Abstract
Osteoarthritis is the most common, incurable joint disease. The rapid pace of the disease has adverse consequences in the quality of patient’s life, while affecting healthcare systems. The research interest is focused on cost-effective and without side-effects methods to reduce the symptoms and improve the quality of life. Several dietary factors have been linked to the health of cartilage tissue, inflammatory processes and the progress of osteoarthritis. Mediterranean diet (MD) is a dietary pattern that was adopted by people living around the Mediterranean sea. This term first appeared in 1950, by Ancel Keys. It’s characterized by high consumption of vegetables, unprocessed grains, fruits, legumes, seeds, modest consumption of fish and poultry and olive oil is the principal fat source. Emerged data emphasize the beneficial effects of MD against chronic inflammation, metabolic complications and chronic diseases. There are few studies investigating the effect of MD against osteoarthritis, but apparent evidence is encouraging, this highlights the need for further research of the relationship between MD and osteoarthritis. The purpose of this work is a literature review between the effect of MD and its components and the progress of osteoarthritis.
Keywords: Osteoarthritis, Mediterranean diet, Prevention, Olive oil, Components of Mediterranean diet
Introduction
Osteoarthritis (OA) is a common incurable chronic rheumatic disease. It is known that prevalence of OA is significantly increased by the advance of age. The characteristics of OA are degradation of the cartilage, inflammation of the synovial membrane and the restructure of the subchondral bone. It’s indicated that inflammation signifies the outcome of OA, that subsequently results in reduced mobility and disability[1]. Recent data show that hip and knee OA is recorded as the 11th most frequent disability factor, worldwide. Taking into account the strong connection between OA and age, it will climb up to the 4th place until 2020[2].
It has been mentioned that interactions of different factors can trigger the development of OA in people with suffered joints or genetic predisposition. These risk factors can be classified into modifiable and non-modifiable, among them obesity, age and mechanical stress appear to have leading role[3,4]. The rapid development of disease has obvious consequences, on the quality of life, while affecting healthcare systems. It seems mandatory to devise methods that are cost-effective to apply and cause minimal or zero side-effects; these methods will improve the quality of life by enhancing mobility and reducing the pain symptoms[3].
The diet has been a major discussion field for the health promotion and prevention of non-communicable diseases. Although there is no clarification on the role of dietary factors in the pathogenesis of OA, data suggest that nutrients affect the health and integrity of cartilage. It has been presented that among patients with OA, the nutritional status is poor, a micronutrient deficiency may be apparent; either as a result of disease or medication[5]. A number of nutrients have been linked to inflammatory processes and especially disease’s progression[3].
Studies indicate negative association between dietary antioxidant intakes with the outcome of OA. It has been presented that low dietary intake of vitamin C was linked with a raised risk of pain and progression of radiological features in the knee. Findings also indicate that increased intake of antioxidants, specifically vitamin C, may decrease the risk of cartilage loss and disease progress in osteoarthritis patients[6].
Johnston County Osteoarthritis Project revealed that the high ratio of alpha/gamma tocopherol linked with 50% lower risk for radiological features’ development in knee osteoarthritis[7]. The role of these two isoforms of tocopherol, is of great scientific interest[7]. In contrast, clinical trial investigating the impact of tocopherol supplementation on symptoms of knee OA failed to show any improvement in OA’s symptoms[8].
The vitamin K is of great importance in tissue mineralization and cartilage[3]. Neogi shown that low levels of plasma phylloquinone (<0,5 nmoles/l) were associated with an increased prevalence of OA, osteophytes in hand and knee. Less clear is the connection between the levels of vitamin K plasma and radiographic features in OA of the knee[3,9].
It has been stated that the combination of lack of iodine and selenium leads to impaired cartilage, bone development and bad outcome of knee OA[12]. While it has been showed that alterations in the expression of collagen type X (ColX) and parathyroid hormone related peptide, was compatible with Kashin-Beck osteoarthropathy, due to selenium and iodine deficiency[10]. Moreover, it has been reported that selenium may help prevent the occurrence of Kashin-Beck osteoarthropathy[11].
Additionally, modifiable metabolic risk factors may lead to raised cartilage loss. One of the factors for metabolic disorders which has been assessed, was the levels of dietary fat intake. It has been proposed that increased consumption of n-6 fatty acids is linked to raise risk of subchondral knee bone deterioration. Other studies noticed that fat consumption is linked to the expression of leptin from the adipose tissue, which may promote nitric oxide synthesis in chondrocytes affected the bad outcome of OA[13].
Mechanisms which nest in OA pathogenesis have not been elucidated yet. Although there is scientific interest for investigating the relationship between OA and risk factors, there is a little bibliography analyzing the MD’s healthy benefits in OA progression. The purpose of this work is a literature review between the effect of MD and the progress of OA.
The characteristics of traditional Mediterranean diet
The term “Mediterranean Diet” was first used by Ancel Keys, in the 50s, in order to describe the eating pattern which was followed by Mediterranean people. The Mediterranean Countries were included in the Seven Countries Study and the cardioprotective effect of Mediterranean dietary habits first demonstrated[14].
