Summary
Background
The major trigger of asthma exacerbations is infection with a respiratory virus, most commonly rhinovirus. Type 2 inflammation is known to be associated with an increased risk of exacerbations in general. Whether type 2 inflammation at baseline increases the risk of future virus‐induced exacerbations is unknown.
Objective
To assess whether type 2 inflammation is associated with an increased risk of virus‐induced exacerbations of asthma.
Methods
Stable asthmatics had spirometry, skin prick test, measurement of FeNO and sputum induced for differential cell counts. Patients were followed up for 18 months, during which they were assessed at the research unit when they had symptoms of an exacerbation. Nasal swabs collected at these assessments underwent viral detection by PCR.
Results
A total of 81 asthma patients were recruited, of which 22 (27%) experienced an exacerbation during the follow‐up period. Of these, 15 (68%) had a respiratory virus detected at exacerbation. Sputum eosinophils >1% at baseline increased the risk of having a subsequent virus‐induced exacerbation (HR 7.6 95% CI: 1.6‐35.2, P=.010) as did having FeNO >25 ppb (HR 3.4 95% CI: 1.1‐10.4, P=.033).
Conclusion and Clinical Relevance
Established type 2 inflammation during stable disease is a risk factor for virus‐induced exacerbations in a real‐life setting. Measures of type 2 inflammation, such as sputum eosinophils and FeNO, could be included in the risk assessment of patients with asthma in future studies.
Keywords: Asthma, eosinophils, exacerbation, FeNO
1. Introduction
Asthma is an inflammatory disease of the airways characterised by variable airflow obstruction and hyperresponsiveness and is one of the most frequent chronic diseases of young adults. Acute exacerbations are the main cause of morbidity and healthcare utilisation1 and thus preventing future exacerbations is a key goal in the clinical management of patients with asthma.
Infection with respiratory viruses, particularly rhinoviruses (RV), is a common trigger of asthma exacerbations, but the mechanisms are still unclear.2, 3, 4 Airway epithelial cells cultured ex vivo from non‐asthmatic subjects produce TSLP, IL‐25 and IL‐33 in response to viral infection, and more so in asthmatic individuals.5, 6, 7 These “alarmins” stimulate T helper cells and innate lymphoid cells to produce IL‐4, IL‐5 and IL‐13,8, 9 which causes recruitment of eosinophils to the airways, increased mucus secretion, bronchial hyperreactivity and class switching by B‐cells to produce IgE.
Whether IL‐5‐mediated eosinophilic inflammation is also a risk factor for virus‐induced exacerbations is unknown, but it has been demonstrated that viruses can activate eosinophils, both directly10 and by signalling through toll like receptors (such as TLR3 and TLR7) resulting in further propagation of inflammation.11 Anti‐Il‐5 reduces the rate of exacerbations, but whether this effect is predominantly related to virus induced, or non‐viral exacerbations, is unknown.12
Adjusting inhaled corticosteroid (ICS) treatment based on maintaining low airway eosinophils is more effective than symptom based management in reducing exacerbation frequency,13, 14 but induction and processing of sputum are not feasible in most clinical settings. Fractional exhaled nitric oxide (FeNO) is produced by airway epithelial cells on stimulation with IL‐4 and IL‐13 and has been associated with increased risk of exacerbation,15 but treatment strategies based on FeNO to reduce the rate of exacerbations have showed conflicting results.16, 17, 18, 19 Trials of anti‐IL‐13 antibodies have shown a reduction in exacerbation rates in patients with high periostin (protein induced by IL‐13), who were receiving treatment with ICS and had poor asthma control.20 To our knowledge, no study has evaluated the association between FeNO or sputum eosinophils and the risk of virus‐induced exacerbations specifically.
We hypothesised that high type 2 inflammatory biomarkers would be associated with a higher risk of virus‐induced asthma exacerbations. To test this, we conducted an observational, prospective cohort study of at heterogeneous population of asthmatics, examining patients at baseline and subsequently during exacerbation.
2. Methods
2.1. Study design
Stable asthma patients were recruited in the period of May 2013 to December 2014 from a respiratory outpatient clinic at Bispebjerg University Hospital in Copenhagen, Denmark. Patients had all been followed for at least 12 months in the specialist outpatient clinic, before enrolment into this study.
