Abstract
Introduction
Large segments of the world population use combustible cigarettes, and our society pays a high price for smoking, through increased healthcare expenditures, morbidity and mortality. The development of combustible cigarette smoking requires the initiation of smoking and a subsequent chain of behavioral transitions from experimental use, to established regular use, to the conversion to addiction. Each transition is influenced by both environmental and genetic factors, and our increasing knowledge about genetic contributions to smoking behaviors opens new potential interventions.
Methods
This review describes the journey from genetic discovery to the potential implementation of genetic knowledge for the treatment of tobacco use disorder.
Results and Conclusions
The field of genetics applied to smoking behaviors has rapidly progressed with the identification of highly validated genetic variants that are associated with different smoking behaviors. The large scale implementation of this genetic knowledge to accelerate smoking cessation represents an important clinical challenge in precision medicine.
Introduction
A revolution in genomic technologies has occurred over the past 15 years, which has allowed the discovery of specific genetic variation that contributes to smoking behaviors and smoking cessation. We are poised at the threshold of integrating this genetic knowledge into the prevention of smoking and treatment of tobacco use disorder, and this journey of discovery to potential implementation is described here in 11 chapters.
Chapter 1: The New Genomic World
In 2004, the completion of the Human Genome Project ushered in a new era in human genomic studies.1 This international, multi-billion dollar project determined the reference DNA sequence that composes the entire human genome. Though some questioned whether the Human Genome Project was worth the cost and effort, skeptics were soon silenced when it became apparent that this project catalyzed genetic discovery.2 With the reference human genome mapped, new studies queried variation across the entire genome for association with a disease. In 2005, the first genome-wide association studies identified specific genetic contributions to a common complex illness, age-related macular degeneration.3–5 Since then, thousands of genetic variants that influence a wide range of complex human traits have been discovered.6 With modern genomic technologies, millions of genetic variants (or single nucleotide polymorphisms; SNPs) can be queried in hundreds of thousands of individuals in a cost-effective manner. In just over a decade, these technological breakthroughs have ushered in studies leading to genetic discovery and paradigm changing knowledge of human traits and diseases.
Chapter 2: Genetic Influences to Smoking Behavior
Combustible cigarette smoking remains one of the leading causes of preventable death worldwide.7 It has long been known that smoking behaviors, particularly heaviness of smoking (defined by number of cigarettes smoked per day) and tobacco use disorder, are influenced by genetic variation. Classic twin studies demonstrate that monozygotic twins, who share all of their genetic makeup, are more similar in their smoking behaviors than dizygotic twins, who share on average half of their genetic makeup.8–10 This knowledge of genetic contributions to smoking behaviors motivated the first genome-wide association studies in search of variation that influences tobacco use disorder and number of cigarettes smoked per day.
Chapter 3: Genomic Studies of Smoking Behavior Identify Variation in Nicotinic Acetylcholine Receptors
In 2007, the first genome-wide analysis of tobacco use disorder was conducted. This initial study identified a correlation between variants in the gene that encodes the α5 nicotinic acetylcholine receptor subunit (CHRNA5) on chromosome 15 and tobacco use disorder.11,12 Because self-reported cigarettes smoked per day is a commonly collected measure in research settings, genomic studies of smoking heaviness quickly grew in size. By 2010, research consortia pooled genomic study results of tens of thousands of individuals and unequivocally confirmed that rs16969968, a variant in the gene CHRNA5, is highly significantly associated with number of cigarettes smoked per day (p = 2.8 × 10–73).13 This finding was then extended across world populations, in European, African, and Asian ancestry groups.14
Chapter 4: Moving From Association to Biologic Function
Once an association between a genetic variant and disease is found, an understanding of the biological mechanism underlying the finding must be pursued. Several lines of evidence point to the specific variant rs16969968 as changing the function of the nicotinic receptor and thus altering a person’s risk of heavy smoking. The α5 nicotinic acetylcholine receptor subunit is one of five subunits that combines to form a pentameric receptor that binds nicotine.15 The variant rs16969968 results in an amino acid change from aspartic acid to asparagine in the α5 subunit protein, and this region in the α5 protein is highly conserved across species—from chimpanzee, to domestic cow, chicken, and mouse—which implies biological importance.16 Experimental studies show that receptors that differ only by the asparagine amino acid substitution have altered biologic response to nicotine agonists.16–19 In addition, the α5 nicotinic acetylcholine receptor subunit is expressed in the important brain regions that are relevant to the development of addiction.20 These converging lines of evidence point to this variant, rs16969968, as changing biologic function of the receptor and altering the risk of developing heavy cigarette smoking and tobacco use disorder.
