The Bruton's tyrosine kinase (BTK) inhibitor ibrutinib has revolutionized the treatment of chronic lymphocytic leukemia (CLL) yielding unprecedented progression-free and overall survival rates in most CLL populations.1 Based on these data, ibrutinib has been widely approved for treatment of CLL and evaluated in various other B-cell malignancies leading to the approval in Waldenström's macroglobulinaemia (WM) and mantle cell lymphoma (MCL). Infections are a common complication in the natural course of CLL. However, they are mostly attributable to defects in T-cell function and humoral immunity. BTK mediates BCR signaling and thereby regulates B-cell activation, differentiation, cell-mediated interaction, and survival.2 It is crucial for development and function of the adaptive immunity: mutations in the encoding gene area result in an almost complete absence of mature B-cells and severe hypogammaglobulinemia as in patients with X-linked (Bruton's) agammaglobulinaemia. Additionally, BTK was identified as a direct regulator of key elements in innate immunity such as Toll-like receptor-mediated recognition of pathogens. It thereby regulates NLRP3 inflammasome activation and recruitment of innate immune cells including neutrophils, monocytes and macrophages.3 It is thus conceivable that therapeutic targeting of BTK in this patient population increases the risk of opportunistic infections. Specifically, invasive fungal infections (IFI) by Aspergillus spp. and other opportunistic infections have recently been observed in several investigations.4–8 Efforts have been made to decipher the exact molecular mechanisms behind this possibly selective defect in immunity. Only recently, in vitro experiments have demonstrated that ibrutinib-associated BTK depletion impairs NFAT and NF-κB responses in macrophages precluding effective eradication of A. fumigatus.9 Comprehensive analyses of ibrutinib-associated IFI and their optimal management are still rare. Hence, systematic and meticulous reporting as well as the identification of further risk factors for the development of IFI under ibrutinib treatment are warranted. We retrospectively reviewed all cases of aspergillosis under treatment with ibrutinib at our institution with the aim to contribute to a better understanding of IFI under BTK inhibition.
We conducted a retrospective database search to identify patients with invasive fungal infections (IFI) under treatment with ibrutinib at the University Hospital of Cologne. We reviewed electronic medical records of all patients receiving ibrutinib monotherapy or combination therapy at our institution from January 2014 to October 2018 and included all patients who had a subsequent diagnosis of IFI by Aspergillus species. All cases were documented by using an electronic case report form (eCRF) designed in EFS Leadership 7.0 Version 1.2 (Questback, Cologne, Germany) accessible through www.clinicalsurveys.net. Data items contained demographics of each patient, the underlying disease, treatment of the underlying disease and data on risk factors, outcome and management of IFI including ibrutinib treatment periods. Risk factors for development of IFI consisted of neutropenia, concomitant corticosteroid usage within the last 30 days, prior anti-CD20 antibody or chemotherapy, diabetes mellitus, liver cirrhosis, renal failure, HIV, and ICU treatment. Neutropenia was defined as an absolute neutrophil count (ANC) ≤500 cells/microliter. IFI were re-assessed by radiologists from our institution as per EORTC 2008 Guidelines.10 Sixteen patients were identified. Five patients with IFI that occurred before treatment with ibrutinib were excluded.
Eleven patients who developed IFI by Aspergillus spp. during ibrutinib treatment were identified at our institution. The median age of patients was 65.9 years (range 48–75) and 10 of 11 patients (91%) were male. Seven patients had CLL, 2 MCL, 1 had diffuse large B-cell lymphoma (DLBCL) and 1 patient had follicular lymphoma (FL). Among CLL patients, there were 3 with a Richter's transformation (2 DLBCL, 1 Hodgkin's lymphoma) (Table 1). Of note, 4 of 7 CLL patients had complex karyotype and 3 showed TP53 mutations. Most patients were intensively pre-treated, the number of previous therapies ranged from 0 to 3 (median 2). Previous treatments included various chemotherapy regimens as well as immunotherapeutic agents such as alemtuzumab, idelalisib, obinutuzumab, pembrolizumab, and rituximab. None of our patients had undergone allogeneic stem cell transplantation (SCT), 1 had undergone autologous SCT 1 year earlier. Prior to IFI diagnosis, 6 of 9 evaluable patients (67%) were neutropenic. Seven patients concomitantly received systemic corticosteroids shortly prior to or at the time point of IFI diagnosis (Table 2). Median time from start of ibrutinib treatment to IFI diagnosis was 1.64 months (range 0–4). 4 of 11 patients received ibrutinib as monotherapy, combination therapy included Alemtuzumab, Idelalisib, Obinutuzumab, Ofotumumab, Pembrolizumab, and Rituximab. A. fumigatus infection was proven in 5 cases, in 5 of 11 patients IFI diagnosis was probable based on radiological findings, host factors and mycological evidence in patient samples, and 1 case was classified as possible aspergillosis. Ten of 11 patients presented with pulmonary focus, and 1 patient with isolated cerebral aspergillosis, while 2 patients had disseminated disease (skin and lungs, CNS and lungs). Diagnosis of IFI prompted discontinuation of ibrutinib in 7 of 11 cases. In 5 of these, ibrutinib treatment was reinitiated at a later time point. First line antifungal treatment consisted of voriconazole in 6 of 11 cases, isavuconazole in 2 patients, amphotericin B in 2 patients and caspofungin in 1 patient. At last follow-up, 6 patients had died, death was attributable to IFI in 3 cases.