The MD is the imprint of interactions among humans, cultures and food. In the Mediterranean area there are differences such as cultural, religious, economic and differences related to agricultural production of the country[15], which characterize people and this results in the formation of different variants of the Mediterranean diet with common aspects[16]. In 1993 the International Conference on the Mediterranean diets, was decided what would be considered as a healthy, traditional Mediterranean dietary pattern. The characteristics of traditional MD[15,17] are the following: 1) Increased consumption of fresh, seasonally and locally grown fruits and vegetables, on a daily basis, 2) Increased consumption of legumes, seeds, unprocessed cereals and their products, tree nuts, olives, on a daily basis, 3) Moderate consumption of poultry, dairy products (mainly yogurt and cheese), 4) Moderate consumption of ethanol, primarily wine, accompanied with meals, 5) Moderate consumption of fish and seafood, 6) Low consumption of meat and meat products and 7) Olive oil is the basic source of fat.
Those characteristics lead to high intake of monounsaturated and polyunsaturated fatty acids, at the same time, low intake of saturated and trans fatty acids, high intake of dietary fiber, minerals, antioxidants, such as b-carotene, tocopherols, vitamin C and phenolic components[15].
Continuously, data highlight the preventive role of MD against CVD, risk factors of metabolic syndrome, inflammatory biomarkers, insulin resistance, diabetes mellitus type 2[18]. Although some aspects of Mediterranean diet’s mechanisms remains to be clarified, it is believed, that patients with OA may benefit from the higher adherence to MD.
The role of Mediterranean diet against osteoarthritis
In the last years, the effects of traditional MD against OA have been documented. A cross sectional study, with data from the Osteoarthritis Initiative, and sample of 4358 participants, examined the association of adherence to MD with the prevalence of OA[19]. The adherence to Mediterranean diet was assessed according to the Mediterranean diet score by Panagiotakos, based on food frequency questionnaire. The consumption of 11 food groups (range 0-55) was taken into account. Higher Med Diet score indicate greater adherence to the MD. The study highlighted that after adjusting for 10 potential confounders, people with the highest adherence to MD had 17% lower prevalence of knee osteoarthritis (OR: 0.83; 95% CIs: 0.69-0.99, p=0.04). Moreover, participants with the greatest adherence to MD significantly reduced Body Mass Index (BMI) and suffered from less morbidity, such as Diabetes Mellitus type 2 (DM2) compared to those with lower adherence to MD. These factors seem to impact the prevalence of knee OA in people with the highest adherence of MD. It was also observed that evaluating the individual elements of Mediterranean diet only the higher cereal consumption was associated with reduced incidence of knee OA, probably through anti-inflammatory and anti-oxidant properties, due to high content of vitamins and minerals[19].
Veronese and his colleagues, this time, revealed that participants with OA and greater adherence to MD had better status of health and quality of life. The consistent MD consumption was associated with less pain and lower disability, suggesting that MD has a beneficial effect on various aspects associated with disability leading to healthier aging[20].
A Clinical trial investigated the effect of MD in cartilage degradation and inflammatory biomarkers, in osteoarthritis patients, pointed out that participants with the MD intervention, had improvements in their dietary habits and this led to weight loss[21]. Research has shown that weight loss is associated with improvements in pain and mobility in patients with OA[21,22]. Besides, there was a reduction of IL-1a and cartilage oligomeric matrix protein in serum “sCOMP” (cartilage degradation significant marker) in the MD intervention group. The study raised concern about effect of MD to OA and the need of analyzing these changes in long term studies[21].
Another study presented that four months dietary intervention with MD to OA patients resulted in a significant improvement of OA biomarkers and a modest reduction sCOMP. According to these results, simple changes in dietary habits may help OA patients to manage the outcomes of their disease and to improve quality of life. However, the effect of MD on the OA pathophysiology and progression is required to be examined in long term and in a larger sample studies[23].
The components of Mediterranean diet as protective mediators in osteoarthritis
Olive Oil
Olive oil is the primary source of fat in the MD and a rich source of polyphenols, monounsaturated fatty acids (oleic acid) and tocopherols. The bibliography analyzing the effect of olive oil components on rheumatoid arthritis (RA) is more extensive and has produced encouraging results. Fewer studies examine the relationship between olive oil and OA with emerging evidence towards proving that components of olive oil exert anti-inflammatory and antioxidant properties against OA[4].
The progress of OA is associated with prolonged production of proinflammatory cytokines that stimulate chondrocytes which in turn produce cartilage-degrading enzymes that lead to the synthesis of nitric oxide (NO) and increase the synthesis of Prostagladin E2 (PGE2) which can be involved with pain in osteoarthritis. NO plays a critical role in OA and it has been noted that the final product of NO, is expressed in the synovial fluid of OA[24].
Study that examined the effect of virgin olive oil components in RA mice model concluded that the oral administration of olive oil exhibited anti-inflammatory properties and minimized the swelling, migration of inflammatory cells, decrease cartilage deterioration and bone erosion. The anti-inflammatory effects of the olive oil components were attributed to phenolic compounds, which appear to modulate the local concentrations of inflammatory cytokines[25]. Another study presented that OA patients have been benefited from 28 weeks administration of olive extract supplement, resulting in reduction of pain, inflammation, and improving quality of life, with anti-inflammatory factors have affected in localized inflammation of joints to OA patients[26].
Olive oil’s phenol secoiridoid oleocanthal has been reported to exhibit anti-inflammatory activity by reducing cytokines, such as granulocyte-macrophage factor (GMCSF), IL-6, IL-1, and tumor necrosis factor-a (TNFa)[4]. Furthermore, oleocanthal seemed to exhibit anti-inflammatory capacity, through reduction of NO, in chondrocytes and normalizes prostaglandin synthesis via reduction of cyclooxygenase (COX) enzymes improving the status of RA and OA[24].
