Summary
Background
Exposures to indoor biological contaminants have been implicated in asthma's aetiology but their effect on lung function is not well quantified.
Objective
The aim of this cross‐sectional study of non‐smoking, asthmatic adults in Scotland was to determine the correlation between the results from a standard spirometry test, forced expiratory volume in one‐second percent (FEV 1%), and quantitative estimates of some biological exposures.
Methods
A population (n = 55) of non‐smoking, adult asthmatics in Scotland was included in this study and each completed a questionnaire that allowed the determination of the Asthma Control Questionnaire scores (ACQ) and St. George's Respiratory Questionnaire scores (SGRQ), as well as corticosteroid use. Spirometry testing was completed and the pre‐bronchodilator FEV 1% value calculated. At about the same time, floor dust samples were collected in the living room and in the bedroom. These dust samples were analysed for mould contamination, as described by the Environmental Relative Moldiness Index (ERMI) values and by (1, 3)‐β‐D‐glucan concentrations, for endotoxin, and for dust mite, cat, and dog allergen concentrations. The asthmatics' FEV 1% values were tested for correlation (Pearson) to questionnaire‐based estimates of health. Also, each biological exposure was tested for correlation (Pearson) to the FEV 1% values.
Results
FEV 1% results were correlated with ACQ scores (ρ −0.586, P < 0.001), SGRQ scores (ρ −0.313, P = 0.020), and weakly with corticosteroid use (ρ −0.221, P = 0.105). The ERMI values in the homes (average 5.3) were significantly correlated with FEV 1% values (ρ −0.378, P = 0.004). There was no correlation between FEV 1% and concentrations of endotoxin, (1, 3)‐β‐D‐glucan, or any of the allergens.
Conclusion and clinical relevance
Although these results do not prove that mould exposures caused the deficit in lung function observed in this study, it might be advisable for asthmatics to avoid high ERMI environments.
Keywords: adults, asthma, ERMI, FEV1%, lung function
Introduction
World‐wide, 300 million people have asthma 1, including approximately 1.1 million children and 4.3 million adults in the UK 2. In Scotland, 1 in 14 people are currently receiving treatment for asthma. Surveys indicate that many patients with severe asthma have poor symptom control and reduced lung function 3, 4, 5. Asthma has been associate with various biological exposures, including mould, endotoxin, and allergens (dust mite, insect, and animal). Depending on the study, each of the agents has been reported to cause, have no effect on, or be protective of the asthma. It is beyond the scope of this paper to review all of this literature. However, only a few studies have examined these biological exposures and their association with lung function as quantified by spirometry testing and the resulting FEV1% values.
Mould exposures have often been estimated using the cell product (1, 3)‐β‐D‐glucan. The studies of (1, 3)‐β‐D‐glucan's link to lung function generally report a lack of a relationship to FEV1% measurements. For example, Thorn and Rylander 6 found no effect of different exposures of 0–19 ng (1, 3)‐β‐D‐glucan per m3 of air on FEV1%. Similarly, Blanc et al. 7 found no correlation between (1, 3)‐β‐D‐glucan concentration in the dust and FEV1% values. However, the high ERMI values in homes of asthmatic children in New Orleans were linked to reduced FEV1% values 8.
Gram‐negative bacterial exposures, estimated by measurement of endotoxin concentrations, were inversely associated with FEV1% values 7. But in other studies, endotoxin exposures were found to be protective in a manner consistent with the hygiene hypothesis 9. But high occupational levels of endotoxin caused FEV1% values to be reduced 10. However, inhalation of 2 μg of endotoxin did not induce any changes in FEV1% values 11.
Dust mite allergen concentrations were not correlated with FEV1% values in a study of adult asthmatics in the U.S. 7. Chiang et al. 12 also found no correlation between the concentration of dust mite allergen antibodies and lung function. However, Omenaas et al. 13 found that exposure of dust mite allergen correlated with reduced FEV1% values in Norwegian adults. These diverse findings might be explained by host genetics. Abdelmotelb et al. 14 showed that the number of copies of the α‐tryptase gene might be critical to the effect of dust mite exposures on lung function.
The effect of exposures to insect and animal allergens on FEV1% values has been examined in some studies. Weiss et al. 15 found that exposures to cockroach, but not dust mite or cat allergens, were correlated with a decline in FEV1% test results. Jaén et al. 16 showed that exposure to cat allergen was associated with lower FEV1 values, but only for women.
