Abstract
Patient: Male, 46-year-old
Final Diagnosis: Chronic tophaceous gout with disseminated cutaneous tophi
Symptoms: Joint pain
Medication: —
Clinical Procedure: —
Specialty: Rheumatology
Objective:
Rare disease
Background:
Gout is a metabolic disease characterized by deposition of monosodium urate (MSU) crystals called tophi. The typical location of tophi is in the joint and will chronically damage the joint. However, there is a rare atypical dermatologic manifestation of tophi that occur extensively in the skin.
Case Report:
A 46-year-old male presented with acute pain in multiple joints. He had a history of gouty arthritis with recurrence attacks, in the past 2 years ago. Over time, he had gradual eruption of multiple tophi and multiple yellowish nodules under his skin which sometimes would ulcerate. Laboratory value showed creatinine 2.3 mg/dL and uric acid 11.5 mg/dL. Ultrasound of the kidney showed nephrocalcinosis appearance. Urate crystal was identified in skin biopsy of the nodules. We diagnosed the patient with chronic tophaceous gout with extensive cutaneous involvement. Given the renal impairment, we gave methylprednisolone 3 doses of 8 mg for 5 days then tapered off, colchicine 0.5 mg every other day and allopurinol 1 dose of 100 mg. The patient had dramatic improvement of his pain and is now being followed up regularly.
Conclusions:
We describe a rare and severe extensive cutaneous manifestation in a chronic tophaceous gout patient.
MeSH Keywords: Gout; Renal Insufficiency, Chronic; Uric Acid
Background
Gout is a crystal arthropathy that occurs because of deposition of monosodium urate (MSU) crystals in and around the joint [1]. Gout is an inflammatory arthritis with varied clinical manifestation, including painful acute flare of joint synovitis and chronic deposition of MSU crystals in joints and other body tissues that can lead to chronic joint damage, renal stone formation, and renal impairment [2]. Gout affects mostly the peripheral joints of the lower limb such as the first metatarsophalangeal joint, ankle, and knee, but it can also affect other joints [3,4]. The prevalence of gout is around 3.9% in the United States, 0.9% in France, 1.4–2.5% in the UK, 1.4% in Germany, and 3.2–26.1% in New Zealand [5]. The incidence of gout in the general population ranges from 0.06 to 2.68 per 1000 person-years [1].
Serum urate level depends on the production and excretion of urate. Urate is produced through de novo biosynthesis, dietary purine intake, and turnover of tissue nucleic acid. Urate is secreted and reabsorbed in the renal proximal tubule and is excreted in the intestine [6]. There is also the role of genetic factors in serum urate homeostasis. For example, purine overproduction might be caused by the deficiency of the enzyme hypoxanthine–guanine phosphoribosyl transferase (HPRT) [7]. The ABCG2 gene plays an important role to the secretion of uric acid, and its dysfunction will lead to excess of serum uric acid [8]. Therefore, analysis of the genetic factors in urate homeostasis might be an important tool to understand better about the pathophysiology of hyperuricemia [6,7].
Urate deposition, or tophus, occurs frequently in avascular tissues over the ears, olecranon and prepatellar bursae, or near tendons [9]. Tophus can also be found in less frequent sites such as in the skin, laryngeal and bronchial wall [10]. There is also an atypical dermatological manifestation of tophi in the form of pustules, ulcerations, and panniculitis [11]. Extensive involvement of tophi in the skin is uncommon. Therefore, this study aimed to report on a rare severe skin manifestation in a patient with chronic tophaceous gout that was complicated with renal impairment
Case Report
A 46-year-old male came to our rheumatology clinic with acute pain in multiple joints since a week before admission to hospital. The pain caused limitation of movement and the patient was unable to walk and was in wheelchair. He had nodules and plaques that were non-tender and firm widespread over the trunk, arms, and legs. Two years ago, he complained of pain and swelling in his joints. He underwent aspiration of joint fluid which showed deposition of gouty crystal. The diagnosis of gouty arthritis was established, but he did not go for regular follow-up to the health facilities and he did not take his medicine regularly. He had recurrent attacks ever since.
