can be diagnosed on nasopharyngeal aspirate, nasal and/or throat swabs, saline throat gargles, sputum samples, tracheal aspirates or broncho‐alveolar lavage (BAL) samples. While collection of nasal and throat swabs or saline throat gargles can be as effective in diagnosing primary viral infections in children when using polymerase chain reaction (PCR) (Blaschke et al, 2011; Tunsjo et al, 2015) and direct immunofluorescent antigen detection (Del et al, 2014), lower sensitivities (Ohrmalm et al, 2010) have been reported in non‐primary and mild infections in immunocompromised patients, suggesting that a nasopharyngeal aspirate or nasal wash should be considered, where possible; it is likely that aspirates or washes provide a more consistent specimen. In patients with symptoms or signs of LRTI, a specimen should be obtained from the lower respiratory tract where possible. This sample might be obtained by induced sputum, tracheal aspirates or BAL sampling. Samples obtained by BAL should also be tested for co‐pathogens (Joos et al, 2007).
Viral culture and immunofluorescence testing have been used in the past for diagnosis of respiratory viruses in this population but have now been superseded by molecular diagnostics. It is currently recommended that the diagnosis of respiratory viral infections is made by quantitative nucleic acid amplification tests (NAATs), generically referred to hereafter as PCR; clinicians should be able to liaise with their virology laboratory colleagues regarding the interpretation of PCR results (reviewed in Hirsch et al, 2013). A panel of viruses should be included for PCR testing, including parainfluenza type 4. Developments in molecular testing may allow for rapid, point of care testing in the future, which would be optimal for this patient population. At present, the assays available for point of care testing are restricted in range or lack sensitivity or specificity for routine use.
Recommendations
It is recommended that the diagnosis of respiratory viral infection is made by polymerase chain reaction (PCR) testing (1A)
It is recommended that the diagnosis of upper respiratory tract infection is made on an upper respiratory tract specimen obtained by nasopharyngeal aspirate or wash (1A) or by collection of nasal and throat swabs (1C).
It is recommended that the diagnosis of lower respiratory tract infection is made on a lower respiratory tract specimen (e.g. bronchoalveolar lavage, tracheal aspirate, induced sputum) where possible (1A)
Risk factors for progression to LRTI and mortality
A number of risk factors have been identified for progression to LRTI and mortality. These are similar for all the common respiratory viruses and include neutropenia, lymphopenia, allogeneic transplant, pre‐engraftment or early post‐engraftment, graft‐versus‐host disease, the presence of co‐pathogens and older age (Walsh et al, 1999, 2008; Ljungman et al, 2001, 2011; Nichols et al, 2001, 2004; Ison et al, 2003; Martino et al, 2005; Johnstone et al, 2008; Schiffer et al, 2009; Choi et al, 2011; Renaud & Campbell, 2011; Shah & Chemaly, 2011; Chemaly et al, 2012a; Mohty et al, 2012; Widmer et al, 2012; Jacobs et al, 2013a; Renaud et al, 2013; Shah et al, 2013; Kim et al, 2014). Table 2 shows the risk factors for progression to LRTI in allogeneic transplant patients with RSV URTI (reviewed in Shah & Chemaly, 2011).
Table 2.
Risk Factors for progression to respiratory syncytial virus lower respiratory tract infection in allogeneic transplant patients (reviewed in Shah & Chemaly, 2011)
|
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Recommendation
It is recommended that patients are assessed for risk factors for progression to lower respiratory tract infection and increased mortality prior to deciding on treatment plan (1B)
Prevention
Vaccination
No vaccines are currently available for RSV, HPIV, HRV or HMPV infection. Patients, their household contacts and staff should be encouraged to receive the influenza vaccine on an annual basis according to the national guidance in the ‘Green Book’ (Public Health England 2013).
Post‐exposure prophylaxis for influenza
Post‐exposure prophylaxis may be an option for immunosuppressed individuals who are in close contact with proven influenza or influenza‐like illness if the exposed person has not been effectively covered by vaccination [National Institute for Health and Care Excellence (NICE, 2008)], or has become immunosuppressed as a result of either underlying disease or conditioning and other ablative treatments used in HSCT. Post exposure prophylaxis could be helpful in patients who have not received vaccination. In addition, it could be helpful in situations where the vaccine administered did not provide protection against the circulating strains of the virus. Patients who have recently received rituximab may also not respond to the vaccine (Berglund et al, 2014).
Recommendations for prophylaxis are updated annually and are available from Public Health England. It is suggested that these websites are reviewed directly to ensure that guidance is up to date (https://www.gov.uk/government/collections/seasonal-influenza-guidance-data-and-analysis; last accessed 1 November 2015). The recommendations provided by Health Protection Scotland are based on these recommendations (http://www.hps.scot.nhs.uk/resp/seasonalinfluenza.aspx; last accessed 1 November 2015) and Public Health Wales also follows similar recommendations (http://www.wales.nhs.uk/sitesplus/888/page/43745; last accessed 5 July 2015). The current recommendations are 75 mg oseltamivir orally for 10 d once daily if the dominant influenza strain is of lower risk for oseltamivir resistance (e.g. A(H3N2). The recommendations provide lower doses for children <13 years of age. Ideally, therapy should be started within 48 h of exposure on the advice of a specialist. Inhaled Zanamivir (10 mg for 10 d once daily for adults and children ≥5 years of age; it is not licensed for children <5 years) is an alternative if the dominant influenza strain is likely to be resistant to oseltamivir e.g. A(H1N1)pdm2009. Ideally, therapy should be started within 36 h of last contact. The use of zanamivir relies on the ability of the adult or child to effectively use the inhaler. Intravenous or nebulized zanamivir is unlicensed but available for compassionate use on a named patient basis.
Infection control – in‐patients
Table 3 highlights infection control measures in the in‐patient and out‐patient settings. Patients with haematological malignancies who are diagnosed with or suspected to have a respiratory virus infection should be isolated in a single room with neutral air pressure and, ideally, an antechamber. It is particularly important that patients with influenza infection or those receiving treatment with ribavirin are not accommodated in rooms with positive pressure. Infection control measures should remain in place until patients no longer have symptoms of acute respiratory virus infection, or have tested negative for the respiratory virus or after discussion with a specialist in clinical virology, microbiology or infectious diseases.
Table 3.
Good practice measures in infection control to prevent the spread of respiratory viruses
| Hand hygiene |
| Wearing protective clothing as per unit policy, e.g., aprons, gloves, masks |
| Safe disposal of oral and nasal secretions |
| Covering the mouth if coughing and sneezing |
| Isolating in‐patients with respiratory symptoms in a neutral pressure room |
| Isolation of out‐patients with respiratory symptoms where possible |
| Advising relatives not to visit if they have respiratory symptoms |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Healthcare workers with URTI or LRTI symptoms should avoid contact with susceptible patients where possible. It is appreciated that this might not always be practical (Hirsch et al, 2013).
Treatment units should have measures in place to stop the spread of infection. This may mean cohorting patients or screening patients in the event of an outbreak. There is insufficient evidence to support routine screening although this may be helpful in the event of an outbreak.
Infection control – out‐patients
Respiratory viral infections are easily transmitted from person to person. Patients with haematological malignancies should be advised to avoid contact with patients with respiratory viral infections in the community where possible (Hirsch et al, 2013).
Out‐patients with URTI or LRTI symptoms should be isolated from other patients in the clinic and ambulatory care facilities where possible (Hirsch et al, 2013).
