Abstract
Patient: Male, 28-year-old
Final Diagnosis: SMART syndrome
Symptoms: Seizure
Medication: —
Clinical Procedure: —
Specialty: Neurosurgery
Objective:
Rare disease
Background:
SMART (Stroke-like Migraine Attacks after Radiation Therapy) syndrome is an uncommon delayed complication of cerebral radiotherapy. Less than 50 cases have been reported in the literature since it was first described in 1995. On average, presentation is about 20 years after radiotherapy, and patients commonly present with headaches, complex seizures, and stroke-like symptoms. The exact pathophysiology of the disease remains poorly understood, but one theory suggests radiation-induced vascular dysfunction.
Case Report:
We present one such case of a 28-year-old man who presented to our Emergency Department with a gradually progressive severe headache and right-sided weakness developing over a few hours. MRI played a central role in the diagnosis of SMART syndrome, with serial studies demonstrating and supporting the theory of vascular dysfunction. The condition is usually self-limiting, and most patients achieve complete recovery of symptoms, as did ours. Its optimal management remains unclear.
Conclusions:
Better understanding of the imaging findings in SMART syndrome may help differentiate it from tumor recurrence, cerebral infections, or vasculitis. Because the diagnosis of this condition portends a significantly better prognosis and substantially alters patient expectation and management, it is important that clinicians are aware of the usual delayed presentation, symptomology, and imaging findings.
MeSH Keywords: Cerebrovascular Disorders, Magnetic Resonance Angiography, Radiotherapy
Background
SMART (Stroke-like Migraine Attacks after Radiation Therapy) syndrome is an uncommon delayed complication of cerebral radiotherapy, occurring on average about 20 years after treatment. Less than 50 cases have been reported in the literature worldwide.
Its pathophysiology remains poorly understood, and patients commonly present with headaches, complex seizures, and stroke-like symptoms.
MRI is the imaging modality of choice for investigation. Our case study stands out as it includes serial MRI perfusion imaging from the time of current presentation through to resolution of symptoms, demonstrating the temporal evolution of the disease and its associated vascular dysfunction appreciated on imaging.
SMART syndrome is self-limiting, and better understanding of the condition by clinicians will help prevent performance of unnecessary interventions such as brain biopsies.
Case Report
A 28-year-old man presented to our Emergency Department with a gradually progressive severe headache and right-sided weakness developing over a few hours. Prior to his presentation, en route to the hospital, he had a single generalised tonic-clonic seizure which terminated following administration of midazolam in the ambulance.
On initial examination, the patient was slightly disoriented and lethargic. There was a right hemiparesis with an MRC power grading of 2/5 globally in his right lower limb and 1/5 in his right upper limb. Apart from a long-standing right homonymous hemianopia due to previous occipital surgery, the remainder of his cranial nerve examination was unremarkable.
All routine blood test results, including full blood count, electrolytes, and inflammatory markers, were within normal ranges. A CT brain demonstrated no acute cause for the presentation.
The initial provisional diagnosis on this presentation was of a migraine accompanied by Todd’s paresis following a seizure. The patient was started on regular analgesia and sodium valproate and was admitted under care of the neurologists. An MRI on the second day of admission demonstrated new left occipital and parietal cortical thickening, T2 hyperintensity, and gyral enhancement surrounding the previous tumor resection bed.
Of relevance to his current presentation, at the age of 16 he had been treated for a left occipital central primitive neuroectodermal tumor (PNET), WHO grade IV. His initial treatment, 12 years prior to the current presentation, included primary gross total surgical resection and adjuvant radiotherapy. He received a WBRT dose of 36 Gy in 20 fractions followed by bursts of 18 Gy in 10 fractions to the left occipital region. Following this initial treatment, he made a good recovery apart from a residual right homonymous hemianopia. Despite the histology of a WHO grade IV tumor, there were no signs of disease recurrence on immediate follow-up MRI over a 5-year period.
