Summary
Background The development of antibiotic resistance by microorganisms is an increasing problem in medicine. In chronic wounds, bacterial colonization is associated with impaired healing. Cold atmospheric plasma is an innovative promising tool to deal with these problems.
Objectives The 5‐min argon plasma treatment has already demonstrated efficacy in reducing bacterial numbers in chronic infected wounds in vivo. In this study we investigated a 2‐min plasma treatment with the same device and the next‐generation device, to assess safety and reduction in bacterial load, regardless of the kind of bacteria and their resistance level in chronic wounds.
Methods Twenty‐four patients with chronic infected wounds were treated in a prospective randomized controlled phase II study with 2 min of cold atmospheric argon plasma every day: 14 with MicroPlaSter alpha device, 10 with MicroPlaSter beta device (next‐generation device) in addition to standard wound care. The patient acted as his/her own control. Bacterial species were detected by standard bacterial swabs and bacterial load by semiquantitative count on nitrocellulose filters. The plasma settings were the same as in the previous phase II study in which wounds were exposed for 5 min to argon plasma.
Results Analysis of 70 treatments in 14 patients with the MicroPlaSter alpha device revealed a significant (40%, P < 0·016) reduction in bacterial load in plasma‐treated wounds, regardless of the species of bacteria. Analysis of 137 treatments in 10 patients with the MicroPlaSter beta device showed a highly significant reduction (23·5%, P < 0·008) in bacterial load. No side‐effects occurred and the treatment was well tolerated.
Conclusions A 2‐min treatment with either of two cold atmospheric argon plasma devices is a safe, painless and effective technique to decrease the bacterial load in chronic wounds.
Chronic ulcers remain a challenge. The most frequent is the venous ulcer, affecting 1–2% of the general population. Venous ulcers require an average of 24 weeks to heal and are associated with considerable patient morbidity; 15% never heal, and up to 71% recur at least once and often multiple times. 1 , 2 They are of considerable socioeconomic importance as they account for 1–2% of the annual healthcare budget in European countries. 3 Bacterial colonization of such wounds is common and is a well‐recognized factor contributing to impaired wound healing. 4 , 5 , 6
Techniques to minimize or avoid the use of antibiotics are required to reduce the development of antibiotic resistance.
Physical science could offer a promising tool. Cold atmospheric plasma (CAP) is the fourth state of matter, in addition to solid, liquid and gas phases. The weakly ionized plasma consists of a highly active mix of oxygen, nitrogen and hydrogen radicals, ions, electrons, photons and ultraviolet (UV) radiation (UVR), and should not be confused with the more familiar blood plasma.
High‐temperature plasmas are already in medical use for the sterilization of equipment, tissue destruction, cutting and cauterizing. 7 , 8 , 9 CAPs have the same advantages as high‐temperature plasmas but without the enormous heat production. Compared with other CAP technologies, the indirect microwave‐driven argon plasma technique has several benefits: no current passes through the patient’s body, an even plasma dose is delivered including to rough surfaces which are common in ulcers, and a large area of up to 6 cm in diameter can be treated in a single application. Because CAPs can be used for in vivo applications without harming surrounding tissue they are of considerable interest in medicine. 10
The physical and chemical characteristics of plasmas allow penetration of small cavities, such as hair follicles, where disinfectants fail to reach. 11 , 12 , 13 Resistance is unlikely to develop to CAPs as plasma is hypothesized to attack pathogens by several processes including reactive species, charging, permeabilization, local energy deposition and electroporation. 11 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21
In 2010 we reported a prospective trial of 5 min argon plasma treatment using a CAP device called MicroPlaSter alpha, that led to a highly significant reduction in bacterial load compared with standard wound care alone. 22 No side‐effects occurred during the clinical trial and the antibacterial effects were independent of the species of bacteria and their drug resistance. A second‐generation device, called MicroPlaSter beta, is more compact, being approximately 25% smaller in total size than the original MicroPlaSter alpha, and has a flexible four‐joint treatment arm, which allows treatment of hard‐to‐reach areas. A rapid clinical improvement has been reported in a patient with Hailey–Hailey disease and secondary infection with Candida albicans and Proteus mirabilis using the new MicroPlaStar beta. 23
In this study a shorter treatment time has been evaluated and the efficacy of the original and second‐generation CAP devices in reducing bacterial load on chronic wounds compared.