The aims of this cross‐sectional study of non‐smoking, adult asthmatics in Scotland were to determine the correlation between FEV1% values and questionnaire‐based measures of respiratory health and to determine the correlation between FEV1% values and some biological exposures in the home. The dust samples and environmental and anonymous clinical data that were obtained in an earlier study 17 were utilized in this analysis.
Methods
Study population
The original study was approved by the Lanarkshire Research Ethics Committee and all participants gave written informed consent. This study was conducted from 2006 to 2008 and the details have been published 17. The original study design was to test whether an added home ventilation system might improve the respiratory health of asthmatic adults who were all allergic to house dust mites. However, because dust samples were collected from each home and because spirometry testing was performed on each adult at baseline (before the intervention), there was an opportunity to use the baseline data and samples in a cross‐sectional study of the effect of some biological exposures on the respiratory health of the asthmatic adults.
A volunteered smoking history was taken and serum samples obtained to determine the cotinine concentration (Cozart Bioscience Ltd, Abingdon, UK) in order to confirm smoking status 17. As smoking is the major exposure affecting pulmonary function 18, smokers were excluded from this evaluation of the relationship between biological exposures and respiratory health.
Persons 16–60 years of age with asthma were recruited for the original study 17. At the time of the initial baseline clinical visit, a health questionnaire was completed and spirometry testing performed (before the use of a bronchodilator) by each person using a Vitalograph Spirometer (Buckingham, UK). The FEV1% of predicted normal was calculated using the European Community for Coal and Steel, 1993 updated reference formula 19 which also adjusts for age, gender, and height and is incorporated into the output from the spirometer.
Sampling
At about the time of the clinical visit, dust samples were collected from the living room floor and separately from the bedroom floor of each home. The open areas (not covered by lamps, furniture etc.) in each room were vacuumed at a rate of 1 m2 per min using a Dyson, 1500 watt, model–DC14 (Dyson, London, UK) vacuum cleaner 17. For example, if there was 2 m2 of open floor space in a room, it was vacuumed for 2 min to collect that sample.
The Dyson DC14 is a bagless vacuum cleaner that uses an extremely effective HEPA filter and captures all particles above 0.3 microns in size, as measured by a laser particle scanner. The dust was collected in the vacuum's reservoir which was meticulously cleaned between each sampling event. The dust in the reservoir was emptied into a sealable bag, placed in a refrigerated cooler, and returned to the laboratory. At the laboratory, the dust samples were screened through a 2‐mm‐pore sieve to remove large particles and then stored at −20°C before further processing and analysis.
Dust analysis
The concentration of dust mite allergens, cat allergen, dog allergen, (1–3)‐β‐glucan, and endotoxin was quantified in each living room and bedroom sample, as previously described 17. For the ERMI analysis, 5.0 ± 0.1 mg of the total, combined living room and bedroom dust samples were extracted using a bead beater (after the internal reference organism was added), as previously described 20.
The 36 ERMI moulds were quantified in each dust extract by QPCR analysis 20. Briefly, the standard reaction assays contained 12.5 μL of Universal Master Mix (Applied Biosystems Inc., Foster City, CA, USA), 1 μL of a mixture of forward and reverse primers at 25 μm each, 2.5 μL of a 400 nm TaqMan probe (Applied Biosystems Inc.), 2.5 μL of 2 mg/mL fraction V bovine serum albumin (Sigma Chemical, St. Louis, MO, USA), and 2.5 μL of DNA‐free water (Cepheid, Sunnyvale, CA, USA). To this mix was added 5 μL of the DNA extract from the sample. All primer and probe sequences used in the assays have been published 21. Primers and probes were synthesized commercially (Applied Biosystems, Inc.).
The ERMI value for each home was calculated, as shown in Eqn. 1, by taking the ‘Sum of the Logs’ of the concentrations of the 26 Group 1 species (s1) and subtracting the ‘Sum of the Logs’ of the concentrations of 10 Group 2 species (s2) 22.
| (Equation 1) |
(The 26 ‘Group 1’ species are found in water‐damaged homes and the 10 ‘Group 2’ species are found in homes independent of water damage and that generally, but not exclusively, come from outdoors.)