On further questioning, the patient reported having a similar presentation to the current one, experiencing a headache and right-sided weakness 5 years after his initial surgery. MRI imaging on this presentation demonstrated similar left occipital cortical thickening and gyral enhancement surrounding the re-section bed. These previous imaging findings had initially been misdiagnosed radiologically as tumor recurrence, but the patient’s symptoms and MRI changes had resolved over a period of 3–4 weeks with conservative treatment.
The patient also had a history of migraines, but it was unclear whether these headaches had preceded surgery and radiotherapy or developed following initial treatment.
A diagnosis of SMART syndrome was made based on the history of previous radiotherapy, typical clinical presentation, and imaging findings. This diagnosis was further supported by the previous identical presentation, which had resolved without treatment. A lumbar puncture and CSF studies ruled out other differentials, including an infective process or cerebral vasculitis, and demonstrated no tumor cells.
He was treated with methylprednisone for 4 days and continued on sodium valproate. His symptoms subsequently resolved completely during 3 weeks.
Serial MRI imaging with perfusion studies
Figures 1–5 presents a temporal series of MRI images including MRI perfusion studies. All MRI perfusion imaging was obtained using standardized contrast volumes, timing, and acquisition parameters on a Siemens 1.5T Aera. Figure 1 (1 year prior), Figure 2A–2C (on day 2), Figure 3A and 3B (9 days later), Figure 4 (4 weeks later) and Figure 5 (4 months later).
