Abstract
Objective
To explore medication safety issues related to use of an electronic medication management system (EMM) in paediatric oncology practice, through the analysis of patient safety incident reports.
Methods
We analysed 827 voluntarily reported incidents relating to oncology patients that occurred over an 18‐month period immediately following implementation of an EMM in a paediatric hospital in Australia. We identified medication‐related and EMM‐related incidents and carried out a content analysis to identify patterns.
Results
We found ~79% (n = 651) of incidents were medication‐related and, of these, ~45% (n = 294) were EMM‐related. Medication‐related incidents included issues with: prescribing; dispensing; administration; patient transfers; missing chemotherapy protocols and information on current stage of patient treatment; coordination of chemotherapy administration; handling or storing medications; children or families handling medications. EMM‐related incidents were classified into four groups: technical issues, issues with the user experience, unanticipated problems in EMM workflow, and missing safety features.
Conclusions
Incidents reflected difficulties with managing therapies rich in interdependencies. EMM, and especially its ‘automaticity’, contributed to these incidents. As EMM impacts on safety in such high‐risk settings, it is essential that users are aware of and attend to EMM automatic behaviours and are equipped to troubleshoot them.
Keywords: computerized provider order entry system, evaluation research, hospital oncology services, medication errors, paediatrics, patient safety
1. INTRODUCTION
Improvements in medication safety in paediatric oncology can be achieved by using electronic medication management (EMM) systems (also known as computerized provider order entry—CPOE) with clinical decision support (CDS; Aita et al., 2013; Bannan & Tully, 2016; Brenner et al., 2016; Chen & Lehmann, 2011; Elsaid, Garguilo, & Collins, 2015; Maaskant et al., 2015; Rinke et al., 2014; van Rosse et al., 2009; Small, Barrett, & Price, 2008). This technology has the potential to improve safety by facilitating the scheduling of chemotherapy cycles and associated monitoring tests, calculating cumulative doses of drugs and supporting medication workflows (Allen et al., 2018; Elsaid et al., 2013; Gandhi, Tyono, Pasetka, & Trudeau, 2014; Hoffman, Baker, Howard, Laver, & Shenep, 2011). However, technology can also introduce new opportunities for error, and lead to unwanted effects on work practices (Elsaid et al., 2015). For example, errors in the configuration of prescribing templates may be ‘automatically incorporated into otherwise error‐free prescriptions’ (Aita et al., 2013). Comprehensive EMM systems for chemotherapy protocols are challenging to implement, both in adult and paediatric settings (Martin, Kaemingk, Frieze, Hendrie, & Payne, 2015; Whalen et al., 2018). They require time‐dependent, patient‐specific dosing functions based on factors such as age, weight and surface area (highly variable over a child's course of treatment), organ function, as well as functionalities for scheduling and timing of interdependent medications and supportive care. The systems must address critical workflow functionalities, such as dual/multiple authorisations and checks of orders by an oncologist and verified by a pharmacist and two nurses (Martin et al., 2015). After implementation, EMM systems need maintenance and updating to address changes in work practices and in chemotherapy protocols (Chen & Lehmann, 2011).
We aimed to explore medication safety issues related to use of EMM in paediatric oncology practice, through the analysis of patient safety incident reports.
2. METHODS
2.1. Setting and EMM
Data derived from voluntary reports of patient safety incidents were gathered from a children's cancer centre within a 350‐bed tertiary paediatric hospital in Sydney, New South Wales (NSW), Australia. The centre introduced an EMM system in oncology covering inpatient/outpatient services and home‐based care. The EMM was integrated in the hospital electronic medical record system (EMR; Cerner Corporation, https://www.cerner.com/) so that patients’ medications, tests orders and results and medical notes are entered and accessed from a single system.
The EMM allows prescribing, recording of drug dispensing, documentation of drug administration and medication reconciliation and monitoring, with all drugs and fluids. However, at the time of study, prescription and administration of blood products and parenteral nutrition were still paper‐based and outpatient prescriptions required a signature on printed copies. The CDS includes links to guidelines, order sets, order sentences, safety alerts (e.g. drug–drug interactions) and dosage calculators. To order chemotherapy with EMM, prescribers are required to select a pre‐populated plan, or if a plan is unavailable (for patients on unique or individual chemotherapy protocols), to create and complete orders using a prebuilt order template. The EMM system provides access to multiple medication screens, including multiple screens for prescribing, a summary view of medications prescribed, as well as a MAR (medication administration record) screen for administration. The system allows for direct links to the chemotherapy protocol attached as a PDF to the patient record. There is ‘automation’ across screens, so that medications prescribed (or ceased) ‘drop’ automatically into the MAR (or are removed from the chart if ceased). Upon prescribers entering orders into the system, the EMM automatically schedules items for administration over set frequencies, days and times. The system provides check points (a ‘ready for chemo’ tick box) for oncologists to approve nurses’ proceeding with administration of chemotherapy. Medications entered in chemotherapy plans must be ‘activated’ first for nurses to be able to administer. Prescribing chemotherapy is restricted to senior medical officers and fellows.