Materials and methods
Patient selection criteria
Patients were invited to participate in the trial if they had at least one chronic infected skin wound large enough for the plasma treatment and a control area of 3 cm2 and who attended the outpatient and inpatient clinics of the Department of Dermatology, Allergology and Environmental Medicine of Hospital Munich Schwabing, Germany. The ethics committee of the Bavarian State Association approved the clinical trial. All patients signed informed consent forms.
Exclusion criteria were patients under 18 years of age, pregnant and lactating women, patients with cancer or dementia and patients who declined or withdrew consent.
Plasma device and configuration
The devices used during the trial were microwave‐driven CAP devices, called MicroPlaSter alpha and beta, designed by the Max Planck Institute for Extraterrestrial Physics, Garching, Germany and manufactured by ADTEC Plasma Technology Co. Ltd (Hiroshima, Japan/London, U.K.). Both devices used the same setting: microwave frequency 2·46 GHz, voltage 50–100 V, power 86 W; argon gas flow 2·2 slm. Figure 1 shows the plasma torch during the treatment of a patient with a chronic ulcer on the medial malleolus of the left ankle.
Figure 1.
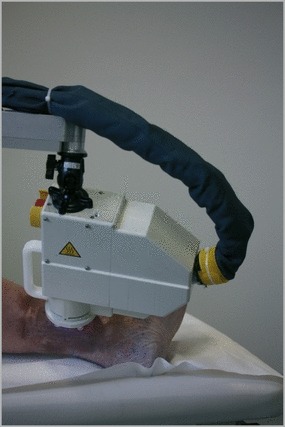
Patient during treatment with MicroPlaSter beta device.
The emission spectrum of MicroPlaSter beta in the UVA, UVB and UVC range was measured using a Hamamatsu UV‐Power Meter C8026 (Hamamatsu Photonics, Hamamatsu, Japan) (Fig. 2). The spectrum of the alpha device is identical.
Figure 2.
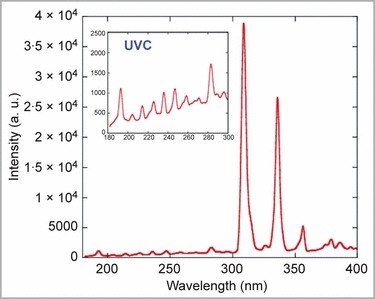
Ultraviolet (UV) spectrum of the MicroPlaSter beta device in the range of 180–400 nm, with a special focus on the UVC spectrum. UV spectrum of the alpha device is identical, due to the same configurations.
Treatment protocol
In addition to standard wound care, patients received a 2‐min cold plasma treatment to the randomized wound(s) using the MicroPlaSter alpha or beta device every day. Control wounds remained undressed during the plasma treatment. The same standard wound care was applied to both plasma‐treated and control areas.
If a fibrin layer developed on the wound, debridement was performed using a scalpel or curette before the plasma therapy or standard wound care.
Assessment of the bacterial load
Once each week, standard bacterial swabs were taken from all control and plasma‐treated areas directly after removal of the dressing and again before redressing for identification of bacterial species present in the wound and antibiotic sensitivity testing. Incubation time and evaluation of swabs was according to the standard operating procedure of the German Society for Hygiene and Microbiology. 24
On all other days of the week, changes in bacterial load were assessed using nitrocellulose filters (Sartorius Stedim Biotech GmbH, Aubagne, France) gently pressed on to the wound before and after treatment 25 then placed on Columbia blood agar plates (Oxoid Ltd, Basingstoke, U.K.) and incubated overnight at 36 °C (according to DIN 58958‐1 26 ) to detect rapidly growing aerobic bacteria. Semiquantitative assessment of the plates was performed by a manual count of the colony‐forming units (CFU) at the Department of Microbiology, Hospital Munich Schwabing, Germany. All colonies were counted if the bacterial load was low (< 300 CFU). If the colony count was higher, one quarter of the nitrocellulose filter boxes on the plate surface were counted, multiplied out then rounded up and down to the nearest full hundreds. The arithmetic mean was formed from both rounded off values and used as the bacterial count for calculations. In cases of very high bacterial load (CFU > 1000/1500) the load was equated to 1000 and 1500, respectively.