Chapter 5: Multiple Variants in the CHRNA5 Region Contribute to Heaviness of Smoking Risk
Though the evidence is unequivocal that the variant rs16969968 in CHRNA5 is associated with heaviness of smoking and tobacco use disorder, smoking behaviors are complex and represent a combination of multiple genetic risks. The chromosome 15 region, which encompasses CHRNA5, demonstrates the strongest genetic association with tobacco use disorder, and there is clear evidence now that multiple variants in this region independently contribute to the risk of heavy smoking. A second distinct association is marked by variant rs880395,21 and functional studies point to another biological mechanism: a several-fold altered α5 nicotine acetylcholine receptor subunit mRNA expression.17,22,23 In addition, low frequency and rare variants that cause other amino acid changes in the α5 nicotinic acetylcholine receptor subunit protein are independently associated with differential risk for tobacco use disorder.24 These many studies point to the importance of the α5 nicotine acetylcholine receptor subunit protein in the differential risk of heavy cigarette smoking and highlight the complex functional effects of this one gene encoding this protein.
Chapter 6: Genetics of Lung Cancer and Other Smoking-Related Diseases
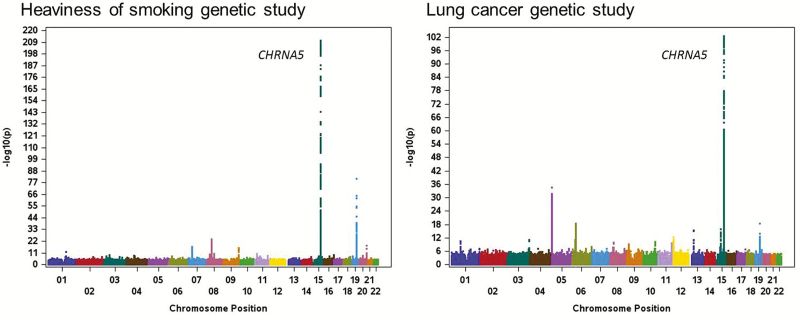
Combustible cigarette smoking is the strongest risk factor for the development of lung cancer and chronic obstructive pulmonary disease (COPD), and there is an intriguing convergence of genetic findings for tobacco use disorder and these medical conditions. Large-scale studies demonstrate that the same genetic variation on chromosome 15 that is associated with heavy smoking is also the strongest genetic risk factor for lung cancer and COPD.25–29Figure 1 demonstrates the similarity of the most up-to-date, large-scale genetic studies for heaviness of smoking and lung cancer.30,31 The convergence of these genetic findings associated with smoking behaviors and smoking-related diseases raises the question of whether the increased risk of lung cancer and COPD can be explained solely through the genetic influences on smoking behaviors (an indirect effect) or if these genetic findings also have a direct effect on the risk of developing these disorders. Growing evidence supports the indirect pathway—genetic risk in CHRNA5 is imparted through smoking behaviors, which in turn drive the risk of developing lung cancer and COPD. The strongest evidence that this CHRNA5 genetic risk indirectly elevates the risk of lung cancer comes from studies that show that variation in the chromosomal 15 region does not increase the risk of lung cancer among nonsmokers.32 These parallel findings for smoking behaviors, lung cancer, and COPD challenge our general paradigms about the relation between smoking and medical disorders. Smoking cannot only be considered an environmental variable in the study of cancer and COPD given that the strongest genetic findings for the development of lung cancer and COPD are the strongest genetic determinants underlying the risk of heavy cigarette smoking.
Figure 1.