2.2. The dataset
The NSW Health Incident Information Management System (NSW Health, 2014) collects staff reported incidents to enable analysis of contributory factors and to develop interventions. In broad terms, incidents are defined as ‘any unplanned event resulting in, or with the potential for, injury, damage or other loss’ (NSW Health, 2014).
A retrospective review was undertaken of all incidents reported by staff related to oncology patients in the hospital in the 18 months immediately following EMM implementation (15 August 2016–15 February 2018). In total, 827 oncology‐related incidents were reported, related to any aspect of patient care, including medications, with or without the use of the EMM system.
The study received ethics approval by the hospital's human research ethics committee. The data were de‐identified prior to review and analysis.
2.3. Methods for analysis
As background for the analysis, we compiled descriptive statistics of the age of the patient involved in the incident and severity of incidents, as classified by the hospital. The incidents were assigned a Severity Assessment Code (SAC) score (NSW Health, 2014) by the hospital. This score, 1 through 4, indicates the severity of any potential or actual consequence and the likelihood of the incident reoccurring (with higher scores indicating less severity). The incidents were assigned either or both an ‘Initial SAC’ (by the reporting person) and ‘Actual SAC’ score (reviewed by manager).
For all incidents in the dataset, we reviewed the Incident description and Contributing factors fields (i.e. free text describing the events). We coded each incident on the basis of two coding schemes—first to identify incidents ‘related, or not, to medication’ (and at which stage of the medication process the incident occurred) and second to identify medication‐related incidents ‘related, or not, to EMM’ (and which type of issue was reported). The two coding schemes, based on established classification systems (Krzyzaniak & Bajorek, 2016; NCC MERP, 2018; Sittig, Classen, & Singh, 2014), were iteratively tested on samples of incident reports and progressively refined during the analysis.
2.3.1. Classification of medication‐related incidents
Medication‐related incidents were those involving at least one medication, regardless of medication type. Incidents involving TPN (Total Parenteral Nutrition), oxygen administration and blood transfusions were also included as these form part of oncology supportive care treatment and follow similar processes of order, supply and administration. Table 1 describes the coding scheme used to classify incidents on the basis of the medication phase. Multiple categories were allowed for each report.
Table 1.
Coding scheme for classification of medication‐related incidents
| Medication phase | Description (inclusion/exclusion criteria) |
|---|---|
| Prescribing | ‘All [incidents] that occur during the decision process and in prescribing/ordering a medication for a patient’ (Krzyzaniak & Bajorek, 2016) |
| Order communication |
All incidents that occur at the stage of communicating the prescription for dispensing to pharmacy Includes also incidents related to the ‘ready for chemo tick’ (EMM tick box used for communicating doctors’ okay to proceed with administration) |
| Dispensing |
All incidents about ‘product labelling, packaging, and nomenclature, compounding, dispensing, distribution’ (NCC MERP, 2018) ‘All [incidents] that occur during the interpretation of medication prescriptions by the pharmacy staff and the subsequent selection, preparation, labelling and distribution of medication’ (Krzyzaniak & Bajorek, 2016) |
| Administration |
‘All [incidents] that occur whilst a medication is being administered to a patient’ (Krzyzaniak & Bajorek, 2016) Includes also:
|
| Education | All incidents that relate to informing the patient or family about the medication |
| Monitoring |
‘All [incidents] associated with the monitoring of clinical and/or laboratory data that assess the patient's response to the administered drug therapy i.e. through therapeutic drug‐monitoring practices’ (Krzyzaniak & Bajorek, 2016) Includes also incidents about monitoring and recording of fluids |
| Use | All incidents that relate to a patient's or families’ use of the medication—for example, giving/taking medications, making decisions about medications |
| Other |
All other incidents where a medication was involved but incident did not occur during one of the above‐listed stages. Includes also:
|
| Unclear | Incidents where there is not enough information to determine at what stage the incident occurred |
| Not applicable | When there is no medication involved in the incident |
2.3.2. Classification of EMM‐related incidents
Medication‐related incidents were then coded as ‘with EMM’ when it was clear, or it could be reasonably inferred from the description of the incident, that the EMM was used at the time the incident occurred. Table 2 outlines the coding scheme used to classify the type of EMM‐related issue reported.
Table 2.