Table 1.
Patient Characteristics
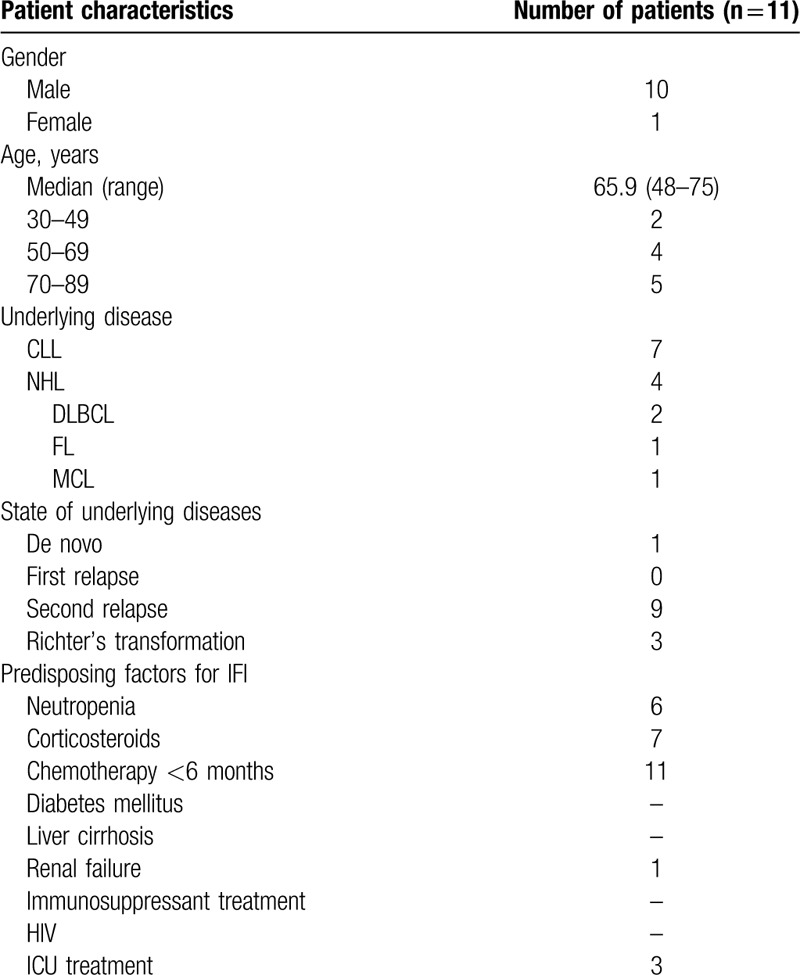
Table 2.
Characteristics and Treatment of IFI.