Prevention
Recommendations
It is recommended that patients undergoing treatment for haematological malignancies or haematopoietic stem cell transplant, as well as staff caring for them and household contacts, receive the influenza vaccine on an annual basis (1A)
It is recommended that the current Public Health England recommendations on post exposure prophylaxis for influenza infection are followed (1B)
It is recommended that allogeneic transplant patients and those undergoing treatment for haematological malignancies with respiratory tract symptoms are isolated from other haemato‐oncology patients in the out‐patient setting where possible (1C)
It is suggested that screening of patients may be considered in the event of an outbreak of respiratory viral infection (2C)
It is recommended that all allogeneic transplant patients and those undergoing treatment for haematological malignancies with respiratory viral infections are isolated in a side room with neutral air pressure and an antechamber while undergoing in‐patient treatment (1C)
Supportive care
Patients with respiratory viral infections are susceptible to co‐infections with other organisms and patients should be actively screened and treated for co‐existent infections. Patients should be monitored closely for signs of respiratory failure including increased respiratory rate and hypoxia and early intervention by the critical care team should be considered in patients with progressive respiratory failure. In patients who are receiving corticosteroids, consideration should be given to tapering the dose where possible. Infection with Pneumocystis jirovecii should be excluded if respiratory infection develops in patients while tapering of steroids is undertaken.
Recommendations
It is recommended that patients with respiratory viruses are actively screened for co‐pathogens including Pneumocystis jirovecii (1C)
It is recommended that patients with respiratory viruses are monitored for signs of respiratory failure and the critical care team is involved where required (1C)
It is recommended that immunosuppression is reduced where possible in patients with respiratory viruses (1C)
Postponement of planned chemotherapy or transplant
Patients with URTI symptoms should be screened prior to treatment. Sequential infection in which a respiratory viral infection may progress to pneumonia or be succeeded by a systemic bacterial or fungal infection is common in immunosuppressed patients and potentially fatal. In addition, the presence of a viral infection, including rhinovirus, prior to HSCT has been linked with poorer outcomes post‐HSCT (Campbell et al, 2015). Therefore, patients with suspected respiratory viral infections should be carefully assessed clinically and have appropriate samples taken for the purpose of diagnostic tests. Postponement of cytotoxic therapy or HSCT until symptoms have resolved must be considered. However, the pace and stage of the underlying disease may make this impractical and case‐by‐case evaluation is necessary (Champlin & Whimbey, 2001; Peck et al, 2004; Tomblyn et al, 2009; Hirsch et al, 2013).
Recommendations
It is recommended that patients with symptoms suggesting upper or lower respiratory tract infection are screened for respiratory viral infections prior to commencing therapy (1C)
It is recommended that consideration be given to delaying cytotoxic therapy or haematopoietic stem cell transplant in patients with active respiratory viral infections (1C)
Treatment
Influenza
Although there are limited data from randomized controlled trials on the utility and correct duration of use of licensed antivirals in immunocompromised individuals, there are some guidelines and clinical reports available.
Almost all observational studies in HSCT and high‐risk leukaemia demonstrate a clinical benefit of antiviral therapy compared with no therapy (Casper et al, 2010; Choi et al, 2011; Fraaij et al, 2011; Ljungman et al, 2011). In a retrospective study of 33 adult patients with leukaemia, 25 patients received neuraminidase inhibitors (NAIs) and 8 patients were untreated. No patients in the treatment group died but 3 of 8 patients who were untreated died (P = 0·001) (Chemaly et al, 2007). In HSCT recipients, a retrospective study including both adults and children showed 6 of 34 untreated patients (18%) developed pneumonia, whereas only 1 of 8 patients treated with rimantadine and 0 of 9 treated with oseltamivir developed pneumonia (i.e. 6% of all treated patients) (Nichols et al, 2004).
NAIs form the mainstay of current recommendations, and their mechanism of action is to interfere with the release of influenza virus particles from infected cells, preventing the spread of infection to other cells.
It is recommended that the Public Health England guidance should be followed regarding the treatment of influenza (https://www.gov.uk/government/collections/seasonal-influenza-guidance-data-and-analysis; last accessed 1 November 2015). The current guidance recommends oseltamivir orally (75 mg twice daily for 5 d for patients ≥13 years of age, doses are provided for younger children in the guidance) as first line treatment where there is a low risk of oseltamivir resistant strains and zanamivir inhaler (Diskhaler) when the dominant strain has a higher risk of resistance e.g. influenza A (H1N1). Treatment should ideally be commenced within 48 h of symptom onset in patients with uncomplicated influenza. In patients with complicated influenza, defined as requiring hospital admission and/or signs of LRTI, central nervous system involvement or exacerbation of underlying medical condition, treatment should be administered regardless of the timing of onset of symptoms. Five days is the minimum duration of treatment and may be continued for longer, depending on response. Second line treatment is indicated in patients with poor gastrointestinal absorption or poor clinical response to first line treatment and inhaled zanamivir (10 mg twice daily for 5 d for adults and children ≥5 years of age) is recommended. Treatment with intravenous or nebulized zanamivir may also be considered in patients who are not able to use inhaled zanamivir effectively. The use of these preparations of zanamivir is unlicensed and they are available on a compassionate use basis for named patients in the UK. Patients should receive treatment when they have either upper or lower respiratory tract symptoms. In patients with suspected complicated influenza, treatment should be commenced on clinical suspicion without waiting for laboratory confirmation. Similarly, in the event of an increase in influenza cases in the community, patients with uncomplicated infection should also be treated at the first sign of symptoms. The most common toxicity of oseltamivir is nausea and vomiting and there have been rare reports of more severe adverse events including anaphylaxis and hepatic dysfunction (https://www.medicines.org.uk/emc/medicine/10446; last accessed 21 June 2015, Dobson et al, 2000). Zanamivir has been occasionally associated with bronchospasm in patients with asthma or chronic obstructive pulmonary disease (http://www.medicines.org.uk/emc/medicine/2608; last accessed 21 June 2015, Williamson & Pegram, 2000).
New agents
Favipiravir (T705, Toyama Chemical) is an investigational antiviral drug that functions as a nucleotide analogue and inhibitor of viral RNA polymerase (Furuta et al, 2009). It has been shown to be effective against oseltamivir‐resistant seasonal and pandemic‐viruses in vitro (Sleeman et al, 2010).
Recommendation
It is recommended that current Public Health England guidance is followed in the management of influenza infection (1A)
Respiratory syncytial virus
Ribavirin is known to be effective against RNA viruses including RSV. This drug is available for aerosolized, intravenous and oral use. At the time of writing access to aerosolized ribavirin in the UK is limited as the licensed product is no longer available. An alternative aerosolized product is manufactured by Valeant pharmaceuticals (Quebec, Canada); it may be available to centres in the UK but it is unlicensed and would need to be imported. Toxicities associated with the administration of aerosolized ribavirin include bronchospasm, cough, claustrophobia, nausea, rash, reduced pulmonary function, mucus plugging and conjunctival irritation (Shah & Chemaly, 2011). There is a potential risk that ribavirin is teratogenic and mutagenic so patients and staff should be made aware of the risks. These are documented in full in the summary of product characteristics (https://www.medicines.org.uk/emc/medicine/866; last accessed 05 April 2015). Intravenous ribavirin has been associated with haemolysis, leucopenia and hyperbilirubinaemia and oral ribavirin has been associated with anaemia and nausea (Shah & Chemaly, 2011). The full toxicity profile of oral ribavirin is available in the summary of product characteristics (https://www.medicines.org.uk/emc/medicine/30881; last accessed 12 December 2015)
A recent adult study compared the use of intermittent versus continuous aerosolized ribavirin administration for 5–10 d and found no statistical difference in progression to LRTI or duration of viral shedding between the two regimens (Chemaly et al, 2012b). The available data on the efficacy of aerosolized ribavirin is largely limited to single centre studies. Boeckh et al (2007) reported a randomized controlled trial of aerosolized ribavirin at a dose of 2 g, 3 times daily compared to supportive care in post‐HSCT patients with upper respiratory tract RSV infection. Only 14 patients (including 2 children) were included due to slow accrual over a 5‐year period. The end point was development of clinical pneumonia, which occurred in 1/9 (11·1%) of ribavirin recipients and 2/5 (40%) of control patients. All patients were alive after 28 d of follow‐up (Boeckh et al, 2007).