Patients were examined as described below and then followed for 18 months from the entry date, during which time patients were instructed to contact the study team, if they experienced any worsening of their asthma symptoms or an increase in beta2‐agonist use, surpassing their usual day‐to‐day variation. Patients were examined within 24 hours of contact with the study team. Exacerbations were defined as increased symptoms that required a change in medication as judged by the attending physician, in line with ATS/ERS recommendations.21 Exacerbations managed by adjusting inhaled therapies were recorded as mild/moderate, whereas exacerbations requiring a course of oral corticosteroids (OCS) or admission to hospital, were considered severe.21 In addition, patients were instructed to perform a nasal swab at home, when experiencing symptoms of a common cold—patients were not examined further on this occasion. The local scientific ethics committee approved the study (journal nr: H‐3‐2011‐121). All subjects were informed and gave written consent.
2.2. Assessments
Spirometry (EasyOne, NDD, Switzerland) was completed according to ERS recommendations,22 using NHANES III as the reference.23 A skin prick test (Soluprick, ALK, Denmark) was completed, and atopy was defined as a positive test (maximum wheal diameter ≥3 mm) to at least one of ten common aeroallergens (grasses, house dust mite, birch, mugwort, dog, cat, horse, cladosporum herbarum and alternaria tenuis). Fractional expiratory nitric oxide (FeNO) was analysed on a NioxMinor (Aerocrine, Solna, Sweden) following the recommendations of the ERS and ATS24 using the mean of two measurements. The ATS‐defined cut‐off of 25 ppb of FeNO was used to define patients with high and low FeNO. Sputum was induced according to the ERS guidelines and processed using a protocol described by Pavord et al.25 Differential cell counts from cytospins were obtained from 400 non‐squamous cells. A cut‐off of 1% for sputum eosinophils was applied to define those with high and low sputum eosinophils, as it has been reported to correspond to the 95th percentile of sputum eosinophils in healthy adults.26
Blood samples were assessed by the hospital clinical service and included a full white blood cell differential count and measurement of C‐reactive protein (CRP) as well as total IgE (Cobas 8000; Roche, Basel, Switzerland). Asthma control was assessed with the Asthma Control Questionnaire (ACQ).27
2.3. Detection of respiratory pathogens
Nasal swabs (Copan Italia) were used to sample nasal fluid and tested using a tandem multiplex real‐time PCR assay detecting a comprehensive range of respiratory viruses: Human adenovirus species B‐D; human bocavirus; coronaviruses OC43, 229E, HKU1 and NL63; influenza viruses A, B and C; parainfluenza viruses 1‐4; KI and WU polyomaviruses; respiratory syncytial virus types A and B and human metapneumovirus.28, 29 RV was detected by a PCR directed at sequences in the 5′UTR genome of RV, and the products were identified to species level as RV‐A, RV‐B or RV‐C by sequencing of this 260 bp product and alignment to known RV sequences using ClustalX software.30, 31 Bispebjerg hospital laboratory completed bacterial cultures on spontaneous expectorates gathered at exacerbation.
2.4. Statistical analyses
The data were analysed with SPSS version 22.0 (IBM SPSS, Armonk, NY, USA). Normally distributed data are reported as mean±standard deviation and analysed using student's t test for continuous variables, and chi‐squared test for categorical variables. Log‐transformed variables are reported as geometric mean (GM) with 95% CI. Non‐normally distributed data (eg, cell counts) are reported as median (range), and comparisons were made using Kruskal‐Wallis test.
To assess the ability of baseline characteristics to predict risk of exacerbation, a proportional hazards model (Cox regression) was used and adjusted for predefined potential confounders of exacerbation risk, including ICS dose, smoking status, ACQ score and % predicted FEV1 (forced expiratory volume in the first second of expiration) at baseline. As the inflammatory markers examined (FeNO, sputum eosinophils) are highly correlated (both in previous studies and in our data), each marker was tested separately in to avoid multicollinearity. For all analyses a P‐value of <.05 was considered significant.
3. Results
A total of 112 patients were approached, of which 81 patients agreed to participate (Figure 1). ICS were taken by 73% of patients, with the remaining 27% using short acting beta2‐agonists alone. In 18 of the 81 recruited patients (22%), FeNO was elevated at baseline (>25 ppb), and among the 56 patients able to produce a valid sputum sample, 16 patients (20%) had sputum eosinophils >1%. Eight patients had both high FeNO and high sputum eosinophils. The clinical characteristics of study subjects are described in Table 1.
