Abstract
Genome replication and assembly of viruses often takes place in specific intracellular compartments where viral components concentrate, thereby increasing the efficiency of the processes. For a number of viruses the formation of ‘factories’ has been described, which consist of perinuclear or cytoplasmic foci that mostly exclude host proteins and organelles but recruit specific cell organelles, building a unique structure. The formation of the viral factory involves a number of complex interactions and signalling events between viral and cell factors. Mitochondria, cytoplasmic membranes and cytoskeletal components frequently participate in the formation of viral factories, supplying basic and common needs for key steps in the viral replication cycle.
Keywords: viral factories, viral morphogenesis, viral replication and assembly
Abbreviations used:
- ASFV
African swine fever virus
- BUNV
bunyamwera virus
- CPV
cytopathic vacuole
- EEV
extracellular enveloped virus
- ER
endoplasmic reticulum
- ERGIC
ER‐Golgi intermediate compartment
- HHV
human herpesvirus
- HSV
herpes simplex virus
- IEV
intracellular enveloped virus
- IF
intermediate filament
- IMV
intracellular mature virion
- IV
immature virus
- NS protein
non‐structural protein
- RER
rough ER
- RUBV
rubella virus
- SFV
Semliki Forest virus
- SINV
sindbis virus
- VV
vaccinia virus
Introduction
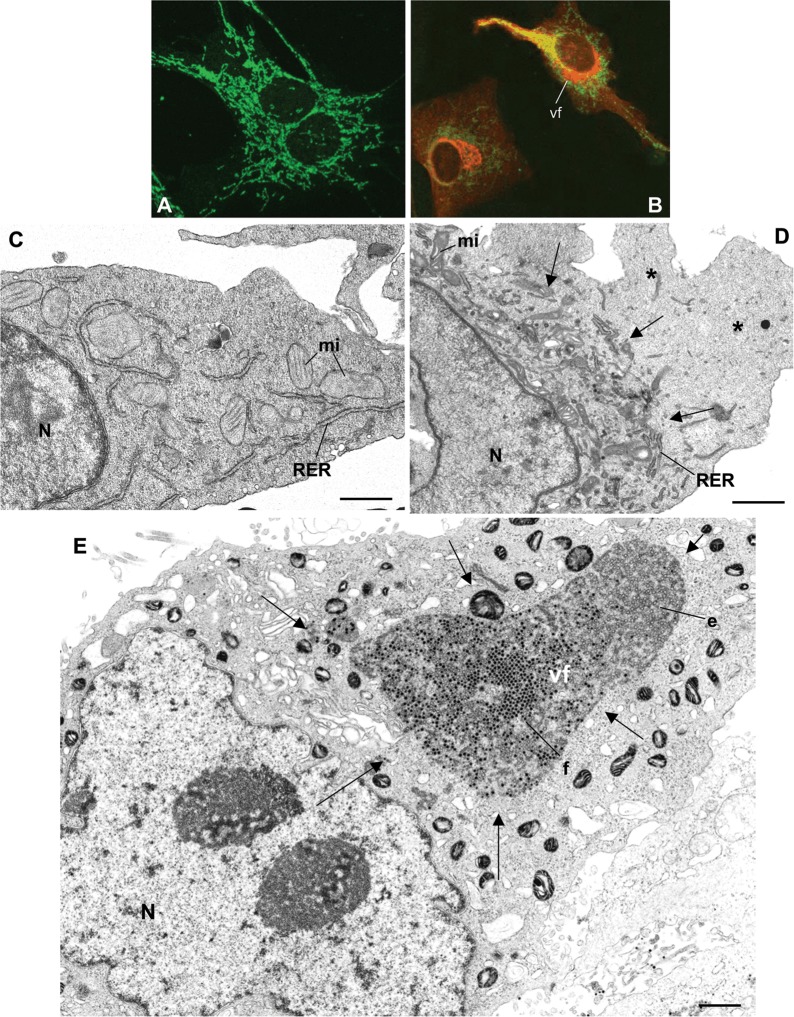
When a virus enters a cell, the subsequent steps in its replication cycle involve interactions between different types of viral components and much more complex pools of host factors. Nowadays much effort is being dedicated to the identification of such host factors, whose characterization is an important field in virology and cell biology. Viruses are obligate intracellular parasites, depending on cell functions for their morphogenesis and propagation (Freed, 2004; van Regenmortel, 2000; Weiss, 2002). The high rates of spontaneous mutation of viral genomes, particularly for RNA viruses (Domingo and Holland, 1997), have created continuous new interactions between viral and cellular elements, some of them rendering useful solutions for efficient viral morphogenesis and propagation. Among the most complex interactions are the signals for formation of structures known as ‘viral factories’. A variety of non‐related viruses have been reported to induce a recruitment of organelles, usually to the perinuclear area, and build a new structure that functions in viral replication, assembly, or both. Well known for complex viruses, such as members of the Poxviridae, Iridoviridae, and Asfarviridae, structures with similar functions are also formed during replication of Herpesviridae, Togaviridae, Bunyaviridae, Flaviviridae, Reoviridae, and potentially also by Coronaviridae, Arteriviridae, and Polioviridae (Table 1). Late in infection, many viruses induce the formation of cytoplasmic inclusions, usually by aggregation of viral structural proteins and/or nucleocapsids. These aggregates accumulate as dead‐end material in inclusion bodies. In contrast, the viral factory is an early functional structure that dramatically alters large areas of the infected cells (Figure 1) and induces changes in composition and organization of cellular compartments depending on the step of the virus replication cycle. Ultrastructural analysis shows that some factories occupy very large regions of the infected cell (Figures 1A–1D) while others are confined to a restricted area (Figure 1E). Viruses use different strategies to create these macro‐structures. It has been suggested that viral factories could form passively as a consequence of localized accumulation of large quantities of protein which is produced in excess during infection. However, there is growing evidence that the generation of particular replication and assembly sites within cells also occurs actively by targeting viral proteins into specific cellular compartments. For example, Pox‐, Irido‐ and Asfar‐like viruses seem to take advantage of a cellular defence mechanism known as the formation of ‘aggresomes’ to concentrate structural components around the microtubule organizing centre (Heath et al., 2001). RNA viruses frequently modify membranes, usually from the secretory pathway, where they anchor their replication complexes and establish assembly sites (Griffiths and Rottier, 1992; Risco and Carrascosa, 1999). We have selected several virus families to describe the best characterized viral factories (Table 1). Members of other families not included in this review probably follow similar principles to build and coordinate the centres of viral replication and assembly inside infected cells.
Table 1.
General characteristics of the virus families included in this reviewIIV‐6, invertebrate iridescent virus 6; FV‐3, frog virus 3; VZV, varicella zoster virus; YFV, yellow fever virus; HCV, hepatitis C virus; BVDV, bovine viral diarrhoea virus; RVFV, Rift valley fever virus; IBV, infectious bronchitis virus; BEV, equine torovirus; MHV, mouse hepatitis virus; EAV, equine arteritis virus; PRRSV, porcine respiratory and reproductive syndrome virus; ds, double‐stranded; ss, single‐stranded; S, M and L denote small, medium and large respectively.
| Virus family (number of members in parentheses) | Representative viruses | Associated diseases | Genome structure | Number of proteins | Virion structure | Replication site | Assembly site |
|---|---|---|---|---|---|---|---|
| Poxviridae (>100) | Vaccinia* Variola | encephalitis smallpox | dsDNA (130–375 kbp) | 150–300 (∼100 in virions) | enveloped, brick‐shaped (>300 nm) | predominantly cytoplasmic (RER‐associated) | cytoplasmic membranes |
| Asfarviridae (1) | ASFV* | severe illness in pigs | dsDNA (170–190 kbp) | ∼150 (∼50 in virions) | enveloped, icosahedral (175–215 nm) | nuclear and cytoplasmic | cytoplasmic membranes |
| Iridoviridae (>20) | IIV‐6* FV‐3 | infections in aquatic animals | dsDNA (140–303 kbp) | >100 (∼36 in virions) | enveloped, icosahedral (120–350 nm) | nuclear | cytoplasmic |
| Herpesviridae (>130) | HHV‐1* VZV | congenital disease, genital infections chicken pox | dsDNA (125–>240 kbp) | 70–>200 (>30 in virions) | enveloped, icosahedral, capsid (125 nm) | nuclear | nuclear and cytoplasmic |
| Togaviridae (25) | SINV* RUBV* | encephalitis polyarthritis, congenital rubella syndrome | ssRNA+(9–11.8 kb) | 7–8 (3–4 in virions) | enveloped, icosahedral (70 nm) | lysosomes endosomes | cytoplasmic Golgi (RUBV) |
| Flaviviridae (∼80) | YFV* HCV* BVDV* | encephalitis hepatitis haemorrhagic fevers | ssRNA+(9.6–11.3 kb) | 9–12 (3–4 in virions) | enveloped, icosahedral (50 nm) | cytoplasmic membranes | RER |
| Bunyaviridae (>350) | BUNV* RVFV* Hantaan* | haemorrhagic fevers, encephalitis | 3 ssRNA− (S=0.9–2.9 kb M=3.6–4.8 kb L=6.4–12.2 kb) | 6 (4 in virions) | enveloped, spherical (80–120 nm) | cytoplasmic membranes | Golgi |
| Coronaviridae (∼18) | IBV* BEV* MHV | gastroenteritis hepatitis respiratory infections | ssRNA+(27.6–31 kb) | 7–10 (4–5 in virions) | enveloped, spherical isometric capsid (120–160 nm) | autophagosomes | pre‐Golgi |
| Arteriviridae (4) | EAV* PRRSV | respiratory disease, abortion, haemorrhagic fever | ssRNA+(12.7–15.7 kb) | 8–9 (6–7 in virions) | enveloped, spherical isometric capsid (45–60 nm) | cytoplasmic double membranes | Golgi |
Type species
Figure 1.
Structural changes in virus‐infected cells during formation of viral factories
Confocal microscopy of uninfected BHK‐21 cells (A) shows normal distribution of mitochondria (green). (B) In bunyavirus‐infected cells, mitochondria (green) have moved to the perinuclear area and surround the Golgi region (red) where the viral factory (vf) is built. Golgi complex was labelled with an anti‐giantin antibody (kindly provided by Dr. M. Renz, Institute of Immunology and Molecular Genetics, Karlsruhe, Germany) while mitochondria were labelled with Mitotracker green (Molecular Probes, Inc.). Ultrastructural analysis shows that the characteristic distribution of organelles in control cells (C) changes completely in infected cells (D) where organelles move to the perinuclear region (arrows), building a large factory and leaving the peripheral areas almost empty (asterisks). (E) In reovirus‐infected cells the vf occupies a large, but apparently more restricted area (marked with arrows) where empty (e) and full (f) viral particles are seen. N, nucleus; mi, mitochondria. Bar=0.5 μm (C) or 1 μm (D and E).
Factories of large DNA viruses I: viral factories resemble aggresomes
The cytoplasm is the site of assembly for several large DNA viruses such as poxviruses, iridoviruses, and the closely related African swine fever virus (ASFV, Asfarviridae). Early in infection there is an exclusion of host proteins and organelles from the region where the factory will be assembled. This creates a rather electronlucent ‘empty’ area close to the nucleus. At the same time large amounts of viral structural proteins and viral DNA accumulate, as well as mitochondria, cytoskeletal filaments and different types of membranous structures. Some of these membranes are used for viral replication while some others will be modified to produce viral envelopes.
Poxviridae
Vaccinia virus (VV) is the best‐characterized member of the Poxviridae family and, due to its complexity, one of the most challenging viruses (Moss, 2001). VV contains a double‐stranded DNA genome of approx. 190 kb encoding for more than 200 proteins (more than 100 are actually incorporated into the infectious virion). This is in contrast with many viruses, which usually encode for approx. 5–12 polypeptides (Flint et al., 2000). A large number of virus‐encoded enzymes and factors are packaged into the virus particle. Poxviruses also encode multiple proteins that interfere with the induction and activity of complement and cytokines (Moss, 2001). Poxviruses are large viruses that can be visualized by light microscopy. VV particles, their largest dimensions reaching 300 nm, are the size of small bacteria. To date, a detailed characterization of VV structure has not been possible due to its size and complexity. Used as an efficient vaccine that eradicated smallpox (caused by the related variola virus) in 1977, and today for designing new vectors and vaccines (Earl et al., 2004; Bisht et al., 2004), this virus has also become the focus of cell biologists, due to the complex interactions that the virus establishes with cellular components (Cudmore et al., 1995; Ploubidou et al., 2000; Sodeik et al., 1993). The replication cycle of VV can be divided into virion entry, early transcription, DNA replication, virus assembly, and egress (Mallardo et al., 2002). All these steps occur in the cytoplasm of the host cell (Figure 2). When VV enters a cell, the viral core is released into the cytoplasm. Early mRNAs are produced inside the core, and subsequently released, by an unknown mechanism. Translation of these mRNAs is required to initiate viral DNA replication. Recent data suggest that core uncoating and the release of the parental DNA occurs close to endoplasmic reticulum (ER) membranes (Welsch et al., 2003). VV replication complexes are then established in distinct cytoplasmic sites that are enclosed by membranes of the rough endoplasmic reticulum (RER; Tolonen et al., 2001). The sites become entirely surrounded by RER membranes until virus assembly is initiated (Figures 2A and 2B). This wrapping, which seems to promote efficient VV replication (Mallardo et al., 2002), could be mediated by a VV protein, the product of the E8R gene. The process shows several interesting analogies to nuclear envelope assembly/disassembly during the cell cycle. VV replication induces the expression of late genes that are required for virion assembly. At that moment the organization of the factory changes completely. RER membranes dissociate from the replication sites and new structures are observed in those areas (Figure 2C). The first characteristic structures are the typical crescent‐shaped membranes that attach to the surface of dense granular aggregations (named viroplasm foci) to form the spherical immature viruses (IVs). Structure and composition of the viroplasm are not completely characterized, but it is known that a number of VV proteins concentrate there (Vanslyke and Hruby, 1994) and membranous structures have sometimes been distinguished inside (Cudmore et al., 1996; Risco et al., 1999). VV morphogenesis occurs in different steps through a series of intermediate structures. Important controversy has traditionally surrounded the origin of the primary membrane that forms VV crescents and IVs. The initial idea of a ‘de novo’ synthesis (Dales and Mosbach, 1968; Hollinshead et al., 1999) has been generally disfavoured and it has been suggested that viral crescents could originate from the transitional elements operating between the ER and the Golgi complex (also known as ERGIC, from ‘ER‐Golgi intermediate compartment’) that are massively recruited to the areas of VV assembly (Krijnse‐Locker et al., 1996; Risco et al., 2002; Rodríguez et al., 1997; Sodeik et al., 1993). Alternatively, it has been recently proposed that viral crescents could directly derive from the ER by a novel mechanism (Husain and Moss, 2003). Approaches to three‐dimensional visualization of IVs strongly suggest that VV builds its first envelope using a unique jigsaw puzzle‐like mechanism, in which individual crescents are put together to build spheres (Risco et al., 2002). This is in contrast with the general processes of budding or wrapping for acquisition of viral envelopes (Garoff et al., 1998; Pornillos et al., 2002; Rouiller et al., 1998).