Assessment of the treatment tolerability
Pain, a possible side‐effect, was specifically asked about and documented according to a standardized World Health Organization (WHO) score from 0 to 10.
Treatment endpoints
The plasma treatment was stopped for one of four reasons: discharge from hospital, three consecutive negative bacterial swabs or nitrocellulose filters, the wound healed (including operative closure by mesh graft) or the patient elected to stop the plasma treatment. Follow‐up ceased after the third consecutive negative bacterial swab/filter or after discharge of the patient.
Data analysis
Data were entered, checked and analysed using SPSS 12.0 (SPSS Inc., Chicago, IL, U.S.A.) and IDL 7.0 (ITT Visual Information Solutions, Boulder, CO, U.S.A.). Results were expressed as median bacterial count reduction in percentage or as log‐return. Statistical tests used were the Mann–Whitney U‐test, the log‐return and the bootstrap test. P < 0·05 was considered as significant and P < 0·01 as highly significant.
Results
Patient characteristics
In total, 24 patients were treated with CAP. Table 1 summarizes the patient demographics, wound characteristics and the use of systemic antibiotics due to clinical signs of infection. Most ulcers were venous in aetiology. Other causes included arterial, arterial‐venous and traumatic.
Table 1.
Patient and wound characteristics in the two treatment groups
| Patient | Age (years) | Sex | Duration of ulcer (months) | Origin of ulcer | Number of ulcers | Antibiotic use |
|---|---|---|---|---|---|---|
| MicroPlaster alpha | ||||||
| 1 | 73 | M | > 6 | Arterial | 2 | Yes |
| 2 | 76 | M | > 3 | Arterial | 1 | Yes |
| 3 | 84 | F | > 6 | Venous | 1 | Yes |
| 4 | 83 | F | > 6 | Arterial | 1 | Yes |
| 5 | 67 | F | > 6 | Venous | 1 | Yes |
| 6 | 84 | F | > 6 | Arterial | 3 | Yes |
| 7 | 67 | M | > 6 | Venous | 1 | Yes |
| 8 | 73 | M | > 6 | Venous | 1 | Yes |
| 9 | 76 | F | > 6 | Venous | > 6 | Yes |
| 10 | 85 | F | > 6 | Venous | 1 | Yes |
| 11 | 67 | M | > 6 | Arterial + venous | 1 | Yes |
| 12 | 73 | M | > 6 | Venous | 1 | Yes |
| 13 | 49 | F | > 3 | Venous | 3 | Yes |
| 14 | 56 | F | > 3 | Venous | 2 | Yes |
| MicroPlaster beta | ||||||
| 1 | 41 | M | > 6 | Venous | 1 | Yes |
| 2 | 70 | F | > 3 | Venous | 2 | Yes |
| 3 | 69 | F | > 6 | Traumatic | 2 | No |
| 4 | 84 | M | > 6 | Venous | 1 | Yes |
| 5 | 87 | M | > 6 | Arterial + venous | 1 | Yes |
| 6 | 75 | F | > 6 | Venous | 2 | No |
| 7 | 74 | M | > 6 | Venous | 2 | Yes |
| 8 | 84 | M | > 6 | Venous | 2 | Yes |
| 9 | 85 | F | > 6 | Venous | 1 | Yes |
| 10 | 88 | F | > 6 | Venous | 1 | Yes |
Fourteen patients (six men and eight women; age range 49–85 years, mean 72·4) were treated with the MicroPlaSter alpha device between February 2009 and August 2009. Two patients in this treatment group were enrolled twice due to two different hospitalizations with the same ulcers.
The MicroPlaSter beta device was used to treat 10 patients (five men and five women; age range 41–88 years, mean 76·0) between December 2009 and May 2011. Two outpatients had particularly long treatments in this subgroup.
The duration of ulcer(s) before enrolment was 3–6 months for four patients (17%); all other patients had ulcer(s) persisting for at least 6 months and ranging up to more than 6 years. Fourteen patients had one large wound that included both plasma and control areas. Ten patients had two or more separate wounds. Figure 3 shows the bacterial diversity detected on wounds of each plasma device group.
Figure 3.