Coding scheme for classification of electronic medication management system (EMM)‐related incidents
| ID | Type of health information technology (HIT) related safety concern | Examples |
|---|---|---|
| HIT 1 | Instances in which HIT fails during use or is otherwise not working as designed | Broken hardware or software ‘bugs’ |
| HIT 2 | Instances in which users describe negative experiences with HIT such as stress, frustration, or confusion (revised definition, modified from original (Sittig et al., 2014; ¥) | Users reporting that the system is too difficult to use, or too slow (example added, not in the original (Sittig et al., 2014; ¥) |
| HIT 3 |
Instances in which HIT is well designed and working correctly, but was not configured, implemented or used in a way anticipated or planned for by system designers and developers. This includes all incidents of EMM use that would be useful to show designers to inform possible improvements to the EMM system—for example, when the drugs ‘fell off’ the chart |
Duplicate order alerts that fire on alternative ‘as needed’ pain medications |
| HIT 4 | Instances in which HIT is working as designed and was configured and used correctly, but interacts with external systems (e.g. via hardware or software interfaces) so that data are lost or incorrectly transmitted or displayed | Medication order for extended‐release morphine inadvertently changed to immediate‐release morphine by error in interface translation table |
| HIT 5 | Instances in which [it is explicitly reported that] specific safety features or functions were not implemented or not available (i.e. HIT could have prevented a safety concern) | Hospitalised patient inadvertently receives 5 g of acetaminophen in 24 hr because maximum daily dose alerting was not available |
| HIT 0 | None of the above (not in original; Sittig et al., 2014) |
Scheme adapted from Sittig et al. (2014) classification of incidents with health information technology (HIT). Italics indicate modifications to the original classification. We modified category HIT2, originally intended to cover HIT usability issues, to make more explicit dimensions related to the user experience of EMM (Usability.gov, 2018). We modified category HIT3 inclusion criteria as it was not possible to determine that anticipated or planned for by designers or developers. Instead, we used HIT3 to classify incidents reporting use of EMM that we assessed would be worthwhile for designers to investigate, in order to improve the design of (or the training on) EMM. We modified HIT5, limiting its use to incident reports providing explicit reference to missing features or functionalities. We added a HIT0 ‘other’ type to code any EMM‐related incidents not otherwise captured by the HIT1‐5 categories.
(¥) Original HIT definition: ‘HIT 2: Instances in which HIT is working as designed, but the design does not meet the user's needs or expectations. Examples: Usability issues’ (Sittig et al., 2014).
Three researchers, with expertise in medication safety and health informatics, coded the incident reports. They initially coded and discussed three samples (84, 51 and 48 reports) to assess consistency in coding and suitability of the coding schemes. Following the three attempts to improve consistency (eventually reaching ~79% agreement for HIT, ~50% for medication stage), the remaining reports were independently coded by two researchers. All inconsistencies in coding were discussed to reach a final consensus.
In order to gain a more refined understanding of the nature and patterns of incidents, we then carried out a qualitative content analysis (Hsieh & Shannon, 2005) of the EMM‐related, medication‐related incidents. This analysis was informed by a human factors’ perspective (Carayon, Wooldridge, Hose, Salwei, & Benneyan, 2018). This is a systemic analytical lens that considers people and technology as interacting in a socio‐technical whole. Errors, or incidents, are understood as resulting from the dynamic interaction of the elements of the sociotechnical system, not from one element alone (i.e. the human or the EMM system).
In reporting the results, we present the quantitative distribution of the incidents as background to the qualitative findings, but note that it is not appropriate to use counts of voluntary incident reports as a measure of incident numbers that have occurred (Schwappach & Gehring, 2015; Westbrook et al., 2015). While we report on all types of medication incidents reported, we focus on those where EMM had a role. In the results, we refer to specific incidents by providing the incident identification number (e.g. id123).
3. RESULTS
The age of the children, whose care the incidents were about, ranged from neonates and babies to young and older children and teenagers (up to 19 years of age). In terms of severity, not all 827 incidents were assigned SAC scores; among those where Initial SAC and/or Actual SAC scores were given, incidents had respective averages 3.3 (n = 433) and 3.4 (n = 628; using the SAC matrix, severity is rated from 1 to 4, with higher scores indicating less severity).
Of the 827 incidents, a total of 651 (~79%) were classified as medication‐related (Figure 1). The phase where the incident originated in the medication ordering/administration process could not be determined with accuracy in 41 of these incidents. Of the remaining 610 incidents, 63 were classified under more than one phase. Most incidents (n = 228, ~35%) occurred during prescribing and 165 (~25%) during administration. A large number of incidents (n = 179, ~27%) were coded as ‘Other’ (Table 3).
Figure 1.
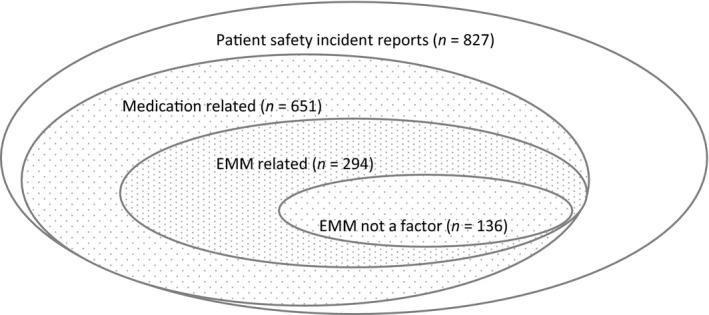
Distribution of the incidents in the dataset—related and non‐related to medication, related and non‐related to the electronic medication management (EMM) system
Table 3.