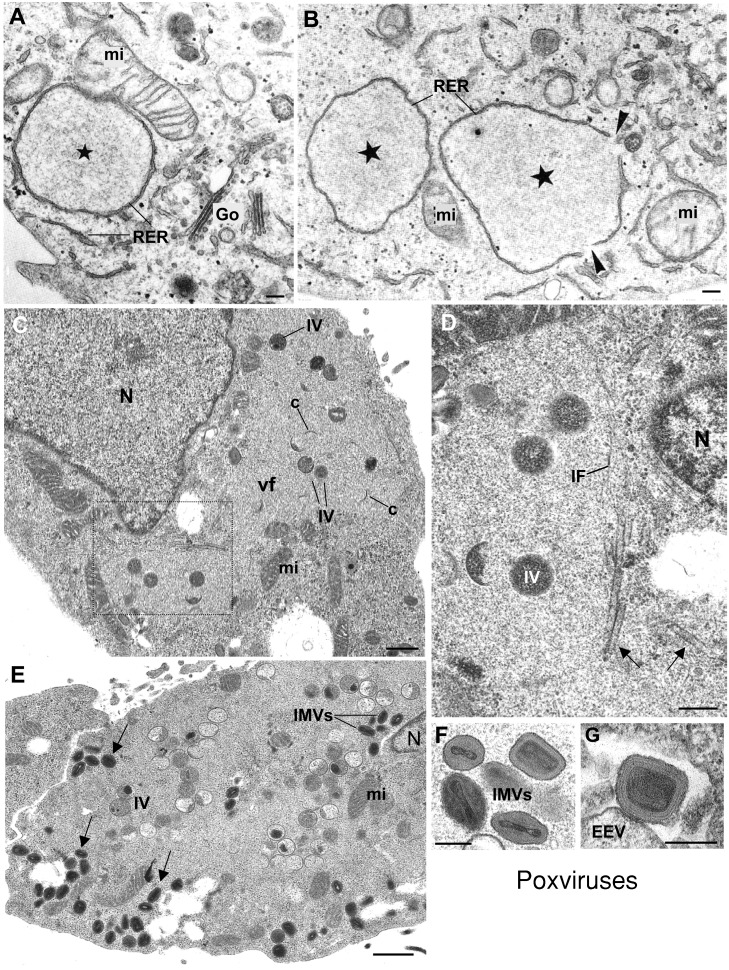
Figure 2.
Structural changes in viral factories of VV‐infected cells
(A and B) Replication complexes (stars) are rapidly formed in the perinuclear region at early times post‐infection. They are enclosed by elements of the RER. Mitochondria (mi) attach to these membranes, which start to open up (arrowheads in B) at the end of the replication phase. (C) When assembly starts, the viral factory (vf) looks like a wide area of low electron density where viral crescents (c) and immature viruses (IV) start to be distinguished. (D) Higher magnification of the area marked in (C), that has been rotated by 90°. Tubular elements of unknown identity (arrows) and IFs surround the factory. (E) At late times post‐infection the factory is filled with both IVs and IMVs, as well as mitochondria. Arrows point to mature virions that have abandoned the perinuclear region to reach the cell periphery before exit. (F and G) Higher magnification electron micrographs showing the two vaccinia infectious forms: the IMV and the EEV, respectively. V V‐infected HeLa cells were kindly provided by Dr. M. Esteban (Centro Nacional de Biotecnología, CSIC, Madrid). (A) and (B) reprinted from Molecular Biology of the Cell (Mol. Biol. Cell 2001 12: 2031–2046) with permission by the American Society for Cell Biology. N, nucleus. Bars=200 nm in A, B, D, F and G; 0.5 μm in C and E.
Vimentin intermediate filaments (IFs) are also recruited to VV factories during the assembly of viroplasm foci. Confocal microscopy shows that these filaments change their distribution in infected cells, building a cage around the VV factory (Risco et al., 2002). A collapse of vimentin IFs around viral factories has also been observed for reoviruses (Sharpe et al., 1982), picornaviruses (Nédellec et al., 1998), iridoviruses (Murti and Goorha, 1983), ASFV (Heath et al., 2001) and human immunodeficiency virus (HIV) (Karczewski and Strebel, 1996). This collapse of vimentin resembles a constitutive cellular process known as aggresome formation that cells use to encapsulate potentially toxic aggregates of misfolded proteins (see below). In addition, ultrastructural characterization of the viroplasm foci within VV factories also suggest that vimentin can participate in the assembly of immature viruses. In fact, vimentin has been localized in viroplasm foci and inside immature viruses. The pattern of immunogold labelling in these structures suggests that vimentin filaments could participate in organizing the interior of the viroplasm foci, facilitating egress of viral crescents and incorporation of viral proteins into immature viral particles (Risco et al., 2002).
Several VV proteins have been found to participate in recruitment and modification of membranes that form the crescents, or in their attachment to viroplasm before formation of IVs (Rodríguez et al., 1997; Wolffe et al., 1996; Traktman et al., 2000). DNA encapsidation seems to trigger IV‐particle maturation, a process that later transforms IVs into infectious, brick‐shaped intracellular mature virions (IMVs) (Sodeik and Krijnse‐Locker, 2002). Particular VV proteins participate in this process (Heljasvaara et al., 2001).
Mitochondria are important elements of the VV factory since they are observed near VV replication sites and in contact with assembling and maturing VV (Risco et al., 2002; Tolonen et al., 2001) (Figures 2A–2C). Since actin has been localized in the viral factories after fowlpox virus infection, a role of actin filaments in morphogenesis of orthopoxvirus has been suggested (Boulanger et al., 2000). In addition, several studies have shown the recruitment of transcription factors, cyclophilins, and chaperonins to viral factories (Broyles et al., 1999; Castro et al., 2003; Hung et al., 2002) pointing to a participation of these factors in viral replication and/or morphogenesis. Ultrastructural analysis shows that when high preservation cryomethods are applied, some elements of unknown identity accumulate in VV factories. These include different types of f ilaments as well as rigid tubes (Figure 2D). These tubular structures exhibit a random distribution in normal, non‐infected cells (C. Risco, unpublished results). Their molecular composition and potential function in the factory remain to be established.
The organization of the VV factory changes again at times late post‐infection. It becomes f illed with viral intermediates and mature virions (Figure 2E). The IV particle transforms into the fully infectious IMV (Figure 2F). A percentage of the IMVs leave the factory along microtubules and travel to the trans‐Golgi network, where virions become enwrapped by a double membrane to form the intracellular enveloped virus (IEV). IEVs move towards the plasma membrane on microtubular tracks and, like some intracellular bacteria, are capable of polymerizing actin tails, which facilitates the release of the extracellular enveloped virus (EEV) into the extracellular space (Rietdorf et al., 2001) (Figure 2G). The process of actin tail formation, first identified for VV and several intracellular bacteria, was later discovered to operate constitutively for vesicular transport in normal cells (Merrifield et al., 1999; Orth et al., 2002; Taunton et al., 2000). The second infectious form of VV, the EEV, contains an additional membrane compared with IMVs and also some additional proteins (Moss, 2001).
In conclusion, the VV factory is a very dynamic structure, where viral and cell factors move in and out depending on the step of the viral life cycle. During the replication phase mitochondria and RER cisternae are recruited and form ‘mini‐nuclei’, that later dissociate when assembly foci and vimentin cages are formed. Assembled infectious virions move along microtubular tracks to the Golgi area, where secondary wrapping takes place, before transport to the plasma membrane and release occurs. These movements probably imply a disruption of the vimentin cage when viruses move en masse to the cell periphery before release into the extracellular medium.
Asfarviridae and Iridoviridae
The only member of the Asfarviridae family, ASFV is the causative agent of a severe disease of pigs. ASFV is an icosahedral double‐stranded DNA virus that replicates in the cytoplasm of porcine monocytic cells where it induces the formation of large virus factories (Brookes et al., 1996). ASFV combines the genomic organization of poxviruses with the striking icosahedral symmetry of the iridoviruses, suggesting an evolutionary connection to both virus families. In fact there are similarities in the organization of the viral factories built by these three virus families. ASFV is a complex virus that encodes approx. 150 potential proteins. Mature virions incorporate more than 50 proteins, including a number of enzymes and factors needed for early mRNA processing (Salas, 1999). Extracellular virions are large enveloped structures of 175–215 nm in diameter. Replication of viral DNA seems to be initiated in the nucleus (Garcia‐Beato et al., 1992), and is followed by a second phase of replication in the cytoplasm, where distinctive viral factories form.
Iridoviruses have only been isolated from poikilothermic animals, usually associated with aquatic environments including marine habitats. Transmission mechanisms are poorly understood for the majority of these viruses (Williams, 1996). Iridoviruses are structurally complex, with up to 36 polypeptides in mature virions, including a number of virion‐associated enzymes. Virions have icosahedral symmetry and are usually 120–200 nm in diameter but may be up to 350 nm. The virion core, which contains a single linear double‐stranded DNA molecule of between 140 to 303 kb, is surrounded by a lipid membrane acquired by budding through the host plasma membrane. Capsid shell is a precise and highly symmetric structure (Yan et al., 2000). Early replication and transcription start inside the nucleus (Schetter et al., 1993). DNA is transported to the cytoplasm in the form of a large branched concatemer whose processing into mature viral DNA could take place during packaging into the virion. Transcription continues in the cytoplasm, where virions assemble in close association with virogenic stroma (Qin et al., 2001).
Figure 3 shows common characteristic features of viral factories formed by ASFV and iridoviruses: in contrast with VV factories, for these viruses there is no description of the specific structure that anchors the viral replication complexes. ASFV factories are distinct from the surrounding cytoplasm (Figure 3), they exclude cellular organelles, but contain ribosomes and polysomes, membranous material, and cytoskeletal elements. Immunofluorescence studies (de Matos and Carvalho, 1993) showed a movement of viral inclusions from a peripheral to a perinuclear location where they fuse to give a single large perinuclear factory enclosed in a vimentin cage (Heath et al., 2001) (Figure 3A). Many mitochondria also accumulate around factories (Figure 3B). Reorganization of other cytoskeletal elements was also demonstrated: microtubules decreased in number and microfilaments were reorganized in association with the plasma membrane (Carvalho et al., 1988). It has been proposed that phosphorylation and dephosphorylation of vimentin could be the mechanism for regulating cage formation during ASFV infection (Heath et al., 2001). The virus factories accumulate immature virus particles, some with and others without a dense nucleoprotein core (Figure 3C). It has been proposed that the nucleoprotein core may be formed in association with a virus factory membrane component and then inserted into a partly formed ‘empty’ particle just before it is completely sealed (Brookes et al., 1996). In the case of ASFV it has been observed that viral particles are formed from precursor membranes that are thought to be cisternal structures derived from the ER (Figures 3D and 3E) (Andrés et al., 1998; Rouiller et al., 1998). Hexagons (200‐nm‐diameter in cross section) and a series of one‐ to six‐sided assembly intermediates are seen in the factories. Morphological evidence indicates that viral membranes become icosahedral by the progressive construction of the outer capsid layer, which is composed mainly of viral protein (Andrés et al., 2002). It has been reported recently that the transmembrane ASFV structural protein p54 is critical for the recruitment and transformation of the ER membranes into the precursors of the viral envelope (Rodríguez et al., 2004).
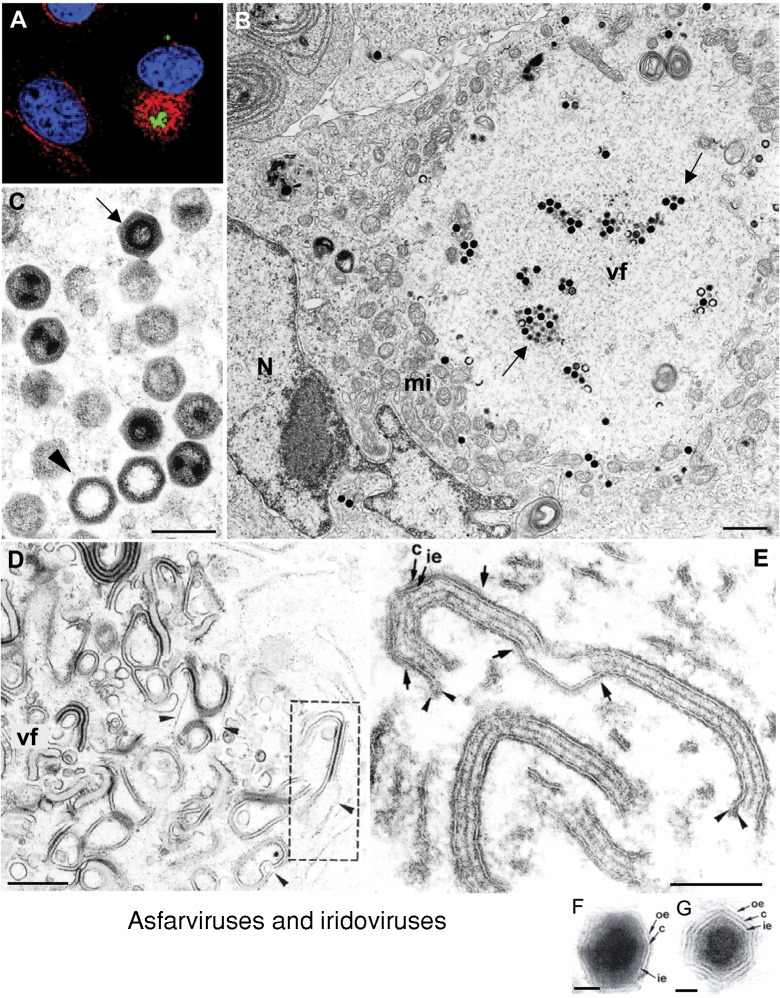
Figure 3.