A year ago, he started noticing gradual eruption of multiple nodules over the finger joints, elbows, ankle, and knee that sometimes would ulcerate. The ulcers would exude white chalk-like materials. The patient often self-medicated himself with steroids, antibiotics, allopurinol, and colchicine whenever he felt pain until the symptoms subside, then he discontinued all his medications. The patient denied any concomitant medical conditions. He also denied family history of gouty arthritis. He had a sedentary lifestyle, was a heavy drinker and was not actively doing physical exercise. He did not particularly pay attention to his diet.
On physical examination, we found multiple subcutaneous non-tender nodules over all extremities and trunk (Figure 1). We also noted multiple hyperpigmented ulcers with erythematous borders on his extremities and trunk (Figures 2, 3). Tender joints were noted in metacarpal phalangeal (MCP) 1 bilaterally, right proximal interphalangeal (PIP) 2 and PIP 4, wrist joint bilaterally, elbow joint bilaterally, knee joint bilaterally, ankle joint bilaterally, and metatarsophalangeal (MTP) 1 bilaterally. Other systemic and neurological physical examination findings were unremarkable.
Figure 1.
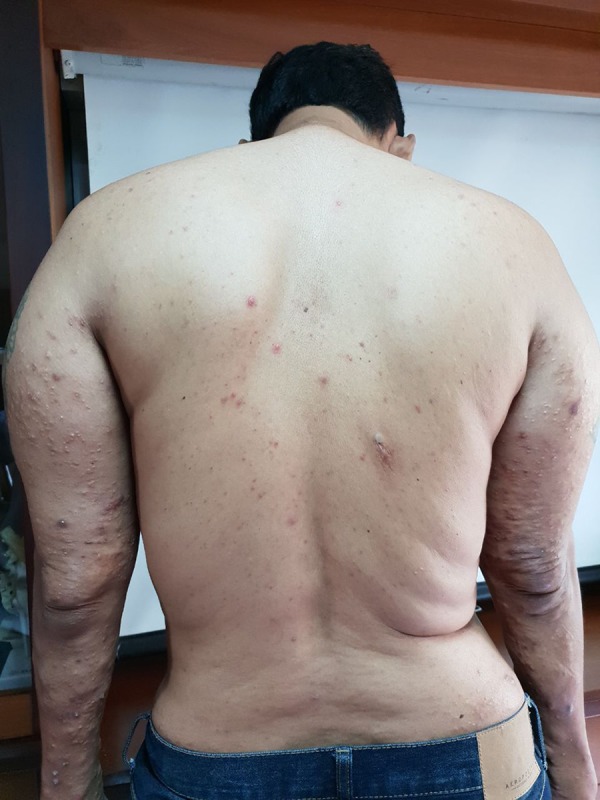
Multiple cutaneous tophi on trunk and upper extremities.
Figure 2.
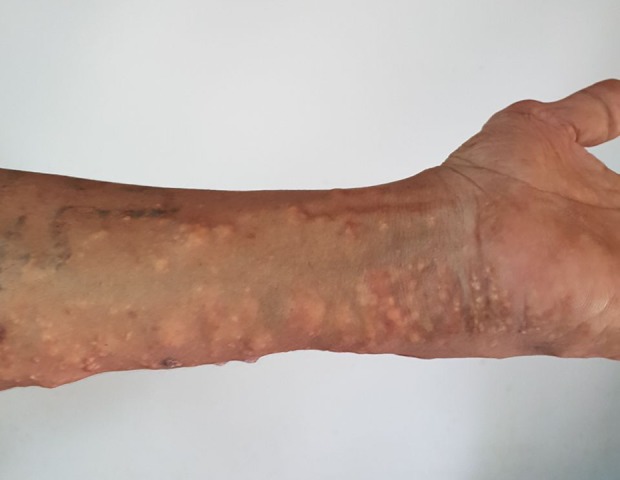
A closer look at tophi and healed ulcers on upper arm.
Figure 3.