Genome-wide association studies of heaviness of smoking and lung cancer. Adapted from Liu et al.31 and McKay et al.30
Chapter 7: Cessation—The Ultimate Goal
One of the strongest predictors of unsuccessful smoking cessation is the level of dependence on nicotine.33,34 Given that genetic variation in CHRNA5 is the strongest genomic risk factor for heavy cigarette smoking and tobacco use disorder, it is not surprising that this genetic variation is also associated with failed smoking cessation. In population-based studies, those with the high-risk genetic variants (rs16969968 genotype – AA) that predict heavy smoking have a later median age of smoking cessation (56 years of age) compared to those with the low-risk genetic variants (rs16969968 genotype – GG) (median age of smoking cessation 52 years of age).35 More recently, the high-risk genetic variants that predict heavy smoking have been shown to also predict failed long-term smoking cessation.31 These findings mirror the epidemiology of smoking behaviors—a genetic risk factor for heavy smoking is also a risk for failed cessation.
Chapter 8: Can Genetic Variants Predict Response to Treatment?
An important purpose of these genetic studies has been to gain insights into smoking cessation. We are in the early stages of testing whether genomic variation can inform and improve smoking cessation treatment. Using genetic variation in CHRNA5 to identify individuals at higher risk of failed smoking cessation and to guide pharmacologic treatment is one promising area for further research. There is some evidence that the same CHRNA5 genetic variation, rs16969968, that predicts heaviness of smoking, failed smoking cessation, and smoking-related diseases, may also predict response to pharmacologic treatment for smoking cessation, but this finding requires further confirmation.36,37 Large, well-powered genomic studies of smoking cessation will be needed to confidently establish genetic predictors that can inform response to pharmacologic treatment.
Chapter 9: Moving Beyond One Gene to Many Genes
These studies of CHRNA5 illustrate the genetic connection between heaviness of smoking, smoking-related diseases, and smoking cessation. However, common complex traits, such as tobacco use disorder, are polygenic in nature, meaning that hundreds to thousands of genes, each with a small effect, contribute to the trait. Recently, hundreds of genetic regions that contribute to smoking initiation, heaviness of smoking measured by cigarettes smoked per day, and successful smoking cessation have been uncovered.31 Variation across many genes, including those that encode different nicotinic receptor subunits and the enzyme that metabolizes nicotine, CYP2A6, contributes to heaviness of smoking and smoking cessation. Differences in the ability to successfully quit smoking are also seen in genes such as dopamine beta-hydroxylase (DBH), which encodes a neurotransmitter. The complex behavior of smoking represents a composite risk of many genes of small effect acting in tandem with environmental influences.
Chapter 10: New Insights and Challenges in Genomics
Genomic studies have provided a new understanding of substance use behaviors. It is now clear that there is significant overlap between many of the genetic risk factors for smoking behaviors and alcohol consumption.31 We are also faced with new questions. Though we have seen an explosion of genetic discovery for smoking-related behaviors, the function of the majority of these associated genetic regions remains undefined. Another challenge is the issue of epigenetics, the turning on and off of genes, which occurs across our lifetimes and in response to different environmental exposures. The field of genomics and smoking has rapidly progressed, and it has raised new questions.
Chapter 11: Precision Medicine
The purpose of these genomic studies is to ultimately provide insights into addiction to nicotine and to reduce the health burden of smoking. The implementation of genomic knowledge into tests to improve health represents the next frontier in science and medicine. For a test to enter mainstream care, it must have analytical validity, clinical validity, clinical utility, and be ethically sound.38 Standard genomic testing can identify these genetic variants that predict the risk of heavy smoking with high analytic reproducibility and validity. The clinical validity of genomic variation in CHRNA5 predicting heavy smoking,13 failed smoking cessation,35,36 lung cancer,28 COPD,39 and mortality is similarly high.40 Evidence for clinical utility is modest, but growing. The ethical implications of genomic testing about smoking behaviors and potential treatment remain essentially unstudied.