Figure 1.
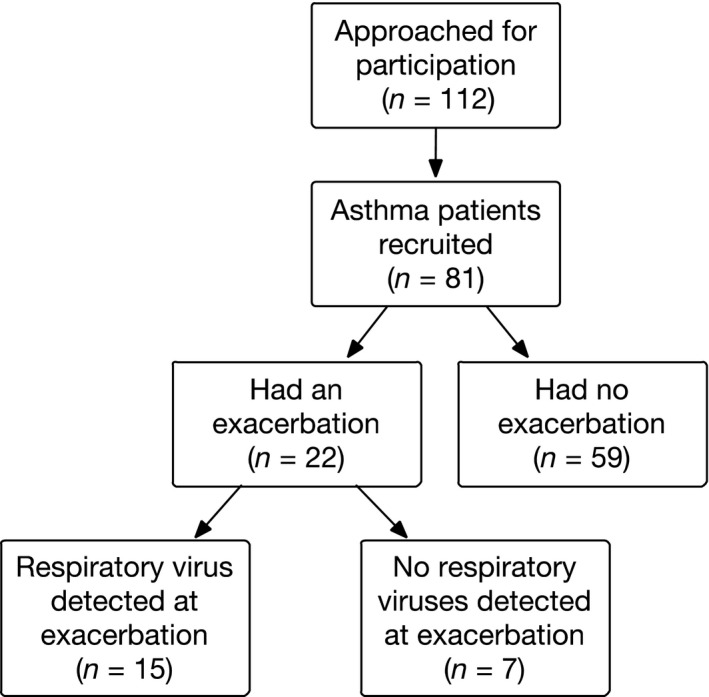
STROBE‐chart of patients in the study
Table 1.
Baseline characteristics of patients who developed an exacerbation during follow‐up (n=22) and those who did not experience any exacerbations (n=59)
| Had an exacerbation (n=22) | Had no exacerbation (n=59) | P‐value | |
|---|---|---|---|
| Age | 35 (16) | 34 (13) | .843 |
| Females, n (%) | 13 (59%) | 33 (56%) | .799 |
| Atopy, n (%) | 10 (46%) | 40 (68%) | .066 |
| Smoking, n (%) | |||
| Current smokers | 0 | 3 (5%) | .559 |
| Former smokers | 7 (32%) | 18 (31%) | |
| Never smokers | 15 (68%) | 38 (64%) | |
| % predicted FEV1 | 90.2 (14.4) | 88.9 (15.5) | .749 |
| % predicted FVC | 94.8 (12.9) | 95.1 (14.8) | .931 |
| ICS use in budesonide equivalent doseb | 800 (0‐1600) | 400 (0‐3200) | .240 |
| ACQ score | 1.4 (1.1) | 0.8 (0.9) | .011 |
| ACQ >1.5, n (%) | 10 (46%) | 11 (19%) | .014 |
| FeNO (ppb)a | 24 (17‐33) | 18 (16‐20) | .048 |
| FeNO >25 ppb, n (%) | 9 (41%) | 9 (15%) | .014 |
| Sputum eosinophils (%)b | 0.5 (0‐17.5) | 0.3 (0‐38.0) | .611 |
| Sputum eosinophils >1%, n (%) | 6 (38%)c | 10 (25%) | .350 |
| Sputum neutrophils (%)b | 40.5 (4.5‐81.3) | 38.6 (6.0‐94.0) | .928 |
| Blood eosinophils (×109 cells/L)b | 0.11 (0.03‐2.02) | 0.14 (0.03‐1.85) | .701 |
| Blood eosinophils >0.3 (×109 cells/L), n (%) | 3 (15%) | 7 (13%) | .803 |
| Blood neutrophils (×109 cells/L)b | 3.2 (1.6‐5.2) | 3.2 (1.3‐5.8) | .530 |
| Total IgE (×103 IU/L)a | 82 (29‐231) | 62 (38‐102) | .584 |
| Total IgE >150 (×103 IU/L), n (%) | 7 (37%) | 17 (32%) | .705 |
Data presented as mean (SD) unless otherwise stated.
Geometric mean (95% CI).
Median (range).