Factories of asfarviruses and iridoviruses
Confocal microscopy shows the factories of ASFV (A) surrounded by a vimentin cage (red) that encloses viral proteins (green; nuclei are stained in blue). (B) Transmission electron microscopy shows the ultrastructure of factories for the related iridoviruses. Numerous mitochondria (mi) accumulate around the factory (vf), where viral particles start to be seen (arrows). (C) Higher magnification shows viral particles at different stages, such as empty capsids (arrowhead) and full viral particles (arrow), the latter containing the viral DNA. (D and E) show modified membranes that accumulate in viral factories (vf) induced by ASFV. These membranes come from the RER and originate the icosahedral viral core (c) and the inner envelope (ie). Extracellular virions (F and G) have an additional outer envelope (oe) taken from the plasma membrane. (A) Reproduced from The Journal of Cell Biology, 2001, 153, 449–455 with copyright permission of the Rockefeller University Press. (D–G) Reproduced from Andrés et al. (1998) J. Virol. 72, 8988–9001 with copyright permission of the American Society for Microbiology. N, nucleus. Bars=1 μm in B; 250 nm in C; 0.5 μm in D; 100 nm in E; 50 nm in F and G.
The complex morphogenesis of these viruses may require a considerable amount of energy. Mitochondria surrounding viroplasm have been described in cells infected with the iridovirus, frog virus 3 (Kelly, 1975). In ASFV it has been demonstrated that the large clusters of mitochondria around the factories are not the result of an increased biogenesis of mitochondria in the infected cell but are due to a mass migration of pre‐existing organelles to the periphery of the viral assembly areas (Rojo et al., 1998). Interestingly, a dramatic shift in the ultrastructure of the mitochondria is detected for those mitochondria surrounding the factories that contain virus particles: condensed ultrastructure, with a marked condensation of the cristae. This has been described as the characteristic state of actively respiring mitochondria (Hackenbrock, 1966; Rojo et al., 1998; Valcarce et al., 1988). However, most of the mitochondria in cells where the viral factories contain only precursor membranes presented the morphology of ‘resting’ mitochondria. Microtubules are probably involved in the transport of the mitochondria to the proximity of the viral factories, as suggested by nocodazole treatment, which prevented their clustering around the viral assembly sites. Mitochondria in viral factories seem to provide the necessary energy for the virus morphogenetic processes; in fact, envelopment and capsid formation depend on calcium gradients and ATP (Cobbold et al., 2000). Intracellular ASFV particles associate with microtubules to reach the plasma membrane (de Matos and Carvalho, 1993). The final product of assembly, the mature extracellular virions, possess an additional envelope derived from the plasma membrane (Figures 3F and 3G).
The described rearrangement of vimentin around the factories, as well as recruitment of mitochondria and chaperons in infected cells, show important similarities with aggresome formation (Heath et al., 2001; Risco et al., 2002). It has been shown that cells respond to the production of high levels of misfolded proteins by transporting them to perinuclear sites known as ‘aggresomes’. In fact, although protein folding and assembly are highly chaperoned processes, their success rate is rather low; overall, up to 30$ of nascent proteins are defective and readily destroyed (Schubert et al., 2000). For wild‐type secretory and membrane proteins synthesized in the ER failure rates of 10–70$ have been reported (Cohen and Taraboulos, 2003). Once formed, aggregates tend to be refractory to proteolysis and may lead to the intracellular deposition of potentially toxic aggregates. Aggregated proteins are specifically delivered to inclusion bodies by dynein‐dependent retrograde transport on microtubules (Kawaguchi et al., 2003; Kopito, 2000). Intracellular and extracellular accumulation of aggregated protein are linked to many diseases, including aging‐related neurodegeneration and systemic amyloidosis.
Aggresomes are located close to centrosomes and are surrounded by a characteristic vimentin cage. It has been proposed that aggresomes recruit mitochondria as an energy source for protein folding and protein degradation. These complexes contain chaperones and the 20 S proteasome, all of which need ATP (García‐Mata et al., 2002; Wigley et al., 1999). The ability of aggresomes to concentrate proteins and cellular chaperones make them highly suitable for facilitating virus assembly. It is possible that a cellular defence mechanism originally designed to reduce the potential toxicity of misfolded proteins is exploited by ASFV, VV and iridoviruses as a means of concentrating structural proteins at sites of virus assembly.
Factories of large DNA viruses II: herpesviruses form nuclear and cytoplasmic factories
Viruses included in the Herpesviridae family encompass enveloped DNA viruses of high complexity which are grouped together based on common biological characteristics and morphology (Roizman and Knipe, 2001). Herpesviruses are widely distributed in nature. Presently, more than 130 virus species are known. One of the important human pathogens and type species of the genus simplexvirus, the human herpesvirus‐1 (HHV‐1), herpes simplex virus‐1 (HSV‐1), infects neonates, children and adults. It produces a wide spectrum of diseases ranging from mild illness to severe and life‐threatening disease (Roizman and Pellett, 2001). The genome of herpesviruses is composed of linear double‐stranded DNA from 125 to more than 240 kb in size. The number of open reading frames contained within herpesvirus genomes ranges from about 70 to more than 200. The polypeptide composition of the mature virion varies greatly among different herpesviruses. HHV‐1, pseudorabies virus, HHV‐3 and varizella zoster virus are the best studied alphaherpesviruses and more than 30 virion proteins have been identified. Virions comprise a complex and characteristic ultrastructure consisting of both symmetric and nonsymmetric components (Homa and Brown, 1997). The virion can be differentiated into a DNA‐containing core, an icosahedral capsid (162 capsomers, 100–110 nm in diameter), the tegument (which surrounds the nucleocapsid), and the envelope. The core consists mainly of the viral genome which is packaged into a preformed capsid. The mature capsid is composed of five proteins, while the tegument contains at least 15 different polypeptides, many of which are not strictly required for virion assembly. The viral envelope contains at least 10 integral membrane glycoproteins, a subset of which is required for adsorption and penetration in the host cell (Spear, 1993). All herpesviruses specify a number of enzymes involved in DNA synthesis and processing of proteins, although the exact array of enzymes may vary among different herpesviruses.
The replication of herpesviruses is a very complex sequence of events that includes the construction of a nuclear factory followed by a cytoplasmic factory (Figure 4). After adsorption and penetration, the incoming nucleocapsids are transported along microtubules to the nuclear pore where the DNA is released into the nucleus (Granzow et al., 1997; Sodeik et al., 1997), while tegument proteins, although many of their functions are unknown, are thought to modify cellular metabolism. Viral transcription, synthesis of viral DNA and capsid assembly occur in the nucleus. Specific viral proteins localize in the nucleus and assemble onto the parental viral DNA molecules in punctate structures called ‘prereplicative sites’ (Figure 4A) located near Nuclear Domain 10 structures (Ishov and Maul, 1996; Uprichard and Knipe, 1996, 1997). As viral DNA synthesis progresses, the progeny DNA molecules and replication complexes accumulate in nuclear globular structures called ‘replication compartments’ (Figure 4B). Viral DNA synthesis, late gene transcription, capsid assembly, and DNA encapsidation all occur within these nuclear factories. The replication structures are dynamic and replication proteins rearrange reversibly to give different patterns, depending on the status of viral DNA replication (Quinlan et al., 1984). The spatial organization of replication structures within the cell nucleus has been studied for HSV‐infected cells by confocal microscopy and three‐dimensional reconstruction of optical series (de Bruyn Kops and Knipe, 1994). Replication structures were shown to extend throughout the nuclear interior in a highly ordered arrangement which appears to be independent of the nuclear lamina. Furthermore, viral DNA replication compartments are assembled within a pre‐existing spatial framework in the cell nucleus, consistent with the view of a highly organized nuclear interior (Baxter et al., 2002; Chubb and Bickmore, 2003). This may reflect targeting of the viral proteins to specific structures in the nucleus or, alternatively, viral proteins may localize to areas where formation of replication complexes is not impeded by existing host structures. Specialized machinery for late gene expression has been detected within replication compartments (McNamee et al., 2000). After synthesis of the capsid proteins, they move into the nucleus, where capsid assembly occurs (Figure 4C). Empty shells containing an internal scaffolding are assembled first, and the scaffolding protein is lost or disassembled upon insertion of viral DNA into the capsid via vertices (Figure 4C, inset).
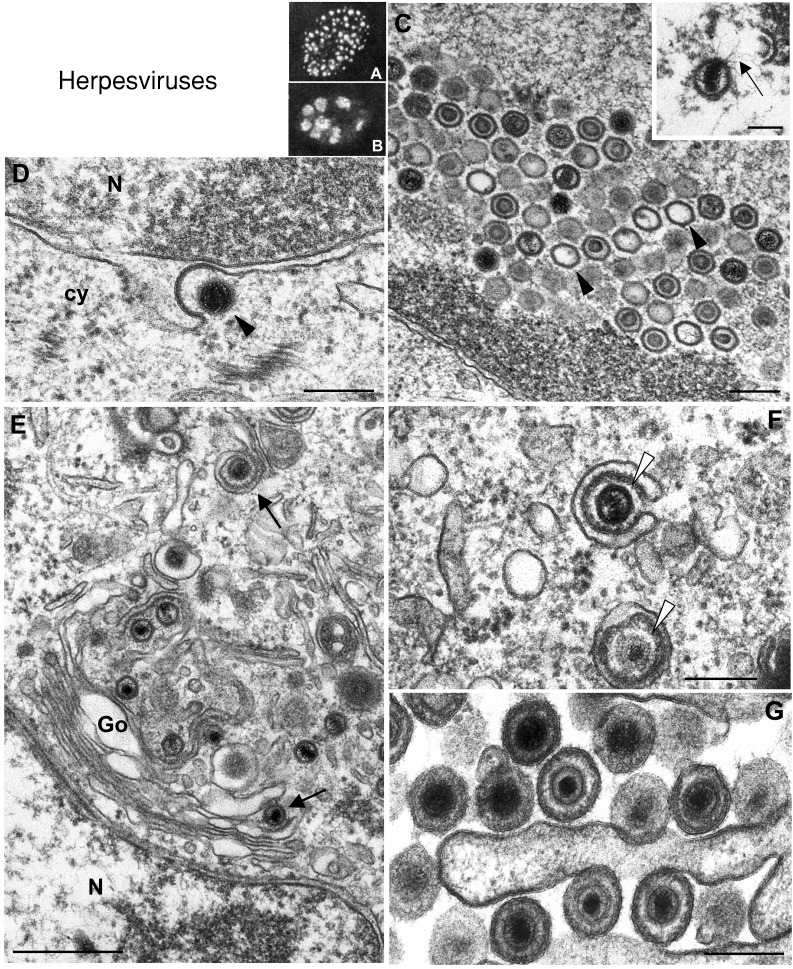
Figure 4.
Nuclear and cytoplasmic factories of herpesviruses
(A) and (B) show the intranuclear location of herpesvirus prereplicative and replicative compartments, respectively, as visualized by immunofluorescence of the viral protein ICP8. (A) Vero cell infected with wild‐type virus in the presence of the viral polymerase inhibitor PAA. (B) Vero cell infected with the wild‐type virus in the absence of PAA. (C) At the ultrastructural level intranuclear empty capsids (arrowheads), as well as capsids packaging DNA (inset), are seen at later times post‐infection. (D) Release of the primary enveloped viral particle from inside the perinuclear space (arrowhead) into the cytoplasm (cy) by fusion of the primary envelope with the outer lamella of the nuclear membrane. (E) Secondary envelopment of nucleocapsids (arrows) in the trans‐Golgi area. (F) Tegumentation adds a layer of viral proteins (arrowheads) to the capsid that acquire an envelope from trans‐Golgi membranes. (G) Extracellular virions after release from cells. (A and B) reproduced from Liptak et al. (1996) J. Virol. 70, 1759–1767 with copyright permission of the American Society for Microbiology. Inset in (C) reproduced from Granzow et al. (1997) J. Virol. 71, 2072–2082, with copyright permission of the American Society for Microbiology. N, nucleus; Go, Golgi. Bars=200 nm in C, D, F, and G; 100 nm in the inset of C; 500 nm in E.
The organization and dynamics of the cytoplasmic factory is not completely understood. While the process of capsid formation has been characterized for HSV in detail (Newcomb and Brown, 1991, 1994; Newcomb et al., 1996; Trus et al., 1996), the following steps of tegumentation and envelopment have been controversial. It is now widely accepted that capsids acquire a primary envelope by budding at the inner leaflet of the nuclear membrane. This primary envelope is lost by fusion with the outer leaflet of the nuclear membrane. Thus, nucleocapsids are released (Figure 4D) by a two‐step process of envelopment/de‐envelopment at different cell compartments (Granzow et al., 1997, 2001; Mettenleiter, 2002; Skepper et al., 2001). The cellular compartment in which tegumentation and secondary envelopment takes place has been identified for pseudorabies virus (Granzow et al., 1997). Tegumentation involves an intricate network of protein—protein interactions and occurs in the cytoplasm during secondary envelopment at membranes of the trans‐Golgi network (Figures 4E and 4F), although some tegument components may be retained or apposed when the capsid exits the nucleus. The first layer of tegument around the capsid seems to be composed of the UL36 protein and a second layer could be made of the UL37 gene product (Mettenleiter, 2002). Subsequent steps in tegumentation are still largely unknown, but it has been proposed that the UL48 protein, a major component of the HSV‐1 and pseudorabies virus tegument, may play a key role in tegument formation (Fuchs et al., 2003; Mossman et al., 2000). Cryo‐electron microscopy analysis showed that at least the innermost part of the tegument adjacent to the icosahedral capsid may also exhibit icosahedral symmetry (Zhou et al., 1999). The three‐dimensional structure of mature HSV‐1 particles has been analysed recently by cryo‐electron tomography (Grünewald et al., 2003). The tegument shows a particulate substructure with short actin‐like filaments. This suggests that actin microfilaments could participate in recruitment of tegument proteins. Interestingly, it has been observed that many tegument proteins are phosphorylated, and therefore protein phosphorylation may play an important role in tegument assembly and function (Mettenleiter, 2002). Secondary envelopment takes place at a Golgi‐derived compartment, the trans‐Golgi network (Figure 4F). Tegumented capsids bud into trans‐Golgi vesicles, where viral glycoproteins accumulate. Re‐envelopment is triggered by interaction of the nucleocapsid and/or attached tegument proteins with glycoprotein cytoplasmic domains of viral membrane glycoproteins. Virions are finally released (Figure 4G) by fusion of the Golgi‐derived vesicle membrane with the cell membrane (Granzow et al., 1997, 2001).