Quality of Fat
The traditional MD is linked to the consumption of fish, vegetables, nuts, which are rich in PUFAs and lead to lower n-6 to n-3 fatty acid ratio[4,17]. In the past, fish consumption has been moderate overall, thought it also used to be proportionate to sea proximity[15]. Moreover olive oil and olives offer MUFAs and especially oleic acid[4,17]. Essential fatty acids (FA) that comprise more than one double bond in their chemical structure are called polyunsaturated fatty acids (PUFAs). They’re precursors of eicosanoids, those are leukotrienes, prostaglandins, thromboxanes. These factors are mechanisms mediators in bone metabolism and inflammation. The COX and lipoxygenases (LOX) mediate the modification of the PUFAs into eicosanoids. N-6 and n-3 series comprise diverse eicosanoids. Eicosanoids derived from n-6 are proinflammatory, contrariwise, eicosanoids came from n-3 are less proinflammatory. The PGE2 are composed from n-6, were linked to the restraining anabolic processes and caused increasing cartilage degradation. Ιt has been stated that control of the precursors of prostaglandins is possible to affect the onset and progress of OA[27].
A diet rich in n-3 FA is beneficial since it may be associated with reduction of the symptoms of both OA cartilage and subchondral bone. Moreover, supplementation of n-3 FA leads to reduction of the OA symptoms. The n-3 FA producing anti-inflammatory resolvins, docosatrienes, etc. Compounds derived from n-3 FA decrease gene expression of proteinases cartilage lesions, inflammatory cytokines and decrease the proliferation of lymphocytes. Conversely, references indicate that high intake of n-6 FA, a Western’s diet characteristic, linked to several inflammatory processes like OA cartilage. Additionally, eicosanoids derivatives of n-3 FA compete with n-6 PUFAs, in receptor level, restrict gene expression of COX-2 but not COX-1[28].
Concerning the bone marrow lesions (BMLs), it has been shown that elevated levels of fat and n-6 PUFAs exist in the bones of patients with OA, while the n-3 PUFAs have been associated with regulation of catabolic factors on cartilage damage[29]. Recent studies show that dietary fat plays an essential role in the development BML. It has been presented that rise intake of MUFA and n-6 PUFAs are linked to BMLs[30]. A Longitudinal study, observed that increased intake of saturated fatty acids was related to incident of BMLs[31]. In order to clarify these effects further research is required.
Fruits, Vegetables, Cereals
The MDP is characterized by daily intake of fruits, vegetables and cereals rich in soluble, insoluble fibers and phytochemicals. They’re also characterized by low fat and cholesterol content and high content in carbohydrates, vitamins A, C, magnesium and selenium (particularly vegetables grown in seleniferous soil, such us carrots, onion, potato, and cabbage).
The bibliography has observed positive relationship between fruit and vegetable consumption and health indicators of bone and joint diseases, although there are few studies in humans. It is believed that soy isoflavones, orange flavonoids, onion’s and tomato’s carotenoids are bioactive ingredients for joint health. Flavonoids have anti-inflammatory and antioxidant activity in animals and cultured cells. Sulphoraphane, an inducer of phase II enzymes from cruciferous vegetables, affects cultured cells by relating it with joint health. Pomegranate extracts contribute to protection of chondrocytes in OA posttraumatic model in rats. It seems that the synergistic action of phytochemicals is associated with the improving health of joints more than the individual components[32]. Studies have indicated the beneficial effect of dietary fiber in the pain of joints in OA. Specifically, the daily intake of 25 g dietary total fiber is related to low risk of moderate and severe pain, in eight years follow up[33]. Other results presented fruits, vegetables consumption is associated with healthier lifestyle[19,33].
Phenolic Compounds
The existing evidence proves that the reduction of both Oxidative stress and inflammatory activity may protect against the outcome of OA via the modulation of the imbalance of catabolic and anabolic activity.
Data highlights that dietary polyphenols protect against the OA development by alleviating chondrocyte inflammation and cartilage destruction. Polyphenols interact with tissues that bind the articulation resulting in reduction of synovial pain.
Literature presents the anti-catabolic effects of tea and pomegranate polyphenols, curcumin and citrus nobiletin. Increased levels of polyphenols in tissues may lead to: 1) reduction of the inflammatory processes by reducing IL1-β, IL-6, IL-8, TNF-α, 2) reduction of the synthesis of NO, COX- 2, PGE2, 3) reduction of expression of chemotactic factors (MCP-1) by reducing NO levels[34,35], 4) raised levels of matrix synthesis and anabolic cytokines, 5) interruption of oxidation by reacting with free radicals and neutralize them and 6) reduction of cell death and enhancement of chondrocyte proliferation.
Polyphenols derived from fruits, vegetables, grains, legumes and wine, appear to be associated with better OA outcome[34-36]. Polyphenols are a promising research field to explain their possible operating results against OA.
Antioxidants
The MD has been proved to increase antioxidant capacity. Research findings highlighted the role of dietary antioxidants in the pathophysiology of OA. Although more research about the role of antioxidants in OA is essential, many promising evidence has been raised about vitamin C, tocopherol, selenium and osteoarthritis.