16 of the 22 patients with an exacerbation had a sputum differential count from baseline.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
During the follow‐up period, 22 participants (27%) developed an exacerbation (Figure 1). No patients had more than one exacerbation during the study period. In 15 of 22 patients with an exacerbation (68%), a respiratory virus was detected at exacerbation, predominantly RV and human coronavirus (HCoV) (Figure 2). A course of oral corticosteroid was prescribed for 36% of patients with an exacerbation, whereas the remaining 64% were managed with a temporary increase in ICS alone. No patients required admission to hospital. Two patients were treated with antibiotics for suspected bacterial pneumonia, although bacterial cultures were later found to be negative.
Figure 2.
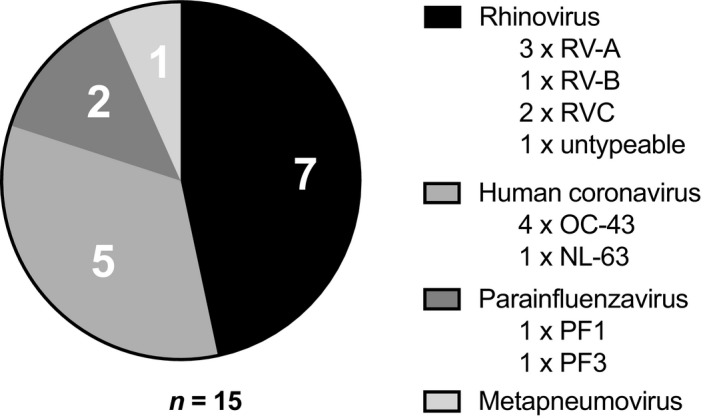
Respiratory viruses detected at exacerbation
Patient characteristics, including age, sex and smoking status, were not different between those that had an exacerbation and those who did not (Table 1). In the exacerbation group, mean FeNO at baseline was higher (24 ppb 95% CI: 17‐33, P=.048) compared to the non‐exacerbation group (18 ppb 95% CI: 16‐20). Exacerbation patients also had a higher ACQ score at baseline (1.4±1.1, P=.011) compared to the non‐exacerbation patients (0.8±0.9). There was a trend for lower prevalence of atopy in the exacerbation group (46% vs 68%, P=.066). Lung function, ICS dose and both sputum and blood differential cell counts were similar between the groups (Table 1).
In patients with high sputum eosinophils at baseline, five patients (29%) had a virus‐positive exacerbation, compared to the eosinophil low group that had six patients (12%) with a virus‐positive exacerbation (P=.10). Among patients with high FeNO at baseline, six patients (27%) experienced a virus‐positive exacerbation, compared to the FeNO low group that had nine patients (12%) with a virus‐positive exacerbation (P=.05).
Unadjusted log‐rank tests showed that patients with high FeNO had a shorter time to exacerbation than those with low FeNO (381 days [95% CI: 292‐470] vs 479 days [95% CI: 445‐513], P=.01), and specifically a shorter time to a virus‐induced exacerbation (410 days [95% CI: 319‐502] vs 500 days [95% CI: 471‐529], P=.04), respectively. A similar difference in time to any exacerbation was seen when comparing patients based on higher vs lower ACQ score. Patients with an ACQ score >1.5 had 392 days (95% CI: 318‐467, P=.01) to their exacerbation vs 480 days (95% CI: 443‐516) for those with an ACQ score ≤1.5. However, there was no difference in time to virus‐induced exacerbation for those with a high vs low ACQ score (447 days [95% CI: 379‐514] vs 490 days [95% CI: 455‐526], P=.12), respectively. In these unadjusted analyses, patients with or without sputum eosinophilia had similar time to exacerbation (P=.26; Figure 3).
Figure 3.