Factories of RNA viruses I: togaviruses induce factories around lysosomes
The association of RNA synthesis with intracellular membranes is a typical feature of positive‐stranded RNA viruses replicating in animal, plant, or insect cells. Many different membranous organelles are used by different RNA viruses to anchor their replication complexes. The family Togaviridae contains two genera, genus Alphavirus and genus Rubivirus (Weaver et al., 2000). The alphaviruses, composed of at least 24 members, are arboviruses (transmitted by arthropods), with a linear positive sense single‐stranded RNA (ssRNA) genome of approx. 9–11.8 kb (Strauss and Strauss, 1994). Rubella virus (RUBV), the only member of the genus Rubivirus, is a human pathogen and has a genome of 10 kb (Frey, 1994). Alphaviruses are transmitted between vertebrates by mosquitoes and other haematophagous arthropods. The type‐specific member of the alphaviruses is sindbis virus (SINV) whose structure and replication have been studied in great detail. In addition, both SINV and Semliki Forest virus (SFV) have provided valuable models for examining synthesis, posttranslational modifications, and localization of membrane glycoproteins. Alphaviruses have a wide host range and nearly worldwide distribution. Neither SINV nor SFV are serious pathogens for adult vertebrates unless virus is injected directly into the brain. In rare infections in humans, SINV and SFV produce arthralgia, rash and fever. Human polyarthritis can result from infection by some SINV strains (Schlesinger, 1999). The infection of cells of vertebrate origin is cytolytic and involves the shutdown of host cell macromolecular synthesis. In mosquito cells, alphaviruses usually establish a noncytolytic infection in which the cells survive and become persistently infected (Schlesinger and Schlesinger, 2001). RUBV, the causative agent of rubella (German measles), is only found in humans and does not replicate in insect cells. This virus is highly teratogenic during the first trimester of pregnancy. Although congenital rubella syndrome is controlled by vaccination in most developed countries, it remains endemic in the developing world and parts of Eastern Europe (Chantler et al., 2001). Apart from the genomic coding strategy, alphaviruses and RUBV are quite dissimilar (Frey, 1994). It has been proposed that RUBV originated by a complicated event including recombination with progenitors of the current alphaviruses, human hepatitis E virus, and possibly plant viruses (Frey and Wolinsky, 1999).
The virions of the two genera are roughly similar in size and structure, they are 70 nm in diameter, spherical, and contain icosahedral nucleocapsids surrounded by a lipid envelope containing heterodimeric glycoprotein spikes composed of two virus glycoproteins. Alphavirus structure has been characterized by cryo‐electron microscopy in combination with X‐ray crystallographic structures (Cheng et al., 1995; Mancini et al., 2000; Zhang et al., 2002). The envelope is tightly organized around an icosahedral nucleocapsid that is 40 nm in diameter. The nucleocapsid is composed of the capsid protein, organized in a T=4 icosahedral symmetry, and the genomic RNA (Kuhn and Strauss, 2003). The three‐dimensional structure of rubella virions has not yet been determined. Particles of RUBV are pleomorphic in nature, indicating that the capsid—glycoprotein interaction is not as tight as it is in virions of alphaviruses (Murphy, 1980).
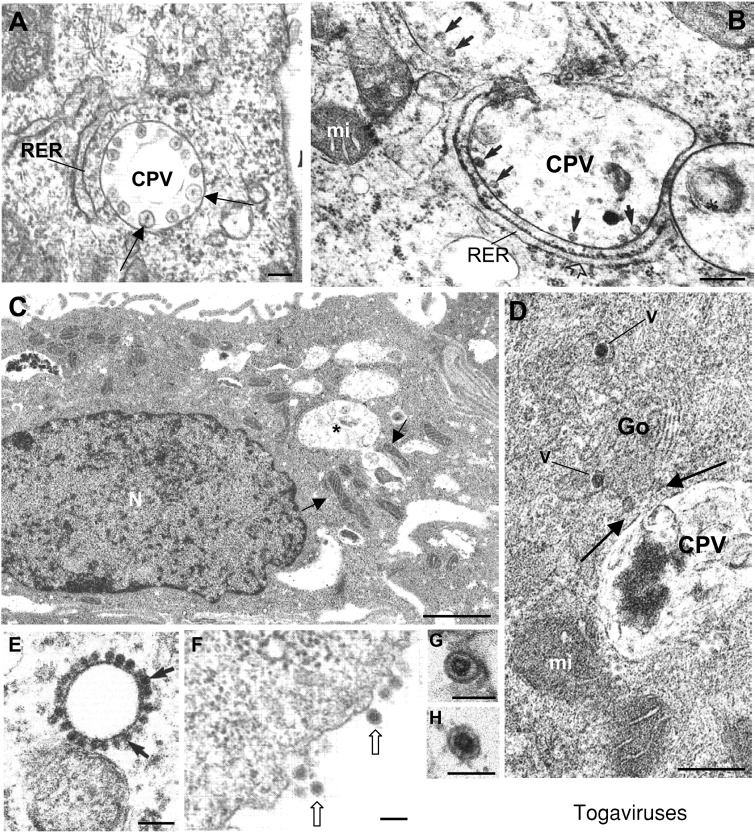
Factories of togaviruses are organized around endosomes and lysosomes (Figure 5). These components of the endocytic pathway have been shown to be involved in the entry and uncoating of many enveloped viruses (Helenius et al., 1989). However, the use of endosomes and lysosomes as sites of viral replication appears to be unique to the togaviruses (Magliano et al., 1998). In infected cells, modified endosomes and lysosomes constitute a special vesicular structure known as cytopathic vacuole (CPV). Similar virus‐induced vesicles have been reported as replication complexes for flaviviruses and picornaviruses (Bienz et al., 1990; Ng et al., 1983). However, the replication complexes of these viruses appear to be derived from the ER rather than from endosomes or lysosomes. Similar vesicular structures support replication of nodaviruses but in this case they assemble in the outer mitochondrial membrane (Miller et al., 2001). For togaviruses CPVs serve as sites of RNA replication and perhaps as sites of RNA translation and nucleocapsid assembly. Immunocytochemistry showed that the majority of CPVs are lysosomal and endosomal and stain positively for a number of specific marker antigens, such as lgp120, lgp110, lgp96, cathepsin L, Rab7, Lamp‐1, and Lamp‐2 (Froshauer et al., 1988; Kujala et al., 2001; Lee et al., 1994). CPVs are 0.6 to 2 μm in diameter and have 50 nm membrane spherules lining the vacuole membrane at regular intervals (Figures 5A and 5B). The viral induced double membrane‐bound vesicles that line the vacuoles of RUBV and alphavirus replication complexes most likely represent the precise sites of viral RNA replication (Kujala et al., 2001; Lee et al., 1994; Zhao et al., 1994). In the case of RUBV the size of spherules varies depending on the cell type, being smaller in Vero cells (Figure 5B) (Magliano et al., 1998; Risco et al., 2003). Viral nonstructural proteins have been localized to the limiting membranes of CPVs and are associated with the cytoplasmic face of the CPVs. Moreover, thread‐like ribonucleoprotein structures extend from the base of the spherules to the cytoplasmic face of the CPVs and form connections with the RER. The viral RNA polymerase is present in large branching granular and thread‐like structures anchored to the cytoplasmic surface of CPVs at the spherules. Antibodies specific for double‐stranded RNA also labelled the CPVs (Lee et al., 1994). The association with the RER suggested that the CPVs are replication factories that constitute sites for RNA replication as well as containing sites for translation of structural proteins and assembly of nucleocapsids. In addition, a number of host‐cell proteins (calreticulin, La antigen, Ro/SS‐A antigen, and the cellular retinoblastoma antigen) have been identified as factors involved in RUBV replication (Lee and Bowden, 2000). They are probably recruited to the CPVs where the membranous structure of the replication complexes could provide a structural framework for the assembly of all the components of replication. Large surface areas in membranes could allow a more rapid synthesis of viral progeny RNA and protect the nascent single‐stranded viral genomic RNA from degradation by cellular RNases (Lyle et al., 2002; Lee and Bowden, 2000).
Figure 5.
Factories of togaviruses
Replication complexes of alphaviruses (A) and RUBV (B) are anchored (arrows) in the internal membrane of modified lysosomes and endosomes known as CPVs surrounded by RER cisternae. (C) Mitochondria (arrows) are seen around CPVs (asterisks) in RUBV‐infected cells. (D) Golgi stacks (Go) containing viruses (V) are seen in contact (arrows) with CPVs of RUBV‐infected cells. (E) Capsids of alphaviruses (arrows) are assembled in the cytoplasm and transported to the plasma membrane before envelopment and release to the extracellular medium (F) where extracellular virions have an homogeneously dense interior (arrows). (G and H) show extracellular mature RUBV particles that exhibit a central core separated from the envelope. (A and F) reproduced from Zhao et al. (1994) EMBO J. 13, 4204–4211, with copyright permission of EMBO J.; (B) Reprinted from Virology, 240, Magliano, Marshall, Bowden, Vardaxis, Meanger and Lee, Rubella virus replication complexes are virus modified lysosomes, pp. 57–63, Copyright (1998), with permission from Elsevier. (C, D, G and H) Reprinted from Virology, 312, Risco, Carrascosa and Frey, Structural maturation of rubella virus in the Golgi complex, pp. 261–269, Copyright (2003), with permission from Elsevier. (E) Reprinted from Virology, 265, Lee, Marshall and Bowden, Localization of rubella virus core particles in Vero cells, pp. 110–119, Copyright (1999), with permission from Elsevier. N, nucleus; mi, mitochondria. Bars=100 nm in A, B, E and F; 1 μm in C; 200 nm in D; 60 nm in G and H.
Mitochondria are seen around CPVs in RUBV‐infected cells (Magliano et al., 1998; Risco et al., 2003) (Figure 5C); this was observed similarly with the SFV replication complexes (Lee et al., 1996). Overexpression of RUBV structural proteins also resulted in clustering of the mitochondria to the perinuclear region (Beatch and Hobman, 2000). Electron‐dense zones associated with mitochondria have been detected in RUBV‐infected cells, findings that were not present in SFV‐infected cells (Lee et al., 1996). RUBV capsid protein has been found to associate with mitochondria in infected Vero cells (Lee et al., 1999). RUBV capsid is in fact a multifunctional protein that has roles in virus—host interactions as well as replication (Chen and Icenogle, 2004; Tzeng and Frey, 2003). It has been reported that RUBV capsid protein interacts with p32, a multi‐compartmental cellular protein capable of binding to a wide range of cellular proteins, which has a putative role in apoptosis through regulation of the mitochondrial permeability transition pore (Jiang et al., 1999). In addition, p32 binds to a variety of virus phosphoproteins that complex with nucleic acids. Rubella capsid protein and p32 interact at the mitochondria (Beatch and Hobman, 2000). The potential role of this interaction in RUBV biology has not been determined.
In RUBV‐infected cells, CPVs establish contacts with the Golgi complex, the site of virus assembly and maturation (Figure 5D). These contacts could facilitate the transfer of replicated genomes from the replication complexes to the assembly sites. On the other hand, the nucleocapsids of alphaviruses appear in the cytoplasm of infected cells well before budding occurs (Figure 5E; Lee et al., 1999; Strauss and Strauss, 1994). Images in which CPVs appeared to fuse with the plasma membrane suggest that nucleocapsids could be transferred directly from the surroundings of CPV to the cell surface for budding and exit (Froshauer et al., 1988), to generate the extracellular mature virions (Figure 5F). Coming from the Golgi, where the virus matures and changes its internal structure (Risco et al., 2003), RUBV mature particles (Figures 5G and 5H) exit the cells inside secretory vesicles. When compared with the alphavirus virions, the core of RUBV particles is clearly separated from the envelope to which it connects with visible stripes (Figure 5H).
Factories of RNA viruses II: flaviviruses
The family Flaviviridae consists of three genera: Flavivirus, Pestivirus, and Hepacivirus. Members of the different genera are only distantly related but share a similar gene order and conserved nonstructural protein motifs (Lindenbach and Rice, 2003). The Flavivirus genus consists of nearly 80 viruses, many of which are arthropod‐borne human pathogens. Flaviviruses cause a variety of diseases including fever, encephalitis, and haemorrhagic fevers. Yellow fever, Dengue, and West Nile viruses are well known pathogens within this group (Lindenbach and Rice, 2003). The positive sense single stranded RNA genome of 11 kb has one long open reading frame giving rise to a single polyprotein comprising three structural proteins and seven nonstructural proteins (Rice et al., 1985). Flaviviruses are spherical enveloped viruses with a diameter of approx. 50 nm, and an electron‐dense core of 30 nm. Virions contain only three structural proteins: E (envelope), prM/M (membrane), and C (capsid). Cryo‐electron microscopy data have shown that the virion envelope and capsid have icosahedral symmetry and that interactions between E proteins are responsible for this structure (Kuhn et al., 2002).