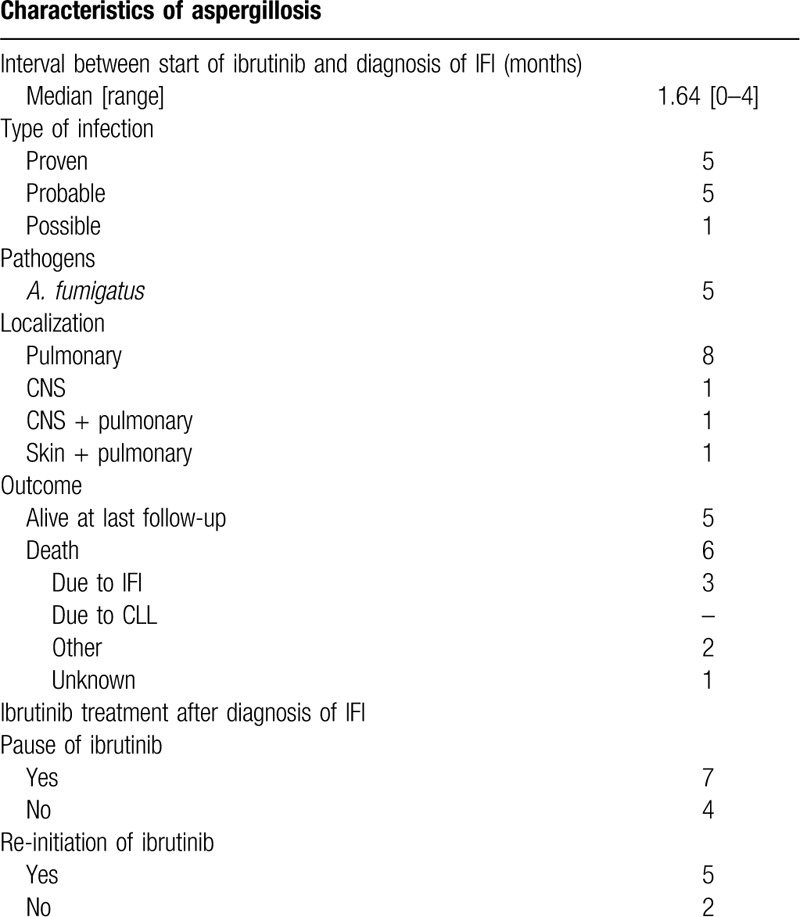
In this case series, we report 11 cases of invasive aspergillosis in patients treated with ibrutinib at the University Hospital of Cologne. Patient characteristics are in line with previous case series of invasive fungal infections5,7,8: A previously published retrospective analysis of 43 patients has established risk factors associated with an increased risk for IFI under ibrutinib treatment. Two statistically significant parameters in this patient population were ≥3 prior treatment regimens and corticosteroid use, which is confirmed by our analysis. Most of our CLL patients (4/7) had high-risk genetics and a substantial number of previous treatment regimens, which is in accordance with other published case series. In contrast to previous studies however, all of our patients had additional typical risk factors for the development of IFI. Apart from neutropenia and ICU stay, a high proportion of patients (7/11) had received high-dosed corticosteroids shortly prior to IFI diagnosis over several days or even weeks. The majority of our patients developed invasive fungal infections early (median time to IFI under ibrutinib: 1.64 months) in the course of ibrutinib treatment. This observation is in line with previously published data. Our data thus support the hypothesis that patients are less susceptible to IFI once the immune system has been partly reconstituted.5 Due to the presence of several different risk factors and previous therapies ibrutinib recipients, it is difficult to clearly associate ibrutinib and the development of IFI. Patients with X-linked agammaglobulinaemia due to mutations of BTK do not typically develop IFI.11 Hence, an off-target effect apart from BTK may be suspected. Besides, the effect of ibrutinib on macrophages appears essential in this context: The inhibition of the macrophage TLR9–BTK–calcineurin–NFAT signaling pathway causes an immune defect rendering the innate immune system susceptible to IFI.12 All patients had pulmonary infiltrates, two had additional cerebral aspergillosis (one shown in Fig. 1) and one had infiltration of the skin. The unusual localizations of IFI in patients treated with ibrutinib have been described earlier.4–7 Treating physicians should be aware of these new disease patterns, particularly in patients with neurological symptoms. To date, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) as well as the European Conference on Infections in Leukaemia (ECIL) have published position papers regarding infections under ibrutinib treatment. However, none gives clear recommendations regarding prophylaxis or treatment of IFI due to lacking evidence for any of both. Our patients received voriconazole in most cases for treatment of IFI and two received isavuconazole. In our patients, TDM was performed in 7 of 11 cases. Here, we saw adequate