Ascorbic acid has been presented to reduce the bone marrow damage and the bone size, significant factors for the pathogenesis of knee osteoarthritis. Lutein and zeaxanthin were also linked to reduced cartilage lesions, regardless of vitamin C. Moreover, beta-cryptoxanthin was associated with tibial plateau bone area reduction[37]. Framingham OA Cohort Study promoted that intake of ascorbic acid reduces the outcome of osteoarthritis in knee, and tocopherol declines the development of OA in males[6]. Other studies examining the effect of vitamin C and E supplementation against OA showed no correlation[38,39].
There are limited data on the role of carotenoids in knee osteoarthritis. The Framingham OA Cohort Study revealed that intake of beta-carotene was associated with reduction of progression of symptomatic knee OA[6]. Another study presented participants with increased levels of serum lutein or beta-cryptoxanthin developed knee OA to a lesser degree, on the contrary, it was more possible participants with the highest concentrations of serum beta-carotene or zeaxanthin to develop knee OA[40].
Suggested protective mechanisms of the Mediterranean diet in osteoarthritis
Anti-inflammatory Properties
The literature describes osteoarthritis as an inflammatory condition. The inflammatory process contributes to the symptoms of OA progression. The most prominent clinical symptoms include joint pain, cracking, stiffness and difficulties through movements. Some radiographic features are the loss of cartilage, formation of osteophytes and modifications in subchondral bone[1]. It has been reported that inflammatory modifications are observed in synovial lining of the synovium. These mediators are macrophages, the proinflammatory cytokines, like TNF-a, NO, metalloproteinases (MMPs), IL-1a, IL-1b, IL-15, IL-17, IL-8 and PGE2.
Traditional Mediterranean diet is characterized by increased consumption of virgin olive oil, fruits, vegetables, legumes, whole grain and fish. Those are sources of MUFA, n-3 PUFA, ascorbic acid, carotenoids, vitamin E, phytochemicals with antinflammatory properties, that lead to protection against inflammatory and oxidative processes[41]. In vitro and in vivo studies, conclude that non-fat and fat components of MD have substantial antinflammatory properties, via modulation of the expression of genes with proinflammatory actions, regulation of arachidonic acid (AA) sequence and the activity of immune system[4].
Figure 1.
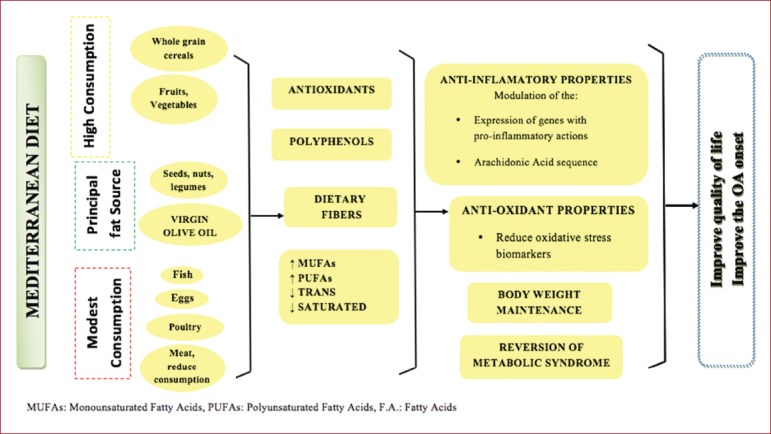
Suggested protective mechanisms of the Mediterranean diet in osteoarthritis.
Significant MD components, which are associated with reduction of inflammation, are n-3 fatty acids and oleic acid (olive oil’s MUFA). The arachidonic acid (AA) is synthesized in human body by the linoleic acid (n-6 PUFAs). The synthesis of AA can reduce inflammation through regulation of eicosanoid production, influencing the tissue arachidonic acid levels[4]. Dietary n-3 fatty acids lead to arachidonic acid content decrease and, in vitro, composition of eicosanoids. They also change plasma phospholipids production for series -3, -5 prostaglandins, in vivo. Oleic acid is referred to “modulate the expression of genes inhibited signal transduction and cytokine production”. Oleic acid’s supplementation in mice model suppressed the synthesis of IL-6, IL-1β and enhanced neutrophil function[42]. Furthermore, it has emerged that MD exhibits immunomodulatory effects through integration of DHA and EPA in cell lipids and through restricted synthesis of arachidonic acid[43].
Virgin olive oil’s non-fatty constituents have a beneficial effect against proinflammatory cytokines. Arginine, dietary fibers, magnesium, phenolic compounds and other phytochemicals have anti-inflammatory properties. The olive oil’s phenol secoiridoid oleocanthal, exhibits significant anti-inflammatory activity by reducing cytokines[4].
Antioxidant Capacity
It has been observed that the increased adherence to the MD results in high antioxidant capacity[44]. Another study documented that consistent consumption of MD lead to increased vitamin C levels and reduced levels of F2-isoprostane, indicator of oxidative stress in healthy individuals[45].
The literature suggests that MD may reduce oxidative stress biomarkers. Τhese biomarkers are affecting the onset of OA, through the rise of levels of collagen type II, while increasing the expression of aggrecan and inhibit the apoptosis of proteins related to the protection of cartilage[46,47].