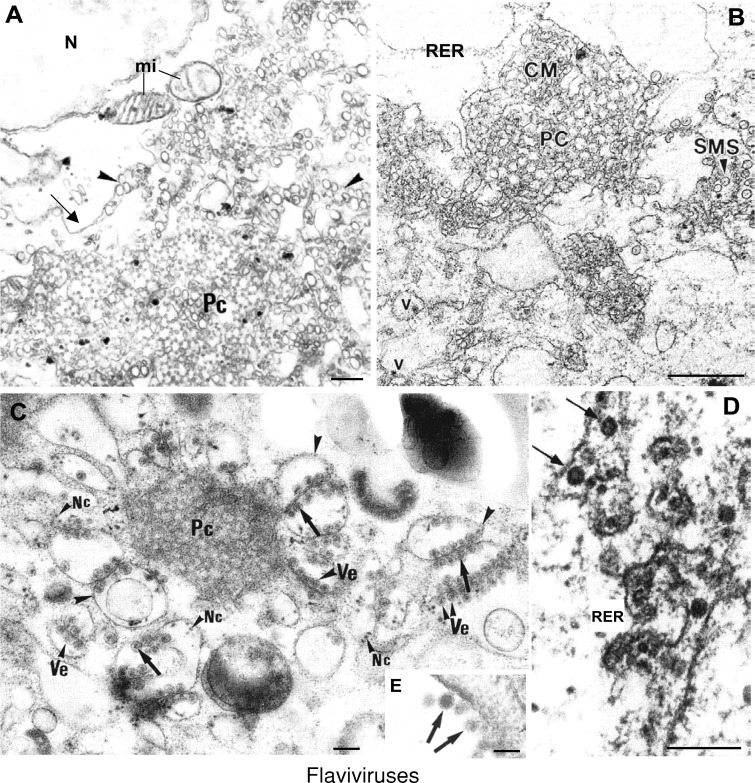
Following translation and processing of the viral proteins, a viral replicase is assembled from non‐structural (NS) proteins, the viral RNA, and presumably some host factors. The replicase associates with membranes, probably through interactions involving the small hydrophobic NS proteins. In fact, flavivirus infection induces rearrangement of cytoplasmic membranes in the perinuclear region, where a factory‐like structure is formed (Figure 6). The earliest of these visible events is proliferation of the RER followed by the appearance of smooth membrane structures around the time of early logarithmic virus production (Ng and Hong, 1989). Smooth membrane vesicles appear as clustered vesicles of 100 to 200 nm (Leary and Blair, 1980; Lindenbach and Rice, 2003; Mackenzie et al., 1999) (Figure 6A). Later after infection, smooth convoluted membranes are found adjacent to vesicle packets. Flavivirus replication is thought to take place in these vesicles, which may be synonymous with ‘smooth membrane vesicle‐like structures’ (SMS) (Mackenzie et al., 2001). These structures appear as randomly folded or ordered membranes (sometimes associated with paracrystalline arrays) that are contiguous with the RER (Figure 6B). Recent work has provided biochemical evidence for a double membrane compartment enclosing the flavivirus replication complexes (Uchil and Satchidanandam, 2003). One study showed that 1,4‐galactosyltransferase, a marker of the trans‐Golgi, was localized to vesicle packets, whereas the RER‐Golgi intermediate compartment‐53 protein was localized within the convoluted membranes, suggesting distinct pathways for the formation of these two structures (Lindenbach and Rice, 2003; Mackenzie et al., 1999). The close physical connections of these structures, RER‐enclosing virions and nearby RER, supports the interpretation that perinuclear foci observed by immunofluorescence microscopy represent virus factories in which translation, RNA replication and virus assembly occur (Westaway et al., 2003). The nonstructural viral proteins NS1, NS2A, NS3, NS4A, and NS5 have all been shown to localize to these vesicles, although only a small proportion of NS3 and NS5 is apparently required for RNA‐dependent RNA polymerase activity. Thus, it appears that along with double‐stranded RNA, proteins implicated in RNA replication associate with vesicle packets. In contrast, the components of the viral serine proteases, NS2B and NS3, colocalized with convoluted membranes. The membrane reorganization that occurs in infected cells might therefore give rise to adjacent, but distinct, subcellular structures where viral polyprotein processing or RNA replication take place (Westaway et al., 2003).
Figure 6.
Factories of flaviviruses
Rearrangement of membranes in the perinuclear region of a Vero cell infected by Kunjin virus. (A) Ordered membranes (referred to as paracrystalline arrays or Pc) and numerous vesicles (arrowheads) are surrounded by distended ER (arrows) and frequently associated with mitochondria (mi). (B) Kunjin virus‐induced Pcs in infected Vero cells are found associated with smooth convoluted membranes (CM) and smooth membrane vesicle‐like structures (SMS). Potential mature virus particles (v) are observed close to these virus‐induced membranous structures. (C) West Nile (Sarafend) virus‐induced paracrystals (Pc) and vesicles (Ve) (sample processed by cryofixation and cryosubstitution). Vesicles containing dense cores are observed around Pc membranes. Membrane bags (arrowheads) have potential nucleocapsids (Nc) associated. (D) Recombinant subviral particles of Tick‐borne encephalitis virus (formed by budding of envelope proteins in the absence of core) observed in the lumen of the RER (arrows) before their transport along the secretory pathway. (E) Extracellular mature particles of West Nile (Sarafend) virus released by infected Vero cells. (A) Reprinted from Hong and Ng (1987) Arch. Virol. 97, 115–121, with permission from Springer—Verlag. (B) Reproduced from Westaway et al. (1997) J. Virol. 71, 6650–6661, with copyright permission of the American Society for Microbiology. (C and E) Reproduced from J. Virol. Methods, 49, Ng, Teong and Tan, Cryosubstitution technique reveals new morphology of flavivirus‐induced structures, pp. 305–314, Copyright (1994), with permission from Elsevier. (D) Reproduced from Lorenz et al. (2003) J. Virol. 77, 4370–4382, with copyright permission of the American Society for Microbiology (original image by Jürgen Kartenbeck). Bars=200 nm in A, C, D; 0.5 μm in B; 50 nm in E.
Studies with Kunjin‐virus‐infected Vero cells showed that perinuclear foci representing virus factories remained largely intact after treatment of cells with cytoskeletal‐disrupting agents that caused only relatively minor reductions in yields of infectious virus (Ng et al., 1983). Mitochondria are frequently seen around flavivirus‐induced membranous structures (Figure 6A) (Mackenzie et al., 1999; Mackenzie and Westaway 2001; Westaway et al., 1997). High preservation methods based on cryofixation followed by cryosubstitution have shown potential nucleocapsids within the membranous bags that surround paracrystals and vesicles (Ng et al., 1994) (Figure 6C). However, because virion particles first become visible in the lumen of the RER (Figure 6D), it is generally believed that particle formation occurs by a still undefined assembly and budding process at the RER membrane (Brinton, 2002; Ng et al., 1994). Confirmation of such a mechanism has been hard to establish because of the difficulty of demonstrating distinct budding processes or of identifying and isolating free core particles from infected cells. However, recent studies with virus‐like particles obtained by expressing the structural proteins of hepatitis C virus have demonstrated budding at the RER membrane (Roingeard et al., 2004). Nascent virus particles pass through the host secretory pathway, where virus maturation occurs, and are released by fusion at the cell membrane (Lindenbach and Rice, 2003). Cryo‐immunoelectron microscopy studies performed with the Kunjin virus, using drugs that inhibit intracellular protein and/or membrane transport, support a maturation model that involves virion assembly in the RER, transport of individual particles to the Golgi apparatus, movement into the trans‐Golgi region, accumulation within secretory vesicles, and release by exocytosis. The carbohydrate side chains in extracellular virions have been shown by endo H digestion or lectin binding to be at least partially of the high mannose type. This incomplete processing could be due to an overloading of the sugar‐modifying enzymes by the large number of glycoproteins on the virion surface and/or their rapid transport through the secretory pathway (Heinz and Allison, 2003). The prM glycoprotein, which is present in immature virions, is cleaved late in the maturation process by a cellular protease to yield fully infectious mature virions. This maturation cleavage of prM is apparently mediated by furin in the trans‐Golgi network (Stadler et al., 1997). Viruses reach the plasma membrane inside secretory vesicles shortly before egress of mature virions (Figure 6E).
Factories of RNA viruses III: bunyaviruses, coronaviruses, and arteriviruses
The capacity of building a viral factory is probably a property of many other enveloped RNA viruses. Evidence found in the bibliography point to that possibility for members of the families Bunyaviridae, Arteriviridae and Coronaviridae.
The family Bunyaviridae contains more than 350 viruses classified in five genera: Bunyavirus, Hantavirus, Nairovirus, Phlebovirus, and Tospovirus (Schmaljohn and Hooper, 2001). Several viruses of the family are considered ‘emerging viruses’ that exhibit a worldwide distribution and cause severe disease in humans, including haemorrhagic fevers and encephalitis (e.g., Rift Valley fever, Hantaan, Oropouche, and Crimean‐Congo haemorrhagic fever viruses) (Elliott, 1997). Virions are spherical, 80 to 120 nm in diameter, and display surface glycoprotein projections of 5 to 10 nm in length anchored in a lipid bilayer (Schmaljohn and Hooper, 2001). Virions contain three single‐stranded RNA genome segments designated large (L), medium (M), and small (S) single‐stranded RNA of negative polarity. Four structural proteins are inserted into mature virions: nucleocapsid (N), polymerase (L), and two glycoproteins (G1 and G2). Bunyamwera virus (BUNV), the prototype of the family, is one of the bunyaviruses that encodes two additional nonstructural proteins synthesized early in infection, named NSs and NSm. NSs acts as a virulence factor and controls the activity of the viral polymerase (Bridgen et al., 2001; Weber et al., 2001). The role of NSm, a protein that accumulates in the Golgi complex (Nakitare and Elliott, 1993) is unknown.
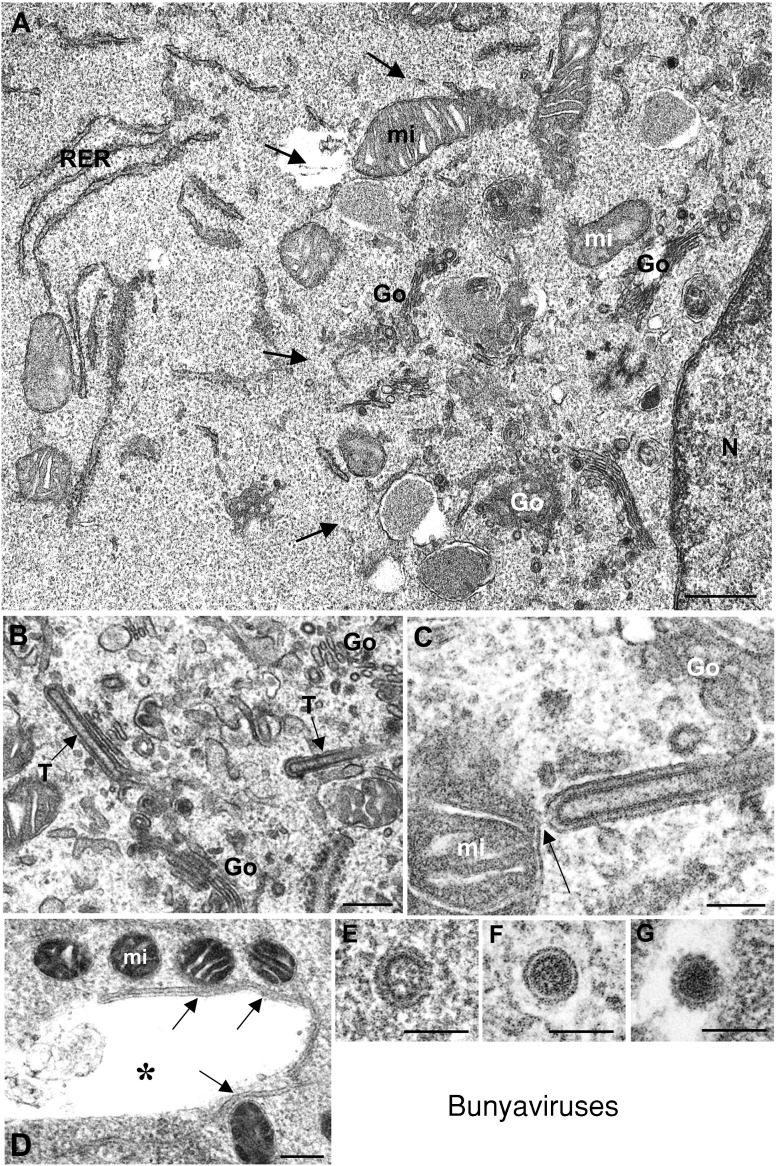
Most bunyaviruses assemble in the Golgi complex and use the secretory pathway to exit the cell. It has been recently observed that a large structure is formed in the perinuclear area of infected cells (Figures 1 and 7). Recruitment of mitochondria to the perinuclear area and establishment of contacts between mitochondria and Golgi stacks have been detected early in infection in BUNV‐infected cells (Figures 1A–1D, 7A, 7C and 7D) (Salanueva et al., 2003). A new viral structure, represented by tubular elements that assemble in Golgi membranes (Figure 7B), often connects with recruited mitochondria (Figure 7C). Preliminary data from in situ molecular detection (Novoa, Calderita and Risco, unpublished results) strongly suggest that these tubular structures represent the replication complexes of the virus, and that they could participate in recruitment and association of mitochondria to the Golgi stacks. Virus assembly takes place in the Golgi complex, where viral immature precursors (Figure 7E) transform into a second viral form (Figure 7F). A functional trans‐Golgi is required for this transformation (Salanueva et al., 2003), which is reminiscent of a similar intra‐Golgi process described for coronaviruses (Salanueva et al., 1999). A second maturation step takes place when the virus exits the cell and the final structure of the coat of spikes is acquired (Figure 7G). The spatial relationships between all these viral and cellular elements and the potential participation of cytoskeletal components in the ‘viral factory’ is under investigation. In particular, three‐dimensional analysis of these large structures will provide insights on the pattern of connections between replication complexes, assembly sites and maturation sites in these perinuclear structures.
Figure 7.
Factories of bunyaviruses
Factories are assembled in the perinuclear area (A), where Golgi stacks (Go) and mitochondria (mi) are seen together. RER cisternae, that are mainly excluded from the factory, are seen in peripheral areas. (B and C) Low and high‐magnification views of viral tubes (T) built in Golgi membranes and frequently seen in contact with mitochondria (arrow in C). (D) Direct contacts between mitochondria and Golgi membranes are also seen (arrows), even after Golgi disruption by monensin treatment. (E–G) Viral forms assembled in infected cells. The immature precursor (shown in E) transforms into the second viral form (F) in a trans‐Golgi dependent manner. Infectious extracellular virion is shown in (G). It acquires the final structure during exit from the cell. (C and E–F) reproduced from Salanueva et al. (2003) J. Virol. 77, 1368–1381, with copyright permission of the American Society for Microbiology. N, nucleus. Bars=0.5 μm in A; 200 nm in B and D; 100 nm in C, and E–G.