A recent detailed review by Shah and Chemaly (2011) included this randomized controlled trial plus 4 prospective studies and 6 retrospective studies of HSCT patients who received aerosolized ribavirin without intravenous immunoglobulin (IVIG). There was a significant reduction in progression to LRTI in those who received ribavirin at the URTI stage compared to those that did not [25% (11/44) compared to 47% (54/116), P = 0·01]. The median duration of therapy varied between studies but was usually 5–7 d although longer in severe infections (Shah & Chemaly, 2011).
The conclusions of this review article are reflected in a more recent study of 280 adult and paediatric allogeneic transplant patients, which also found that aerosolized ribavirin at the URTI stage was the most important single factor in reducing progression to LRTI and mortality (Shah et al, 2013). This retrospective study reviewed the outcome of all patients who had laboratory‐confirmed RSV infections from January 1996 to May 2009. Patients were treated with aerosolized ribavirin and/or IVIG at the discretion of the treating physician. The same group has subsequently published an Immunodeficiency Scoring Index based on the data from the 237 patients in the study who presented with URTI symptoms (Shah et al, 2014). Patients were stratified into low, medium and high‐risk groups based on a scoring system (see Table 4). Patients who scored 0–2 were classified as low‐risk, those who scored 3–6 were classified as moderate risk and those who scored 7–12 were classified as high‐risk (Shah et al, 2014). High‐risk patients were significantly more at risk of LRTI and mortality than moderate or low‐risk patients (P < 0·001). Patients in the high‐risk group also had the greatest benefit from ribavirin administered at the URTI stage. This scoring system has not yet been validated in a prospective cohort of patients.
Table 4.
Immunodeficiency Scoring Index for patients with respiratory syncytial virus infection post‐allogeneic haematopoietic stem cell transplant (Shah et al, 2014)
| Risk factor | Score |
|---|---|
| Neutrophil count <0·5 × 109/l | 3 |
| Lymphocyte count <0·2 × 109/l | 3 |
| Age ≥40 years | 2 |
| Myeloablative conditioning | 1 |
| Graft‐versus‐host disease (acute/chronic) | 1 |
| Corticosteroids | 1 |
| Pre‐engraftment/within 30 d of transplant | 1 |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Aerosolized ribavirin and immunomodulator treatment
A number of studies have investigated the role of immunodulatory agents in the treatment of RSV. These include the use of IVIG and monoclonal antibodies. RSV specific immunoglobulin (RespiGam) was withdrawn by the manufacturers in March 2004 and is no longer available for use. Palivizumab is a humanized anti‐RSV monoclonal antibody that has been used in animal models (Ottolini et al, 2002) and was found to be safe for use in HSCT recipients in a small phase 1 study (Boeckh et al, 2001). A larger series of 40 adults and children treated with palivizumab following HSCT showed no evidence of improved survival or prevention of progression to LRTI (de Fontbrune et al, 2007). Although no randomized data exist in the stem cell transplant population, there are randomized data supporting the use of palivizumab prophylaxis in selected high‐risk children (The Impact‐RSV study Group, 1998). Palivizumab is only licensed for this indication and not for treatment of RSV in patients with haematological malignancies or post‐HSCT. Palivizumab is not recommended for the treatment of adults with RSV infection but may be considered in children.
Shah and Chemaly (2011) reviewed thirteen studies that included 407 post‐HSCT adults treated with aerosolized ribavirin plus either IVIG, Palivizumab or RSV‐specific IVIG. Similarly, the risk of progression to LRTI was lower in those treated at the URTI stage (12% compared to 45%). There was a trend towards a lower rate of progression to LRTI in recipients of dual therapy than in those patients that received aerosolized ribavirin alone (12% compared to 25%, P = 0·13). The RSV‐specific mortality rate in patients with LRTI was significantly lower in the combination group (24% vs. 50%, P < 0·001) (Shah & Chemaly, 2011). It is recommended that patients who have undergone allogeneic transplantation and have risk factors for progression to LRTI with RSV should receive therapy with aerosolized ribavirin and IVIG. If aerosolized ribavirin is not available, oral ribavirin may be an alternative. The authors note that IVIG may not be available for use in all patients due to demand management guidelines in the United Kingdom (http://www.ivig.nhs.uk/documents/dh_129666.pdf; last accessed 12 December 2015) An algorithm for treatment is outlined in Fig 1.
Figure 1.
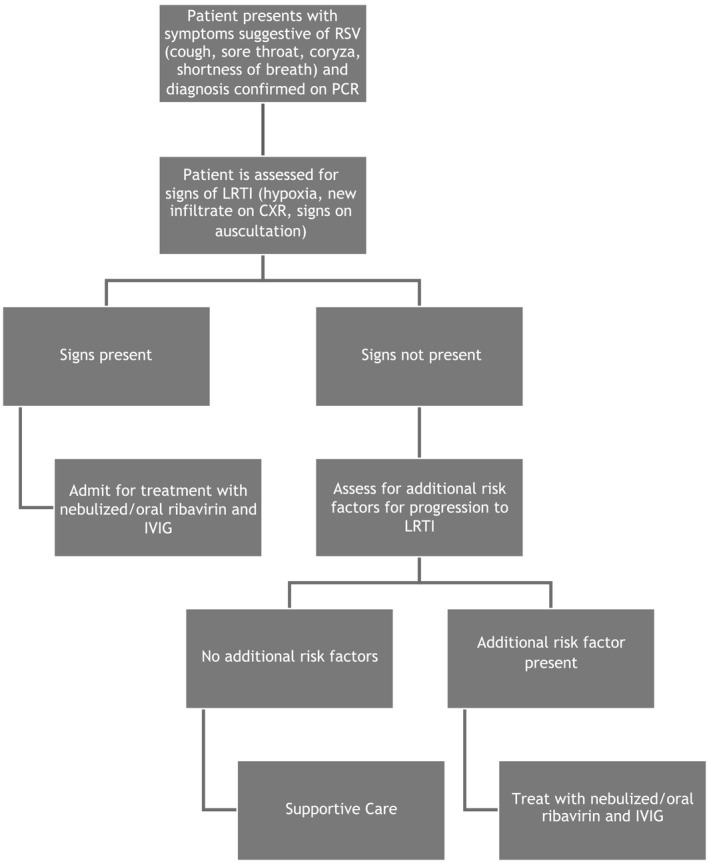
Proposed algorithm for treatment for patients with RSV following allogeneic transplantation. RSV, respiratory syncytial virus; PCR, polymerase chain reaction; LRTI, lower respiratory tract infection; CXR, chest X‐ray; IVIG, intravenous immunoglobulin.
Oral ribavirin
In view of the recent lack of availability of aerosolized ribavirin, there has been considerable interest in the use of oral ribavirin in RSV infection in HSCT recipients. Oral ribavirin is not licensed for the treatment of respiratory viruses but has been used in several reports. Khanna et al (2008) reported a retrospective review of 34 adult patients with RSV (22 with URTI and 12 with LRTI). Twenty‐four patients (71%) had undergone allogeneic HSCT. Twenty‐five patients received treatment with oral ribavirin with IVIG or oral ribavirin plus palivizumab (14 for URTI, 11 for LRTI). Oral ribavirin was administered at a loading dose of 10 mg/kg, which was then increased to 400 mg three times daily on day 2, and 600 mg three times daily on day 3. Four patients required mechanical ventilation and received IV ribavirin using the same dosing regimen. RSV‐attributable mortality was 18% (6/34). Five patients developed haemolysis (Khanna et al, 2008).