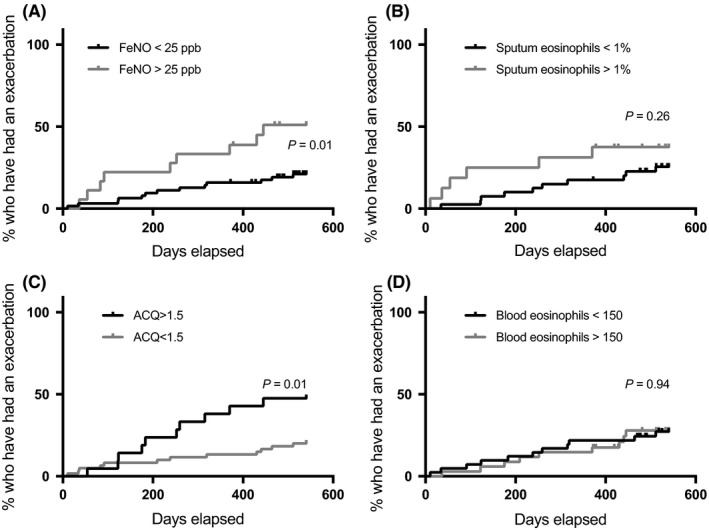
Log‐rank test of time to exacerbation in groups based on FeNO (A), sputum eosinophils (B), ACQ score (C) and blood eosinophils (D)
Proportional hazards models were constructed adjusting for known determinants of exacerbation risk (amount of ICS usage, current smoking, ACQ score and % predicted FEV1) at baseline. All demographic variables (sex, age, BMI) had a P‐value >.3 in univariate analyses and were not included in the models. Each inflammatory marker was assessed separately for predicting risk of exacerbation (summarised in Table 2). There were too few patients in the virus‐negative group for this analysis.
Table 2.
Proportional hazards models of inflammatory markers at baseline and risk (hazard ratio, HR) of exacerbation (all exacerbations, and virus‐induced exacerbations)
| Any exacerbation (n=22) | Virus pos. exacerbation (n=15) | |||
|---|---|---|---|---|
| HR (95% CI) | P‐value | HR (95% CI) | P‐value | |
| FeNO >25 ppb | 3.1 (1.2‐7.8) | .015 | 3.4 (1.1‐10.4) | .033 |
| Sputum eosinophils >1% | 4.1 (1.2‐13.8) | .024 | 7.6 (1.6‐35.2) | .010 |
| Blood eosinophils >150 (×109 cells/L) | 1.2 (0.5‐2.9) | .720 | 1.0 (0.3‐3.0) | .941 |
| Atopy (positive SPT) | 0.5 (0.2‐1.2) | .129 | 0.6 (0.2‐1.6) | .269 |
| IgE >150 (×103 IU/L) | 1.3 (0.5‐3.3) | .648 | 1.4 (0.4‐4.5) | .555 |
Each HR in the table represents a regression model adjusted for ICS dose, smoking status, ACQ score and % predicted FEV1 at baseline.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
High FeNO at baseline increased the risk of any subsequent exacerbation (HR 3.1 [95% CI: 1.2‐7.8], P=.015) and increased the risk of virus‐positive exacerbations (HR 3.4 [95% CI: 1.1‐10.4], P=.033) compared to those with low FeNO. High sputum eosinophils also increased the risk of any exacerbation (HR 4.1 [95% CI: 1.2‐13.8], P=.024), and particularly virus‐positive exacerbations (HR 7.6 [95% CI: 1.6‐35.2], P=.010) compared to those with low eosinophils (Table 2). In other words, patients with FeNO >25 ppb had a 3.4‐fold increased risk of virus‐induced exacerbation compared to patients with lower FeNO, and patients with sputum eosinophils >1% had a 7.6‐fold increased risk, compared to patients with lower eosinophil percentage when disease control, lung function, ICS use and tobacco consumption at baseline were kept the same.
Other potentially relevant risk factors relating to type 2 inflammation were also examined with adjustment for the same potential confounders as above: Atopic status, blood eosinophils >150×109 cells/L and total IgE >150×103 IU. None of these variables predicted the risk of exacerbation (Table 2).
A total of 21 patients sent in a swab during symptoms of a common cold, without any signs of an exacerbation. Of these 11 (52%) had a detectable respiratory virus (5 RV‐A, 1 RV‐B, 4 RV‐C and 1 enterovirus). Sputum eosinophils and mean FeNO at baseline were not different between those with no exacerbation or cold (n=38), those who experienced a virus‐positive cold (n=11) and those with a virus‐positive exacerbation (n=15; Table 3). However, the proportion of patients with FeNO >25 was significantly different, and lowest in those with a virus‐positive cold and highest in those with a virus‐positive exacerbation (Table 3). Patients with a virus‐positive exacerbation did not have a higher FeNO at exacerbation (GM 22 ppb 95% CI: 15‐32) than a follow‐up (GM 22 ppb 95% CI: 13‐35, .905).
Table 3.