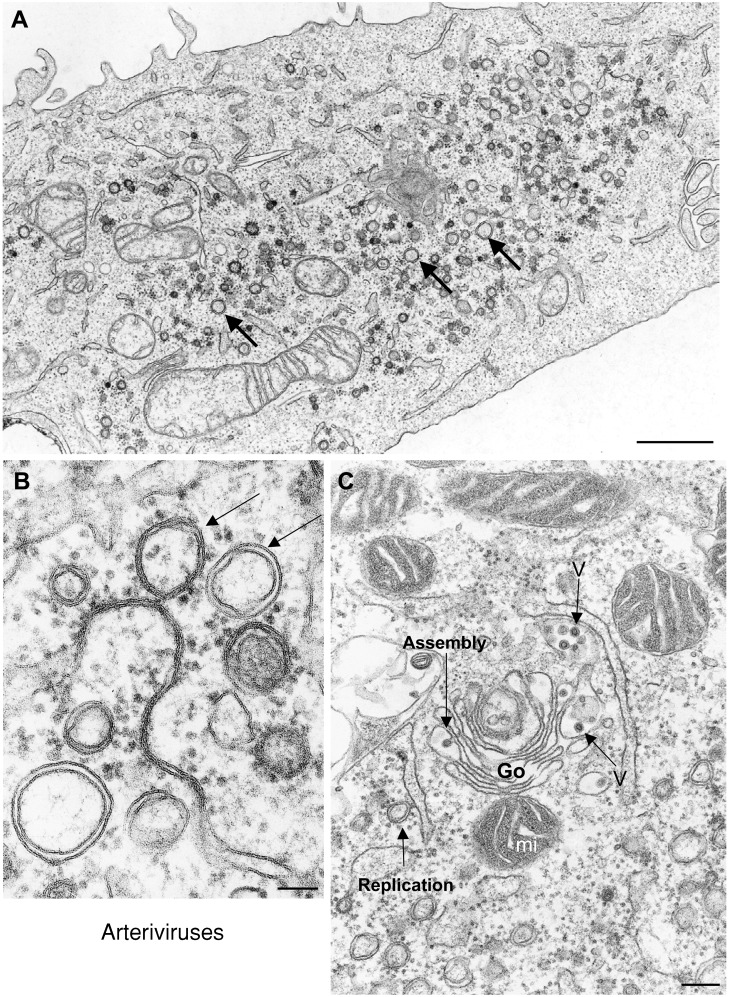
Coronaviruses and arteriviruses have been classified as separate families in the order Nidovirales (Lai and Holmes, 2001), whose members share a unique feature: they all synthesize a nested set of multiple subgenomic mRNAs (Cavanagh, 1997). Coronaviruses are enveloped, single‐stranded positive‐sense RNA viruses that replicate in the cytoplasm of infected cells. The coronavirus genome (27 to 31.5 kb) is the largest among known RNA viruses. It encodes for four structural proteins (the nucleocapsid N, and the envelope proteins M, E and S), as well as nonstructural proteins required for virus replication and transcription, that are encoded within gene 1. Coronaviruses cause severe diseases in many domestic animals and are a common cause of human colds. A new human coronavirus has been recently identified as the causative agent of the severe acute respiratory syndrome (SARS) (Drosten et al., 2003; Peiris et al., 2003). The family Arteriviridae comprises four enveloped, positive‐stranded RNA viruses that infect only animals. The replicase genes of coronaviruses and arteriviruses are assumed to share common ancestry. The consequences of arterivirus infection can range from an asymptomatic persistent or acute infection to abortion or lethal haemorrhagic fever (Snijder and Muelenberg, 2001). Arteriviruses are spherical, 40 to 60 nm in diameter, that contain an isometric, possibly icosahedral nucleocapsid of 25 to 35 nm composed of the 12.7 to 15.7 kb RNA genome and the nucleocapsid protein N. The envelope contains five or six envelope proteins, an unusually large number compared with other positive‐stranded RNA viruses. The replicase gene of members of the order Nidovirales encodes two polyproteins of extraordinary size and complexity. Arterivirus replication was found to be associated with RER or intermediate compartment membranes, which are modified into vesicular structures with a double membrane (van der Meer et al., 1998). These structures (Figures 8A and 8B) have been suggested to originate in cellular autophagosomes (Pedersen et al., 1999), similarly to poliovirus replication complexes (Suhy et al., 2000). Recent data for coronaviruses strongly suggest that their replication also occurs on autophagosomes. It has been reported that autophagy, a cellular stress response that functions to recycle proteins and organelles (Klionsky and Emr, 2000), is required for formation of double‐membrane bound murine hepatitis virus replication complexes, and that RER is implicated as the possible source of membranes for replication complexes (Prentice et al., 2004).
Figure 8.
Factories of arteriviruses
Cells infected with arteriviruses (A and B) contain large amounts of double‐membranes vesicles (arrows) that represent the replication complexes of the virus and derive from the ER. (C) Replication sites are close to assembly sites in Golgi stacks (Go), where viral particles are distinguished (V). These areas are also surrounded by mitochondria (mi). Bars=1 μm in (A); 100 nm in (B); 200 nm in (C).
Components of the coronavirus replication complex, the helicase and N proteins, translocate between 6 and 8 h post‐infection from sites of RNA replication to sites of viral assembly in the RER—Golgi intermediate compartment (Bost et al., 2000). Arteriviruses acquire their envelope by budding of preformed nucleocapsids into the lumen of the Golgi (Stueckemann et al., 1982). Most arterivirus envelope proteins are retained in intracellular membranes, but their specific roles in assembly have not yet been established. Genome encapsidation may occur or begin at the site of viral RNA synthesis (Molenkamp et al., 2000). Assembling viruses are seen in pre‐Golgi and Golgi membranes, near replication complexes. Both replication complexes and Golgi stacks with assembling viruses are seen surrounded by mitochondria (Figures 8A and 8C) which suggests, as with other viruses described in this review, a specific recruitment of this organelle to supply the energy needed for replication and/or assembly. After budding, coronaviruses and arteriviruses use vesicles from the secretory pathway to reach the plasma membrane before release. For coronaviruses, it has been reported that immature precursors change their structure and mature when reaching the trans‐Golgi subcompartment (Salanueva et al., 1999).
Conclusion and perspective
The study of the viral life cycle has traditionally provided unique information about cellular functions, including signalling. The characterization of viral factories demonstrates that different, non‐related viruses are able to interfere with cell processes to recruit and form a new complex structure where replication complexes and assembly sites are established. A number of viruses not described in this review most probably form factories as well. Picorna‐, rhabdo‐, reo‐ and birna‐viruses are all good candidates. New tools will help to understand the signals and mechanisms involved in the construction of viral factories. Among them, the analysis of cells expressing viral structural and non‐structural proteins, replicons, virus‐like particles, and infectious clones will help to identify key viral factors (Johnson and Chiu, 2000; Tzeng et al., 2001; Yount et al., 2003). On the other hand, the use of fluorescent tags in living cells and small interfering RNA technology (Lippincott‐Schwartz et al., 2003; Fraile‐Ramos et al., 2003; Déctor et al., 2002) will be very useful to characterize signals acting in recruitment and interaction between cellular and viral components. New methods for three‐dimensional analysis of cellular structures, such as electron tomography (Medalia et al., 2002; Grünewald et al., 2003) will help us to understand the architecture and dynamics of these unique structures, and to clarify key aspects such as how replication and assembly get functionally connected.
Acknowledgements
We express our gratitude to Professor Dr T.C. Mettenleiter, president of the Federal Research Centre for Virus Diseases of Animals, Germany, for many helpful discussions and critical reading of the manuscript. R.R.N. is the recipient of a fellowship for postgraduate students (FPI Program) from the Ministerio de Ciencia y Tecnología of Spain. This work has been supported by grants BMC2000‐0555 and BMC2003‐01630 from the Ministerio de Ciencia y Tecnología of Spain and 07B/0039/2002 from the Comunidad de Madrid (to C.R.).
Footnotes
Inclusion body:Aggregated, dead‐end material (usually unused viral proteins) that accumulates in the cytoplasm of infected cells at late times post‐infection.
Viroplasm foci:Electron‐dense masses seen in infected cells at early times post‐infection representing early accumulation of viral and cellular factors involved in viral replication and morphogenesis.
Poikilothermic animal:Animal with a body temperature that varies with the external environment (also known as ‘cold blooded’).
T=4 icosahedral symmetry:The viral core protein can adopt up to four quasi‐equivalent conformations in the icosahedral capsid.
References: Articles of special interest
- Andrés G. García‐Escudero R. Simón‐Mateo C. Viñuela E. African swine fever virus is enveloped by a two‐membraned collapsed cisterna from the endoplasmic reticulum J. Virol. 1998. 72 8988–9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés G. García‐Escudero R. Salas M.L. Rodríguez J.M. Repression of African swine fever virus polyprotein pp220‐encoding gene leads to the assembly of icosahedral core‐less particles J. Virol. 2002. 76 2654–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J. Merkenschlager M. Fisher A.G. Nuclear organisation and gene expression Curr. Opin. Cell Biol. 2002. 14 372–376. [DOI] [PubMed] [Google Scholar]
- Beatch M.D. Hobman T.C. Rubella virus capsid associates with host cell protein p32 and localizes to mitochondria J. Virol. 2000. 74 5569–5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz K. Egger D. Troxler M. Pasamontes L. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region J. Virol. 1990. 64 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht H. Roberts A. Vogel L. Bukreyev A. Collins P.L. Murphy B.R. Subbarao K. Moss B. Severe acute respiratory syndrome coronavirus spike protein expressed by attenuated vaccinia virus protectively immunizes mice Proc. Natl. Acad. Sci. U.S.A. 2004. 101 6641–6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bost A.G. Carnahan R.H. Lu X.T. Denison M.R. Four proteins processed from the replicase gene polyprotein of mouse hepatitis virus colocalize in the cell periphery and adjacent to sites of virion assembly J. Virol. 2000. 74 3379–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger D. Smith T. Skinner M.A. Morphogenesis and release of fowlpox virus J. Gen. Virol. 2000. 81 675–687. [DOI] [PubMed] [Google Scholar]
- Bridgen A. Friedemann W. Fazakerley J.K. Elliott R.M. Bunyamwera bunyavirus nonstructural protein NSs is a nonessential gene product that contributes to viral pathogenesis Proc. Natl. Acad. Sci. U.S.A. 2001. 98 664–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton M.A. The molecular biology of West Nile Virus: a new invader of the Western hemisphere Annu. Rev. Microbiol. 2002. 56 371–402. [DOI] [PubMed] [Google Scholar]
- Brookes S.M. Dixon L.K. Parkhouse R.M.E. Assembly of African swine fever virus: quantitative ultrastructural analysis in vitro and in vivo Virology 1996. 224 84–92. [DOI] [PubMed] [Google Scholar]
- Broyles S.S. Liu X. Zhu M. Kremer M. Transcription factor YY1 is a vaccinia virus late promoter activator J. Biol. Chem.. 1999. 274 35662–35667. [DOI] [PubMed] [Google Scholar]
- Carvalho Z.G. de Matos A.P. Alves Rodrigues‐Pousada C. Association of African swine fever virus with the cytoskeleton Virus Res. 1988. 11 175–192. [DOI] [PubMed] [Google Scholar]
- Castro A.P.V. Carvalho T.M.U. Moussatché N. Damaso C.R.A. Redsitribution of Cyclophilin A to viral factories during vaccinia virus infection and its incorporation into mature particles J. Virol. 2003. 77 9052–9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae Arch. Virol. 1997. 142 629–633. [PubMed] [Google Scholar]
- Chantler J. Wolinsky J.S. Tingle A. Knipe D.M. Holey P.M. Rubella virus Fields Virology 2001. Philadelphia Lippincott Williams and Wilkins; 963–990. [Google Scholar]
- Chen M.‐H. Icenogle J.P. Rubella virus capsid protein modulates viral genome replication and virus infecivity J. Virol. 2004. 78 4314–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R.H. Kuhn R.J. Olson N.H. Rossmann M.G. Choi H.K. Smith T.J. Baker T.S. Nucleocapsid and glycoprotein organization in an enveloped virus Cell (Cambridge, Mass.) 1995. 80 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb J.R. Bickmore W.A. Considering nuclear compartmentalization in the light of nuclear dynamics Cell (Cambridge, Mass.) 2003. 112 403–406. [DOI] [PubMed] [Google Scholar]
- Cobbold C. Brookes S.M. Wileman T. Biochemical requirements of virus wrapping by the endoplasmic reticulum: involvement of ATP and endoplasmic reticulum calcium store during envelopment of African swine fever virus J. Virol. 2000. 74 2151–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E. Taraboulos A. Scrapie‐like prion protein accumulates in aggresomes of cyclosporin A‐treated cells EMBO J. 2003. 22 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudmore S. Cossart P. Griffiths G. Way M. Actin‐based motility of vaccinia virus Nature (London) 1995. 378 636–638. [DOI] [PubMed] [Google Scholar]
- Cudmore S. Blasco R. Vincentelli R. Esteban M. Sodeik B. Griffiths G. Krijnse‐Locker J. A vaccinia virus core protein, p39, is membrane associated J. Virol. 1996. 70 6909–6921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales D. Mosbach E.H. Vaccinia as a model for membrane biogenesis Virology 1968. 35 564–583. [DOI] [PubMed] [Google Scholar]
- de Kops A. Bruyn Knipe D.M. Pre‐existing nuclear architecture defines the intranuclear location of herpesvirus DNA replication structures J. Virol. 1994. 68 3512–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Déctor M.A. Romero P. López S. Arias C.F. Rotavirus gene silencing by small interfering RNAs EMBO Rep. 2002. 3 1175–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Matos A.P. Carvalho Z.G. African swine fever virus interaction with microtubules Biol. Cell. 1993. 78 229–234. [DOI] [PubMed] [Google Scholar]
- Domingo E. Holland J.J. RNA virus mutations and fitness for survival Annu. Rev. Microbiol. 1997. 51 151–178. [DOI] [PubMed] [Google Scholar]
- Drosten C. Gunther S. Preiser W. van der Werf S. Brodt H.R. Becker S. Rabenau H. Panning M. Kolesnikova L. Fouchier R.A., et al., Identification of a nobel coronavirus in patients with severe acute respiratory syndrome N. Engl. J. Med.. 2003. 348 1967–1976. [DOI] [PubMed] [Google Scholar]
- Earl P.I. Americo J.L. Wyatt L.S. Eller L.A. Whitbeck J.C. Coehn G.H. Eisenberg R.J. Hartmann C.J. Jackson D.L. Kulesh D.A., et al., Immunogenicity of a highly attenuated MVA smallpox vaccine and protection against monkeypox Nature (London) 2004. 428 182–185. [DOI] [PubMed] [Google Scholar]
- Elliott R.M. Emerging viruses: the Bunyaviridae Mol. Med.. 1997. 3 572–577. [PMC free article] [PubMed] [Google Scholar]
- Flint S.J. Enquist L.W. Frug R.M. Racaniello V.R. Skalka A.M. Virus Structure Principles of Virology: Molecular Biology, Pathogenesis and Control 2000. Washington D.C. ASM Press; 58–98. [Google Scholar]
- Fraile‐Ramos A. Kohout T.A. Waldhoer M. Marsh M. Endocytosis of the viral chemokine receptor US28 does not require beta‐arrestins but is dependent on the clathrin‐mediated pathway Traffic 2003. 4 243–253. [DOI] [PubMed] [Google Scholar]
- Freed E.O. HIV‐1 and the host cell: an intimate association Trends Microbiol. 2004. 12 170–177. [DOI] [PubMed] [Google Scholar]
- Frey T.K. Molecular biology of rubella virus Adv. Virus Res. 1994. 44 69–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey T.K. Wolinsky J.S. Granoff A. Webster R.G. Rubella virus (Togaviridae) Encyclopedia of Virology 1999. San Diego Academic Press; 1592–1601. [Google Scholar]