A more recent study used oral ribavirin in 13 adult patients with RSV infection post‐HSCT (Casey et al, 2013). Seven patients had URTI and 6 had LRTI. Patients received oral ribavirin at a dose of 10 mg/kg/d in four divided doses, which was escalated dependent on clinical response to a total dose of 60 mg/kg/d along with IVIG. Three patients (3/13, 23%) died of RSV‐related respiratory infection. All seven patients with URTI progressed to LRTI at the starting dose of 10 mg/kg/d but six subsequently responded at higher doses. The median duration of therapy was 25·5 d (range 10–33 d). No patients developed haemolysis. Marcelin et al (2014) also recently reported on the use of oral ribavirin in a heterogeneous patient group of 34 patients including some HSCT recipients. No patients died of RSV infection but one developed haemolytic anaemia and lactic acidosis and another developed altered mental status (Marcelin et al, 2014). Although evidence is limited, oral ribavirin appears to be safe and cost‐effective for the treatment of URTI in HSCT recipients. A starting dose higher than 10 mg/kg/d, e.g 15–20 mg/kg/d, may be more effective (Casey et al, 2013).
Intravenous ribavirin
Intravenous ribavirin is not licensed for the treatment of respiratory viruses but has also been used in several small series. Shah and Chemaly (2011) reported on a review of four retrospective studies and two prospective studies using oral or intravenous ribavirin with or without IVIG. Of the 48 adult patients who received treatment for LRTI, 26 (54%) died (Shah & Chemaly, 2011). There are limited data available on patients who received intravenous ribavirin at the URTI stage. It is suggested that the use of intravenous ribavirin should be restricted to patients who require RSV‐specific therapy and are unable to receive oral or aerosolized ribavirin. Intravenous ribavirin may be helpful in patients who are receiving mechanical ventilation.
Treatment of non‐allogeneic transplant patients with RSV infection
There is limited evidence for the efficacy of treatment of RSV in patients undergoing autologous transplantation or chemotherapy treatment. Anaissie et al (2004) reported on 190 adults undergoing chemotherapy with or without autologous transplantation. All patients were screened pre‐treatment and a large number of patients (71 patients, 37%) were found to be RSV positive. No patients received RSV‐specific treatment. There was no significant increase in serious respiratory complications in the patients with RSV. It is suggested that patients who are undergoing chemotherapy treatment or autologous transplantation would not generally require RSV specific therapy but this decision should be made on a case‐by‐case basis.
Recommendations
It is recommended that aerosolized ribavirin is administered to allogeneic transplant patients with lower respiratory tract infection with respiratory syncytial virus (1B)
Oral ribavirin may be an alternative in allogeneic transplant patients with lower respiratory tract infection with RSV if aerosolized ribavirin is not available (1C)
It is recommended that intravenous immunoglobulin is administered to allogeneic transplant patients with respiratory syncytial virus infection (1B)
It is recommended that aerosolized/oral ribavirin is administered to allogeneic transplant patients with upper respiratory tract infection with respiratory syncytial virus and multiple risk factors for progression to lower respiratory tract infection (1B)
Human metapneumovirus
Most patients with HMPV will require no therapy, although prolonged asymptomatic shedding may occur and may be an infection control issue requiring confirmation of negative viral PCR or discussion with clinical virology prior to relaxation of infection control measures. URTI should not be treated as there is no evidence this reduces risk of LRTI. Occurrences of LRTI should be assessed on a case‐by‐case basis and the Immunodeficiency Scoring Index (Shah et al, 2014) may be useful here. However, it must be appreciated that LRTI HMPV in adults, when compared to RSV and influenza, is associated with similar complication rates, including intensive care unit (ICU) admission, mechanical ventilation, length of hospitalization and length of stay in the ICU (Widmer et al, 2012; Jain et al, 2014).
The high prevalence of previous infection in the population means that relatively high titres of HMPV neutralizing antibodies are found in pooled IVIG. In vitro studies have shown similar efficacy of IVIG against RSV and HMPV (Wyde et al, 2003). IVIG at a dose of 0·4 g/kg weekly has been used in several case studies and small series to treat patients with HMPV (Shachor‐Meyouhas et al, 2012; Kitanovski et al, 2013).
Published studies using ribavirin to treat HMPV have been somewhat inconclusive but ribavirin is a valid treatment option in selected high‐risk cases. Several case studies report resolution of infection in patients with advanced LRTI using intravenous (Safdar, 2008; Bonney et al, 2009), oral (Egli et al, 2012) or nebulized ribavirin (Shahda et al, 2011; Renaud et al, 2013), usually in combination with IVIG. Recently, the Seattle group have published their experience in treating LRTI caused by HMPV (n = 23), and compared the outcomes to those with RSV LRTI (n = 23). Mortality was similar at 43% (Renaud et al, 2013). While there was no impact on outcome of using nebulized ribavirin +/− IVIG there were important limitations in this study. In addition to being a small retrospective series, the study populations were imbalanced with only half of the HMPV patients receiving IVIG or ribavirin or both. There was a delay in time to start ribavirin treatment in the HMPV‐treated group, the patients who received therapy had more advanced disease and presented earlier post‐transplant than the untreated group.
Humanized monoclonal antibodies that target the HMPV fusion show promise (Ulbrandt et al, 2008; Hamelin et al, 2010; Corti et al, 2013). Candidate peptide antivirals are also in development (Deffrasnes et al, 2008), however, at present, none of these approaches are commercially available. Currently, no effective vaccines are available.
At present there is uncertainty about the role of ribavirin in HMPV and it is not routinely recommended. It may be used in selected high‐risk patients at the discretion of the treating physician.
Parainfluenza
Therapy of HPIV infection is generally supportive together with respiratory isolation. Given that corticosteroid therapy is a risk factor for progression to LRTI, reduction of steroid dosage where appropriate and feasible may be a valid approach to therapy (Ison, 2007).
No proven anti‐viral agent exists. Anecdotal reports have suggested a role for ribavirin whether in aerosolized, oral or intravenous form (Sparrelid et al, 1997; Chakrabarti et al, 2000; Dignan et al, 2006; Stankova et al, 2007; Shima et al, 2008; Casey et al, 2013). However, in patients diagnosed by direct fluorescent antibody or culture, ribavirin given as nebulizer has not been found to improve viral shedding or survival after HPIV‐LRTI, regardless of the concurrent use of immunoglobulins or the absence of co‐pathogens (Ison, 2007). There remains uncertainty about the use of ribavirin in HPIV infection but it may be considered in selected high risk patients with LRTI.
Alternative antiviral agents are in pre‐clinical or early phase clinical trial development (Alymova et al, 2005; Chen et al, 2011; Barik & Lu, 2015). Vaccine development has been on‐going for several decades and a number of live attenuated vaccines are now undergoing clinical trials. While these are unlikely to be directly applicable to immunosuppressed patients they may prove useful in the context of vaccination of household contacts (Rubin et al, 2014). Reduction in risk of initial viral infection of the immunosuppressed may reduce the risk of secondary bacteria or fungal infection (Alymova et al, 2005; Bosch et al, 2013).
Human rhinovirus infection
There is currently no specific treatment available for HRV infection. The capsid‐binding agent Pleconaril has been studied in picornavirus‐infected patients but not specifically in the setting of HSCT. Limited effects on reduction of symptom duration were observed with this drug and, ultimately, acquired viral resistance was detected (Hayden et al, 2003; Pevear et al, 2005). Another capsid‐binding drug, BTA‐798, is under evaluation in a Phase II study enrolling asthma‐sufferers (NCT01175226). Inhaled interferon‐β 1a (SNG001) is similarly being investigated in asthmatics (NCT01126177). Neither of these agents has as yet been tested in HSCT recipients. Vaccination against HRV remains a challenging prospect, due mainly to the presence of >100 HRV serotypes. It is recommended that supportive care only should be used in the management of HRV infection due to insufficient evidence to support specific therapies. A recent study confirmed increased post‐HSCT mortality in patients with HRV infection pre‐transplant, underlying the need for research and better intervention for HRV infections (Campbell et al, 2015).