The MD components hydroxytyrosol, resveratrol and oleuropein show antioxidant action in vitro, as they constrain the activation of transcription NF-kB factors and AP-1 and decrease the expression of endothelial adhesion molecules[48]. The relative expression of COX-2 and the production of prostanoids and metalloproteinases have significant importance in angiogenesis, pathogenic mechanism connected to chronic arthritis and atherosclerotic vascular diseases[49]. It has been also reported that olive’s polyphenols provide protection against oxidative stress by reducing the reactive oxygen species. The available evidence indicates that the MD, through direct supply the body with antioxidants, brings great benefits, reducing oxidative stress.
Body Weight maintenance
Evidence suggests obesity as an important risk factor for the progression of knee OA. Findings of the Framingham Study show obesity precedes from knee OA[50]. Other studies link obesity with radiological findings of knee OA[51,52]. Twins research study demonstrated that it isn’t genetic factors that cause the relationship between Body Mass Index (BMI) and knee OA, data indicating that environmental modifications in BMI may benefit patients with OA[53].
Several studies indicate that increased adherence to the Mediterranean diet is linked to a decreased incidence of obesity[54,55]. In contrast, there have been studies that show no correlation[56]. However, most of the available evidence promotes the benefits of MD in BMI regulation.
Strong evidence suggests that dietary fiber intake is associated with satiety and reduced calorie intake. Clinical studies show that high intake of dietary fiber increases satiety and reduce hunger compared to the low fiber intake. Additionally, the literature examining the effect of high fiber diet on caloric intake and weight loss conclude to the reduction of both[57,58]. There is an evidence that MD, a rich source of soluble and insoluble dietary fibers is associated with body weight regulation[59]. However, the relationship between MD and the progress of OA through the regulation of body weight needs further investigation.
Reversion of the Metabolic Syndrome Status
Metabolic Syndrome (MS) is a combination of abdominal obesity, dyslipidemia, elevated blood pressure levels and impaired glucose tolerance[60]. MS is considered as a major risk factor for morbidity and mortality from cardiovascular disease.
Several research data link OA with the components of MS. It has been demonstrated the connection between diastolic pressure and knee OA. Another study shown OA hand was significantly correlated with high serum cholesterol levels, in females. Other studies suggest that people with OA appeared to have high glucose levels, while it’s been stating that metabolic components, such as increased blood glucose levels, hypertension, hypercholesterolemia associated with knee osteoarthritis[61,62]. It’s suggested that knee osteoarthritis shares components with MS, such as age and obesity[63-65]. At the same time data indicates that cardiovascular diseases are frequent among people with OA[29].
The pathophysiology of cardiovascular diseases aggravates the OA. It has been observed the decrease in blood flow through the vessel to the subchondral bone because of venous obstruction, impaired venous circulation based cartilage plate, hypercoagulability and hypertension. The previous findings lead to subchondral bone ischemia, reduced supply of nutrients and oxygen to the cartilage plate leading on the death of osteocytes. Thereafter bone resorption occurs, affecting adversely the viability of the subchondral bone. Histologically, it is observed osteonecrosis, disorders in bone remodeling and swelling[29,66,67].
Framingham Offspring Study, with 2834 volunteers showed that the consumption of cereals and whole grains products reduce the risk of metabolic syndrome[68]. The ATTICA study[69], revealed that higher adherence to MD linked with 20% lower risk for metabolic syndrome, after adjustment for confounding factors. It has been demonstrated that the combination of MD components protects against the onset of MS[70]. A recent study with 4358 individuals pointed that people with high adherence to MD significantly improves BMI and showed fewer morbidities, such as type 2 diabetes compared to those with lower adherence. These individuals had also lower prevalence of knee osteoarthritis[19].
The above findings raise the need for long term research to examine the role of Mediterranean diet through the reverse of MS, by modifying the components of MS, in progress of OA.
Conclusion
Mediterranean diet is investigated at times in prevention and treatment of chronic diseases. The role of MD in arthritis has been researched mainly against Rheumatoid Arthritis with promising results. The research landscape that describes the effect of MD in OA progress is still unclear. However, emerging data reveal that anti-inflammatory and antioxidant properties of MD are promising parts against Osteoarthritis progression. This highlights the need for further research between mechanisms actions of MD and the outcome of OA.
Mediterranean diet is not only an adequate dietary pattern but a holistic balanced and healthy lifestyle with benefits to the quality of life. In any case, MD could be an integral part of everyday lives of OA sufferers.