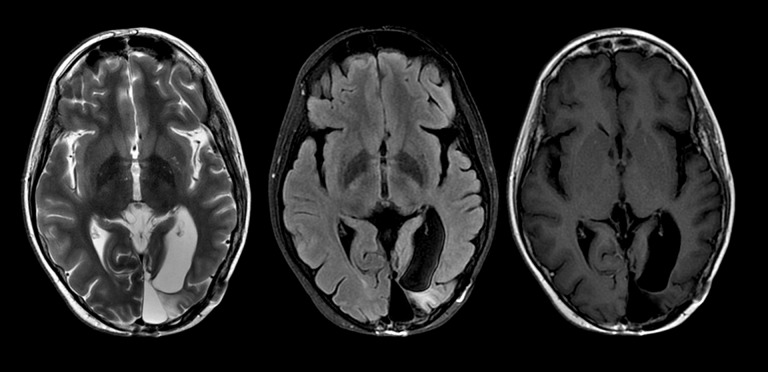
Figure 1.
Routine follow-up MRI 11 years after treatment with selected axial T2, FLAIR, and T1 post-contrast images at the level of the resection cavity demonstrating focal left mesial occipital encephalomalacia without residual mass, cortical thickening, or gyral enhancement.
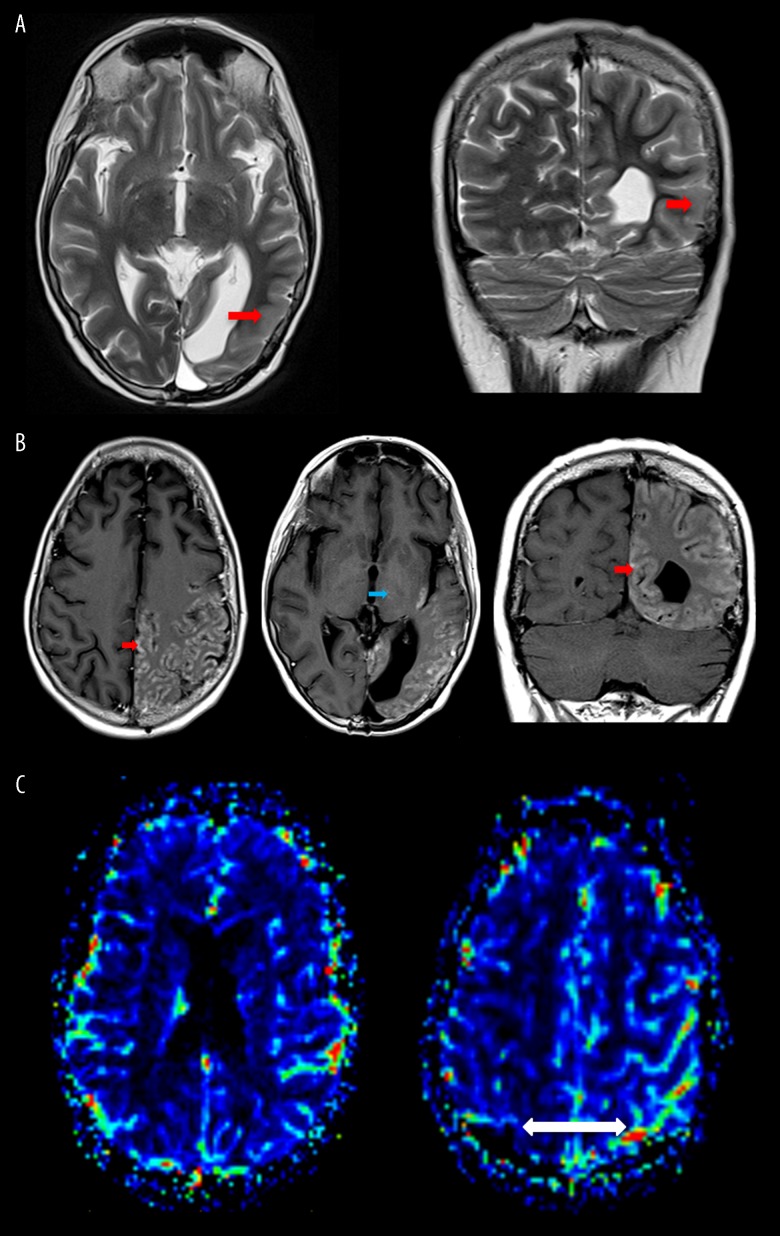
Figure 2.
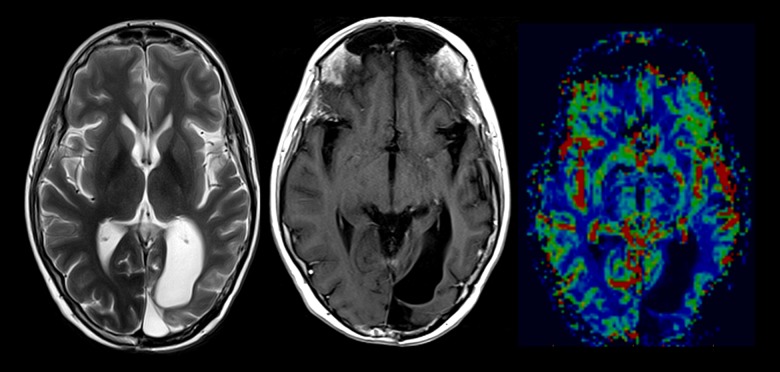
(A) Axial and coronal T2 images at the level of the previous left occipital resection cavity demonstrates marked posterior temporal, parietal and occipital cortical thickening, and increased T2 signal (red arrows). (B) Selected axial and coronal T1 post-contrast images demonstrating marked predominantly cortical gyriform enhancement in association with regions of cortical thickening and increased T2 signal (red arrows). A few further small foci of subcortical enhancement and minor enhancement are seen within the left thalamus (blue arrow). (C) Dynamic susceptibility contrast-enhanced MR perfusion cerebral blood volume maps after DCE gadolinium preloading. Cortical enhancement is not associated with significant relative change in cerebral volume as compared to the contralateral side with slight congestion of overlying pial vessels (white arrow).
Figure 3.
(A) Persisting relatively similar cortical high T2 signal and thickening, but a reduction in cortical enhancement is noted. (B) Although there is a reduction in enhancement at this stage, there is an approximate 4-fold increase in relative cerebral blood volume within the left parietal and occipital cortex compared to the contralateral side (white arrows).