Recommendations
It is recommended that supportive care only is used in the management of rhinovirus as there is insufficient evidence to support specific treatments (1B)
Treatment with ribavirin is not recommended for patients with upper respiratory tract infection with parainfluenza or metapneumovirus (2C)
Summary of key recommendations
-
It is recommended that:
the diagnosis of respiratory viral infection is made by polymerase chain reaction (PCR) testing (1A).
the diagnosis of upper respiratory tract infections is made on an upper respiratory tract specimen obtained by nasopharyngeal aspirate or wash (1A) or by collection of nasal and throat swabs (1C).
the diagnosis of lower respiratory tract infection is made on a lower respiratory tract specimen (e.g. bronchoalveolar lavage, tracheal aspirate, induced sputum) where possible (1A).
patients are assessed for risk factors for progression to lower respiratory tract infection prior to deciding on treatment plan (1B).
patients undergoing treatment for haematological malignancies or haematopoietic stem cell transplantation, as well as staff caring for them and household contacts, receive the influenza vaccine on an annual basis (1A).
the current Public Health England recommendations on post exposure prophylaxis for influenza infection are followed (1B).
that allogeneic transplant patients and those undergoing treatment for haematological malignancies, with respiratory tract symptoms, are isolated from other haemato‐oncology patients in the out‐patient setting where possible (1C).
It is suggested that screening of patients may be considered in the event of an outbreak of respiratory viral infection (2C).
It is recommended that all allogeneic transplant patients and those undergoing treatment for haematological malignancies with respiratory viral infections are isolated in a side room with neutral air pressure and an antechamber while undergoing in‐patient treatment (1C).
-
It is recommended that:
patients with respiratory viruses are actively screened for co‐pathogens including Pneumocystis jirovecii (1C).
patients with respiratory viruses are monitored for signs of respiratory failure and the critical care team is involved where required (1C).
immunosuppression is reduced where possible in patients with respiratory viruses (1C).
patients with upper or lower respiratory tract symptoms are screened for respiratory viral infections prior to commencing therapy (1C).
consideration be given to delaying cytotoxic therapy or haematopoietic stem cell transplantation in patients with active respiratory viral infections (1C).
current Public Health England guidance is followed in the management of influenza infection (1A).
aerosolized ribavirin is administered to allogeneic transplant patients with lower respiratory tract infection with respiratory syncytial virus (1B).
oral ribavirin may be an alternative in allogeneic transplant patients with lower respiratory tract infection with RSV if aerosolized ribavirin is not available (1C).
intravenous immunoglobulin is administered to allogeneic transplant patients with respiratory syncytial virus infection (1B).
aerosolized/oral ribavirin is administered to allogeneic transplant patients with upper respiratory tract infection with respiratory syncytial virus and multiple risk factors for progression to lower respiratory tract infection (1B).
supportive care only is used in the management of rhinovirus as there are no specific treatments (1B).
Treatment with ribavirin is not recommended for patients with upper respiratory tract infection with parainfluenza or metapneumovirus (2C).
Disclaimer
While the advice and information in these guidelines is believed to be true and accurate at the time of going to press, neither the authors, the British Society for Haematology, the British Society of Blood and Marrow Transplantation, the UK Clinical Virology Network nor the publishers accept any legal responsibility for the content of these guidelines.
Conflicts of interest
None declared.
Acknowledgements
FLD reviewed the literature and wrote the initial draft of the manuscript and chaired the writing group. AC represented BCSH, reviewed the literature and revised the manuscript. MNP represented BSBMT, reviewed the literature and revised the manuscript. CA, MG, VJ, PK, AP, BES, RS, AT, RW and PC reviewed the literature and revised the manuscript. The authors are grateful to the BCSH task force and BSBMT executive committee for their support in preparing these guidelines.
References
- Alymova, I.V. , Portner, A. , Takimoto, T. , Boyd, K.L. , Babu, Y.S. & McCullers, J.A. (2005) The novel parainfluenza virus hemagglutinin‐neuraminidase inhibitor BCX 2798 prevents lethal synergism between a paramyxovirus and Streptococcus pneumoniae. Antimicrobial Agents Chemotherapy, 49, 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anaissie, E.J. , Mahfouz, T.H. , Aslan, T. , Pouli, A. , Desikan, R. , Fassas, A. & Barlogie, B. (2004) The natural history of respiratory syncytial virus infection in cancer and transplant patients: implications for management. Blood, 103, 1611–1617. [DOI] [PubMed] [Google Scholar]
- Barik, S. & Lu, P. (2015) Therapy of respiratory viral infections with intranasal siRNAs. Methods in Molecular Biology, 1218, 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund, A. , Willén, L. , Grödeberg, L. , Skattum, L. , Hagberg, H. & Pauksens, K. (2014) The response to vaccination against influenza A(H1N1) 2009, seasonal influenza and Streptococcus pneumoniae in adult outpatients with ongoing treatment for cancer with and without rituximab. Acta Oncologica, 53, 1212–1220. [DOI] [PubMed] [Google Scholar]
- Blaschke, A.J. , Allison, M.A. , Meyers, L. , Rogatcheva, M. , Heyrend, C. , Mallin, B. , Carter, M. , Lafleur, B. , Barney, T. , Poritz, M.A. , Daly, J.A. & Byington, C.L. (2011) Non‐invasive sample collection for respiratory virus testing by multiplex PCR. Journal of Clinical Virology, 52, 210–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckh, M. , Berrey, M.M. , Bowden, R.A. , Crawford, S.W. , Balsley, J. & Corey, L. (2001) Phase 1 evaluation of the respiratory syncytial virus‐specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. Journal of Infectious Diseases., 184, 350–354. [DOI] [PubMed] [Google Scholar]
- Boeckh, M. , Englund, J. , Li, Y. , Miller, C. , Cross, A. , Fernandez, H. , Kuypers, J. , Kim, H. , Gnann, J. & Whitley, R. ; NIAID Collaborative Antiviral Study Group . (2007) Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upperrespiratory tract infection in hematopoietic cell transplant recipients. Clinical Infectious Diseases, 44, 245–249. [DOI] [PubMed] [Google Scholar]
- Bonney, D. , Razali, H. , Turner, A. & Will, A. (2009) Successful treatment of human metapneumovirus pneumonia using combination therapy with intravenous ribavirin and immune globulin. British Journal of Haematology, 145, 667–669. [DOI] [PubMed] [Google Scholar]