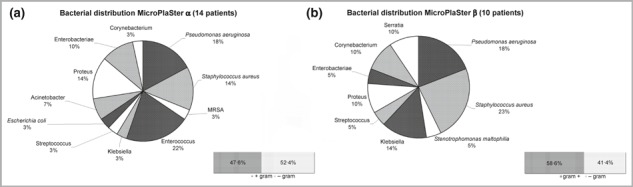
Species/genera and Gram‐distribution of bacteria detected on wounds by weekly wound swabs (a) in MicroPlaSter alpha group and (b) in MicroPlaSter beta group. MRSA, methicillin‐resistant Staphylococcus aureus.
Treatment
All 24 patients received standard wound care to all wounds; 22 patients also received systemic antibiotics (92%). In the MicroPlaSter alpha subgroup, a mean of 7·6 cold argon plasma treatments was administered per randomized wound (range 1–17; total treatments n = 70). In the MicroPlaSter beta subgroup a mean of 15·9 cold argon plasma treatments was administered per randomized wound (range 1–71; total treatments n = 137). Where patients had only one large wound, the control and plasma‐treated areas were separated by at least 0·5 cm.
Outcomes
The bacterial load, as determined by the CFU count before and after MicroPlaSter alpha device treatments (n = 70), was significantly reduced (40%, P < 0·016), regardless of the species of bacteria identified on weekly swabs (Fig. 4, left). Treatment with the MicroPlaSter beta device (n = 137) resulted in a highly significant reduction (23·5%, P < 0·008) of bacterial load (Fig. 4, right).
Figure 4.
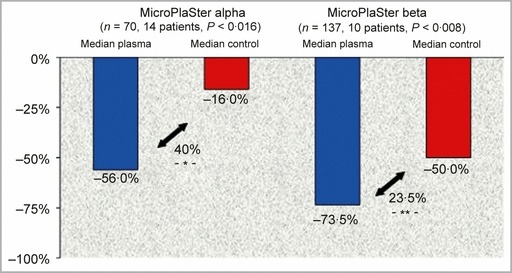
Left panel: MicroPlaSter alpha device. Significant reduction in bacterial count (40%, P < 0·016) in plasma‐treated area (blue bar) compared with standard wound care alone (red bar). Right panel: MicroPlaSter beta device. Highly significant reduction in bacterial count (23·5%, P < 0·008) in plasma‐treated area (blue bar) compared with standard wound care alone (red bar).
The reduction in bacterial count was also calculated using the log‐return to avoid a possible statistical problem due to percentage changes. With the MicroPlaSter alpha device there was a highly significant (P < 0·002) median log reduction of −1·15 in the treated area compared with −0·26 in the control area. The MicroPlaSter beta device also showed a highly significant (P < 0·002) median log reduction of −1·1 in the treated area vs. −0·69 in the control area (Fig. 5).
Figure 5.
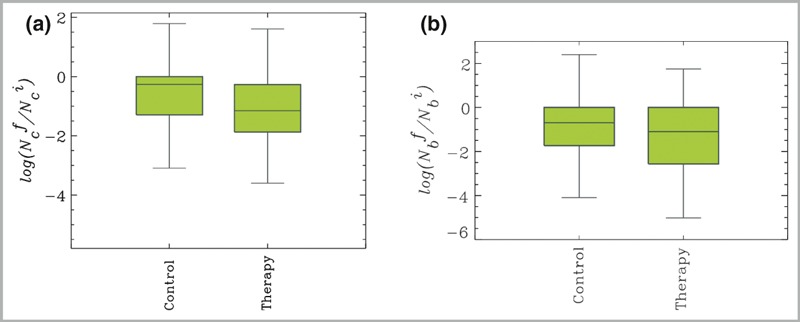
(a) Box plots of the log‐returns of the number of colonies of MicroPlaSter alpha group, N
c indicating the reduction in bacterial count: −1·15 log reduction of bacterial load in plasma‐treated area compared with a −0·26 reduction in control area (P < 0·002). The y‐axis is defined as 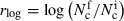 , where
, where 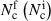 . is the number of colonies after (before) treatment, respectively. (b) MicroPlaSter beta group with median log reduction of −1·1 in treated area vs. a −0·69 reduction in control area (P < 0·002).