- Froshauer S. Kartenbeck J. Helenius A. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes J. Cell Biol. 1988. 107 2075–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs W. Granzow H. Mettenleiter T.C. A pseudorabies virus recombinant simultaneously lacking the major tegument proteins encoded by the UL46, UL47, UL48, and UL49 genes is viable in cultured cells J. Virol. 2003. 77 12891–12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Beato R. Salas M.L. Viñuela E. Salas J. Role of the host cell nucleus in the replication of African swine fever virus Virology 1992. 188 637–649. [DOI] [PubMed] [Google Scholar]
- García‐Mata R. Gao Y.‐S. Sztul E. Hassles with taking out the garbage: aggravating aggresomes Traffic 2002. 3 388–396. [DOI] [PubMed] [Google Scholar]
- Garoff H. Hewson R. Opstelten D.‐J.E. Virus maturation by budding Microbial. Mol. Biol. Rev.. 1998. 62 1171–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzow H. Weiland F. Jöns A. Klupp B.G. Karger A. Mettenleiter T.C. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment J. Virol. 1997. 71 2072–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granzow H. Klupp B.G. Fuchs W. Veits J. Osterrieder N. Mettenleiter T.C. Egress of alphaherpesviruses: comparative ultrastructural study J. Virol. 2001. 75 3675–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G. Rottier P. Cell biology of viruses that assemble along the biosynthetic pathway Semin. Cell Biol. 1992. 3 367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünewald K. Desai P. Winkler D.C. Heymann J.B. Belnap D.M. Baumeister W. Steven A.C. Three‐dimensional structure of herpes simplex virus from cryo‐electron tomography Science (Washington, D.C.) 2003. 302 1396–1398. [DOI] [PubMed] [Google Scholar]
- Hackenbrock C. Ultrastructural basis for metabolically linked mechanical activity of mitochondria. I. Reversible ultrastructural changes with change in metabolic steady state in isolated liver mitochondria J. Cell Biol. 1966. 30 269–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath C.M. Windsor M. Wileman T. Aggresomes resemble sites specialized for virus assembly J. Cell Biol. 2001. 153 449–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz F.X. Allison S.L. Chambers T.M. Monath T.P. Flavivirus structure and membrane fusion Advances in Virus Research. The Flaviviruses: Structure, Replication and Evolution 2003. San Diego Elsevier Academic Press; 63–97. [DOI] [PubMed] [Google Scholar]
- Helenius A. Kielian M. Mellman I. Schmid S. Compans R.W. Helenius A. Oldstone M.B.A. Entry of enveloped viruses into their host cells Cell biology of virus entry, replication, and pathogenesis 1989. New York AR Liss; 145–161. [Google Scholar]
- Heljasvaara R. Rodríguez D. Risco C. Carrascosa J.L. Esteban M. Rodríguez J.R. The major core protein P4a (A10L gene) of vaccinia virus is essential for correct assembly of viral DNA into the nucleoprotein complex to form immature viral particles J. Virol. 2001. 75 5778–5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinshead M.A. Vanderplasschen A. Smith G.L. Vaux D.J. Vaccinia virus intracellular mature virions contain only one lipid membrane J. Virol. 1999. 73 1503–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa F.L. Brown J.C. Capsid assembly and DNA packaging in herpes simplex virus Rev. Med. Virol. 1997. 7 107–122. [DOI] [PubMed] [Google Scholar]
- Hong S.S. Ng M.L. Involvement of microtubules in Kunjin virus replication Arch. Virol. 1987. 97 115–121. [DOI] [PubMed] [Google Scholar]
- Hung J.J. Chung C.‐S. Chang W. Molecular chaperone Hsp90 is important for vaccinia virus growth in cells J. Virol. 2002. 76 1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M. Moss B. Evidence against an essential role of COPII‐mediated cargo transport to the endoplasmic reticulum‐Golgi intermediate compartment in the formation of the primary membrane of vaccinia virus J. Virol. 2003. 77 11754–11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishov A.M. Maul G.G. The periphery of nuclear domain 10 (ND10) as site of DNA virus deposition J. Cell Biol. 1996. 134 815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. Zhang Y. Krainer A.R. Xu R.M. Crystal structure of human p32, a doughnut‐shaped acidic mitochondrial matrix protein Proc. Natl. Acad. Sci. U.S.A. 1999. 96 3572–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.E. Chiu W. Structures of viruses and virus‐like particles Curr. Opin. Struct. Biol.. 2000. 10 229–235. [DOI] [PubMed] [Google Scholar]
- Karczewski M.K. Strebel K. Cytoskeleton association and virion incorporation of the human immunodeficiency virus type 1 Vif protein J. Virol. 1996. 70 494–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Kovacs J.J. McLaurin A. Vance J.M. Ito A. Yao T.‐P. The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress Cell (Cambridge, Mass.) 2003. 115 727–738. [DOI] [PubMed] [Google Scholar]
- Kelly D.C. Frog virus 3 replication: electron microscope observations on the sequence of infection in chick embryo fibroblasts J. Gen. Virol. 1975. 26 71–86. [DOI] [PubMed] [Google Scholar]
- Klionsky D.J. Emr S.D. Autophagy as a regulated pathway of cellular degradation Science (Washington, D.C.) 2000. 290 1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito R.R. Aggresomes, inclusion bodies and protein aggregation Trends Cell Biol. 2000. 10 524–530. [DOI] [PubMed] [Google Scholar]
- Krijnse‐Locker J. Schleich S. Rodríguez D. Goud B. Snijder E.J. Griffiths G. The role of a 21‐kDa viral membrane protein in the assembly of vaccinia virus from the intermediate compartment J. Biol. Chem.. 1996. 271 14950–14958. [DOI] [PubMed] [Google Scholar]
- Kuhn R.J. Strauss J.H. Chiu W. Johnson J.E. Enveloped viruses Advances in protein chemistry. Virus Structure 2003. San Diego Academic Press; 363–377. [DOI] [PubMed] [Google Scholar]
- Kuhn R.J. Zhang W. Rossman M.G. Pletner S.V. Corver J. Lenches E. Jones C.T. Mukhopadhyay S. Chipman P.R. Strauss E.G. Baker T.S. Strauss J.H. Structure of dengue virus. Implications for flavivirus organization, maturation, and fusion Cell (Cambridge, Mass.) 2002. 108 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujala P. Ikaheimonen A. Ehsani N. Vihinen H. Auvinen P. Kaarianen L. Biogenesis of the Semliki Forest virus RNA replication complex J. Virol. 2001. 75 3873–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M.C. Holmes K.V. Knipe D.M. Holey P.M. Coronaviridae: the viruses and their replication Fields Virology 2001. Philadelphia Lippincott Williams and Wilkins; 1163–1185. [Google Scholar]
- Leary K. Blair C.D. Sequential events in the morphogenesis of Japanese encephalitis virus J. Ultrastruct. Res.. 1980. 72 123–129. [DOI] [PubMed] [Google Scholar]
- Lee J.‐Y. Bowden D.S. Rubella virus replication and links to teratogenicity Clin. Microbiol. Rev.. 2000. 13 571–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.‐Y. Bowden D.S. Marshall J.A. Membrane junctions associated with rubella virus infected cells J. Submicrosc. Cytopathol. 1996. 28 101–108. [PubMed] [Google Scholar]
- Lee J.‐Y. Marshall J.A. Bowden D.S. Characterization of Rubella virus replication complexes using antibodies to double‐stranded RNA Virology 1994. 200 307–312. [DOI] [PubMed] [Google Scholar]
- Lee J.Y. Marshall J.A. Bowden D.S. Localization of rubella virus core particles in vero cells Virology 1999. 265 110–119. [DOI] [PubMed] [Google Scholar]
- Lindenbach B.D. Rice C.M. Chiu W. Johnson J.E. Molecular biology of Flaviviruses Advances in virus research. The Flaviviruses: Structure, Replication and Evolution 2003. London Elsevier Academic Press; 23–61. [DOI] [PubMed] [Google Scholar]
- Lippincott‐Schwartz J. Altan‐Bonnet N. Patterson G.H. Photobleaching and photoactivation: following protein dynamics in living cells Nat. Cell Biol. 2003. Suppl. S7–S14. [PubMed] [Google Scholar]
- Liptak L.M. Uprichard S.L. Knipe D.M. Functional order of assembly of herpes simplex virus DNA replication proteins into prereplicative site structures J. Virol. 1996. 70 1759–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz I.C. Kartenbeck J. Mezzacasa A. Allison S.L. Heinz F.X. Helenius A. Intracellular assembly and secretion of recombinant subviral particles from tick‐borne encephalitis virus J. Virol. 2003. 77 4370–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyle J.M. Bullitt E. Bienz K. Kirkegaard K. Visualization and functional analysis of RNA‐dependent RNA polymerase lattices Science (Washinton, D.C.) 2002. 296 2218–2222. [DOI] [PubMed] [Google Scholar]
- Mackenzie J.M. Westaway E.G. Assembly and maturation of the flavivirus Kunjin virus appear to occur in the rough endoplasmic reticulum and along the secretory pathway, respectively J. Virol. 2001. 75 10787–10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J.M. Jones M.K. Westaway E.G. Markers for the trans‐Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in Flavivirus‐infected cells J. Virol. 1999. 73 9555–9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J.M. Khromykh A.A. Westaway E.G. Stable expression of noncytopathic Kunjin replicons simulates both ultrastructural and biochemical characteristics observed during replication of Kunjin virus Virology 2001. 279 161–172. [DOI] [PubMed] [Google Scholar]
- Magliano D. Marshall J.A. Bowden D.S. Vardaxis S. Meanger J. Lee J.Y. Rubella virus replication complexes are virus modified lysosomes Virology 1998. 240 57–63. [DOI] [PubMed] [Google Scholar]
- Mallardo M. Leithe E. Schleich S. Ross N. Doglio L. Locker J.K. Relationship between vaccinia virus intracellular cores, early mRNAs, and DNA replication sites J. Virol. 2002. 76 5167–5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini E.J. Clarke M. Gowen B.E. Rutten T. Fuller S.D. Cryoelectron microscopy reveals the functional organization of an enveloped virus, Semliki Forest virus Mol. Cell. 2000. 5 255–266. [DOI] [PubMed] [Google Scholar]
- McNamee E.E. Taylor T.J. Knipe D.M. A dominant‐negative herpesvirus protein inhibits intranuclear targeting of viral proteins: effects on DNA replication and late gene expression J. Virol. 2000. 74 10122–10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia O. Weber I. Frangakis A.S. Nicastro D. Gerish G. Baumeister W. Macromolecular architecture in eukaryotic cells visualized by cryoelectron tomography Science (Washinton, D.C.) 2002. 298 1209–1213. [DOI] [PubMed] [Google Scholar]
- Merrifield C.J. Moss S.E. Ballestrem C. Imhof B.A. Giese G. Wunderlich I. Almers W. Endocytic vesicles move at the tips of actin tails in cultured mast cells Nat. Cell Biol. 1999. 1 72–74. [DOI] [PubMed] [Google Scholar]
- Mettenleiter T.C. Herpesvirus assembly and eggress J. Virol. 2002. 76 1537–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.J. Schwartz M.D. Ahlquist P. Flock house virus RNA replicates on outer mitochondrial membranes in Drosophila cells J. Virol. 2001. 75 11664–11676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenkamp R. van Tol H. Rozier B.C.D. van der Meer Y. Spaan W.J. Snijder E.J. The arterivirus replicase is the only viral protein required for genome replication and subgenomic mRNA transcription J. Gen. Virol. 2000. 81 2491–2496. [DOI] [PubMed] [Google Scholar]
- Moss B. Knipe D.M. Holey P.M. Poxviridae: the viruses and their replication Fields Virology 2001. Philadelphia Lippincott Williams and Wilkins; 2849–2883. [Google Scholar]
- Mossman K. Sherburne R. Lavery C. Duncan J. Smiley J. Evidence that herpes simplex virus VP16 is required for viral egress dowstream of the initial envelopment event J. Virol. 2000. 74 6287–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F.A. Schlesinger R.W. Togavirus morphology and morphogenesis The Togaviruses 1980. New York Academic Press; 1223–1227. [Google Scholar]
- Murti K.G. Goorha R. Interaction of frog virus‐3 with the cytoskeleton. I. Altered organization of microtubules, intermediate filaments, and microfilaments J. Cell Biol. 1983. 96 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakitare G.W. Elliott R.M. Expression of the Bunyamwera virus M genome segment and intracellular localization of NSm Virology 1993. 195 511–520. [DOI] [PubMed] [Google Scholar]
- Nédellec P. Vicart P. Laurent‐Winter C. Martinat C. Prévost M.C. Brahic M. Interaction of Theiler's virus with intermediate filaments of infected cells J. Virol. 1998. 72 9553–9560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W.W. Brown J.C. Structure of the herpes simplex virus capsid: effects of extraction with guanidine hydrochloride and partial reconstitution of extracted capsids J. Virol. 1991. 65 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W.W. Brown J.C. Induced extrusion of DNA from the capsid of herpes simplex virus type 1 J. Virol. 1994. 68 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W.W. Homa F.L. Thomsen D.R. Booy F.P. Trus B.L. Steven A.C. Spencer J.V. Brown J.C. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell‐free capsid formation J. Mol. Biol.. 1996. 263 432–446. [DOI] [PubMed] [Google Scholar]
- Ng M.L. Hong S.S. Flavivirus infection: essential ultrastructural changes and association of Kunjin virus NS3 protein with microtubules Arch. Virol. 1989. 106 103–120. [DOI] [PubMed] [Google Scholar]
- Ng M.L. Pedersen J.S. Toh B.H. Westaway E.G. Immunofluorescent sites in Vero cells infected with the flavivirus Kunjin Arch. Virol. 1983. 78 177–190. [DOI] [PubMed] [Google Scholar]
- Ng M.L. Teong F.M. Tan S.H. Cryosubstitution technique reveals new morphology of flavivirus‐induced structures J. Virol. Methods 1994. 49 305–314. [DOI] [PubMed] [Google Scholar]
- Orth J.D. Krueger E.W. Cao H. McNiven M.A. The large GTPase dynamin regulates actin comet formation and movement in living cells Proc. Natl. Acad. Sci. U.S.A. 2002. 99 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K.W. van der Meer Y. Roos N. Snijder E.J. ORF1a‐encoded subunits of the arterivirus replicase induce endoplasmic reticulum‐derived double membrane vesicles which carry the viral replication complex J. Virol. 1999. 73 2016–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris J.S. Yuen K.Y. Osterhaus A.D. Stohr K. The severe acute respiratory syndrome N. Engl. J. Med.. 2003. 349 2431–2441. [DOI] [PubMed] [Google Scholar]