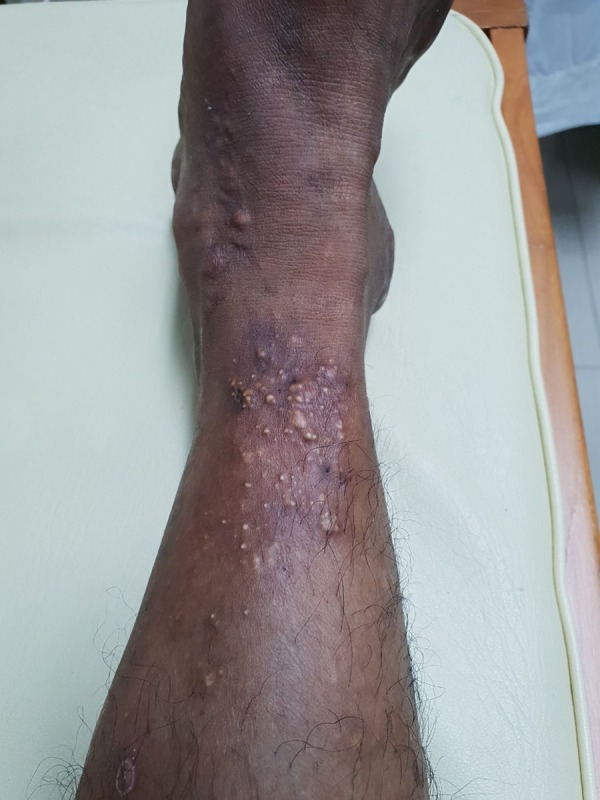
Multiple cutaneous tophi and ulcers on lower leg.
Laboratory values showed elevated uric acid of 11.5 mg/dL (normal range 3.4–7.0 mg/dL) and erythrocyte sedimentation rate (ESR) of 118 (normal range 0–20 mm). The patient also had elevated creatinine of 2.3 mg/dL (normal range 0.8–1.3 mg/dL) thus a decrease in estimated glomerular filtration rate to 32.8 mL/min/1.73 m2. C-reactive protein (CRP) was elevated to 254.1 mg/L (normal <5.0 mg/L). Ultrasound of the kidney showed nephrocalcinosis. Skin biopsy of the nodules taken from left arm showed eosinophilic deposition with needle shape aggregates with surrounding granulomatous reaction (Figures 4, 5). We diagnose the patient as having acute flare of chronic tophaceous gout with disseminated cutaneous tophi and renal impairment.
Figure 4.
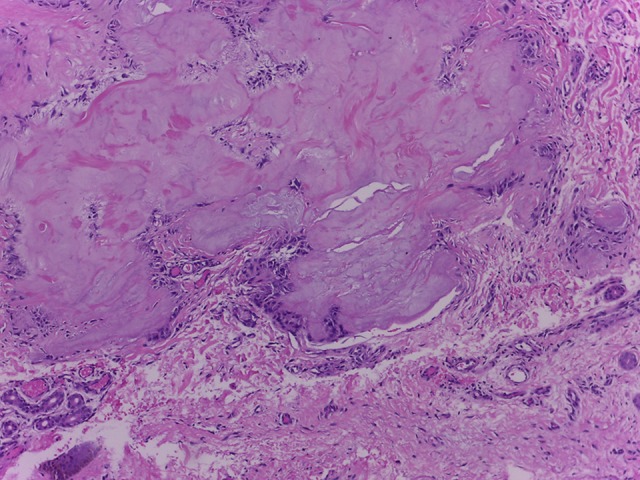
Eosinophilic amorph mass, with granulomatous (hematoxylin and eosin staining, 100×).
Figure 5.
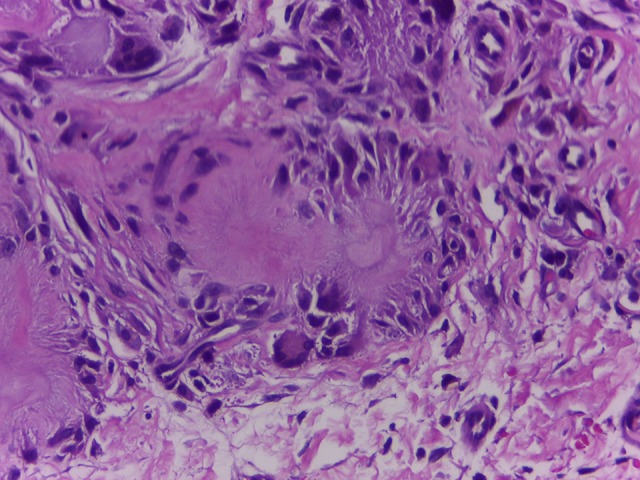
Eosinophilic amorph deposition with needle shape aggregates with surrounding granulomatous reaction (hematoxylin and eosin staining, 400×).