Footnotes
Edited by: George Lyritis
References
- 1.Rahmatia M, Mobasheri A, Mozafari M. Inflammatory mediators in osteoarthritis:A critical review of the state-of-the-art.Current prospects and future challenges. Bone. 2016;85:81–90. doi: 10.1016/j.bone.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni K, Karssiens T, Kumar T, Pandit H. Obesity and osteoarthritis. Maturitas. 2016;89:22–28. doi: 10.1016/j.maturitas.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Johnson VL, Bach App. Honours, David J. Hunter. The epidemiology of osteoarthritis. Best Practice & Research Clinical Rheumatology. 2014:5–15. doi: 10.1016/j.berh.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Oliviero F, Spinella P, Fiocco U, et al. How the Mediterranean diet and some of its components modulate inflammatory pathways in arthritis, Review article. Swiss Medical Weekly. 2015;145:w14190. doi: 10.4414/smw.2015.14190. [DOI] [PubMed] [Google Scholar]
- 5.Lister CE, Skinner MA, Hunter DC. Fruits, vegetables and their phytochemicals for bone and joint health. Current topics in nutraceutical research. 2007;5:67–82. [Google Scholar]
- 6.McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39:648–58. doi: 10.1002/art.1780390417. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Jordan JM. Epidemiology of Osteoarthritis. Clin Geriatr Med. 2010;26(3):355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wluka AE, Stuckey S, Brand C, Cicuttini FM. Supplementary vitamin E does not affect the loss of cartilage volume in knee osteoarthritis:a 2 year double blind randomized placebo controlled study. J Rheumatol. 2002;29(12):2585–91. [PubMed] [Google Scholar]
- 9.Neogi T, Booth SL, Zhang YQ, et al. Low vitamin K status is associated with osteoarthritis in the hand and knee. Arthritis Rheum. 2006;54:1255–61. doi: 10.1002/art.21735. [DOI] [PubMed] [Google Scholar]
- 10.Ren Master FL, et al. Effects of selenium and iodine deficiency on bone, cartilage growth plate and chondrocyte differentiation in two generations of rats. Osteoarthritis and Cartilage. 2007;15:1171–1177. doi: 10.1016/j.joca.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigo Moreno-Reyes, Françoise Mathieu, Marleen Boelaert, et al. Selenium and iodine supplementation of rural Tibetan children affected by Kashin-Beck osteoarthropathy. Am J Clin Nutr. 2003;78:137–44. doi: 10.1093/ajcn/78.1.137. [DOI] [PubMed] [Google Scholar]
- 12.Jordan JM, Fang F, Arab L, et al. Low selenium levels are associated with increased risk for osteoarthritis of the knee. Arthritis Rheum. 2005;52:s455. [Google Scholar]
- 13.Jungmann PM, et al. Association of Metabolic Risk Factors With Cartilage Degradation Assessed by T2 Relaxation Time at the Knee:Data From the Osteoarthritis Initiative. Arthritis Care & Research. 2013:1942–1950. doi: 10.1002/acr.22093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucker KL, Dallal GE, Rush D. Dietary patterns of the elderly Boston-area residents defined by cluster analysis. J. Am. Diet. Assoc. 1992;92:1487–1491. [PubMed] [Google Scholar]
- 15.Trichopoulou A, Lagiou P. Healthy traditional Mediterranean diet:an expression of culture, history, and lifestyle. Nutrition Reviews. 1997;55:383–389. doi: 10.1111/j.1753-4887.1997.tb01578.x. [DOI] [PubMed] [Google Scholar]
- 16.Hu FB. The Mediterranean diet and mortality - olive oil and beyond. Engl J Med. 2003;348:2595–2596. doi: 10.1056/NEJMp030069. [DOI] [PubMed] [Google Scholar]
- 17.Faig Anna Bach, Berry Elliot M, Lairon Denis, et al. Mediterranean diet pyramid today. Science and cultural Updates, Public Health Nutrition. 2011;14(12A):2274–2284. doi: 10.1017/S1368980011002515. [DOI] [PubMed] [Google Scholar]
- 18.Salas-Salvado J, Martinez-Gonzalez M, Bullo Ros E. The role of diet in the prevention of type 2 diabetes. Nutrition, Metabolism & Cardiovascular Diseases. 2011;21:B32–B48. doi: 10.1016/j.numecd.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Veronese N, Stubbs B, Noale M, et al. Adherence to a Mediterranean diet is associated with lower prevalence of osteoarthritis:Data from the osteoarthritis initiative. Clinical Nutrition. 2016:1–6. doi: 10.1016/j.clnu.2016.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veronese N, Stubbs B, Noale M, et al. Adherence to the Mediterranean diet is associated with better quality of life:data from the Osteoarthritis Initiative. Am J Clin Nutr. 2016;104(5):1403–1409. doi: 10.3945/ajcn.116.136390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dyer J, Davison G, Marcora SM, Mauger AR. Effect Of A Mediterranean Type Diet On Inflammatory And Cartilage Degradation Biomarkers In Patients With Osteoarthritis. J Nutr Health Aging. 2016:1–5. doi: 10.1007/s12603-016-0806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartels EM, Christensen R, Christensen P, et al. Effect of a 16 weeks weight loss program on osteoarthritis biomarkers in obese patients with knee osteoarthritis:a prospective cohort study. Osteoarthritis Cartilage. 2014;22(11):1817–25. doi: 10.1016/j.joca.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Davison G, Dyer R, Marcora M, Mauger R. The efficacy of a Mediterranean type diet on symptoms of osteoarthritis-a pilot study. Proceedings of the Nutrition Society. 2015;74(OCE1):E142. [Google Scholar]