Statistical analyses
Associations between FEV1% and other measures of respiratory health, that is ACQ, SGRQ scores, and corticosteroid use, were determined via their respective Pearson correlation coefficients. Likewise, relationship between FEV1% results and the biological exposures, that is ERMI values, allergens from dust mite, cat, and dog, (1, 3)‐β‐D‐glucan, and endotoxin, were determined via their respective Pearson correlation coefficients. In addition, multiple linear‐regression analysis was used to further investigate the relationship between FEV1% and the combination ERMI score and living room endotoxin levels, given the latter's marginally significant relationship with FEV1%. Regression analysis of FEV1% on ERMI was performed and graphed along with the corresponding 95% confidence interval. Analyses were performed in sas version 9.3 (SAS Institute, Cary, NC, USA) and r version 2.14 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The demographic, clinical or home characteristics of the non‐smoking, adult asthmatics and their homes are shown in Table 1. The FEV1% test results were correlated with ACQ scores (ρ −0.586, P < 0.001), SGRQ scores (ρ −0.313, P = 0.020), and weakly with corticosteroid use (ρ −0.221, P = 0.105) (Table 2). The ERMI values in the homes were significantly correlated with FEV1% test results (ρ −0.378, P = 0.004) (Table 3). There was no correlation between FEV1% and the concentrations of endotoxin, (1, 3)‐β‐D‐glucan, or any of the allergens (Table 3).
Table 1.
Baseline demographic, clinical and home characteristics
| Demographic | Mean ± SD or % of total |
|---|---|
| Age (years) | 42 ± 23 |
| Gender (female) | 65 |
| Race (Caucasian) | 98 |
| Cotinine (ng/mL serum) | 3.1 ± 2.6 |
| Clinical | |
| Duration of asthma (years) | 20 ± 13 |
| Atopic dermatitis (positive) | 15 |
| Allergic hay fever (positive) | 78 |
| Allergic eczema (positive) | 29 |
| Dose of inhaled corticosteroid (μg)a | 715 ± 412 |
| Home | |
| Age of home (years) | 43 ± 9.5 |
| With carpets (positive) | 85 |
| With cat (positive) | 13 |
| With dog (positive) | 17 |
Beclomethasone equivalent.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 2.
Pearson correlations of the forced expiratory volume in one‐second percent predicted (FEV1%) and other with other measures of respiratory health. All spirometry tests were completed before use of a bronchodilator
| Median (IQR) | Pearson's ρ | P‐value | |
|---|---|---|---|
| FEV1% | 88 (74, 99) | 1.0 | Not applicable |
| ACQ score | 1.4 (1.1, 2.4) | −0.586 | <0.001 |
| SGRQ score | 27.3 (16.3, 39.6) | −0.313 | 0.020 |
| Dose of inhaled corticosteroid (μg)a | 800 (400, 1000) | −0.221 | 0.105 |
IQR, interquartile range; ACQ, Asthma Control Questionnaire score; SGRQ, St. George's Respiratory Questionnaire score.
Beclomethasone equivalent.
P‐values highlighted in bold indicate statistical significance.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Table 3.
Pearson correlation between forced expiratory volume in one‐second percent predicted (FEV1%) and exposure variables (median)
| Median (IQR) | Pearson's ρ | P‐value | |
|---|---|---|---|
| ERMI | 5.26 (2.6, 8.6) | −0.378 | 0.004 |
| Living Room Der p 1 (μg/gm) | 0.6 (0.2, 3.7) | 0.006 | 0.967 |
| Living Room Der p 2 (μg/gm) | 0.2 (0.1, 1.6) | 0.045 | 0.755 |
| Living Room Fel d 1 (μg/gm) | 0.4 (0.1, 1.6) | −0.026 | 0.856 |
| Living Room Can f 1 (μg/gm) | 4.6 (1.1, 27.5) | −0.093 | 0.521 |
| Bedroom Der p 1 (μg/gm) | 0.3 (1.0, 1.5) | 0.017 | 0.910 |
| Bedroom Der p 2 (μg/gm) | 0.2 (0.0, 1.9) | 0.074 | 0.618 |
| Bedroom Fel d 1 (μg/gm) | 0.4 (0.1, 5.8) | −0.068 | 0.646 |
| Bedroom Can f 1 (μg/gm) | 4.1 (0.5, 20.6) | 0.016 | 0.911 |
| Living room endotoxin (EU/g dust) | 10.4 (5.2, 16.7) | −0.271 | 0.063 |
| Bedroom endotoxin (EU/g dust) | 10.0 (5.2, 16.0) | −0.204 | 0.169 |
| Living room glucan (ng/g dust) | 435 (279, 631) | −0.173 | 0.240 |
| Bedroom glucan (ng/g dust) | 363 (277, 579) | −0.107 | 0.472 |
IQR, interquartile range; ERMI, Environmental Relative Moldiness Index; Der p 1 and Der p 2, Dermatophagoides pteronyssinus allergens 1 and 2; Fel d 1, cat allergen; Can f 1, dog allergen; EU, endotoxin units.