- Ploubidou A. Moreau V. Ashman K. Reckmann I. González C. Way M. Vaccinia virus infection disrupts microtubule organization and centrosome function EMBO J. 2000. 19 3932–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pornillos O. Garrus J.E. Sundquist W.I. Mechanisms of enveloped RNA virus budding Trends Cell Biol. 2002. 12 569–579. [DOI] [PubMed] [Google Scholar]
- Prentice E. Jerome W.G. Yoshimori T. Mizushima M. Denison M.R. Coronavirus replication complex formation utilizes components of cellular autophagy J. Biol. Chem.. 2004. 279 10136–10141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q.W. Lam T.J. Sin Y.M. Shen H. Chang S.F. Ngoh G.H. Chen C.L. Electron microscopic observations of a marie fish iridovirus isolated from brown‐spotted grouper, Epinephelus tauvina J. Virol. Methods 2001. 98 17–24. [DOI] [PubMed] [Google Scholar]
- Quinlan M.P. Chen L.B. Knipe D.M. The intranuclear location of a herpes virus DNA‐binding protein is determined by the status of viral DNA replication Cell (Cambridge, Mass.) 1984. 36 857–868. [DOI] [PubMed] [Google Scholar]
- Rice C.M. Lenches E.M. Eddy S.R. Shin S.J. Sheets R.L. Strauss J.H. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution Science (Washinton, D.C.) 1985. 229 726–735. [DOI] [PubMed] [Google Scholar]
- Rietdorf J. Ploubidou A. Reckmann I. Holmstrom A. Frieschnecht F. Zettl M. Zimmermann T. Way M. Kinesin‐dependent movement on microtubules precedes actin‐based motility of vaccinia virus Nat. Cell Biol. 2001. 3 992–1000. [DOI] [PubMed] [Google Scholar]
- Risco C. Carrascosa J.L. Visualization of viral assembly in the infected cell Histol. Histopathol. 1999. 14 905–926. [DOI] [PubMed] [Google Scholar]
- Risco C. Rodríguez J.R. Demkovicz W. Heljasvaara R. Carrascosa J.L. Esteban M. Rodríguez D. The vaccinia virus 39‐KDa protein forms a stable complex with the p4a/a major core protein early in morphogenesis Virology 1999. 265 375–386. [DOI] [PubMed] [Google Scholar]
- Risco C. Rodríguez J.R. López‐Iglesias C. Carrascosa J.L. Esteban M. Rodríguez D. Endoplasmic reticulum‐Golgi intermediate compartment membranes and vimentin filaments participate in vaccinia virus assembly J. Virol. 2002. 76 1839–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risco C. Carrascosa J.L. Frey T.K. Structural maturation of rubella virus in the Golgi complex Virology 2003. 312 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez J.M. García‐Escudero R. Salas M.L. Andrés G. African swine fever virus structural protein p54 is essential for the recruitment of envelope precursors to assembly sites J. Virol. 2004. 78 4299–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez J.R. Risco C. Carrascosa J.L. Esteban M. Rodríguez D. Characterization of early stages in vaccinia virus membrane biogenesis: implication of the 21‐kilodalton (A14L) protein and a newly identified 15‐kilodalton envelope protein J. Virol. 1997. 71 1821–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roingeard P. Hourioux C. Blanchard E. Brand D. Ait‐Goughoulte M. Hepatitis C virus ultrastructure and morphogenesis Biol. Cell. 2004. 96 103–108. [DOI] [PubMed] [Google Scholar]
- Roizman B. Knipe D. Knipe D.M. Holey P.M. Herpes simplex viruses and their replication Fields Virology 2001. Philadelphia Lippincott Williams and Wilkins; 2399–2460. [Google Scholar]
- Roizman B. Pellett P.E. Knipe D.M. Holey P.M. The family Herpesviridae: A brief introduction Fields Virology 2001. Philadelphia Lippincott Williams and Wilkins; 2381–2397. [Google Scholar]
- Rojo G. Chamorro M. Salas M.L. Viñuela E. Cuezva J.M. Salas J. Migration of mitochondria to viral assembly sites in African Swine Fever Virus‐infected cells J. Virol. 1998. 72 7583–7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller I. Brookes S.M. Hyatt A.D. Windsor M. Wileman T. African swine fever virus is wrapped by the endoplasmic reticulum J. Virol. 1998. 72 2373–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanueva I.J. Carrascosa J.L. Risco C. Structural maturation of the transmissible gastroenteritis coronavirus J. Virol. 1999. 73 7952–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanueva I.J. Novoa R.R. Cabezas P. López‐Iglesias C. Carrascosa J.L. Elliott R.M. Risco C. Polymorphism and structural maturation of Bunyamwera virus in Golgi and post‐Golgi compartments J. Virol. 2003. 77 1368–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas M.L. Granoff A. Webster R.G. African Swine Fever Virus (Asfarviridae) Encyclopedia of Virology 1999. San Diego and London Academic Press; 30–31. [Google Scholar]
- Schetter C. Grunemann B. Holker I. Doerfler W. Patterns of frog virus 3 DNA methylation and DNA methyltransferase activity in nuclei of infected cells J. Virol. 1993. 67 6973–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M.J. Granoff A. Webster R.G. Sindbis and Semliki Forest Viruses (Togaviridae) Encyclopedia of Virology 1999. San Diego and London Academic Press; 1656–1663. [Google Scholar]
- Schlesinger S. Schlesinger M.J. Knipe D.M. Holey P.M. Togaviridae: the viruses and their replication Fields Virology 2001. Philadelphia Lippincott Williams and Wilkins; 523–539. [Google Scholar]
- Schmaljohn S. Hooper J.W. Knipe D.M. Holey P.M. Bunyaviridae: The viruses and their replication Fields Virology 2001. Philadelphia Lippincott Williams and Wilkins; 1581–1602. [Google Scholar]
- Schubert U. Anton L.C. Gibbs J. Norbury C.C. Yewdell J.W. Bennink J.R. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes Nature (London) 2000. 404 770–774. [DOI] [PubMed] [Google Scholar]
- Sharpe A.H. Chen L.B. Fields B.N. The interaction of mammalian reoviruses with the cytoskeleton of monkey kidney CV‐1 cells Virology 1982. 120 399–411. [DOI] [PubMed] [Google Scholar]
- Skepper J. Whiteley A. Browne H. Minson A. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopement‐deenvelopement‐reenvelopement pathway J. Virol. 2001. 75 5697–5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E.J. Meulenberg J.J.M. Knipe D.M. Holey P.M. Arteriviruses Fields Virology 2001. Philadelphia Lippincott Williams and Wilkins; 1205–1220. [Google Scholar]
- Sodeik B. Krijnse‐Locker J. Assembly of vaccinia virus revisited: de novo membrane synthesis or acquisition from the host? Trends Microbiol. 2002. 10 15–24. [DOI] [PubMed] [Google Scholar]
- Sodeik B. Doms R.W. Ericsson M. Hiller G. Machamer C.E. van't Hof W. van Mer G. Moss B. Griffiths G. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks J. Cell Biol. 1993. 121 521–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeik B. Ebersold M. Helenius A. Microtubule‐mediated transport of incoming herpes simplex virus 1 capsids to the nucleus J. Cell Biol. 1997. 136 1007–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P.G. Entry of alphaherpesviruses into cells Semin. Virol. 1993. 4 167–180. [Google Scholar]
- Stadler K. Allison S.L. Schlich J. Heinz F.X. Proteolytic activation of tick‐borne encephalitis virus by furin J. Virol. 1997. 71 8475–8481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J.H. Strauss E.G. The alphaviruses: gene expression, replication, and evolution Microbiol. Rev.. 1994. 58 491–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueckemann J.A. Holth M. Swart W.J. Kowalchyk K. Smith M.S. Wolstenholme A.J. Cafruny W.A. Plagemann P.G. Replication of lactate dehydrogenase‐elevating virus in macrophages. 2. Mechanism of persistent infection in mice and cell culture J. Gen. Virol. 1982. 59 263–272. [DOI] [PubMed] [Google Scholar]
- Suhy D.A. Giddings T.H., Jr Kirkegaard K. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy‐like origin for virus‐induced vesicles J. Virol. 2000. 74 8953–8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taunton J. Rowning B.A. Coughlin M.L. Wu M. Moon R.T. Mitchison T.J. Larabell C.A. Actin‐dependent propulsion of endosomes and lysosomes by recruitment of N‐WASP J. Cell Biol. 2000. 148 519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolonen N. Doglio L. Schleich S. KrijnseLocker J. Vaccinia virus DNA replication occurs in ER‐enclosed cytoplasmic mini‐nuclei Mol. Biol. Cell. 2001. 12 2031–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traktman P. Liu K. DeMasi R. Rollins R. Jesty S. Unger B. Elucidating the essential role of the A14 phosphoprotein in vaccinia virus morphogenesis: construction and characterization of a tetracycline‐inducible recombinant J. Virol. 2000. 74 3682–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus B.L. Booy F.P. Newcomb W.W. Brown J.C. Homa F.L. Thomsen D.R. Steven A.C. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19c and VP23 in assembly J. Mol. Biol.. 1996. 263 447–462. [DOI] [PubMed] [Google Scholar]
- Tzeng W.‐P. Frey T.K. Complementation of a deletion in the rubella virus P150 nonstructural protein by the viral capsid protein J. Virol. 2003. 77 9502–9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng W.P. Chen M.‐H. Derdeyn C.A. Frey T.K. Rubella virus DI RNAs and replicons: requirement for nonstructural proteins acting in cis for amplification by helper virus Virology 2001. 289 63–73. [DOI] [PubMed] [Google Scholar]
- Uchil P.D. Satchidanandam V. Architecture of the flaviviral replication complex J. Biol. Chem.. 2003. 278 24388–24398. [DOI] [PubMed] [Google Scholar]
- Uprichard S.L. Knipe D.M. Herpes simplex virus ICP27 mutant viruses exhibit reduced expression of specific DNA replication genes J. Virol. 1996. 70 1969–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uprichard S.L. Knipe D.M. Assembly of herpes simplex virus replication proteins at two distinct intranuclear sites Virology 1997. 229 113–125. [DOI] [PubMed] [Google Scholar]
- Valcarce C. Navarrete R.M. Encabo P. Loeches E. Satrústegui J. Cuezva J.M. Postnatal development of rat liver mitochondrial functions. The role of protein synthesis and of adenine nucleotides J. Biol. Chem.. 1988. 263 7767–7775. [PubMed] [Google Scholar]
- van der Meer Y. van Tol H.G. Locker J. Krijnse Snijder E.J. ORF1a‐encoded replicase subunits are involved in membrane association of the arterivirus replication complex J. Virol. 1998. 72 6689–6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Regenmortel M. Introduction to the virus species concept in Virus Taxonomy Virus Taxonomy 2000. San Diego Academic Press; 3–16 Seventh report of the international committee on taxonomy of viruses.. [Google Scholar]
- Vanslyke J.K. Hruby D.E. Immunolocalization of vaccinia virus structural proteins during virion formation Virology 1994. 198 624–635. [DOI] [PubMed] [Google Scholar]
- Weaver S.C. Dalgarno L. Frey T.K. Huang H.V. Kinney R.M. Rice C.M. Roehring J.T. Shope R.E. Strauss E.G. van Regenmortel M.H.V. Family Togaviridae Virus Taxonomy, Seventh report of the international committee on taxonomy of viruses 2000. San Diego Academic Press; 879–888. [Google Scholar]
- Weber F. Dunn E.F. Bridgen A. Elliott R.M. The bunyamwera virus nonstructural protein NSs inhibits viral RNA polymerase in a minireplicon system Virology 2001. 281 67–74. [DOI] [PubMed] [Google Scholar]
- Weiss R.A. Virulence and pathogenesis Trends Microbiol. 2002. 10 314–317. [DOI] [PubMed] [Google Scholar]
- Welsch S. Doglio L. Schleich S. Krijnse‐Locker J. The vaccinia virus I3L gene product is localized to a complex endoplasmic reticulum‐associated structure that contains the viral parental DNA J. Virol. 2003. 77 6014–6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway E.G. Mackenzie J.M. Kenney M.T. Jones M.K. Khromykh A.A. Ultrastructure of Kunjin virus‐infected cells: colocalization of NS1 and NS3 with double‐stranded RNA, and of NS2B with NS3, in virus‐induced membrane structure J. Virol. 1997. 71 6650–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westaway E.G. Mackenzie J.M. Khromykh A.A. Chambers T.M. Monath T.P. Kunjin RNA replication and applications of Kunjin replicons Advances in Virus Research. The Flaviviruses: Structure, Replication and Evolution 2003. San Diego Elsevier Academic Press; 99–140. [DOI] [PubMed] [Google Scholar]
- Wigley W.C. Fabunmi R.P. Lee M.G. Marino C.R. Muallen S. DeMartino G.N. Thomas P.J. Dynamic association of proteasomal machinery with the centrosome J. Cell Biol. 1999. 145 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T. The iridoviruses Adv. Virus Res. 1996. 46 347–412. [DOI] [PubMed] [Google Scholar]
- Wolffe E.J. Moore D.M. Peters P.J. Moss B. Vaccinia virus A17L open reading frame encodes an essential component of nascent viral membranes that are required to initiate morphogenesis J. Virol. 1996. 70 2797–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X. Olson N.H. Van Etten J.L. Bergoin M. Rossmann M.G. Baker T.S. Structure and assembly of large lipid‐containing dsDNA viruses Nature Struct. Biol.. 2000. 7 101–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount B. Curtis K.M. Fritz E.A. Hensley L.E. Jahrling P.B. Prentice E. Denison M.R. Geisbert T.W. Baric R.S. Reverse genetics with a full‐length cDNA of severe acute respiratory syndrome coronavirus Proc. Natl. Acad. Sci. U.S.A. 2003. 100 12995–13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. Mukhopadhyay S. Pletnev S. Baker T.S. Kuhn R.J. Rossmann M.G. Placement of the structural proteins of Sindbis virus J. Virol. 2002. 76 11645–11658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. Lindqvist B. Garoff H. von Bonsdorff C.‐H. Liljeström P. A tyrosine‐based motif in the cytoplasmic domain of the alphavirus envelope protein is essential for budding EMBO J. 1994. 13 4204–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z. Chen D. Jakana J. Rixon F.J. Chiu W. Visualization of tegument‐capsid interactions and DNA in intact herpes simplex virus type I virions J. Virol. 1999. 73 3210–3218. [DOI] [PMC free article] [PubMed] [Google Scholar]