Medication‐related incidents
| Medication phase | Medication‐related incidents | |
|---|---|---|
| No. of incidents | % (¥) | |
| Prescribing | 228 | 35 |
| Other | 179 | 27 |
| Administration | 165 | 25 |
| Dispensing | 31 | 5 |
| Monitoring | 34 | 5 |
| Order communication | 25 | 4 |
| Use | 8 | 1 |
| Education | 3 | 0 |
| Unclear/phase could not be identified | 41 | 6 |
(¥) More than one category possible for each incident, total >100%.
Of the 651 medication‐related incidents, 294 (~45%) were classified as EMM‐related (Table 4). For approximately half of these (136, ~46%), we determined that the use of EMM was not a factor in the incidents reported (coded as HIT 0). The distribution of the remaining 158 EMM‐related incidents across medication phases is detailed in Table 5.
Table 4.
Medication and electronic medication management system (EMM)‐related incidents
| Category | No. of incidents | % of total |
|---|---|---|
| HIT 1 | 37 | 13 |
| HIT 2 | 5 | 2 |
| HIT 3 | 113 | 38 |
| HIT 4 | 0 | 0 |
| HIT 5 | 3 | 1 |
| HIT 0 | 136 | 46 |
| Total | 294 | 100 |
Table 5.
Distribution of medication and electronic medication management system (EMM)‐related incidents across medication phases
| Medication phase | Medication‐related incidents related to EMM | |||||||
|---|---|---|---|---|---|---|---|---|
| HIT1 | HIT2 | HIT3 | HIT4 | HIT5 | HIT0 | HIT totals (¥) | % (¥) | |
| Prescribing | 11 | 3 | 77 | 1 | 52 | 144 | 43 | |
| Other | 6 | 3 | 26 | 34 | 69 | 20 | ||
| Administration | 19 | 14 | 1 | 36 | 70 | 21 | ||
| Dispensing | 1 | 2 | 5 | 8 | 2 | |||
| Monitoring | 3 | 1 | 7 | 11 | 3 | |||
| Order communication | 6 | 15 | 21 | 6 | ||||
| Use | 0 | |||||||
| Education | 0 | |||||||
| Unclear/phase could not be identified | 2 | 3 | 9 | 14 | 4 | |||
| 100 | ||||||||
(¥) More than one category possible for each incident, totals may differ from Table 4.
3.1. All medication‐related incidents (related or unrelated to EMM)
3.1.1. Prescribing, dispensing and administration
Prescribing incidents included the following: incorrect drug (id88), dose for a patient weight (id78) or days/frequency (id315); not changing a default dose in an ‘order sentence’ (id70); or prescribing for the incorrect patient (id314). At the time of administration, incidents included administration of the incorrect dose of a pain medication (id223), medication overdose (id480) or signing for a dose as given when it was not (id424). At the time of dispensing, incidents included the wrong formulation of a drug dispensed (morphine 5 mg instead of morphine 5 mg slow release; id206).
3.1.2. Patient transfers
Incidents were also noted to occur at the time of handovers or transfer between hospital areas, including from ED (id471), operating theatre (id54) or ICU (id612) to the oncology wards, as well as transfers between hospitals (id616). Incidents revealed transfers to be problematic because of the absence of a suitable interface between the hospital and the separate medication systems in these clinical areas (e.g. id476).
3.1.3. Information on chemotherapy protocols
We identified several incidents reporting the lack (or currency) of the protocol in the patient record (e.g. id62, id771), lack of information about the current stage of therapy (cycle, dose) (e.g. id72, id122) and difficulty in identifying ‘where patients are up to’ in their treatment (id219). This reflected in part the absence of an electronic road map (a ‘summary’ of the chemotherapy protocol; Allen et al., 2018) to guide scheduling of therapy, admissions and investigations.
3.1.4. Chemotherapy coordination
Incidents were also related to failure to complete a check point (‘ready for chemo tick’), allowing staff to proceed with therapy despite the patient being ‘ready’ to receive chemotherapy (e.g. id302, id641), or clinicians erroneously progressing with the activation or administration of the medicine without the confirmation that the patient was ‘ready’ (e.g. id364, id114). In one incident (id302) a child remained under general anaesthetic for longer than required while waiting for a missing check point to be rectified.
3.1.5. ‘Handling’ or storing the medication
Errors in the preparation of medication products, pumps, syringes or lines were frequently reported, including shattered ampoules (id664, id148), leaking bags (id129), occluded (id238) or accidentally disconnected (id316) central venous access lines, storing a ‘do not refrigerate’ medication in the fridge (id529) or a ‘keep refrigerated’ drug out of the fridge (id744).
3.1.6. Patients’ ‘use’ of the medication
We coded a small number of incidents under the category ‘patient use’. Three of these involved children disconnecting lines, and two incidents related to family management of a child's medication (id198, id755), one of these at home (id198).