Baseline FeNO and sputum eosinophils in patients with either: (i) no cold symptoms or exacerbation during the study, (ii) only a cold during the study or (iii) an exacerbation during the study
| No cold or exacerbation (n=38) | Virus‐positive cold (n=11) | Virus‐positive exacerbation (n=15) | P‐value | |
|---|---|---|---|---|
| FeNO (ppb), GM (95% CI) | 18 (16‐22) | 17 (14‐20) | 22 (13‐35) | .470 |
| FeNO >25 ppb, n (%) | 8 (21%) | 0 | 6 (40%) | .046 |
| Sputum eosinophils (%), median (range)a | 0.4 (0‐28.0) | 0.8 (0‐38.0) | 0.8 (0‐17.5) | .781 |
| Sputum eosinophils >1%, n (%)a | 7 (27%) | 3 (43%) | 5 (45%) | .480 |
Number of patients with a sputum sample in each group were 26, 11 and 7, respectively.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
4. Discussion
In this study, both FeNO >25 ppb and sputum eosinophils >1% were associated with an increased risk of developing a virus‐induced asthma exacerbation. Furthermore, patients who developed a virus‐induced exacerbation were more likely to have FeNO >25 ppb at baseline, than patients who only reported upper respiratory symptoms during respiratory viral infection. While both FeNO and sputum eosinophils have been associated with a higher risk of exacerbation in general,13, 14, 15 to our knowledge, this is the first study to assess type 2 airway inflammation as a risk factor specifically for virus‐induced asthma exacerbations.
The rate of exacerbation was lower than previous studies with a similar design, and because the majority of exacerbations were virus induced, there were too few patients with non‐viral exacerbations to rule out an association between type 2 inflammation at baseline and risk of exacerbation in this group. Indeed, given that there was a similar proportion of virus‐positive vs virus‐negative exacerbations in both the low and high group of each type 2 marker, it seems plausible that the effect is not limited to virus‐induced exacerbations. Nevertheless, we have identified an association between type 2 inflammation and virus‐induced exacerbations, suggesting this area warrants further investigation.
Other limitations include a lack of surveillance of patients (eg, peak flow monitoring), hence registration of exacerbations was depended on the patients contacting the research unit when they experienced symptoms surpassing their daily variation. However, patients were regularly contacted by phone to remind them of their participation in the study. Finally, it is possible the results might be different in a cohort of more severe asthmatics, with a higher exacerbation rate and potentially more severe exacerbations. The strength of this study is that it prospectively followed a heterogeneous population of asthmatics, representative of the everyday clinical scenario experienced by most respiratory clinicians.
A causal role of type 2 inflammation in asthma exacerbations has been supported by studies of antibodies inhibiting IL‐5 and IL‐13 signalling. Phase II and III studies of the monoclonal anti‐IL‐5 antibody mepolizumab have shown a reduction in exacerbation frequency by approximately half32, 33—an effect that was lost within 6 months of treatment cessation.34 Likewise, treatment with the anti‐IL‐13 antibody Lebrikizumab, reduced the rate of exacerbations by 60% in moderate to severe asthmatics with elevated periostin.20 Whether the exacerbations that can be prevented with these strategies are predominantly viral or non‐viral have yet to be reported, but our data suggests that an effect on virus‐induced exacerbations is likely.
None of the participants who only developed upper respiratory tract symptoms had FeNO above 25 ppb, while 40% of those who developed an exacerbation had elevated FeNO. This is in line with our finding that high FeNO increased the risk of virus‐induced exacerbation, and suggests that patients with well‐controlled type 2 inflammation are more resistant to lower airways involvement when infected with common respiratory viruses. However, for this exploratory outcome the numbers were low, and FeNO levels were not different when analysed as a continuous variable and therefore this needs further exploration in larger studies.
A synergistic effect of allergic sensitisation and viral infection on risk of hospital admission has been described in children.35 In our study, we did not observe an increased risk of exacerbation (viral or non‐viral) in patients sensitised to common aeroallergens, indicating that this association might be more pronounced in paediatric populations.
Studies have found that cross‐linking of the high affinity IgE‐receptor (FcεR1) results in blunted interferon responses to RV,36, 37 which in turn is associated with ineffective viral clearing38 and more pronounced asthma symptoms.39, 40 Furthermore, the anti‐IgE antibody omalizumab has been shown to eliminate the return‐to‐school peaks of exacerbations in a large cohort of asthmatic children, suggestive of a reduction of virus‐induced exacerbations.12 Taken together this indicates that high IgE might play a role in virus‐induced asthma exacerbations; however, in our study, high IgE was not associated with an increased risk of virus‐induced exacerbations.