Many important questions about clinical utility remain, and these questions represent a next generation of research studies about smoking behaviors and improved smoking cessation interventions. Will knowledge of genetic variants in CHRNA5 and other genes predict response to pharmacologic treatment? Will these genomic tests related to smoking be effective in routine clinical care settings? Will a person’s knowledge of one’s own genetic predispositions and susceptibility alter behavior? These questions remain unanswered. Pragmatic clinical trials that undertake a practical approach to test genomically informed interventions for smoking cessation integrated into routine medical care will provide the greatest information about clinical utility. This next phase of research will test how knowledge of genetic variation that predicts heavy cigarette smoking measured by cigarettes smoked per day, tobacco use disorder, smoking-related disease outcomes, and potentially pharmacogenomic responses to smoking cessation medications can further accelerate the reduction of combustible cigarette smoking. Clearly genomic variation beyond CHRNA5 contributes to smoking behaviors and smoking-related illnesses; how we summarize risk across a genome remains an open question that has active ongoing research.
While these questions remain unanswered, efforts funded by private companies in the genomic health space, such as 23andMe (https://www.23andme.com), are pushing forward research, products, and policies for affordable genomics implementation. Individual knowledge about genetic background and risk is desired by many, as demonstrated by the 5 million people who have had testing through 23andMe.41 The use of genomic testing and feedback to aid in smoking cessation is an appealing area for implementation that can potentially save many lives. All public and private efforts aimed at reducing combustible cigarette smoking must continue to be actively pursued given the large number of smoking-related deaths that occur each year. An important component of the foundation of precision prevention needs to focus on combustible cigarette smoking, as long as smoking remains one of the foremost causes of preventable death worldwide.
Acknowledgments
The participants in many of these studies generously contributed their time, smoking history information, and genomic data to researchers worldwide. They form the foundation for genomic discoveries related to smoking and I thank them for their participation and generosity.
Funding
LJB is supported by National Institute on Drug Abuse (NIDA) grant R01DA036583 and National Cancer Institute (NCI) grant P30CA091842.
Declaration of Interests
LJB is listed as an inventor on Issued U.S. Patent 8,080,371, “Markers for Addiction” covering the use of certain SNPs in determining the diagnosis, prognosis, and treatment of addiction.
References
- 1. International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature. 2004;431(7011):931–945. [DOI] [PubMed] [Google Scholar]
- 2. Daiger SP. Genetics. Was the Human Genome Project worth the effort? Science. 2005;308(5720):362–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Edwards AO, Ritter R III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. [DOI] [PubMed] [Google Scholar]
- 4. Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308(5720):419–421. [DOI] [PubMed] [Google Scholar]
- 5. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MacArthur J, Bowler E, Cerezo M, et al. The new NHGRI-EBI Catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res. 2017;45(D1):D896–D901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control. Smoking & tobacco use: fast facts 2018. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/index.htm?s_cid=osh-stu-home-spotlight-001. Accessed November 26, 2018.
- 8. Carmelli D, Swan GE, Robinette D, Fabsitz R. Genetic influence on smoking–a study of male twins. N Engl J Med. 1992;327(12):829–833. [DOI] [PubMed] [Google Scholar]
- 9. Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychol Med. 1999;29(2):299–308. [DOI] [PubMed] [Google Scholar]
- 10. Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98(1):23–31. [DOI] [PubMed] [Google Scholar]
- 11. Bierut LJ, Madden PA, Breslau N, et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saccone SF, Hinrichs AL, Saccone NL, et al. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16(1):36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet. 2010;42(5):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen LS, Saccone NL, Culverhouse RC, et al. Smoking and genetic risk variation across populations of European, Asian, and African American ancestry–a meta-analysis of chromosome 15q25. Genet Epidemiol. 2012;36(4):340–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Purves D, Augustine GJ, Fitzpatrick D, et al. , eds. Neuroscience. 5th ed. Sutherland, MA: Sinauer Associates; 2011. [Google Scholar]