- Bosch, A.A. , Biesbroek, G. , Trzcinski, K. , Sanders, E.A. & Bogaert, D. (2013) Viral and bacterial interactions in the upper respiratory tract. PLoS Pathogens., 9, e1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, A.P. , Guthrie, K.A. , Englund, J.A. , Farney, R.M. , Minerich, E.L. , Kuypers, J. , Corey, L. & Boeckh, M. (2015) Clinical outcomes associated with respiratory virus detection before allogeneic hematopoietic stem cell transplant. Clinical Infectious Diseases, 61, 192–202. Apr 5. pii: civ272 DOI: 10.1093/cid/civ272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, J. , Morris, K. , Narayana, M. , Nakagaki, M. & Kennedy, G.A. (2013) Oral ribavirin for treatment of respiratory syncitial virus and parainfluenza 3 virus infections post allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplantation, 48, 1558–1561. [DOI] [PubMed] [Google Scholar]
- Casper, C. , Englund, J. & Boeckh, M. (2010) How I treat influenza in patients with hematologic malignancies. Blood, 115, 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti, S. , Collingham, K.E. , Holder, K. , Oyaide, S. , Pillay, D. & Milligan, D.W. (2000) Parainfluenza virus type 3 infections in hematopoietic stem cell transplant recipients: response to ribavirin therapy. Clinical Infectious Diseases, 31, 1516–1518. [DOI] [PubMed] [Google Scholar]
- Champlin, R.E. & Whimbey, E. (2001) Community respiratory virus infections in bone marrow transplant recipients: the M.D. Anderson Cancer Center experience. Biology of Blood and Marrow Transplantation, 7, 8S–10S. [DOI] [PubMed] [Google Scholar]
- Chemaly, R.F. , Torres, H.A. , Aguilera, E.A. , Mattiuzzi, G. , Cabanillas, M. , Kantarjian, H. , Gonzalez, V. , Safdar, A. & Raad, I.I. (2007) Neuraminidase inhibitors improve outcome of patients with leukemia and influenza: an observational study. Clinical Infectious Diseases, 44, 964–967. [DOI] [PubMed] [Google Scholar]
- Chemaly, R.F. , Torres, H.A. , Munsell, M.F. , Shah, D.P. , Rathod, D.B. , Bodey, G.P. , Hosing, C. , Saifan, C. , Raad, I.I. & Champlin, R.E. (2012a) An adaptive randomized trial of an intermittent dosing schedule of aerosolized ribavirin in patients with cancer and respiratory syncytial virus infection. Journal of Infectious Diseases, 206, 1367–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemaly, R.F. , Hanmod, S.S. , Rathod, D.B. , Ghantoji, S.S. , Jiang, Y. , Doshi, A. , Vigil, K. , Adachi, J.A. , Khoury, A.M. , Tarrand, J. , Hosing, C. & Champlin, R. (2012b) The characteristics and outcomes of parainfluenza virus infections in 200 patients with leukemia or recipients of hematopoietic stem cell transplantation. Blood, 119, 2738–2745. [DOI] [PubMed] [Google Scholar]
- Chen, Y.B. , Driscoll, J.P. , McAfee, S.L. , Spitzer, T.R. , Rosenberg, E.S. , Sanders, R. , Moss, R.B. , Fang, F. & Marty, F.M. (2011) Treatment of parainfluenza 3 infection with DAS181 in a patient after allogeneic stem cell transplantation. Clinical Infectious Diseases, 53, e77–e80. [DOI] [PubMed] [Google Scholar]
- Choi, S.M. , Boudreault, A.A. , Xie, H. , Englund, J.A. , Corey, L. & Boeckh, M. (2011) Differences in clinical outcomes after 2009 influenza A/H1N1 and seasonal influenza among hematopoietic cell transplant recipients. Blood, 117, 5050–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien, J.P. , George, D. , Shaman, J. , Chitale, R.A. & McKenzie, F.E. (2014) Influenza forecasting in human populations: a scoping review. PLoS ONE, 9, e94130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti, D. , Bianchi, S. , Vanzetta, F. , Minola, A. , Perez, L. , Agatic, G. , Guarino, B. , Silacci, C. , Marcandalli, J. , Marsland, B.J. , Piralla, A. , Percivalle, E. , Sallusto, F. , Baldanti, F. & Lanzavecchia, A. (2013) Cross‐neutralization of four paramyxoviruses by a human monoclonal antibody. Nature, 501, 439–443. [DOI] [PubMed] [Google Scholar]
- Deffrasnes, C. , Hamelin, M.E. , Prince, G.A. & Boivin, G. (2008) Identification and evaluation of a highly effective fusion inhibitor for human metapneumovirus. Antimicrobial Agents Chemotherapy, 52, 279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del, P.P. , Abarca, K. , Concha, I. & Cerda, J. (2014) Concordance of nasal swabs and nasopharyngeal swabs in the detection of respiratory viruses by direct immunofluorescence. Revista Chilena de Infectologia, 31, 160–164. [DOI] [PubMed] [Google Scholar]
- Dignan, F. , Alvares, C. , Riley, U. , Ethell, M. , Cunningham, D. , Treleaven, J. , Ashley, S. , Bendig, J. , Morgan, G. & Potter, M. (2006) Parainfluenza type 3 infection post stem cell transplant: high prevalence but low mortality. Journal of Hospital Infection., 63, 452–458. [DOI] [PubMed] [Google Scholar]
- Dobson, J. , Whitley, R.J. , Pocock, S. & Monto, A.S. (2000) Oseltamivir treatment for influenza in adults: a meta‐analysis of randomised controlled trials. The Lancet, 385, 1729–1737. [DOI] [PubMed] [Google Scholar]
- Egli, A. , Bucher, C. , Dumoulin, A. , Stern, M. , Buser, A. , Bubendorf, L. , Gregor, M. , Servida, P. , Sommer, G. , Bremerich, J. , Gratwohl, A. , Khanna, N. , Widmer, A.F. , Battegay, M. , Tamm, M. , Hirsch, H.H. & Halter, J.P. (2012) Human metapneumovirus infection after allogeneic hematopoietic stem cell transplantation. Infection, 40, 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fontbrune, F.S. , Robin, M. , Porcher, R. , Scieux, C. , de Latour, R.P. , Ferry, C. , Rocha, V. , Boudjedir, K. , Devergie, A. , Bergeron, A. , Gluckman, E. , Azoulay, E. , Lapalu, J. , Socié, G. & Ribaud, P. (2007) Palivizumab treatment of respiratory syncytial virus infection after allogeneic hematopoietic stem cell transplantation. Clinical Infectious Diseases, 45, 1019–1024. [DOI] [PubMed] [Google Scholar]
- Fraaij, P.L. , van der Vries, E. , Beersma, M.F. , Riezebos‐Brilman, A. , Niesters, H.G. , van der Eijk, A.A. , de Jong, M.D. , Reis Miranda, D. , Horrevorts, A.M. , Ridwan, B.U. , Wolfhagen, M.J. , Houmes, R.J. , van Dissel, J.T. , Fouchier, R.A. , Kroes, A.C. , Koopmans, M.P. , Osterhaus, A.D. & Boucher, C.A. (2011) Evaluation of the antiviral response to zanamivir administered intravenously for treatment of critically ill patients with pandemic influenza A (H1N1) infection. Journal of Infectious Diseases., 2011, 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry, A.M. , Curns, A.T. , Harbour, K. , Hutwagner, L. , Holman, R.C. & Anderson, L.J. (2006) Seasonal trends of human parainfluenza viral infections: United States, 1990‐2004. Clinical Infectious Diseases, 43, 1016–1022. [DOI] [PubMed] [Google Scholar]
- Furuta, Y. , Takahashi, K. , Shiraki, K. , Sakamoto, K. , Smee, D.F. , Barnard, D.L. , Gowen, B.B. , Julander, J.G. & Morrey, J.D. (2009) T‐705 (favipiravir) and related compounds: novel broad‐spectrum inhibitors of RNA viral infections. Antiviral Research, 82, 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, L.E. , Thijsen, S.F. , van Elden, L. & Heemstra, K.A. (2013) Human metapneumovirus in adults. Viruses, 5, 87–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin, M.E. , Gagnon, C. , Prince, G.A. , Kiener, P. , Suzich, J. , Ulbrandt, N. & Boivin, G. (2010) Prophylactic and therapeutic benefits of a monoclonal antibody against the fusion protein of human metapneumovirus in a mouse model. Antiviral Research, 88, 31–37. [DOI] [PubMed] [Google Scholar]