3.2. Medication‐related incidents determined to be EMM‐related
3.2.1. Technical issues with EMM (HIT 1)
Most of the incidents describing technical issues with EMM were related to the underlying automation across medication screens, scheduling or charting of medications. For example, ordered medications not automatically migrating from the prescribing screen to the administration chart (id263), ceased medications remaining on activation screens (id155), EMM issues with scheduling midnight doses (id520) or the system not updating the display of the status of a medication after administration (id300). Other technical problems related to data visualisation/design (such as visibility of complete order sentences—id116), and saving/updating/locking records, system response, or availability/accessibility of the system (e.g. EMM ‘was down’ – id334).
… I think charting the time at 2400 or 0000hrs makes the second lot of boluses drop off the MAR for some reason (id520)
3.2.2. User experience with EMM (HIT 2)
Incidents described clinicians’ experiences of confusion or frustration associated with how data were entered or displayed in EMM. For example, a chemotherapy cycle plan not broken down into weeks (id64), or the need to ‘scroll through’ a plan to determine where the patient is up to in terms of treatment (id204). It appears that clinicians at times managed to overcome impediments (e.g. in rescheduling and then activating doses) without understanding where the problem was (e.g. id108).
… Nursing staff had difficulty activating the MTX [methotrexate] order, error message kept stating that MTX blood forms needed to be rescheduled. The Night staff re‐scheduled the date of these blood forms and still could not activate the order. ? unsure of the issue, it was finally resolved and the order was activated… (id108)
3.2.3. Unanticipated problems in EMM workflow (HIT 3)
Several incidents pertained to the task of scheduling doses and the timing of medications. An incident outlined contributing factors to such events, including complex patient conditions (i.e. requiring ICU admission and extra monitoring), a difficult EMM ‘to instruct’, distributed work among different doctors and working under pressure:
Difficulty in juggling template plan days vs patient's clinical condition and moving certain drugs accordingly. Multiple/overlapping plans and different prescribers. Rapidly reading dosing tables as under pressure to order plan while managing clinical loa[d]’ [truncated] (id289)
Incidents resulted when prescribers entered medication orders in the EMM in different ways, some of which had flow‐on effects to other users (doctors, or nurses) who could not progress, complete, document or sign off with their tasks in the system (e.g. id115, id323 and id200) possibly leading to delays and frustration. Modes of prescribing also had effects on documentation of chemotherapy, which would not be complete or accurate (id86, id119 and id276), and on the linked decision support functions (i.e. calculations of the cumulative doses; id86).
When a patient was transferred to or from another ward not using the same EMM system (e.g. ICU), if prescribers did not withhold (or ‘cease’) the medications on the patient's record, then all medications would become automatically ‘overdue’, with the result that it was difficult to determine the history of treatment (e.g. id214, id227). Recurrent incidents involved duplicated, or multiple, prescriptions of a medication, for example resulting from omitting to cease a previous order once a new one was added, the former continuing to be scheduled automatically on MAR (id188).
Incidents also occurred, which reflected incompatibilities of the EMM with existing workflows. As an example, nurses signed that a dose had been given, prior to (rather than after) administering the medication to the patient. This was problematic if for some reason the patient then did not, or could not, take the dose or took it later than documented. The dose already signed for on the EMM could not be ‘undone’ (id488), countersigned by a second nurse (id298) and/or its timing changed (id25), with risks for time compression of doses (risk of toxicity) or a dose not being administered.
Another recurrent example related to the activation of the drugs for administration—how this was done (or not done) affected subsequent tasks. For example, medications were missing from MAR because ‘block of inpatient chemo not being activated as a whole but rather day by day’ in the outpatient clinic (id41). Finally, reported incidents described doses ‘falling off’ the medication chart. One of the reasons for this was medications prescribed with shorter durations than needed (id81, id109).
Few incidents related to EMM data configuration or implementation, including missing prescribing plans (id135–140, id144).
3.2.4. Missing EMM safety features (HIT 5)
Three incidents suggested that a contributing factor to the events was the absence of EMM features, such as specific decision support (id23), the availability of information (conversion rate for an unusual chemotherapy unit—id674) and space for recording monitoring activity:
We used to check syringes every hour and record how much was left in the syringe, but since EMR [EMM] started this practice ceased as there was nowhere to record this information. (id425)
4. DISCUSSION
Incident reports offer a valuable bird's eye view of the events those working at the front line consider important for the safety of patients. Our analysis showed that medication safety incidents in paediatric oncology occurred in prescribing, dispensing, administration and monitoring tasks, consistent with those found in adult oncology and other clinical settings (Ferner & Aronson, 1999, 2006; Fyhr & Akselsson, 2012; Schwappach & Wernli, 2010). However, they also occurred in other tasks, those related to care coordination, to the handling or storing of medications and to children or families handling medications. This was also reflected in the large number of incidents in the ‘Other’ category (27%) in our classification of the reported incidents by medication stage. Incidents related to medication handling can impact patients and families, as well as staff if appropriate cytotoxic protections are not in place. Incidents occurring while medications are managed by patients or families, also highlight difficulties typical for paediatric oncology patients given the close involvement of families in patients’ care (Walsh et al., 2013; Walsh, Ryan, Daraiseh, & Pai, 2016).