levels of voriconazole in 6 of 7 patients. Ibrutinib is cleared by cytochrome P450 (CYP) 3A4 and thus concomitant treatment with CYP3A inhibitors such as voriconazole has been shown to increase ibrutinib exposure. On this basis, the concomitant administration of voriconazole and ibrutinib is currently not recommended. However, sparse PK data from uncontrolled phase 2 studies with moderate CYP3A inhibitors showed a lower magnitude of drug-drug interactions (DDI) than observed in studies with healthy subjects or in vitro.13 Due to its lower potential for drug-drug-interactions with ibrutinib, isavuconazole may be a more suitable treatment or prophylaxis option for these patients in the future.14,15 To date, isavuconazole is not established as prophylactic treatment regimen, but investigated as such in ongoing trials. First results indicate a lack of efficacy in antifungal prophylaxis, however, further clinical trials are warranted to confirm this.16 Limitations of our analysis are its retrospective nature and the limited number of patients. Potential IFI diagnosed and treated outside of our institution could not be identified and hence not be included. In summary, this study supports the previously described risk factors for the development of IFI and particularly Aspergillus spp. in patients treated with ibrutinib. It is yet too early to recommend any specific prophylaxis in all patients, but treating physicians should be aware of this potential risk and screen their patients carefully. Further prospective clinical trials need to assess the actual risk of IFI in patients treated with ibrutinib or other BTK inhibitors. The need for antifungal prophylaxis in ibrutinib recipients needs to be re-evaluated, at least for patients with additional risk factors as concomitant corticosteroid medication or diabetes mellitus. Further evidence by prospective clinical trials is warranted not only in ibrutinib recipients, but recipients of all novel targeted antineoplastic agents.
Figure 1.
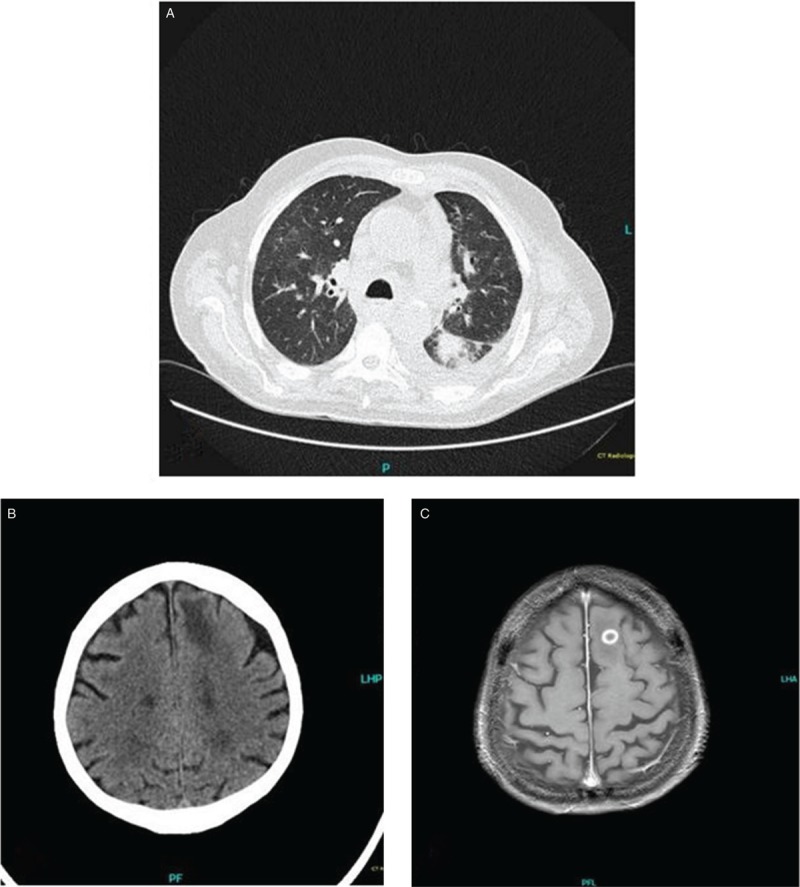
From pulmonary to cerebral aspergillosis. This Patient with a history of CLL was diagnosed with Richter Transformation. Treatment was initiated with Prednisolon 100 mg for 5 days and then switched to Ibrutinib 420 mg due to uneligibility for intensive therapy. The patient developed neutropenic fever after 12 days of ibrutinib-treatment. CT scan showed multiple pulmonary nodules consistent with pulmonary aspergillosis (A). Blood cultures and BAL were positive for A. fumigatus. Antifungal treatment was initiated. Fifteen days later, the patient showed a change in behaviour and confusion. A cCT was performed which showed a frontal lesion consistent with an abscess (B). Consecutive MRI showed a solitary abscess in the left frontal lobe consistent with aspergillosis in T1-weighted imaging with gadolinium injection (C).