. is the number of colonies after (before) treatment, respectively. (b) MicroPlaSter beta group with median log reduction of −1·1 in treated area vs. a −0·69 reduction in control area (P < 0·002).
Tolerability
No side‐effects were reported and the treatment was well tolerated in all cases. One patient reported wound pain both before and after the plasma treatment, but this was not aggravated by the treatment.
Discontinuation
One patient discontinued the plasma treatment because of ‘too much effort’ for a daily treatment. One patient was not able to continue the treatment as a physical handicap limited his ability to keep the area in a fixed position for the 2‐min treatment. The patient who reported pain before and after the treatment discontinued because of general pain during the whole hospitalization period.
Discussion
This study clearly demonstrates that a short 2‐min plasma treatment is sufficient to achieve a significant or even highly significant reduction in the bacterial load on chronic infected wounds in vivo. Efficacy and tolerability were demonstrated with both generations of devices (MicroPlaSter alpha and MicroPlaSter beta). The effects of the plasma treatment on bacterial load did not depend on the cause of the chronic wound or on the bacterial species present. This study could not assess the effects on wound healing.
The exact numbers calculated cannot be compared directly across the two devices or the two treatment times as these are in vivo studies involving three patient groups with some differences in patient and wound characteristics. The alpha group had more patients with fewer treatments compared with the beta group with fewer patients but more treatments. This difference occurred due to the availability of the two devices defining the recruitment periods. The total number of patients treated with the MicroPlaSter alpha device was smaller in the 2‐min group than in the previously reported 5‐min group, which may account for the lower level of significance despite an apparently greater reduction in bacterial load. The bacterial genera and species present in the wounds may also be relevant. The inclusion of both inpatients and outpatients may influence the calculations, as outpatients did not attend on weekends for a dressing change compared with inpatients who had daily dressing changes.
The reduction in bacterial load on control areas between removal of the dressing and redressing may have been due to the mechanical debridement caused by the nitrocellulose filter technique and drying effect when the wound was open to the air, which decreases the ability of microorganisms to stick to the filter surface. We cannot exclude a possible overflow of the active agents to the control area in patients with a single large wound divided into plasma‐treated and control areas, although a safety zone of at least 0·5 cm was kept between the two areas. Although most of the patients in this study were treated with systemic antibiotics as well, the antibiotics would affect both the control and plasma‐treated wound equally.
These results are consistent with those previously published for the 5‐min treatment regimen. 22 The 2‐min plasma treatment was evaluated for two reasons. The first was to assess whether reduced application time compromised efficacy, as a shorter time would make treatment more convenient for the patient and less time‐ and argon gas‐consuming for wound care facilities, important issues in terms of cost efficiency.
The second reason was to reduce the exposure to UVR. CAP therapy is a new technique so there are as yet no regulations and limits for safety issues, a situation comparable with the first studies with medical lasers decades ago. The technique to generate plasma is the same in both devices and they operate with the same technical specifications, so there are no differences in UV emission. We calculated the total integrated erythemal‐weighted irradiance of the MicroPlaSter alpha device as follows:
The maximum recommended dose for intact skin according to the WHO guidelines of the International Commission on Non‐Ionizing Radiation Protection is 0·30 W m−2. 27 Thus the dose in this trial is below the limit for intact skin. However, as we have been treating chronic wounds rather than intact skin, we can only refer to recommendations of a European Commission Report (Scientific Committee on Consumer Products Report 0949/05 28 ) for open wounds or unprotected skin and use a modified erythema action spectrum to calculate the total erythemal weighted irradiance: 28
This value is still below the WHO limit of 0·30 W m−2 and is therefore still safe for chronic wounds.
The MicroPlaStar optical emission spectrum includes the UVA, UVB and, to a lesser extent, UVC range (Fig. 2), which is known to be carcinogenic due to its capacity to dimerize adjacent thymines in DNA and thereby inhibit the replication of DNA. 29 , 30 The optical emission spectra of the UVR produced by the MicroPlaSter alpha/beta was compared with those emitted by the sun during sun peak hours based on the annual mean in Garching (Munich, Germany), which has the same altitude/latitude as the Department of Dermatology in Munich. One minute of MicroPlaSter treatment gives the same UVC dose as 5 min of sunlight. For UVB, a 1‐min application is equivalent to 1 min solar exposure and for UVA 1 min of treatment corresponds to 10 s of sun exposure.