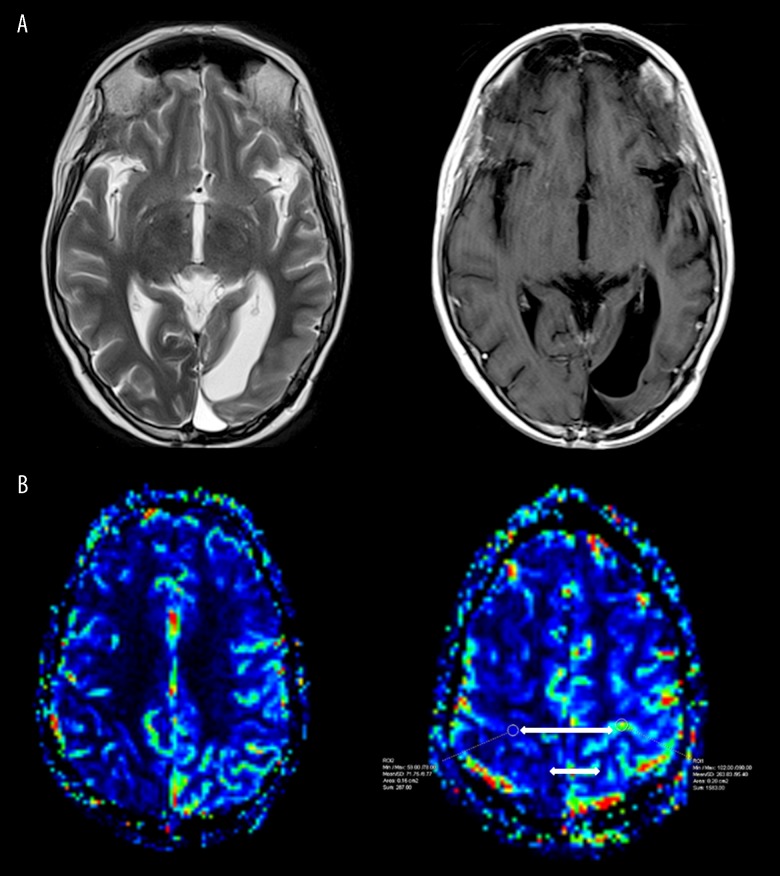
Figure 4.
Axial T2, T1 post-contrast. and T2 DSC perfusion CBV map, with very subtle persisting high T2 cortical signal involving the left posterior temporal, parietal, and occipital cortex, but with resolution of most of the cortical enhancement. A few subtle persisting foci of cortical enhancement with resolution of previous relatively increased cerebral blood volume are seen within the left cerebral hemisphere (white arrow).
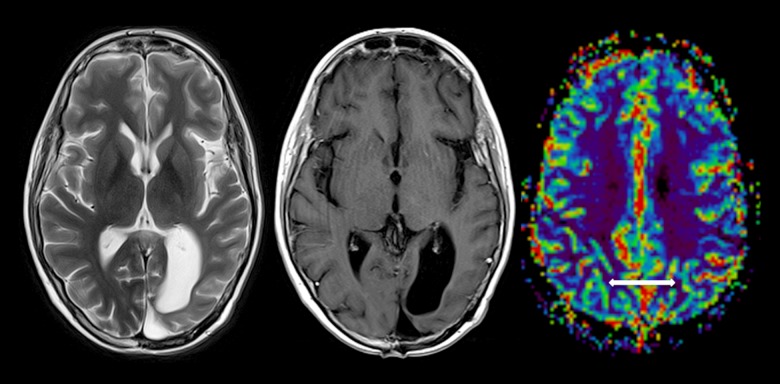
Figure 5.
Resolution of cortical T2 thickening and enhancement throughout the posterior left cerebral hemisphere surrounding the resection cavity. There are a few subtle left parieto-occipital, left thalamic, and cerebellar foci of enhancement, which have waxed and waned over a few studies, but are not progressive. There is relatively symmetrical T2 perfusion cerebral volume, allowing for reduction along the margins of the gliosis and the resection cavity.
Discussion
SMART syndrome is an uncommon delayed complication of cerebral radiotherapy, typically occurring many years after treatment [1–3]. Less than 50 cases have been reported in the literature since it was first described in 1995 [4]. A large range has been reported, but presentation on average is 20 years after radiotherapy. Patients commonly present with headaches, complex seizures, and stroke-like symptoms [5,6].