Footnotes
Citation: Fürstenau M, Simon F, Cornely OA, Hicketier T, Eichhorst B, Hallek M, Mellinghoff SC. Invasive Aspergillosis in Patients Treated With Ibrutinib. HemaSphere, 2020;4:2(e309). http://dx.doi.org/10.1097/HS9.0000000000000309
OAC has received research grants from Actelion, Amplyx, Astellas, Basilea, Cidara, Da Volterra, F2G, Gilead, Janssen Pharmaceuticals, Medicines Company, MedPace, Melinta Therapeutics, Merck/MSD, Pfizer, Scynexis, is a consultant to Actelion, Allecra Therapeutics, Amplyx, Astellas, Basilea, Biosys UK Limited, Cidara, Da Volterra, Entasis, F2G, Gilead, Matinas, MedPace, Menarini Ricerche, Merck/MSD, Octapharma, Paratek Pharmaceuticals, Pfizer, PSI, Rempex, Scynexis, Seres Therapeutics, Tetraphase, Vical, and received lecture honoraria from Astellas, Basilea, Gilead, Grupo Biotoscana, Merck/MSD and Pfizer. BE received research grants from Abbvie, Gilead, Janssen, Roche, Beigene; honoraria and advisory boards from the same companies and Celgene and Novartis in addition. MH received research support from Roche, Gilead, Mundipharma, Janssen, Celgene, Pharmacyclics, Abbvie. Speakers bureau and Advisory Board: Roche Gilead, Mundipharma, Janssen, Celgene, Pharmacyclics, Boehringer. SCM has been a consultant to Octapharma.
MF, FS, and TH declare no conflicts of interest.
References
- 1.Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet (London, England). 2018;391:1524–1537. [DOI] [PubMed] [Google Scholar]
- 2.Satterthwaite AB, Witte ON. The role of Bruton's tyrosine kinase in B-cell development and function: a genetic perspective. Immunolog Rev. 2000;175:120–127. [PubMed] [Google Scholar]
- 3.Weber ANR, Bittner Z, Liu X, et al. Bruton's tyrosine kinase: an emerging key player in innate immunity. Front Immunol. 2017;8:1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grommes C, Younes A. Ibrutinib in PCNSL: the curious cases of clinical responses and aspergillosis. Cancer Cell. 2017;31:731–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghez D, Calleja A, Protin C, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood. 2018;131:1955–1959. [DOI] [PubMed] [Google Scholar]
- 6.Chamilos G, Lionakis MS, Kontoyiannis DP. Call for action: invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin Infect Dis. 2018;66:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varughese T, Taur Y, Cohen N, et al. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin Infect Dis. 2018;67:687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rogers KA, Mousa L, Zhao Q, et al. Incidence of opportunistic infections during ibrutinib treatment for B-cell malignancies. Leukemia. 2019;33:2527–2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bercusson A, Colley T, Shah A, et al. Ibrutinib blocks Btk-dependent NF-κB and NFAT responses in human macrophages during Aspergillus fumigatus phagocytosis. Blood. 2018;132:1985–1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46:1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochs HD, Smith CI. X-linked agammaglobulinemia. A clinical and molecular analysis. Medicine. 1996;75:287–299. [DOI] [PubMed] [Google Scholar]
- 12.Herbst S, Shah A, Mazon Moya M, et al. Phagocytosis-dependent activation of a TLR9–BTK–calcineurin–NFAT pathway co-ordinates innate immunity to Aspergillus fumigatus. EMBO Mol Med. 2015;7:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Jong J, Hellemans P, De Wilde S, et al. A drug-drug interaction study of Ibrutinib with moderate and strong CYP3A inhibitors in patients with B-Cell malignancy. Blood. 2016;128:3964. [Google Scholar]
- 14.Mellinghoff SC, Panse J, Alakel N, et al. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Ann Hematol. 2017;97:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummins KC, Cheng MP, Kubiak DW, et al. Isavuconazole for the treatment of invasive fungal disease in patients receiving ibrutinib. Leuk Lymphoma. 2019;60:527–530. [DOI] [PubMed] [Google Scholar]
- 16.Rausch CR, DiPippo AJ, Bose P, et al. Breakthrough fungal infections in patients with leukemia receiving isavuconazole. Clin Infect Dis. 2018;67:1610–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]