Some of the EMM‐related incidents reported were not unique to oncology. For example, EMM use in other settings has been associated with an increase in duplicated orders (Wetterneck et al., 2011). However, we identified incidents that were specific to oncology, in particular in connection with protocol‐mandated care. In the literature, safety events related to protocols, also known as roadmap errors (Hatton, 2018; Watts & Parsons, 2013), usually involve incorrect protocols or sequencing of cycles. In addition to these types of incidents, we found the absence of the protocol in patients’ EMM records to be an issue. The need to co‐ordinate order, verification, approval and delivery of varying therapy and interdependent doses, timed to coincide with clinical and laboratory parameters, as well as the health of the patient, required insertion of steps and check points in the EMM. Incidents surrounding these check points (the ‘ready for chemo tick’) are examples of medication safety events also found to be associated with approval processes and communication in other oncology settings (Hatton, 2018; Ranchon et al., 2011).
EMM‐related incidents also appeared to reflect difficulties with technology automated behaviours. ‘Automaticity’ contributed to : negative user experiences, ‘incorrect use’ of the system, and concealing software issues. It is likely that specific EMM design features contributed to negative user experiences and incorrect use (Ratwani et al., 2018). Some users had difficulty understanding EMM functionality and at times managed to overcome blocks without being able to appreciate the source of the problem, reducing their ability to learn. The hospital has incorporated lessons learned from incidents within the training of new clinical staff, especially that received by doctors on rotation. During EMM training, clinicians are made aware of EMM automatic behaviours and of the downstream consequences of their actions for others using the system in the medication process. Ongoing pharmacy assistance on site, and a training manual, equips users with strategies to prevent or troubleshoot common issues associated with ‘incorrect’ use of the system. Thus, when technology cannot be improved by redesign, problems can be avoided through reflective practice and careful use. However, this requires continuous efforts of learning and improvement at the organisational level.
4.1. Limitations
The incidents we studied are not an accurate representation of all incidents in this hospital nor of those that are EMM related. Absence of EMM from the reports is not evidence that EMM was not a factor in the incident. The reports contained often limited information. The field Contributing factors was empty in 55% (456) of reports, and some descriptions were truncated. The limited information included in incident descriptions also affected our coding. We were only able to reach ~50% agreement for medication stage. We addressed this limitation by having two reviewers to discuss all incidents until consensus was reached. The scope of this study is limited to what ‘goes wrong’ with EMM, as it is unlikely that staff will report on the benefits and value of EMM in an incident report.
5. CONCLUSION
We analysed patient safety incident reports with paediatric oncology patients following an implementation of EMM. A large proportion of incidents related to medications. They reflected protocol‐based therapies rich in interdependencies, especially complex in paediatrics, with tasks distributed across clinicians over time. Standard classifications of medication incidents do not seem to capture this complexity in medication use and workflow. EMM informs and coordinates a patient's treatment, in part through the automation of interdependencies—such as the scheduling of doses and tests, workflows’ rules and check points. However, this ‘automaticity’ also contributed to incidents in different ways. To ensure medication safety with EMM in oncology, it is essential that clinicians are aware of and attend to EMM automatic behaviours and are equipped to troubleshoot them.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTION
VL, MB, PG contributed to study design and data analysis. LDP assisted in interpreting and analysing site‐specific and clinical data. All authors contributed to drafting and critically revising the manuscript.
ACKNOWLEDGEMENTS
We gratefully acknowledge the assistance received from the study hospital in retrieving, and sharing with us, the patient safety incident data used in this study. The first author (VL) received funding from the European Union's Horizon 2020 research and innovation programme under the Marie‐Skłodowska Curie Grant Agreement number 740131. This research was supported by the Australian National Health and Medical Research Council Partnership Grant 1094878.
Lichtner V, Baysari M, Gates P, Dalla‐Pozza L, Westbrook JI. Medication safety incidents in paediatric oncology after electronic medication management system implementation. Eur J Cancer Care. 2019;28:e13152 10.1111/ecc.13152
[The copyright line for this article was changed on 18 March 2020 after original online publication.]