The exact pathophysiology of the disease remains poorly understood. Some of the proposed theories include radiation-induced vascular dysfunction leading to a faulty autoregulatory state and neuronal dysfunction with impairment or a lowered threshold for cortical spreading depression [7].
MRI is the imaging modality of choice for investigation. Our patient demonstrated the hallmarks of SMART syndrome: cortical thickening, high T2 signal with mild mass effect, and prominent unilateral gyral enhancement in the expected field of previous radiotherapy [1,8].
In 2006, Black et al. proposed the revised diagnostic criteria for SMART syndrome [8]. This included:
Remote history of cranial radiation;
Prolonged, reversible unilateral cortical signs and symptoms beginning years after radiation with manifestations including visuo-spatial deficit, confusion, hemiparesis, aphasia, seizures, headaches, and antecedent migraine with or without aura;
Transient, diffuse, unilateral cortical gray matter enhancement sparing white matter;
Not attributed to any other disorder.
SMART syndrome is usually self-limiting, and most patients achieve complete recovery of symptoms. This is, however, not always the case. One case series of 11 patients reported complete recovery in only about 55% at the 2-month mark [2]. Patients with incomplete recovery in this series were noted to have areas of cortical laminar necrosis and infarction/gliosis on follow-up MRI. Our patient had complete resolution of neurologic symptoms, but there were persisting waxing and waning subtle foci of enhancement up until the 4-month mark.
The diagnosis of SMART syndrome is in general a clinical one in tandem with typical imaging appearances. However, the presentation and particularly the imaging in SMART syndrome has considerable overlap with those of patients without a history of previous radiotherapy presenting with seizures or hemiplegic migraines. In addition, differentiation from recurrent tumor and associated new seizures is important to avoid unnecessary interventions, including biopsy. While there are other case reports of SMART syndrome which include both CT and MRI perfusion studies, to the best of our knowledge, this is one of the few to include serial MRI perfusion imaging from the time of current presentation through to resolution of symptoms.
In our case, the patient’s neurologic symptoms were maximal within the first 5 days, at which point gyral enhancement and cortical thickening were noted to be most marked on day 2. This agreed with other imaging case series, but, interestingly, marked gyral enhancement was not associated with a significant relative increase in cerebral blood volume. After 9 days, the patient’s symptoms had improved, at which time there was far less gyral enhancement and cortical swelling on the follow-up MRI. Despite this reduction in enhancement, there was a 4-fold increase in cerebral blood volume on the affected side, far more marked compared to imaging at initial presentation (day 2) when there was more prominent enhancement. On further follow-up at 4 weeks and 4 months, the patient’s neurologic symptoms had completely resolved, while imaging demonstrated small persisting foci of enhancement but symmetrical cerebral blood volume.
Seizures are well documented to cause alterations in cerebral perfusion, likely related to vasodilation, often with an increase in cerebral blood flow and cerebral blood volume in the acute phase [9]. In the post-ictal phase, there may be reduced cerebral blood volume with cerebral hypoperfusion [10]. While the initial imaging appearances in our case may be attributed to the single documented seizure prior to presentation, the later prolonged enhancement over weeks despite resolution of symptoms and increased cerebral blood volume at 9 days are atypical for seizure. There are also case reports of SMART syndrome in patients who have presented with headache, cerebral hyperperfusion, and gyral enhancement, but without prior seizures [11].
Hemiplegic migraine has also been reported to be associated with alterations in cerebral perfusion. Cerebral hypoperfusion is more often documented, thought to be due to spreading cortical depression with reduced cerebral blood flow and cerebral blood volume [12,13]. There are, however, also reports of hyperperfusion in this condition, likely related to vasodilation [14].
Absence of increased cerebral hyperperfusion in the presence of marked enhancement makes high-grade tumor recurrence less likely, unless imaging findings are attributed solely to coexisting seizures.
Conclusions
Our patient had previously been provisionally diagnosed with a terminal illness, only to make a complete recovery. Better understanding of the temporal evolution of imaging findings and cerebral perfusion in SMART syndrome may help differentiate the entity from tumor recurrence, averting unnecessary interventions such as brain biopsies, with their possible risks and complications. It may also better elucidate the underlying pathophysiology of the disease, which remains poorly understood. Because the diagnosis of this condition portends a significantly better prognosis and substantially alters patient expectation and management, it is important that clinicians are aware of the usual delayed presentation, symptomology, and imaging findings.
