Pediatric acute myeloid leukemia (pedAML) is a rare hematological disease accounting for 20% of all pediatric leukemias.1 Current chemotherapeutic regimens have reached a survival plateau around 70%.2,3 Still 30% to 40% of the good responders experience relapse, and especially patients with fms-like tyrosine kinase receptor-3 internal tandem duplications (FLT3-ITD) show a detrimental outcome.2 These observations have driven the development of alternate therapeutic strategies, including targeting antibodies and chimeric antigen receptor (CAR)- or T-cell receptor (TCR)-transgenic cytotoxic T-cells (CTLs). However, besides FLT3 inhibitor-based therapies4,5 and CD33-directed agents,6 targeted strategies have not yet found their way into treatment protocols.
We recently identified the TCR γ chain alternate reading frame protein (TARP) as an immunotherapeutic target in leukemic blasts (L-blast) and in leukemic stem cells (LSC) of adults and children with AML.7 TARP was previously only reported in androgen-sensitive prostate and breast adenocarcinoma.8
Although TARP was upon its discovery described as a truncated TCRγ transcript encoding the first TCR γ chain constant domain (TRGC1), we found that an AML-exclusive, TRGC2-encoding TARP transcript co-exists in AML.7 The high sequence homology between TRGC1 and TRGC2 hampers distinction through conventional techniques. Here, we used mRNA sequencing to demonstrate both TARP transcripts in four wild type (WT) AML cell lines with documented TARP expression.7 We confirmed that TRGC1 and TRGC2 transcript are highly expressed in MV4;11, next to a moderate expression in HL-60 and THP-1, while negative in OCI-AML3 (Fig. S1A). To gain insight into the biological relevance of both transcripts, transgenic TARP knockdown (TARP-KD) cell lines were generated for 2/4 AML cell lines, HL-60 and MV4;11, by retroviral transduction of TARP-targeting short-hairpin (shRNA) encoding viral particles, next to a mock construct.7 In MV4;11, both TRGC1 and TRGC2 transcripts were suppressed upon TARP knockdown. In HL-60, only the TRGC2 transcript was significantly downregulated compared to mock and WT, while TRGC1 showed a two-fold decrease (Fig. S1B, Table S1). Altogether, these data confirm that both TRGC1- and TRGC2-encoded TARP transcripts co-exist in AML cell lines, and are targetable.
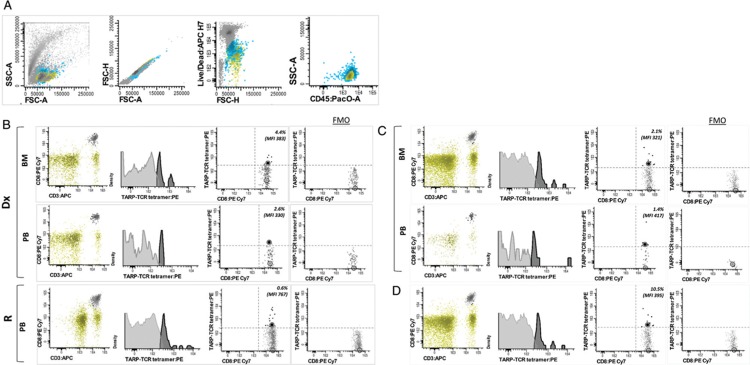
Our previous data suggested that TARP peptides are adequately MHC-presented, as leukemic cells could be targeted in vitro by cytotoxic T-cells (CTLs) retrovirally transduced with a TCR directed against the HLA-A2 enhanced affinity TARP(P5L)4-13 epitope.7 However, whether recognition of tumor TARP peptide-MHC complexes (p-MHCs) by TCR-bearing CTLs is able to trigger antigen-specific immune responses in vivo still needs to be elucidated. To this end, TARP-TCR expression on CTLs isolated from pedAML patients was measured using HLA-A∗0201-restricted, PE-conjugated tetramers directed against the TARP(P5L)4-13 epitope, kindly provided by the NIH Tetramer Core Facility. Lymphocytes from TARP-high (n = 5, 3/5 HLA-A∗0201 positive) and TARP-low (n = 6, 5/6 HLA-A∗0201 positive) pedAML patients were surface- and tetramer-stained and measured on a FACSCanto II flow cytometer (BD Biosciences).