REFERENCES
- Aita, M. , Belvedere, O. , De Carlo, E. , Deroma, L. , De Pauli, F. , Gurrieri, L. , … Fasola, G. (2013). Chemotherapy prescribing errors: An observational study on the role of information technology and computerized physician order entry systems. BMC Health Services Research, 13, 522–522. 10.1186/1472-6963-13-522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, S. W. , Hayashi, R. J. , Jones, S. J. , Drozda, M. H. , Brown, R. L. , Lackey, I. T. , … Huang, F. S. (2018). Development of electronic chemotherapy roadmaps for pediatric oncology patients. Journal of Pediatric Oncology Nursing, 35(5), 314–319. 10.1177/1043454218767876 [DOI] [PubMed] [Google Scholar]
- Bannan, D. F. , & Tully, M. P. (2016). Bundle interventions used to reduce prescribing and administration errors in hospitalized children: A systematic review. Journal of Clinical Pharmacy and Therapeutics, 41(3), 246–255. 10.1111/jcpt.12398 [DOI] [PubMed] [Google Scholar]
- Brenner, S. K. , Kaushal, R. , Grinspan, Z. , Joyce, C. , Kim, I. , Allard, R. J. , … Abramson, E. L. (2016). Effects of health information technology on patient outcomes: A systematic review. Journal of the American Medical Informatics Association, 23(5), 1016–1036. 10.1093/jamia/ocv138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carayon, P. , Wooldridge, A. R. , Hose, B.‐Z. , Salwei, M. , & Benneyan, J. (2018). Challenges and opportunities for improving patient safety through human factors and systems engineering. Health Affairs, 37(11), 1862–1869. 10.1377/hlthaff.2018.0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, A. R. , & Lehmann, C. U. (2011). Computerized provider order entry in pediatric oncology: Design, implementation, and outcomes. Journal of Oncology Practice, 7(4), 218–222. 10.1200/jop.2011.000344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaid, K. A. , Garguilo, S. , & Collins, C. M. (2015). Chemotherapy e‐prescribing: Opportunities and challenges. Integrated Pharmacy Research and Practice, 4, 39–48. 10.2147/iprp.s84232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsaid, K. , Truong, T. , Monckeberg, M. , McCarthy, H. , Butera, J. , & Collins, C. (2013). Impact of electronic chemotherapy order forms on prescribing errors at an urban medical center: Results from an interrupted time‐series analysis. International Journal for Quality in Health Care, 25(6), 656–663. 10.1093/intqhc/mzt067 [DOI] [PubMed] [Google Scholar]
- Ferner, R. E. , & Aronson, J. K. (1999). Errors in prescribing, preparing, and giving medicines: Definition, classification, and prevention In Aronson J. K., & Elis J. (Eds.), Side effects of drugs annual (Vol. 22, pp. xxiii–xxxvi). Elsevier, Amsterdam: Elsevier. [Google Scholar]
- Ferner, R. E. , & Aronson, J. K. (2006). Clarification of terminology in medication errors. Drug Safety, 29(11), 1011–1022. 10.2165/00002018-200629110-00001 [DOI] [PubMed] [Google Scholar]
- Fyhr, A. , & Akselsson, R. (2012). Characteristics of medication errors with parenteral cytotoxic drugs. European Journal of Cancer Care, 21(5), 606–613. 10.1111/j.1365-2354.2012.01331.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi, S. , Tyono, I. , Pasetka, M. , & Trudeau, M. (2014). Evaluating an oncology systemic therapy computerized physician order entry system using international guidelines. Journal of Oncology Practice, 10(2), e14–e25. 10.1200/jop.2013.000914 [DOI] [PubMed] [Google Scholar]
- Hatton, J. (2018). An evaluation of medication errors in paediatric cytotoxic chemotherapy, prior to electronic prescribing implementation. Archives of Disease in Childhood, 103(2), e1 10.1136/archdischild-2017-314584.14 [DOI] [Google Scholar]
- Hoffman, J. M. , Baker, D. K. , Howard, S. C. , Laver, J. H. , & Shenep, J. L. (2011). Safe and successful implementation of CPOE for chemotherapy at a children's cancer center. Journal of the National Comprehensive Cancer Network, 9(Suppl. 3), S‐36–S‐50. 10.6004/jnccn.2011.0131 [DOI] [PubMed] [Google Scholar]
- Hsieh, H.‐F. , & Shannon, S. E. (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15(9), 1277–1288. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- Krzyzaniak, N. , & Bajorek, B. (2016). Medication safety in neonatal care: A review of medication errors among neonates. Therapeutic Advances in Drug Safety, 7(3), 102–119. 10.1177/2042098616642231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maaskant, J. M. , Vermeulen, H. , Apampa, B. , Fernando, B. , Ghaleb, M. A. , Neubert, A. , … Soe, A. (2015). Interventions for reducing medication errors in children in hospital. Cochrane Database of Systematic Reviews, 3, CD006208 10.1002/14651858.CD006208.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, D. B. , Kaemingk, D. , Frieze, D. , Hendrie, P. , & Payne, T. H. (2015). Safe implementation of computerized provider order entry for adult oncology. Applied Clinical Informatics, 6(4), 638–649. 10.4338/ACI-2015-03-RA-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCC MERP . (2018). About medication errors. What is a medication Error? Retrieved from https://www.nccmerp.org/about-medication-errors