The p‐value highlighted in bold indicates statistical significance.
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The average ERMI value in these homes in Scotland was 5.3 (standard deviation 4.5), and the average FEV1% test result was 85.4% (standard deviation 18.6%). The regression analysis scatter plot of FEV1% test results on ERMI values showed their inverse relationship (Fig. 1). Living room endotoxin levels alone were marginally related to FEV1% values (P = 0.063) and their inclusion in the multiple linear‐regression analysis, along with ERMI values, only increased the R 2 of the regression from 14% to 17%.
Figure 1.
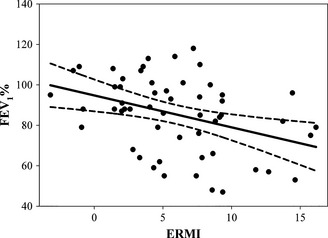
Scatter plot of Environmental Relative Moldiness Index (ERMI) values (n = 55) and the regression line (solid black) through the corresponding forced expiratory volume in one‐second percent predicted (FEV 1%) values and the 95% confidence interval (dashed lines).
Discussion
The demographic, clinical or home characteristics of the non‐smoker, adult asthmatics and their homes are shown in Table 1. Except for cotinine concentrations, these values were comparable to the entire cohort (smokers and non‐smokers) in the original study (data not given) 17.
We found that FEV1%, as an objective measure of respiratory health, was correlated with other measures of respiratory health which are based on recall in answering a questionnaire, that is corticosteroid use, ACQ and SGRQ scores. The ACQ score does include the FEV1% value, so it is not surprising that the FEV1% values are the most highly correlated with ACQ scores. The mould exposure, as described by the ERMI values in each home, was the only biological assessment that was correlated with FEV1% results.
The average ERMI value in these Scottish homes of asthmatic adults was 5.3. In a study of asthmatic adults in the U.S., specifically California, the average ERMI value was 6.0 23. No other study of adult asthmatics has utilized the ERMI metric. However, the homes of asthmatic children in three cities (Boston, Kansas City and San Diego) in the U.S., had an average ERMI value of 8.7 24. Also, infants exposed to homes with ERMI values above 5 were found to be twice as likely to develop asthma by age seven 25. So the average ERMI value measured in the homes of asthmatic adults in Scotland was consistent with results from the homes of asthmatic adults and children in the US.
We recognize that asthma has a complex aetiology and there are likely many causes. However, if mould is causing some cases of asthma, then elucidating the agent's causal mechanism would help clarify the relationship.
Recently, Millien et al. 26 demonstrated that some moulds can cause chronic airway surface mycotic infections (ASMI) in a mouse model of asthma. These ASMI result in lung damage, which includes enhanced airway epithelial and vascular endothelial cell permeability. ASMI set into motion a cascade of events that led to asthma‐like symptoms in these mice. If this model is confirmed in humans, then it would help to explain why high mould exposures are linked to some cases of asthma.
Many of the previous studies of the relationship between mould exposures and asthma have utilized methods that are not reliably quantified, for example visual inspection or mouldy odour. The ERMI is a standardized metric developed by the U.S. Environmental Protection Agency, in conjunction with the U.S. Department of Housing and Urban Development, to describe mould contamination in U.S. homes 22. Its wider geographic applicability remains to be seen.
Although mould exposures have been linked to asthma in many studies 27, 28, 29, the linkage to lung function, as quantified by spirometry testing, has not often been included in such studies. Norbäck et al. 30 did show that the presence of dampness in homes was a risk factor for lung function decline, especially in women. Although our results in Scotland suggest that there is a quantitative link between mould exposures and reduced lung function, this study cannot be considered as causal proof because of the study's many limitations.
The limitations of this study included the relatively small sample size, the fact that the sampled population was not randomly obtained (but part of earlier study), and many chemicals associated with decreased lung function 31 were not measured. Also, we did not quantify every possible biological exposure, including specific bacteria 32, viruses 33, or horse allergens 34 that might affect FEV1% values.
In addition, dust samples were used in this study, as opposed to air samples, which might have provided different results 35. Also, we recognize that the ERMI scale was created for US housing and improvements to the scale might be made for Europe by a random European sampling of homes, as was carried out in the US to create the ERMI 36.