Due to his renal impairment, we gave moderate dose of steroid and adjusted the dose of colchicine and allopurinol. We gave methylprednisolone 3 doses of 8 mg for 5 days, colchicine 0.5 mg every other day and allopurinol 1 dose of 100 mg. We also collaborated with the dermatology department for treatment of his ulcers and skin lesion. The patient had dramatic improvement of his pain and ulcers and is now being followed regularly in the outpatient clinic.
The patient was given an explanation about his disease and was strongly urged to comply to the treatment given. We also asked him to abstain from alcohol. His steroid medication was slowly tapered off by 4 mg every week. After a month of treatment, his serum uric acid decreased to 9.9 mg/dL, and creatinine to 2.2 mg/dL. The ulcers have healed, and a few of the tophi in his skin became smaller in size. Currently we are regularly following the patient and our goal for the patient is to lower the hyperuricemia, maintain renal function, and prevent future tophi formation.
Discussion
Gout is a highly prevalent inflammatory arthritis caused by deposition of MSU crystals with a classic history and clinical course [1]. The characteristics of gout are as follows [12]: 1) hyperuricemia; 2) recurrent flare of acute arthritis; 3) deposit of monosodium urate crystals (tophi); 3) renal impairment; and 4) uric acid nephrolithiasis.
The prevalence and incidence of gout has increased in recent times because of aging, metabolic-related diseases (hypertension, obesity, metabolic syndrome) and diet-related causes (alcoholism, high intake of proteins, and a high fructose-enriched food). Genetic studies and meta-analyses showed that there might be an important role of genetic factor in the pathophysiology of gout [13]. There are specific genes, such as ABCG2 gene, which influence the serum uric acid level through renal uric acid excretion by encoding urate transporters such as URAT1 (SLC22A12) and GLUT9 (SLC2A9). Dysfunction of the ABCG2 gene would result in a decrease of uric acid excretion that leads to increased level of uric acid serum, therefore the ABCG2 gene is considered a significant genetic factor for gout [14]. In a recent study, genetic variants of the ABCG2 gene showed it to have a significant effect on earlier onset of gout and familial gout [15].
Decreased renal function and impaired capacity of urate clearance by the kidney also contribute to hyperuricemia and progression of gout stages. As a chronic disease, gout arthritis may progress to tophaceous gout if not treated effectively. The aim of long-term management is to achieve low level of serum uric acid and to prevent disability due to chronic joint damage.
There are 3 stages of gout arthritis: 1) asymptomatic hyper-uricemia, 2) intercritical or interval gout, and 3) chronic gouty arthritis (formation of tophi) [12]. The diagnosis of gout can be established using the 2015 ACR/EULAR classification criteria for gout, which consist of clinical, laboratory, and imaging domain [5]. The entry criterion for the classification criteria is occurrence of at least 1 episode of gout attack. Then, if MSU crystals in synovial fluid or a tophus are found, then it is sufficient to make diagnosis of gout. The gold standard for diagnosis of gout in the chronic stage is skin biopsy and the presence of chalk-like material on the open skin and around joints [5,12,16].
Our patient came to us already in the third stage of gout, which is chronic tophaceous gout. He had already been diagnosed with gout before and had undergone synovial fluid analysis, which showed positivity for MSU crystals. Therefore, we did not perform further imaging examination, such as musculo-skeletal ultrasound or dual energy computed tomography scan (DECT) to find MSU crystals.
The usual locations of tophi are around the joint, synovium, olecranon, knee and tragus, but our patient presented with multiple tophi deposits and ulceration all over the skin. We performed skin biopsies and the result was supportive of the MSU crystal deposition, thus we assessed this patient as having acute flare of chronic gouty arthritis with disseminated cutaneous tophi. Extensive cutaneous involvement of gout is relatively rare, with several cases described in literature [11,17–20].
The first goal of gout treatment is to manage the acute attack, which is usually very painful. The first-line drugs for gouty flare include colchicine, nonsteroidal anti-inflammatory drugs (NSAIDs), and steroids. To prevent flares, the therapeutic options for prophylaxis is colchicine given with urate lowering therapy such as allopurinol, febuxostat and probenecid [9,16]. A novel uricosuric agent, lesinurad, might be combined with allopurinol or febuxostat, but this is not yet a standard recommendation [21]. The aim of chronic gout management is to reach and maintain serum uric acid at sub-saturating levels, which is under 6 mg/dL for all patients, or under 5 mg/dL for patients with severe gout (tophi, chronic arthropathy, frequent attacks). Long-term achievement of this serum uric acid target might lower incidence of gouty flares and facilitate dissolution of tophi [9,21].