With the advent of further antibody treatment options in the coming years, there is a widespread intent to enhance personalised asthma management, by tailoring medication choice and dosage according to the inflammatory phenotype. These treatments are likely to prevent some, but not all, exacerbations. Given that respiratory viruses are a major trigger of exacerbations, understanding how each pathway of airway inflammation influences the risk of virus‐induced exacerbation will be a critical step in understanding how to optimise the use of antibody therapies to achieve the best individual patient outcomes.
In conclusion, we have shown that high FeNO and sputum eosinophils at baseline are risk factors for virus‐induced exacerbations in a real‐life setting. If confirmed in larger studies, this could have implications on the risk assessment of asthma patients.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
The study was funded by Bispebjerg University Hospital, the University of Copenhagen and by an unrestricted grant from the Lundbeck Foundation.
Bjerregaard A, Laing IA, Backer V, et al. High fractional exhaled nitric oxide and sputum eosinophils are associated with an increased risk of future virus‐induced exacerbations: A prospective cohort study. Clin Exp Allergy. 2017;47:1007–1013. 10.1111/cea.12935
References
- 1. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2015. Available from: http://www.ginasthma.org.
- 2. Corne JM, Marshall C, Smith S, et al. Frequency, severity, and duration of rhinovirus infections in asthmatic and non‐asthmatic individuals: a longitudinal cohort study. Lancet. 2002;359:831‐834. [DOI] [PubMed] [Google Scholar]
- 3. Nicholson KG, Kent J, Ireland DC. Respiratory viruses and exacerbations of asthma in adults. BMJ. 1993;307:982‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Message SD, Laza‐Stanca V, Mallia P, et al. Rhinovirus‐induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL‐10 production. Proc Natl Acad Sci USA. 2008;105:13562‐13567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee H‐C, Headley MB, Loo Y‐M, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus‐infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol. 2012;130:1187‐1196.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaiko GE, Phipps S, Angkasekwinai P, Dong C, Foster PS. NK cell deficiency predisposes to viral‐induced Th2‐type allergic inflammation via epithelial‐derived IL‐25. J Immunol. 2010;185:4681‐4690. [DOI] [PubMed] [Google Scholar]
- 7. Jackson DJ, Makrinioti H, Rana BMJ, et al. IL‐33‐dependent type 2 inflammation during rhinovirus‐induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190:1373‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zoltowska AM, Lei Y, Fuchs B, Rask C, Adner M, Nilsson GP. The interleukin‐33 receptor ST2 is important for the development of peripheral airway hyperresponsiveness and inflammation in a house dust mite mouse model of asthma. Clin Exp Allergy. 2016;46:479‐490. [DOI] [PubMed] [Google Scholar]
- 9. Iijima K, Kobayashi T, Hara K, et al. IL‐33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J Immunol. 2014;193:1549‐1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davoine F, Cao M, Wu Y, et al. Virus‐induced eosinophil mediator release requires antigen‐presenting and CD4+ T cells. J Allergy Clin Immunol. 2008;122:69‐77. e1–2. [DOI] [PubMed] [Google Scholar]
- 11. Månsson A, Cardell L‐O. Role of atopic status in Toll‐like receptor (TLR)7‐ and TLR9‐mediated activation of human eosinophils. J Leukocyte Biol. 2009;85:719‐727. [DOI] [PubMed] [Google Scholar]
- 12. Busse WW, Morgan WJ, Gergen PJ, et al. Randomized Trial of Omalizumab (Anti‐IgE) for Asthma in Inner‐City Children. N Engl J Med. 2011;364:1005‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Green RH, Brightling CE, McKenna S, et al. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. The Lancet. 2002;360:1715‐1721. [DOI] [PubMed] [Google Scholar]
- 14. Jayaram L, Pizzichini MM, Cook RJ, et al. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J. 2006;27:483‐494. [DOI] [PubMed] [Google Scholar]
- 15. Zeiger RS, Schatz M, Zhang F, et al. Elevated exhaled nitric oxide is a clinical indicator of future uncontrolled asthma in asthmatic patients on inhaled corticosteroids. J Allergy Clin Immunol. 2011;128:412‐414. [DOI] [PubMed] [Google Scholar]