- Hayden, F.G. , Herrington, D.T. , Coats, T.L. , Kim, K. , Cooper, E.C. , Villano, S.A. , Liu, S. , Hudson, S. , Pevear, D.C. , Collett, M. & McKinlay, M. ; Pleconaril Respiratory Infection Study Group . (2003) Efficacy and safety of oral pleconaril for treatment of colds due to picornaviruses in adults: results of 2 double‐blind, randomized, placebo‐controlled trials. Clinical Infectious Diseases, 36, 1523–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrickson, K.J. (2003) Parainfluenza viruses. Clinical Microbiology Reviews, 16, 242–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch, H.H. , Martino, R. , Ward, K.N. , Boeckh, M. , Einsele, H. & Ljungman, P. (2013) Fourth European Conference on Infections in Leukaemia (ECIL‐4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clinical Infectious Diseases, 56, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison, M.G. (2007) Respiratory viral infections in transplant recipients. Antiviral Therapy, 12(4 Pt B), 627–638. [PubMed] [Google Scholar]
- Ison, M.G. (2011) Epidemiology, prevention, and management of influenza in patients with hematologic malignancy. Infectious Disorders ‐ Drug Targets, 11, 34–39. [DOI] [PubMed] [Google Scholar]
- Ison, M.G. , Hayden, F.G. , Kaiser, L. , Corey, L. & Boeckh, M. (2003) Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clinical Infectious Diseases, 36, 1139–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, S.E. , Soave, R. , Shore, T.B. , Satlin, M.J. , Schuetz, A.N. , Magro, C. , Jenkins, S.G. & Walsh, T.J. (2013a) Human rhinovirus infections of the lower respiratory tract in hematopoietic stem cell transplant recipients. Transplant Infectious Disease, 15, 474–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, S.E. , Lamson, D.M. , St George, K. & Walsh, T.J. (2013b) Human rhinoviruses. Clinical Microbiology Reviews., 26, 135–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain, B. , Singh, A.K. , Dangi, T. , Agarwal, A. , Verma, A.K. , Dwivedi, M. , Singh, K.P. & Jain, A. (2014) High prevalence of human metapneumovirus subtype B in cases presenting as severe acute respiratory illness: an experience at tertiary care hospital. The Clinical Respiratory Journal, 8, 225–233. [DOI] [PubMed] [Google Scholar]
- Johnstone, J. , Majumdar, S.R. , Fox, J.D. & Marrie, T.J. (2008) Viral infection in adults hospitalized with community‐acquired pneumonia: prevalence, pathogens, and presentation. Chest, 134, 1141–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos, L. , Chhajed, P.N. , Wallner, J. , Battegay, M. , Steiger, J. , Gratwohl, A. & Tamm, M. (2007) Pulmonary infections diagnosed by BAL: a 12‐year experience in 1066 immunocompromised patients. Respiratory Medicine, 101, 93–97. [DOI] [PubMed] [Google Scholar]
- Khanna, N. , Widmer, A.F. , Decker, M. , Steffen, I. , Halter, J. , Heim, D. , Weisser, M. , Gratwohl, A. , Fluckiger, U. & Hirsch, H.H. (2008) Respiratory syncytial virus infection in patients with hematological diseases: single‐center study and review of the literature. Clinical Infectious Diseases, 46, 402–412. [DOI] [PubMed] [Google Scholar]
- Kim, Y.J. , Guthrie, K.A. , Waghmare, A. , Walsh, E.E. , Falsey, A.R. , Kuypers, J. , Cent, A. , Englund, J.A. & Boeckh, M. (2014) Respiratory syncytial virus in hematopoietic cell transplant recipients: factors determining progression to lower respiratory tract disease. Journal of Infectious Diseases, 209, 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitanovski, L. , Kopriva, S. , Pokorn, M. , Dolničar, M.B. , Rajić, V. , Stefanović, M. & Jazbec, J. (2013) Treatment of severe human metapneumovirus (hMPV) pneumonia in an immunocompromised child with oral ribavirin and IVIG. Journal of Pediatric Hematology/oncology, 35, e311–e313. [DOI] [PubMed] [Google Scholar]
- Lessler, J. , Reich, N.G. , Brookmeyer, R. , Perl, T.M. , Nelson, K.E. & Cummings, D.A. (2009) Incubation periods of acute respiratory viral infections: a systematic review. The Lancet Infectious Disease, 9, 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungman, P. , Ward, K.N. , Crooks, B.N.A. , Parker, A. , Martino, R. , Shaw, P.J. , Brinch, L. , Brune, M. , De La Camara, R. , Dekker, A. , Pauksen, K. , Russell, N. , Schwarer, A.P. & Cordonnier, C. (2001) Respiratory virus infections after stem cell transplantation: a prospective study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplantation, 28, 479–484. [DOI] [PubMed] [Google Scholar]
- Ljungman, P. , de la Camara, R. , Perez‐Bercoff, L. , Abecasis, M , Campuzano, J.B.N. , Cannata‐Ortiz, M.J. , Cordonnier, C. , Einsele, H. , Gonzalez‐Vicent, M. , Espigado, I. , Halter, J. , Martino, R. , Mohty, B. , Sucak, G. , Ullmann, A.J. , Vázquez, L. , Ward, K.N. & Engelhard, D. ; for the Infectious Diseases Working Party of the European Group, for Blood and Marrow Transplantation (EBMT), and the Infectious Complications Subcommittee of the Spanish Group of Haematopoietic Stem‐cell Transplantation (GETH) . (2011) Outcome of pandemic H1N1 infections in hematopoietic stem cell transplant recipients. Haematologica, 96, 1231–1235.21546495 [Google Scholar]
- Marcelin, J.R. , Wilson, J.W. & Razonable, R.R. (2014) Oral ribavirin therapy for respiratory syncytial virus infections in moderately to severely immunocompromised patients. Transplant Infectious Disease, 16, 242–250. [DOI] [PubMed] [Google Scholar]
- Martino, R. , Porras, R.P. , Rabella, N. , Williams, J.V. , Rámila, E. , Margall, N. , Labeaga, R. , Crowe, J.E. Jr , Coll, P. & Sierra, J. (2005) Prospective study of the incidence, clinical features, and outcome of symptomatic upper and lower respiratory tract infections by respiratory viruses in adult recipients of hematopoietic stem cell transplants for hematologic malignancies. Biology of Blood and Marrow Transplantation, 11, 781–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohty, B. , Thomas, Y. , Vukicevic, M. , Nagy, M. , Levrat, E. , Bernimoulin, M. , Kaiser, L. , Roosnek, E. , Passweg, J. & Chalandon, Y. (2012) Clinical features and outcome of 2009‐influenza A (H1N1) after allogeneic hematopoietic SCT. Bone Marrow Transplantation, 47, 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, M.L. , Stokes, K.L. & Hartert, T.V. (2013) The impact of viral genotype on pathogenesis and disease severity: respiratory syncytial virus and humanrhinoviruses. Current Opinion in Immunology., 25, 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICE . (2008) Oseltamivir, amantadine (review) and zanamivir for the prophylaxis of influenza. NICE technology appraisal guidance [TA158], Guidance section. © National Institute for Health and Care Excellence. https://www.nice.org.uk/guidance/ta158/chapter/1-Guidance (last accessed 15 November 2015).