The challenge in treating our patient was the disease’s chronic complications. Gout patients with tophi formations and recurrent flares should be treated aggressively, and the target of uric acid is below 5 mg/dL [21]. The aggressive treatment is aimed to facilitate dissolution of crystals and prevent future flares. However, our patient had renal impairment, as evidenced by the low estimated glomerular filtration rate (eGFR); the renal impairment made aggressive treatment recommended for a patient with normal kidney function not suitable for our patient.
The pathophysiology of renal impairment in a patient with gout is due to chronic hyperuricemia. Hyperuricemia will lead to endothelial dysfunction, stimulation of the renin-angiotensin system, and oxidative stress. These will in turn cause proliferation of smooth muscle cells of the afferent arterioles and lower perfusion of the kidney [14]. In patients with renal impairment, treatment of gout should be done very carefully so as to not further deteriorate kidney function.
There are 4 principles in the management of gout follows regardless of the presence of kidney impairment: 1) manage the hyperuricemia and lower the serum urate level, 2) prophylaxis therapy while initiating uric acid lowering therapy, 3) manage the flare symptoms, and 4) optimize dietary and lifestyle factors. Pharmacologic options for gout patients with renal impairment should be given cautiously, and there might be a need to adjust the dose according to eGFR [13,15].
Regarding renal impairment, the safest option for gout attack is glucocorticoids that is given short-term. The usual starting dose 0.5 mg/kg of body weight per day for the first few days, then taper progressively. If colchicine is already used for prophylaxis, the usual loading dose of 1 mg continued with 0.5 mg an hour later should not be used for gout flare in patients with renal impairment [13,15,16]. For all patients starting on allopurinol, the starting dose should be low and adjusted to eGFR. The dosage of 50 mg/day for patients with chronic kidney disease (CKD) stage 4 or 5, and not more than 100 mg/day in all others can be used. The daily dose can be up-titrated every 2 to 5 weeks to achieve the serum urate target level [15]. Allopurinol can be used in patients with hemodialysis or peritoneal dialysis who still need urate lowering therapy.
Our patient had eGFR of 32.8 and was classified as having CKD stage 3b. In accordance with his renal impairment, for his flare he was given methylprednisolone 3 doses of 8 mg for 5 days then slowly tapered off by 4 mg every week. We avoided giving colchicine for flare dose because he had already used it for prophylaxis, instead we continue giving colchicine 0.5 mg every other day for prophylaxis. We planned to give febuxostat for a faster and safer lowering of uric acid serum, but the patient refused since the drug is expensive, not widely available, and not covered by the national insurance system in our country. Previous case reports showed that allopurinol administration up to 600 mg daily might result in continual reduction of the intradermal tophi size and number [9,22]. The patient had already taken allopurinol before, so the allopurinol dose was maintained at 100 mg daily and was up titrated by 100 mg during his follow-up visits based on the serum urate levels.
Conclusions
We report a case of a rare and severe cutaneous manifestation in a patient with chronic tophaceous gout.
Footnotes
Conflict of interest
None.