- 24.Lucas L, Russell A, Russell K. Molecular Mechanisms of Inflammation. Anti-Inflammatory Benefits of Virgin Olive Oil and the Phenolic Compound Oleocanthal. Current Pharmaceutical Design. 2011;17:754–768. doi: 10.2174/138161211795428911. [DOI] [PubMed] [Google Scholar]
- 25.Rosillo MA, Alcaraz MJ, Hidalgo MS, et al. Anti-inflammatory and joint protective effects of extra-virgin olive-oil polyphenol extract in experimental arthritis. Journal of Nutritional Biochemistry. 2014;25:1275–1281. doi: 10.1016/j.jnutbio.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Bitler CM, Matt K, Irving K, et al. Olive extract supplement decreases pain and improves daily activities in adults with osteoarthritis and decreases plasma homocysteine in those with rheumatoid arthritis. Nutrition Research. 2007;27:470–477. [Google Scholar]
- 27.Knotty L, Avery NC, Hollanderz AP, Tarlton JF. Regulation of osteoarthritis by omega-3 polyunsaturated fatty acids in a naturally occurring model of disease. Osteoarthritis and Cartilage. 2011;19:1150–1157. doi: 10.1016/j.joca.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurst S, Zainal Z, Caterson B, et al. Dietary fatty acids and arthritis, Prostaglandins, Leukotrienes and Essential Fatty Acids. 2010;80:315–318. doi: 10.1016/j.plefa.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 29.DoréD Hoog J, Giles G, et al. A longitudinal study of the association between dietary factors, serum lipids, and bone marrow lesions of the knee. Arthritis Research & Therapy. 2012;14:R13. doi: 10.1186/ar3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Wluka AE, Hodge AM, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:579–83. doi: 10.1016/j.joca.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Davies-Tuck ML, Wluka AE, et al. Dietary fatty acid intake affects the risk of developing bone marrow lesions in healthy middle-aged adults without clinical knee osteoarthritis:a prospective cohort study. Arthritis Res Ther. 2009;11:R63. doi: 10.1186/ar2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lister CE, Skinner MA, Hunter DC. Fruits, vegetables and their phytochemicals for bone and joint health. Current Topics In Nutraceutical Research. 2007;5:67–82. [Google Scholar]
- 33.Zhaoli Dai, Na Lu, Jingbo Niu, et al. Dietary intake of fiber in relation to knee pain trajectories. Arthritis Care & Research. 2016:2–29. [Google Scholar]
- 34.Aherne SA, O'Brien NM. Dietary flavonols:chemistry, food content, and metabolism. Nutrition. 2002;18(1):75–81. doi: 10.1016/s0899-9007(01)00695-5. [DOI] [PubMed] [Google Scholar]
- 35.Ross JA, Kasum CM. Dietary flavonoids:bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 36.Shen CL, Smith JB, Lo DF, et al. Dietary polyphenols and mechanisms of osteoarthritis. Journal of Nutritional Biochemistry. 2012;23:1367–1377. doi: 10.1016/j.jnutbio.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Hodge MA, Wluka EA, et al. Effect of antioxidants on knee cartilage and bone in healthy, middle-aged subjects:a cross-sectional study. Arthritis Research & Therapy. 2007;9:R66. doi: 10.1186/ar2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraus VB, Huebner JL, Stabler T, et al. Ascorbic acid increases the severity of spontaneous knee osteoarthritisin a guinea pig model. Arthritis Rheum. 2004;50:1822–1831. doi: 10.1002/art.20291. [DOI] [PubMed] [Google Scholar]
- 39.Wluka AE, Stuckey S, Brand C, Cicuttini FM. Supplementary vitamin E does not affect the loss of cartilage volume in knee osteoarthritis:a 2 year double blind randomized placebo controlled study. J Rheumatol. 2002;29:2585–2591. [PubMed] [Google Scholar]
- 40.De Roos AJ, Arab L, Renner JB, et al. Serum carotenoids and radiographic knee osteoarthritis:the Johnston County Osteoarthritis Project. Public Health Nutr. 2001;4:935–942. doi: 10.1079/phn2001132. [DOI] [PubMed] [Google Scholar]
- 41.Bullo M, Casas-Agustench P, Amigo-Correig P, et al. Inflammation, obesity and comorbidities:the role of diet. Public Health Nutr. 2007;10(10A):1164–72. doi: 10.1017/S1368980007000663. [DOI] [PubMed] [Google Scholar]
- 42.Magdalon J, Vinolo MA, Rodrigues HG, et al. Oral administration of oleic or linoleic acids modulates the production of inflammatory mediators by rat macrophages. Lipids. 2012;47:803–12. doi: 10.1007/s11745-012-3687-9. [DOI] [PubMed] [Google Scholar]
- 43.Volker DH, Fitzgerald PE, Garg ML. The eicosapentaenoic to docosahexaenoic acid ratio of diets affects the pathogenesis of arthritis in Lew/SSN Rats. J Nutr. 2000;130:559–65. doi: 10.1093/jn/130.3.559. [DOI] [PubMed] [Google Scholar]
- 44.Pitsavos C, Panagiotakos DB, Tzima N, et al. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults:the ATTICA study. Am J Clin Nutr. 2005;82(3):694–9. doi: 10.1093/ajcn.82.3.694. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez-Moreno C, Cano MP, de Ancos B, et al. Mediterranean vegetable soup consumption increases plasma vitamin C and decreases F(2)-isoprostanes, prostaglandin E(2) and monocyte chemotactic protein-1 in healthy humans. J Nutr Biochem. 2006;17:183–9. doi: 10.1016/j.jnutbio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Chatzianagnostou K, Del Turco S, Pingitore A, et al. The Mediterranean lifestyle as a non-pharmacological and natural antioxidant for healthy aging. Antioxidants. 2015;4:719–36. doi: 10.3390/antiox4040719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziskoven C, Jager M, Zilkens C, et al. Oxidative stress in secondary osteoarthritis:from cartilage destruction to clinical presentation? Orthop Rev. 2010;2:23. doi: 10.4081/or.2010.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carluccio MA, Siculella L, Ancora MA, et al. Olive oil and red wine antioxidant polyphenols inhibit endothelial activation:antiatherogenic properties of Mediterranean Diet phytochemicals. Arterioscler Thromb Vasc Biol. 2003;23:622–9. doi: 10.1161/01.ATV.0000062884.69432.A0. [DOI] [PubMed] [Google Scholar]