Even though the UV exposure was within the safe range with the 5 min treatment protocol, possible long‐term effects may be limited by reducing treatment time, especially for patients requiring long‐term plasma treatment.
The antibacterial mechanisms of plasmas are not yet fully understood. However, bacterial resistance is unlikely to develop due to the complex chemical and physical diversity of plasmas and the direct effect on the bacteria’s natural environment.
In conclusion, this innovative technique offers dermatologists and wound care specialists a short‐duration, safe and painless treatment to decrease the bacterial load of chronic wounds effectively, independent of the colonizing species.
What’s already known about this topic?
-
•
A 5‐min cold atmospheric argon plasma treatment results in a highly significant reduction of the bacterial load in chronic infected wounds.
-
•
The effect is independent of bacterial species and resistance level.
What does this study add?
-
•
A 2‐min treatment is sufficient for significant bactericidal effect.
-
•
The effect is demonstrated in two generations of plasma devices.
-
•
Plasma treatment is safe concerning ultraviolet radiation dose.
Acknowledgments
We thank ADTEC Plasma Technology Co. Ltd, Hiroshima for the allocation of the MicroPlaSter alpha and beta devices. We are indebted to Delwyn Dyall‐Smith, FACD, for proofreading the manuscript.
Funding sources The phase II study for the treatment of infected wounds with cold plasma was supported by the Max Planck Institute for Extraterrestrial Physics, Munich. There were no funding sources related to the writing of this original article.
Conflicts of interest The following authors are designated inventors of the patent of the plasma applicator and corresponding method: G.I., G.M., H.‐U.S., T.S., B.S. and W.S.
References
- 1. Heit JA. Venous thromboembolism epidemiology: implications for prevention and management. Semin Thromb Hemost 2002; 28 (Suppl. 2):3–13. [DOI] [PubMed] [Google Scholar]
- 2. Kurz X, Kahn SR, Abenhaim L et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence‐based report of the VEINES task force. Venous Insufficiency Epidemiologic and Economic Studies. Int Angiol 1999; 18:83–102. [PubMed] [Google Scholar]
- 3. Etufugh CN, Phillips TJ. Venous ulcers. Clin Dermatol 2007; 25:121–30. [DOI] [PubMed] [Google Scholar]
- 4. Breidenbach WC, Trager S. Quantitative culture technique and infection in complex wounds of the extremities closed with free flaps. Plast Reconstr Surg 1995; 95:860–5. [PubMed] [Google Scholar]
- 5. Robson MC. Wound infection. A failure of wound healing caused by an imbalance of bacteria. Surg Clin North Am 1997; 77:637–50. [DOI] [PubMed] [Google Scholar]
- 6. Robson MC, Heggers JP. Delayed wound closure based on bacterial counts. J Surg Oncol 1970; 2:379–83. [DOI] [PubMed] [Google Scholar]
- 7. Bogle MA, Arndt KA, Dover JS. Evaluation of plasma skin regeneration technology in low‐energy full‐facial rejuvenation. Arch Dermatol 2007; 143:168–74. [DOI] [PubMed] [Google Scholar]
- 8. Elsaie ML, Kammer JN. Evaluation of plasma skin regeneration technology for cutaneous remodeling. J Cosmet Dermatol 2008; 7:309–11. [DOI] [PubMed] [Google Scholar]
- 9. Kilmer S, Semchyshyn N, Shah G et al. A pilot study on the use of a plasma skin regeneration device (Portrait PSR3) in full facial rejuvenation procedures. Lasers Med Sci 2007; 22:101–9. [DOI] [PubMed] [Google Scholar]
- 10. Heinlin J, Isbary G, Stolz W et al. Plasma applications in medicine with a special focus on dermatology. J Eur Acad Dermatol Venereol 2012; 25:1–11. [DOI] [PubMed] [Google Scholar]
- 11. Kong MG, Kroesen G, Morfill G et al. Plasma medicine: an introductory review. New J Phys 2009; 11:115012. [Google Scholar]