References:
- 1.Mutlu A, Dündar E, İşeri M, et al. An unusual presentation of gout: Tophi in the middle ear. J Int Adv Otol. 2016;12(2):216–18. doi: 10.5152/iao.2016.1293. [DOI] [PubMed] [Google Scholar]
- 2.Wijnands JMA, Viechtbauer W, Thevissen K, et al. Determinants of the prevalence of gout in the general population: A systematic review and meta-regression. Eur J Epidemiol. 2015;30(1):19–33. doi: 10.1007/s10654-014-9927-y. [DOI] [PubMed] [Google Scholar]
- 3.Naredo E, Uson J, Jiménez-Palop M, et al. Ultrasound-detected musculoskeletal urate crystal deposition: Which joints and what findings should be assessed for diagnosing gout? Ann Rheum Dis. 2014;73(8):1522–28. doi: 10.1136/annrheumdis-2013-203487. [DOI] [PubMed] [Google Scholar]
- 4.Di Matteo A, Filippucci E, Cipolletta E, et al. The popliteal groove region: A new target for the detection of monosodium urate crystal deposits in patients with gout. An ultrasound study. Jt Bone Spine. 2019;86(1):89–94. doi: 10.1016/j.jbspin.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Neogi T, Jansen TLTA, Dalbeth N, et al. 2015 Gout classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2015;74(10):1789–98. doi: 10.1136/annrheumdis-2015-208237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keenan RT, Nowatzky J, Pillinger MH. Etiology and pathogenesis of hyper-uricemia and gout. Kelley’s Textb Rheumatol. 2013;(1):1553.e5. [Google Scholar]
- 7.Petru L, Pavelcova K, Sebesta I, Stiburkova B. Genetic background of uric acid metabolism in a patient with severe chronic tophaceous gout. Clin Chim Acta. 2016;460:46–49. doi: 10.1016/j.cca.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 8.Stiburkova B, Miyata H, Závada J, et al. Novel dysfunctional variant in ABCG2 as a cause of severe tophaceous gout: Biochemical, molecular genetics and functional analysis. Rheumatology (Oxford) 2016;55(1):191–94. doi: 10.1093/rheumatology/kev350. [DOI] [PubMed] [Google Scholar]
- 9.Lo TEN, Racaza GZ, Penserga EG. “Golden kernels within the skin”: Disseminated cutaneous gout. BMJ Case Rep. 2013;2013:bcr2013009735. doi: 10.1136/bcr-2013-009735. : pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero JBC. Acute respiratory failure due to chronic tophaceous gout with laryngeal and bronchial involvement: An unusual complication. Arch Bronconeumol. 2018;54(7):399–400. doi: 10.1016/j.arbres.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Orzan O. Severe disseminated cutaneous gout. Acta Endocrinol. 2012;8(1):131. [Google Scholar]
- 12.Burns CM, Wortmann R. Clinical features and treatment of gout. In: Firestein G, Budd R, Gabriel S, et al., editors. Textbook of Rheumatology Tenth Edit. Elsevier Ltd; 2017. pp. 1620–44. [Google Scholar]
- 13.Köttgen A, Albrecht E, Teumer A, et al. Genome-wide association analyses identify 18 new loci associated with serum urate concentrations. Nat Genet. 2013;45(2):145–54. doi: 10.1038/ng.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takada T, Ichida K, Matsuo H, et al. ABCG2 dysfunction increases serum uric acid by decreased intestinal urate excretion. Nucleosides, Nucleotides Nucleic Acids. 2014;33(4–6):275–81. doi: 10.1080/15257770.2013.854902. [DOI] [PubMed] [Google Scholar]
- 15.Stiburkova B, Pavelcova K, Zavada J, et al. Functional non-synonymous variants of ABCG2 and gout risk. Rheumatology (Oxford) 2017;56(11):1982–92. doi: 10.1093/rheumatology/kex295. [DOI] [PubMed] [Google Scholar]
- 16.Richette P, Bardin T. Gout. Lancet. 2010;375(9711):318–28. doi: 10.1016/S0140-6736(09)60883-7. [DOI] [PubMed] [Google Scholar]
- 17.Hung TL, Wang WM, Chiang CP. Miliarial gout: A rare presentation of extensive cutaneous tophi. QJM. 2016;109(12):811–12. doi: 10.1093/qjmed/hcw169. [DOI] [PubMed] [Google Scholar]
- 18.Aguayo RS, Baradad M, Soria X, et al. Unilateral milia-type intradermal tophi associated with underlying urate subcutaneous deposition: An uncommon cutaneous presentation of gout. Clin Exp Dermatol. 2013;38(6):622–25. doi: 10.1111/ced.12084. [DOI] [PubMed] [Google Scholar]
- 19.Olazagasti J, Wang AS, Isseroff R. Multiple firm nodules and tender, indurated plaques. JAMA Dermatol. 2014;150(5):569–70. doi: 10.1001/jamadermatol.2013.8734. [DOI] [PubMed] [Google Scholar]
- 20.Mireku KA, Burgy JR, Davis LS. Miliarial gout: A rare clinical presentation. J Am Acad Dermatol. 2014;71(1):e17–18. doi: 10.1016/j.jaad.2014.01.851. [DOI] [PubMed] [Google Scholar]
- 21.Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. 2017;76(1):29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 22.Shukla R, Vender RB, Alhabeeb A, et al. Miliarial gout (a new entity) J Cutan Med Surg. 2007;11(1):31–34. doi: 10.2310/7750.2007.00002. [DOI] [PubMed] [Google Scholar]