- 49.Scoditti E, Calabriso N, Massaro M, et al. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells:a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys. 2012;527:81–9. doi: 10.1016/j.abb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis. The Framingham Study. Annals of Internal Medicine. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 51.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly:the Framingham Study. Arthritis and Rheumatism. 1997;40:728–733. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 52.Spector TD, Hart DJ, Doyle DV. Incidence and progression of osteoarthritis in women with unilateral knee disease in the general population:the effect of obesity. Annals of the Rheumatic Diseases. 1994;53:565–568. doi: 10.1136/ard.53.9.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manek NJ, Hart D, Spector TD, et al. The association of body mass index and osteoarthritis of the knee joint:an examination of genetic and environmental influences. Arthritis and Rheumatism. 2003;48:1024–102. doi: 10.1002/art.10884. [DOI] [PubMed] [Google Scholar]
- 54.Sanchez-Villegas A, Bes-Rastrollo M, Martinez-Gonzalez MA, Serra-Majem L. Adherence to a Mediterranean dietary pattern and weight gain in a follow-up study:the SUN cohort. Int J Obes. 2006;30:350–358. doi: 10.1038/sj.ijo.0803118. [DOI] [PubMed] [Google Scholar]
- 55.Schroder H, Marrugat J, Vila J, et al. Adherence to the traditional Mediterranean diet is inversely associated with body mass index and obesity in a Spanish population. J Nutr. 2004;134:3355–3361. doi: 10.1093/jn/134.12.3355. [DOI] [PubMed] [Google Scholar]
- 56.Trichopoulou A, Naska A, Orfanos P, Trichopoulos D. Mediterranean diet in relation to body mass index and waist-to-hip ratio:the Greek European Prospective Investigation into Cancer and Nutrition Study. Am J Clin Nutr. 2005;82:935–940. doi: 10.1093/ajcn/82.5.935. [DOI] [PubMed] [Google Scholar]
- 57.Howarth NC, Saltzman E, Roberts SB. Dietary fiber and weight regulation. Nutr Rev. 2001;59(5):129–39. doi: 10.1111/j.1753-4887.2001.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 58.Slavin JL. Dietary fiber and body weight. Nutrition. 2005;21(3):411–8. doi: 10.1016/j.nut.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 59.Schroder Helmut. Protective mechanisms of the Mediterranean diet in obesity and type 2 diabetes. Reviews:current topics. Journal of Nutritional Biochemistry. 2007;18:149–160. doi: 10.1016/j.jnutbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 60.Esposito K, Kastorini C.M, Panagiotakos D.B, Giugliano D. Mediterranean diet and metabolic syndrome:An updated systematic review. Rev Endocr Metab Disord. 2013;14(3):255–63. doi: 10.1007/s11154-013-9253-9. [DOI] [PubMed] [Google Scholar]
- 61.Cimmino MA, Cutolo M. Plasma glucose concentration in symptomatic osteoarthritis:a clinical and epidemiological survey. Clin Exp Rheumatol. 1990;8:251–7. [PubMed] [Google Scholar]
- 62.Hart DJ, Doyle DV, Spector TD. Association between metabolic factors and knee osteoarthritis in women:the Chingford study. J Rheumatol. 1995;22:1118–23. [PubMed] [Google Scholar]
- 63.Sharma L, Kapoor D. Osteoarthritis:Diagnosis and Medical/Surgical Management. 4th edn. Philadelphia: Lippincott Williams and Wilkins; 2007. Epidemiology of Osteoarthritis. [Google Scholar]
- 64.Felson DT, Anderson JJ, Naimark A, et al. Obesity and knee osteoarthritis:the Framingham study. Ann Intern Med. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 65.Muraki S, Akune T, Oka H, et al. Association of occupational activity with radiographic knee osteoarthritis and lumbar spondylosis in elderly patients of population-based cohorts:a large-scale population-basedstudy. Arthritis Rheum. 2009;61:779–86. doi: 10.1002/art.24514. [DOI] [PubMed] [Google Scholar]
- 66.Findlay DM. Vascular pathology and osteoarthritis. Rheumatology. 2007;46:1763–8. doi: 10.1093/rheumatology/kem191. [DOI] [PubMed] [Google Scholar]
- 67.Winet H, Hsieh A, Bao JY. Approaches to study of ischemia in bone. J Biomed Mater Res. 1998;43:410–21. doi: 10.1002/(sici)1097-4636(199824)43:4<410::aid-jbm8>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 68.McKeown NM, Meigs JB, Liu S, et al. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care. 2004;27:538–5346. doi: 10.2337/diacare.27.2.538. [DOI] [PubMed] [Google Scholar]
- 69.Panagiotakos DB, Pitsavos CH, Chrysohoou C, et al. The impact of lifestyle habits on the prevalence of the metabolic syndrome among Greek adults from the ATTICA study. Am Heart J. 2004;147:106–112. doi: 10.1016/s0002-8703(03)00442-3. [DOI] [PubMed] [Google Scholar]
- 70.Baxter AJ, Coyne T, McClintock C. Dietary pattern and metabolic syndrome:a review of epidemiological evidence. Asia Pac J Clin Nutr. 2006;15:134–142. [PubMed] [Google Scholar]