References:
- 1.Ramanathan SR, Sreedher G, Malhotra K, et al. Unusual case of recurrent SMART (stroke-like migraine attacks after radiation therapy) syndrome. Ann Indian Acad Neurol. 2016;19(3):399–401. doi: 10.4103/0972-2327.168634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black DF, Morris JM, Lindell EP, et al. Stroke-like migraine attacks after radiation therapy (SMART) syndrome is not always completely reversible: A case series. Am J Neuroradiol. 2013;34(12):2298–303. doi: 10.3174/ajnr.A3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pruitt A, Dalmau J, Detre J. Episodic neurologic dysfunction with migraine and reversible imaging findings after radiation. Neurology. 2006;67:676–78. doi: 10.1212/01.wnl.0000228862.76269.62. [DOI] [PubMed] [Google Scholar]
- 4.Khanipour S, Salmela MB, McKinney AM. Susceptibility-weighted imaging in stroke-like migraine attacks after radiation therapy syndrome. Neuroradiology. 2015;57(11):1103–9. doi: 10.1007/s00234-015-1567-8. [DOI] [PubMed] [Google Scholar]
- 5.Caplan LR. Caplan’s Stroke A clinical approach. Philadelphia (Penn): Saunders; 2009. [Google Scholar]
- 6.Bartleson JD, Krecke KN, O’neill BP. Reversible, stroke-like migraine attacks in patients with previous radiation therapy. Neurooncology. 2003;5(2):121–27. doi: 10.1215/S1522-8517-02-00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farid K, Meissner WG. Normal cerebrovascular reactivity in stroke like migraine attacks after radiation therapy syndrome. Clin Nucl Med. 2010;35:583–85. doi: 10.1097/RLU.0b013e3181e4db6f. [DOI] [PubMed] [Google Scholar]
- 8.Black DF, Bartleson JD, Bell ML, Lachance DH. SMART: Stroke-like migraine attacks after radiation therapy. Cephalalgia. 2006;26(9):1137–42. doi: 10.1111/j.1468-2982.2006.01184.x. [DOI] [PubMed] [Google Scholar]
- 9.Lie C-H, Seifert M, Poggenborg J, et al. Perfusion computer tomography helps to differentiate seizure and stroke in acute setting. Clin Neurol Neurosurg. 2011;113:925–27. doi: 10.1016/j.clineuro.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Gelfand JM, Wintermark M, Josephson SA. Cerebral perfusion-CT patterns following seizure. Eur J Neurol. 2010;17:594–601. doi: 10.1111/j.1468-1331.2009.02869.x. [DOI] [PubMed] [Google Scholar]
- 11.Olsen AL, Miller JJ, Bhattacharyya S, et al. Cerebral perfusion in stroke-like migraine attacks after radiation therapy syndrome. Neurology. 2016;86(8):787–89. doi: 10.1212/WNL.0000000000002400. [DOI] [PubMed] [Google Scholar]
- 12.Shah L, Rana S, Valeriano J, Scott TF. Reversible CT perfusion abnormalities in patient with migraine variant: A two-phase process. Clin Neurol Neurosurg. 2013;115:830–32. doi: 10.1016/j.clineuro.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Jiménez-Hoyuela JM, Amrani-Raissouni T, Gallardo-Tur A, et al. Impact of 99mTc-HMPAO brain perfusion scan in the diagnosis of hemiplegic migraine. Clin Nucl Med. 2013;38:103–5. doi: 10.1097/RLU.0b013e31826c0cf1. [DOI] [PubMed] [Google Scholar]
- 14.Mourand I, Menjot de Champfleur N, Carra-Dallière C, et al. Perfusion-weighted MR imaging in persistent hemiplegic migraine. Neuroradiology. 2012;54(3):255–60. doi: 10.1007/s00234-011-0946-z. [DOI] [PubMed] [Google Scholar]