- NSW Health . (2014). NSW health incident management policy. Retrieved from https://www1.health.nsw.gov.au/pds/ActivePDSDocuments/PD2014_004.pdf
- Ranchon, F. , Salles, G. , Späth, H.‐M. , Schwiertz, V. , Vantard, N. , Parat, S. , … Rioufol, C. (2011). Chemotherapeutic errors in hospitalised cancer patients: Attributable damage and extra costs. BMC Cancer, 11, 478 10.1186/1471-2407-11-478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratwani, R. M. , Savage, E. , Will, A. , Fong, A. , Karavite, D. , Muthu, N. , … Rising, J. (2018). Identifying Electronic health record usability and safety challenges in pediatric settings. Health Affairs, 37(11), 1752–1759. 10.1377/hlthaff.2018.0699 [DOI] [PubMed] [Google Scholar]
- Rinke, M. L. , Bundy, D. G. , Velasquez, C. A. , Rao, S. , Zerhouni, Y. , Lobner, K. , … Miller, M. R. (2014). Interventions to reduce pediatric medication errors: A systematic review. Pediatrics, 134(2), 338–360. 10.1542/peds.2013-3531 [DOI] [PubMed] [Google Scholar]
- Schwappach, D. L. , & Gehring, K. (2015). Frequency of and predictors for withholding patient safety concerns among oncology staff: A survey study. European Journal of Cancer Care, 24(3), 395–403. 10.1111/ecc.12255 [DOI] [PubMed] [Google Scholar]
- Schwappach, D. L. , & Wernli, M. (2010). Medication errors in chemotherapy: Incidence, types and involvement of patients in prevention. A review of the literature. European Journal of Cancer Care, 19(3), 285–292. 10.1111/j.1365-2354.2009.01127.x [DOI] [PubMed] [Google Scholar]
- Sittig, D. F. , Classen, D. C. , & Singh, H. (2014). Patient safety goals for the proposed Federal Health Information Technology Safety Center. Journal of the American Medical Informatics Association, 22(2), 472–478. 10.1136/amiajnl-2014-002988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small, M. D. C. , Pharm, B. , Barrett, A. , & Price, G. M. (2008). The impact of computerized prescribing on error rate in a department of Oncology/Hematology. Journal of Oncology Pharmacy Practice, 14(4), 181–187. 10.1177/1078155208094453 [DOI] [PubMed] [Google Scholar]
- Usability.gov . (2018). User experience basics. Retrieved from https://www.usability.gov/what-and-why/user-experience.html
- van Rosse, F. , Maat, B. , Rademaker, C. M. , van Vught, A. J. , Egberts, A. C. , & Bollen, C. W. (2009). The effect of computerized physician order entry on medication prescription errors and clinical outcome in pediatric and intensive care: A systematic review. Pediatrics, 123(4), 1184–1190. 10.1542/peds.2008-1494 [DOI] [PubMed] [Google Scholar]
- Walsh, K. E. , Mazor, K. M. , Roblin, D. , Biggins, C. , Wagner, J. L. , Houlahan, K. , … Weingart, S. N. (2013). Multisite parent‐centered risk assessment to reduce pediatric oral chemotherapy errors. Journal of Oncology Practice, 9(1), e1–e7. 10.1200/jop.2012.000601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, K. , Ryan, J. , Daraiseh, N. , & Pai, A. (2016). Errors and non‐adherence in pediatric oral chemotherapy use. Oncology, 91(4), 231–236. 10.1159/000447700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts, R. G. , & Parsons, K. (2013). Chemotherapy medication errors in a pediatric cancer treatment center: Prospective characterization of error types and frequency and development of a quality improvement initiative to lower the error rate. Pediatric Blood & Cancer, 60(8), 1320–1324. 10.1002/pbc.24514 [DOI] [PubMed] [Google Scholar]
- Westbrook, J. I. , Li, L. , Lehnbom, E. C. , Baysari, M. T. , Braithwaite, J. , Burke, R. , … Day, R. O. (2015). What are incident reports telling us? A comparative study at two Australian hospitals of medication errors identified at audit, detected by staff and reported to an incident system. International Journal for Quality in Health Care, 27(1), 1–9. 10.1093/intqhc/mzu098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetterneck, T. B. , Walker, J. M. , Blosky, M. A. , Cartmill, R. S. , Hoonakker, P. , Johnson, M. A. , … Carayon, P. (2011). Factors contributing to an increase in duplicate medication order errors after CPOE implementation. Journal of the American Medical Informatics Association, 18(6), 774–782. 10.1136/amiajnl-2011-000255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen, K. , Lynch, E. , Moawad, I. , John, T. , Lozowski, D. , & Cummings, B. M. (2018). Transition to a new electronic health record and pediatric medication safety: Lessons learned in pediatrics within a large academic health system. Journal of the American Medical Informatics Association, 25(7), 848–854. 10.1093/jamia/ocy034 [DOI] [PMC free article] [PubMed] [Google Scholar]