- Nichols, W.G. , Corey, L. , Gooley, T. , Davis, C. & Boeckh, M. (2001) Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood, 98, 573–578. [DOI] [PubMed] [Google Scholar]
- Nichols, W.G. , Guthrie, K.A. , Corey, L. & Boeckh, M. (2004) Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clinical Infectious Disease, 39, 1300–1306. [DOI] [PubMed] [Google Scholar]
- Ohrmalm, L. , Wong, M. , Rotzen‐Ostlund, M. , Norbeck, O. , Broliden, K. & Tolfvenstam, T. (2010) Flocked nasal swab versus nasopharyngeal aspirate for detection of respiratory tract viruses in immunocompromised adults: a matched comparative study. BMC Infectious Diseases, 10, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini, M.G. , Curtis, S.R. , Mathews, A. , Ottolini, S.R. & Prince, G.A. (2002) Palivizumab is highly effective in suppressing respiratory syncytial virus in an immunosuppressed animal model. Bone Marrow Transplantation, 29, 117–120. [DOI] [PubMed] [Google Scholar]
- Peck, A.J. , Corey, L. & Boeckh, M. (2004) Pretransplantation respiratory syncytial virus infection: impact of a strategy to delay transplantation. Clinical Infectious Diseases, 39, 673–680. [DOI] [PubMed] [Google Scholar]
- Pevear, D.C. , Hayden, F.G. , Demenczuk, T.M. , Barone, L.R. , McKinlay, M.A. & Collett, M.S. (2005) Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses. Antimicrobial Agents and Chemotherapy, 49, 4492–4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pica, N. & Bouvier, N.M. (2012) Environmental factors affecting the transmission of respiratory viruses. Current Opinion in Virology., 2, 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health England . (2013) Influenza In: Immunisation Against Infectious Disease (Eds: Salisbury, D., Ramsay, M. Chapter 19. © Crown Copyright. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/456568/2904394_Green_Book_Chapter_19_v10_0.pdf (last accessed 1 November 2015). [Google Scholar]
- Renaud, C. & Campbell, A.P. (2011) Changing epidemiology of respiratory viral infections in hematopoietic cell transplant recipients and solid organ transplant recipients. Current Opinion in Infectious Diseases, 24, 333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud, C. , Xie, H. , Seo, S. , Kuypers, J. , Cent, A. , Corey, L. , Leisenring, W. , Boeckh, M. & Englund, J.A. (2013) Mortality rates of human metapneumovirus and respiratory syncytial virus lower respiratory tract infections in hematopoietic cell transplantation recipients. Biology of Blood and Marrow Transplantation, 19, 1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, L.G. , Levin, M.J. , Ljungman, P. , Davies, E.G. , Avery, R. , Tomblyn, M. , Bousvaros, A. , Dhanireddy, S. , Sung, L. , Keyserling, H. & Kang, I. (2014) Infectious diseases society of America. Clinical Infectious Disease, 58, 309–318. [DOI] [PubMed] [Google Scholar]
- Safdar, A. (2008) Immune modulatory activity of ribavirin for serious human metapneumovirus disease: early i.v. therapy may improve outcomes in immunosuppressed SCT recipients. Bone Marrow Transplantation, 41, 707–708. [DOI] [PubMed] [Google Scholar]
- Schiffer, J.T. , Kirby, K. , Sandmaier, B. , Storb, R. , Corey, L. & Boeckh, M. (2009) Timing and severity of community acquired respiratory virus infections after myeloablative versus non‐myeloablative hematopoietic stem cell transplantation. Haematologica, 94, 1101–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomacker, H. , Schaap‐Nutt, A. , Collins, P.L. & Schmidt, A.C. (2012) Pathogenesis of acute respiratory illness caused by human parainfluenza viruses. Current Opinion in Virology., 2, 294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shachor‐Meyouhas, Y. , Ben‐Barak, A. & Kassis, I. (2012) Treatment with oral ribavirin and IVIG of severe human metapneumovirus pneumonia (HMPV) in immune compromised child. Pediatric Blood & Cancer, 57, 350–351. Erratum in: Pediatr Blood Cancer., 58, 319. [DOI] [PubMed] [Google Scholar]
- Shah, J.N. & Chemaly, R.F. (2011) Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood, 117, 2755–2763. [DOI] [PubMed] [Google Scholar]
- Shah, D.P. , Ghantoji, S.S. , Shah, J.N. , El Taoum, K.K. , Jiang, Y. , Popat, U. , Hosing, C. , Rondon, G. , Tarrand, J.J. , Champlin, R.E. & Chemaly, R.F. (2013) Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. Journal of Antimicrobial Chemotherapy, 68, 1872–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah, D.P. , Ghantoji, S.S. , Ariza‐Heredia, E.J. , Shah, J.N. , El Taoum, K.K. , Shah, P.K. , Nesher, L. , Hosing, C. , Rondon, G. , Champlin, R.E. & Chemaly, R.F. (2014) Immunodeficiency scoring index to predict poor outcomes in hematopoietic cell transplant recipients with RSV infections. Blood, 123, 3263–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahda, S. , Carlos, W.G. , Kiel, P.J. , Khan, B.A. & Hage, C.A. (2011) The human metapneumovirus: a case series and review of the literature. Transplant Infectious Diseases, 13, 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima, T. , Yoshimoto, G. , Nonami, A. , Yoshida, S. , Kamezaki, K. , Iwasaki, H. , Takenaka, K. , Miyamoto, T. , Harada, N. , Teshima, T. , Akashi, K. & Nagafuji, K. (2008) Successful treatment of parainfluenza virus 3 pneumonia with oral ribavirin and methylprednisolone in a bone marrow transplant recipient. International Journal of Hematology, 88, 336–340. [DOI] [PubMed] [Google Scholar]
- Sleeman, K. , Mishin, V.P. , Deyde, V.M. , Furuta, Y. , Klimov, A.I. & Gubareva, L.V. (2010) In vitro antiviral activity of favipiravir (T‐705) against drug‐resistant influenza and 2009 A(H1N1) viruses. Antimicrobial Agents and Chemotherapy, 54, 2517–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrelid, E. , Ljungman, P. , Ekelöf‐Andström, E. , Aschan, J. , Ringdén, O. , Winiarski, J. , Wåhlin, B. & Andersson, J. (1997) Ribavirin therapy in bone marrow transplant recipients with viral respiratory tract infections. Bone Marrow Transplantation, 19, 905–908. [DOI] [PubMed] [Google Scholar]
- Stankova, J. , Carret, A.S. , Moore, D. , McCusker, C. , Mitchell, D. , Davis, M. , Mazer, B. & Jabado, N. (2007) Long‐term therapy with aerosolized ribavirin for parainfluenza 3 virus respiratory tract infection in an infant with severe combined immunodeficiency. Pediatric Transplantation, 11, 209–213. [DOI] [PubMed] [Google Scholar]
- The IMpact‐RSV Study Group . (1998) Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high‐risk infants. Pediatrics, 1998, 531–537. [PubMed] [Google Scholar]
- Tomblyn, M. , Chiller, T. , Einsele, H. , Gress, R. , Sepkowitz, K. , Storek, J. , Wingard, J.R. , Young, J.A. & Boeckh, M.J. ; Center for International Blood and Marrow Research, National Marrow Donor program, European Blood and MarrowTransplant Group, American Society of Blood and Marrow Transplantation, Canadian Blood and Marrow Transplant Group; Infectious Diseases Society of America, Society for Healthcare Epidemiology of America, Association of Medical Microbiology and Infectious Disease Canada, Centers for Disease Control and Prevention . (2009) Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biology of Blood and Marrow Transplantation, 15, 1143–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunsjo, H.S. , Berg, A.S. , Inchley, C.S. , Roberg, I.K. & Leegaard, T.M. (2015) Comparison of nasopharyngeal aspirate with flocked swab for PCR‐detection of respiratory viruses in children. APMIS, 123, 473–477. 2015 Apr 23 doi: 10.1111/apm.12375 [DOI] [PubMed] [Google Scholar]
- Ulbrandt, N.D. , Ji, H. , Patel, N.K. , Barnes, A.S. , Wilson, S. , Kiener, P.A. , Suzich, J. & McCarthy, M.P. (2008) Identification of antibody neutralization epitopes on the fusion protein of humanmetapneumovirus. Journal of General Virology, 89, 3113–3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, E.E. , Falsey, A.R. & Hennessey, P.A. (1999) Respiratory syncytial and other virus infections in persons with chronic cardiopulmonary disease. American Journal of Respiratory and Critical Care Medicine, 160, 791–795. [DOI] [PubMed] [Google Scholar]
- Walsh, E.E. , Peterson, D.R. & Falsey, A.R. (2008) Human metapneumovirus infections in adults: another piece of the puzzle. Archives of Internal Medicine, 168, 2489–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whimbey, E. , Englund, J.A. & Couch, R.B. (1997) Community respiratory virus infections in immunocompromised patients with cancer. American Journal of Medicine, 102, 10–18; discussion 25‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer, K. , Zhu, Y. , Williams, J.V. , Griffin, M.R. , Edwards, K.M. & Talbot, H.K. (2012) Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. Journal of Infectious Diseases, 206, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson, J.C. & Pegram, P.S. (2000) Respiratory distress associated with zanamivir. New England Journal of Medicine, 342, 661–662. [DOI] [PubMed] [Google Scholar]
- Wyde, P.R. , Chetty, S.N. , Jewell, A.M. , Boivin, G. & Piedra, P.A. (2003) Comparison of the inhibition of human metapneumovirus and respiratory syncytial virus by ribavirin and immune serum globulin in vitro. Antiviral Research, 60, 51–59. [DOI] [PubMed] [Google Scholar]