- 16. Honkoop PJ, Loijmans RJB, Termeer EH, et al. Symptom‐ and fraction of exhaled nitric oxide‐driven strategies for asthma control: a cluster‐randomized trial in primary care. J Allergy Clin Immunol. 2015;135:682‐688. e11. [DOI] [PubMed] [Google Scholar]
- 17. Kupczyk M, ten Brinke A, Sterk PJ, et al. Frequent exacerbators–a distinct phenotype of severe asthma. Clin Exp Allergy. 2014;44:212‐221. [DOI] [PubMed] [Google Scholar]
- 18. Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician‐, biomarker‐, and symptom‐based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308:987‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Syk J, Malinovschi A, Johansson G, et al. Anti‐inflammatory treatment of atopic asthma guided by exhaled nitric oxide: a randomized, controlled trial. J Allergy Clin Immunol Pract. 2013;1:639‐648. e1–8. [DOI] [PubMed] [Google Scholar]
- 20. Hanania NA, Noonan M, Corren J, et al. Lebrikizumab in moderate‐to‐severe asthma: pooled data from two randomised placebo‐controlled studies. Thorax. 2015;70:1‐9: thoraxjnl–2014–206719–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59‐99. [DOI] [PubMed] [Google Scholar]
- 22. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319‐338. [DOI] [PubMed] [Google Scholar]
- 23. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179‐187. [DOI] [PubMed] [Google Scholar]
- 24. American Thoracic Society, European Respiratory Society . ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912‐930. [DOI] [PubMed] [Google Scholar]
- 25. Pavord ID, Pizzichini MM, Pizzichini E, Hargreave FE. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52:498‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006;11:54‐61. [DOI] [PubMed] [Google Scholar]
- 27. Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902‐907. [DOI] [PubMed] [Google Scholar]
- 28. Chidlow GR, Harnett GB, Shellam GR, Smith DW. An economical tandem multiplex real‐time PCR technique for the detection of a comprehensive range of respiratory pathogens. Viruses. 2009;1:42‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chidlow GR, Laing IA, Harnett GB, et al. Respiratory viral pathogens associated with lower respiratory tract disease among young children in the highlands of Papua New Guinea. J Clin Virol. 2012;54:235‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee W‐M, Kiesner C, Pappas T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One. 2007;2:e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bochkov YA, Grindle K, Vang F, Evans MD, Gern JE. Improved molecular typing assay for rhinovirus species A, B, and C. Journal of Clinical Microbiology. American Society for. Microbiology. 2014;52:2461‐2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198‐1207. [DOI] [PubMed] [Google Scholar]
- 33. Pavord ID, Korn S, Howarth P, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double‐blind, placebo‐controlled trial. Lancet. 2012;380:651‐659. [DOI] [PubMed] [Google Scholar]
- 34. Haldar P, Brightling CE, Singapuri A, et al. Outcomes after cessation of mepolizumab therapy in severe eosinophilic asthma: a 12‐month follow‐up analysis. J Allergy Clin Immunol. 2014;133:921‐923. [DOI] [PubMed] [Google Scholar]
- 35. Murray CS. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax. 2006;61:376‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Durrani SR, Montville DJ, Pratt AS, et al. Innate immune responses to rhinovirus are reduced by the high‐affinity IgE receptor in allergic asthmatic children. J Allergy Clin Immunol. 2012;130:489‐495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sykes A, Edwards MR, Macintyre J, et al. Rhinovirus 16‐induced IFN‐α and IFN‐β are deficient in bronchoalveolar lavage cells in asthmatic patients. J Allergy Clin Immunol. 2012;129:1506‐1514. [DOI] [PubMed] [Google Scholar]
- 38. Parry DE, Busse WW, Sukow KA, Dick CR, Swenson C, Gern JE. Rhinovirus‐induced PBMC responses and outcome of experimental infection in allergic subjects. J Allergy Clin Immunol. 2000;105:692‐698. [DOI] [PubMed] [Google Scholar]
- 39. Contoli M, Message SD, Laza‐Stanca V, et al. Role of deficient type III interferon‐λ production in asthma exacerbations. Nat Med. 2006;12:1023‐1026. [DOI] [PubMed] [Google Scholar]
- 40. Gern JE, Vrtis R, Grindle KA, Swenson C, Busse WW. Relationship of upper and lower airway cytokines to outcome of experimental rhinovirus infection. Am J Respir Crit Care Med. 2000;162:2226‐2231. [DOI] [PubMed] [Google Scholar]