- 12. Lademann O, Kramer A, Richter H et al. Antisepsis of the follicular reservoir by treatment with tissue‐tolerable plasma (TTP). Laser Phys Lett 2011; 8:313–17. [Google Scholar]
- 13. Lademann O, Richter H, Meinke MC et al. Drug delivery through the skin barrier enhanced by treatment with tissue‐tolerable plasma. Exp Dermatol 2011; 20:488–90. [DOI] [PubMed] [Google Scholar]
- 14. Kotnik T, Mir LM, Flisar K et al. Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses – Part I. Increased efficiency of permeabilization. Bioelectrochemistry 2001; 54:83–90. [DOI] [PubMed] [Google Scholar]
- 15. Leduc M, Guay D, Leask RL, Coulombe S. Cell permeabilization using a non‐thermal plasma. New J Phys 2009; 11:115021. [Google Scholar]
- 16. Liburdy RP, Vanek PF. Microwaves and the cell‐membrane. 2. Temperature, plasma, and oxygen mediate microwave‐induced membrane‐permeability in the erythrocyte. Radiat Res 1985; 102:190–205. [PubMed] [Google Scholar]
- 17. Morfill GE, Kong MG, Zimmermann JL. Focus on plasma medicine. New J Phys 2009; 11:115011. [Google Scholar]
- 18. Nosenko T, Shimizu T, Morfill GE. Designing plasmas for chronic wound disinfection. New J Phys 2009; 11:115013. [Google Scholar]
- 19. Stoffels E, Sakiyama Y, Graves DB. Cold atmospheric plasma: charged species and their interactions with cells and tissues. IEEE Trans Plasma Sci 2008; 36:1441–57. [Google Scholar]
- 20. Yonson S, Coulombe S, Leveille V et al. Cell treatment and surface functionalization using a miniature atmospheric pressure glow discharge plasma torch. J Phys D 2006; 39:3508–13. [Google Scholar]
- 21. Kushner MJ, Babaeva NY. Models for the Interaction of Dielectric Barrier Discharges with Exposed Cells and Tissues Under Liquids. Presented at 3rd International Conference on Plasma Medicine (ICPM‐3), Greifswald, Germany, 2010. [Google Scholar]
- 22. Isbary G, Morfill G, Schmidt HU et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br J Dermatol 2010; 163:78–82. [DOI] [PubMed] [Google Scholar]
- 23. Isbary G, Morfill G, Zimmermann J et al. Cold atmospheric plasma: a successful treatment of lesions in Hailey–Hailey disease. Arch Dermatol 2011; 147:388–90. [DOI] [PubMed] [Google Scholar]
- 24. Podbielski A, Herrmann M, Kniehl E et al. MiQ: Quality Standards in Microbiological/Infectiological Diagnostics. Amsterdam: Elsevier GmbH, 2007; 1–58. [Google Scholar]
- 25. Craythorn JM, Barbour AG, Matsen JM et al. Membrane filter contact technique for bacteriological sampling of moist surfaces. J Clin Microbiol 1980; 12:250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. German Institute for Standardization . Medical Microbiology – Microbiological Urine Analysis – Part 1. Rapidly Growing Bacteria and Yeasts Which can be Cultured Aerobically. DIN 58958‐1:2008. Berlin: German Institute for Standardization, 2008. [Google Scholar]
- 27. International Commission on Non‐Ionizing Radiation Protection (ICNIRP) . Guidlines on Limits of Exposure to Ultraviolet Radiation of Wavelengths Between 180 nm and 400 nm (Incoherent Optical Radiation). Munich: ICNIRP, 2004. [DOI] [PubMed] [Google Scholar]
- 28. European Commission . Biological Effects of Ultraviolet Radiation Relevant to Health with Particular Reference to Sunbeds for Cosmetic Purposes. Scientific Committee on Consumer Products Report 0949/05. Brussels: European Commission, 2006. [Google Scholar]
- 29. Mitchell DL, Jen J, Cleaver JE. Sequence specificity of cyclobutane pyrimidine dimers in DNA treated with solar (ultraviolet B) radiation. Nucleic Acids Res 1992; 20:225–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Xue Y, Nicholson WL. The two major spore DNA repair pathways, nucleotide excision repair and spore photoproduct lyase, are sufficient for the resistance of Bacillus subtilis spores to artificial UV‐C and UV‐B but not to solar radiation. Appl Environ Microbiol 1996; 62:2221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