In spite of these many limitations, this study in Scotland adds to the growing scientific literature linking mould exposure to poor respiratory health and asthma. Therefore, it might be prudent for asthmatics to avoid high ERMI environments.
Conflict of interest
The U.S. Environmental Protection Agency (EPA) through its Office of Research and Development collaborated in the research described here. Although this work was reviewed by EPA and approved for publication, it may not necessarily reflect official EPA policy. Mention of trade names or commercial products does not constitute endorsement or recommendation by the EPA for use. As mould‐specific quantitative PCR technology is patented by the US EPA, the Agency has a financial interest in its commercial use.
Acknowledgements
We thank Teresa Ruby for preparation of the figure, Miss Lisa Jolly and Mrs Iona Donnelly for laboratory biomarker measurements, the Lanarkshire GPs and practice staff for their help with patient recruitment, and the study participants and their families for their helpful participation. The original study 17 was funded by MRC, UK. The development of the Multiplex Array for Indoor Allergens (MARIA) has been supported in part by the National Institute of Environmental Health Sciences, Small Business Innovation Research contract ES55545.
McSharry C., Vesper S., Wymer L., Howieson S., Chaudhuri R., Wright G. R. and Thomson N. C., Clinical & Experimental Allergy, 2015. (45) 902–907.
References
- 1. Masoli M, Fabian D, Holt S et al Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004; 59:469–78. [DOI] [PubMed] [Google Scholar]
- 2. ASTHMA, UK . http://www.asthma.org.uk/asthma-facts-and-statistics. (last accessed 10 March 2014).
- 3. Illi S, von Mutius E, Lau S et al Perennial allergen sensitization early in life and chronic asthma in children: a birth cohort study. Lancet 2006; 368:763–70. [DOI] [PubMed] [Google Scholar]
- 4. Newby C, Agbetile J, Hargadon B et al Lung function decline and variable airway inflammatory pattern: longitudinal analysis of severe asthma. J Allergy Clin Immunol 2014; 134:287–94. [DOI] [PubMed] [Google Scholar]
- 5. Witt CA, Sheshadri A, Carlstrom L et al Severe Asthma Research Program (SARP). Longitudinal changes in airway remodeling and air trapping in severe asthma. Acad Radiol 2014; 21:986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thorn J, Rylander R. Airways inflammation and glucan in a row house area. Am J Respir Crit Care Med 1998; 157:1798–803. [DOI] [PubMed] [Google Scholar]
- 7. Blanc PD, Eisner MD, Katz PP et al Impact of the home indoor environment on adult asthma and rhinitis. J Occup Environ Med 2005; 47:362–72. [DOI] [PubMed] [Google Scholar]
- 8. Vesper SJ, Wymer L, Kennedy S et al Decreased pulmonary function measured in children exposed to high environmental relative moldiness index homes. Open Respir Med J 2014; 7:83–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Morcos MM, Morcos WM, Ibrahim MA et al Environmental exposure to endotoxin in rural and urban Egyptian school children and its relation to asthma and atopy. Minerva Pediatr 2011; 63:19–26. [PubMed] [Google Scholar]
- 10. Shi J, Mehta AJ, Hang JQ et al Chronic lung function decline in cotton textile workers: roles of historical and recent exposures to endotoxin. Environ Health Perspect 2010; 118:1620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sohy C, Pons F, Casset A et al Low‐dose endotoxin in allergic asthmatics: effect on bronchial and inflammatory response to cat allergen. Clin Exp Allergy 2006; 36:795–802. [DOI] [PubMed] [Google Scholar]
- 12. Chiang CH, Wu KM, Wu CP et al Evaluation of risk factors for asthma in Taipei City. J Chin Med Assoc 2005; 68:204–9. [DOI] [PubMed] [Google Scholar]
- 13. Omenaas E, Bakke P, Eide GE, Elsayed S, Gulsvik A. Serum house‐dust‐mite antibodies and reduced FEV1 in adults of a Norwegian community. Am J Respir Crit Care Med 1995; 152(4 Pt 1):1158–63. [DOI] [PubMed] [Google Scholar]
- 14. Abdelmotelb AM, Rose‐Zerilli MJ, Barton SJ et al Alpha‐tryptase gene variation is associated with levels of circulating IgE and lung function in asthma. Clin Exp Allergy 2014; 44:822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weiss ST, O'Connor GT, DeMolles D, Platts‐Mills T, Sparrow D. Indoor allergens and longitudinal FEV1 decline in older adults: the Normative Aging Study. J Allergy Clin Immunol 1998; 101(6 Pt 1):720–5. [DOI] [PubMed] [Google Scholar]