- 16. Bierut LJ, Stitzel JA, Wang JC, et al. Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry. 2008;165(9):1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang JC, Cruchaga C, Saccone NL, et al. ; COGEND collaborators and GELCC collaborators Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet. 2009;18(16):3125–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tammimäki A, Herder P, Li P, et al. Impact of human D398N single nucleotide polymorphism on intracellular calcium response mediated by α3β4α5 nicotinic acetylcholine receptors. Neuropharmacology. 2012;63(6):1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sciaccaluga M, Moriconi C, Martinello K, et al. Crucial role of nicotinic α5 subunit variants for Ca2+ fluxes in ventral midbrain neurons. FASEB J. 2015;29(8):3389–3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fowler CD, Tuesta L, Kenny PJ. Role of alpha5* nicotinic acetylcholine receptors in the effects of acute and chronic nicotine treatment on brain reward function in mice. Psychopharmacology (Berl). 2013;229( 3): 503–513. doi:10.1007/s00213-013-3235-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saccone NL, Culverhouse RC, Schwantes-An TH, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet. 2010;6(8):e1001053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Falvella FS, Galvan A, Colombo F, Frullanti E, Pastorino U, Dragani TA. Promoter polymorphisms and transcript levels of nicotinic receptor CHRNA5. J Natl Cancer Inst. 2010;102(17):1366–1370. [DOI] [PubMed] [Google Scholar]
- 23. Smith RM, Alachkar H, Papp AC, et al. Nicotinic α5 receptor subunit mRNA expression is associated with distant 5’ upstream polymorphisms. Eur J Hum Genet. 2011;19(1):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Olfson E, Saccone NL, Johnson EO, et al. Rare, low frequency and common coding variants in CHRNA5 and their contribution to nicotine dependence in European and African Americans. Mol Psychiatry. 2016;21(5):601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amos CI, Wu X, Broderick P, et al. Genome-wide association scan of tag SNPs identifies a susceptibility locus for lung cancer at 15q25.1. Nat Genet. 2008;40(5):616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hung RJ, McKay JD, Gaborieau V, et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature. 2008;452(7187):633–637. [DOI] [PubMed] [Google Scholar]
- 27. Liu P, Vikis HG, Wang D, et al. Familial aggregation of common sequence variants on 15q24-25.1 in lung cancer. J Natl Cancer Inst. 2008;100(18):1326–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thorgeirsson TE, Geller F, Sulem P, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature. 2008;452(7187):638–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Broderick P, Wang Y, Vijayakrishnan J, et al. Deciphering the impact of common genetic variation on lung cancer risk: a genome-wide association study. Cancer Res. 2009;69(16):6633–6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McKay JD, Hung RJ, Han Y, et al. ; SpiroMeta Consortium Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat Genet. 2017;49(7):1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu M, Jiang Y, Wedow R, et al. ; 23andMe Research Team; HUNT All-In Psychiatry Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51(2):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lips EH, Gaborieau V, McKay JD, et al. Association between a 15q25 gene variant, smoking quantity and tobacco-related cancers among 17 000 individuals. Int J Epidemiol. 2010;39(2):563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3(3-4):235–241. [DOI] [PubMed] [Google Scholar]
- 34. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 35. Chen LS, Hung RJ, Baker T, et al. CHRNA5 risk variant predicts delayed smoking cessation and earlier lung cancer diagnosis--a meta-analysis. J Natl Cancer Inst. 2015;107(5):pii: djv100. doi:110.1093/jnci/djv1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen LS, Baker TB, Piper ME, et al. Interplay of genetic risk factors (CHRNA5-CHRNA3-CHRNB4) and cessation treatments in smoking cessation success. Am J Psychiatry. 2012;169(7):735–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schuit E, Panagiotou OA, Munafò MR, Bennett DA, Bergen AW, David SP. Pharmacotherapy for smoking cessation: effects by subgroup defined by genetically informed biomarkers. Cochrane Database Syst Rev. 2017;9:CD011823. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. National Academies of Sciences, Engineering, and Medicine. An Evidence Framework for Genetic Testing. Washingon, DC: The National Academies Press; 2017. [PubMed] [Google Scholar]
- 39. Pillai SG, Ge D, Zhu G, et al. ; ICGN Investigators A genome-wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009;5(3):e1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Joshi PK, Fischer K, Schraut KE, Campbell H, Esko T, Wilson JF. Variants near CHRNA3/5 and APOE have age- and sex-related effects on human lifespan. Nat Commun. 2016;7:11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Geggel L. 23andMe is Sharing Its 5 Million Clients’ Genetic Data with Drug Giant GlaxoSmithKline. Livescience. 2018. https://www.livescience.com/63173-23andme-partnership-glaxosmithkline.html. Accessed February 14, 2019. [Google Scholar]