- 16. Jaén A, Sunyer J, Basagaña X et al Specific sensitization to common allergens and pulmonary function in the European Community Respiratory Health Survey. Clin Exp Allergy 2002; 32:1713–9. [DOI] [PubMed] [Google Scholar]
- 17. Wright GR, Howieson S, McSharry C et al Effect of improved home ventilation on asthma control and house dust mite allergen levels. Allergy 2009; 64:1671–80. [DOI] [PubMed] [Google Scholar]
- 18. Valsamis C, Krishnan S, Dozor AJ. The effects of low‐level environmental tobacco smoke exposure on pulmonary function tests in preschool children with asthma. J Asthma 2014; 51:685–90. [DOI] [PubMed] [Google Scholar]
- 19. Miller M, Hankinson J, Brusasco V et al ATS/ERS Task force: standardisation of lung function testing. 2. Standardisation of Spirometry. Eur Respir J 2005; 26:319–38. [DOI] [PubMed] [Google Scholar]
- 20. Haugland RA, Varma M, Wymer LJ et al Quantitative PCR of selected Aspergillus, Penicillium and Paecilomyces species. Syst Appl Microbiol 2004; 27:198–210. [DOI] [PubMed] [Google Scholar]
- 21. Haugland RA, Vesper SJ. Identification and quantification of specific fungi and bacteria.U. S. Patent and Trademark Office, Patent number 6,387,652. May 2002.
- 22. Vesper SJ, McKinstry C, Haugland RA et al Development of an environmental relative moldiness index for homes in the U.S. J Occup Environ Med 2007; 49:987–90. [DOI] [PubMed] [Google Scholar]
- 23. Blanc PB, Quinlan PJ, Katz PP et al Higher environmental relative moldiness index values measured in homes of adult asthmatics. Environ Res 2013; 122:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vesper S, Barnes C, Ciaccio CE et al Higher Environmental Relative Moldiness Index (ERMI) values measured homes of asthmatic children in Boston, Kansas City and San Diego. J Asthma 2013; 50:155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reponen T, Vesper S, Levin L et al High Environmental Relative Moldiness Index during infancy as a predictor of age seven asthma. Ann Allergy Asthma Immunol 2011; 107:120–6. [DOI] [PubMed] [Google Scholar]
- 26. Millien VO, Lu W, Shaw J et al Cleavage of fibrinogen by proteinases elicits allergic responses through Toll‐like receptor 4. Science 2013; 341:792–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. World Health Organization, Europe . WHO guidelines for indoor air quality: dampness and mould. Copenhagen, Denmark: WHO; 2009. [PubMed] [Google Scholar]
- 28. Norbäck D, Zock JP, Plana E et al Mould and dampness in dwelling places, and onset of asthma: the population‐based cohort ECRHS. Occup Environ Med 2013; 70:325–31. [DOI] [PubMed] [Google Scholar]
- 29. Denning DW, Pashley C, Hartl D et al Fungal allergy in asthma‐state of the art and research needs. Clin Transl Allergy 2014; 4:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Norbäck D, Zock JP, Plana E et al Lung function decline in relation to mould and dampness in the home: the longitudinal European Community Respiratory Health Survey ECRHS II. Thorax 2011; 66:396–401. [DOI] [PubMed] [Google Scholar]
- 31. Padula AM, Balmes JR, Eisen EA et al Ambient polycyclic aromatic hydrocarbons and pulmonary function in children. J Expo Sci Environ Epidemiol 2014. DOI: 10.1038/jes.2014.42. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vandenplas O, D'Alpaos V, Evrard G et al Asthma related to cleaning agents: a clinical insight. BMJ Open 2013; 3:e003568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Green BJ, Wiriyachaiporn S, Grainge C et al Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One 2014; 9:e100645. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zomer‐Kooijker K, van der Ent CK, Ermers MJ et al Increased risk of wheeze and decreased lung function after respiratory syncytial virus infection. PLoS One 2014; 9:e87162. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Konradsen JR, Nordlund B, Onell A et al Severe childhood asthma and allergy to furry animals: refined assessment using molecular‐based allergy diagnostics. Pediatr Allergy Immunol 2014; 25:187–92. [DOI] [PubMed] [Google Scholar]
- 36. Vesper S. Traditional mould analysis compared to a DNA‐based method of mould analysis. Crit Rev Microbiol 2011; 37:15–24. [DOI] [PubMed] [Google Scholar]