All three TARP-high/HLA-A∗0201-positive pedAML patients showed a positive tetramer staining within the CD3+/CD8+ compartment (median 2.4%, median MFI 357) (Fig. 1). MFI values were comparable between BM and PB as evaluated for 2/3 patients. TARP-TCR expression was higher at relapse compared to diagnosis in both PB and BM, as measured in one patient. Higher intensity tetramer staining can be associated with a higher TCR and/or co-receptor expression, and/or to a higher TCR avidity. This observation may relate to an achieved immunity for the tumor epitope, that is, the presence of resting CD8+ memory T-cells that rapidly reactivate and upregulate TCR expression upon p-MHC re-stimulation,8 or the presence of a T-stem cell pool generated during the initial immune response.9 If this hypothesis holds true, CTL priming by TARP vaccination after/during chemotherapy could be a therapeutic strategy. More diagnosis-relapse couples are needed to confirm this finding. Tetramer-positive CTLs from pedAML patients showed a fivefold lower TARP-TCR expression than the positive control (median MFI = 1793). No tetramer-positive population could be measured in the CTL compartment from TARP-high/HLA-A∗0201 negative patients (n = 2), TARP-low/HLA-A∗0201 positive patients (n = 5) or TARP-low/HLA-A∗0201 negative (n = 1) patients.
Figure 1.
Tetramer staining of TARP-TCR+ CTLs from pedAML patients. The lymphocyte gating strategy, described in Supplemental Materials, is illustrated in (A). The total lymphocyte compartment is indicated in green, other white blood cells in blue en non-viable cells or doublets in dark grey. Subsequent patient-individual gating of the CD3+/CD8+ compartment, and tetramer staining within this compartment, is shown for three TARP-high/HLA-A∗0201 positive patients in (B-D). Tetramer-positive events, defining TARP-TCR+ CTLs, are indicated in black, and tetramer-negative CTLs in light grey. Non-CD3+/CD8+ cells within the lymphocyte gate are indicated in green. Median MFI values are indicated by circles. Tetramer positivity was gated for each patient individually based on sample-specific FMO controls. For two out of the three patients (B-C), both BM and PB were analysed. For one of the three patients (B), lymphocytes from both diagnosis and relapse could be evaluated. BM = bone marrow, CTL = cytotoxic T-cell, FMO = fluorescence-minus-one, PB = peripheral blood, TARP = T-cell receptor γ chain alternate reading frame protein, TCR = T-cell receptor.
Altogether, these data suggest that a pedAML patient's native immune response is triggered by HLA-presentation of TARP antigenic peptides in vivo, but, apparently insufficiently to eradicate the leukemic cells. Both leukemic cell resistance and lymphocyte quiescence may account for this finding.10 Also, due to T-cell ignorance, tumor-specific CTLs may be present but not primed by the antigen, or priming may be inefficient.11 Gaining a deeper understanding of the underlying mechanisms may contribute to therapeutic targeting of TARP in pedAML.
We previously showed that TARP expression is associated with FLT3-ITD in pedAML. Unfortunately, the initial pediatric sample cohort was too small to evaluate a possible clinical impact of TARP expression. We here evaluated TARP expression in a larger cohort of LSC (n = 24) and L-blast (n = 29) cells sorted from pedAML patients using real-time quantitative PCR (qPCR), and compared expression levels to those measured in HSC (n = 25) and C-blast (n = 28) sorted from healthy controls. Data analysis is described in Supplemental, and expression values were expressed as calibrated normalised relative quantities (CNRQ).
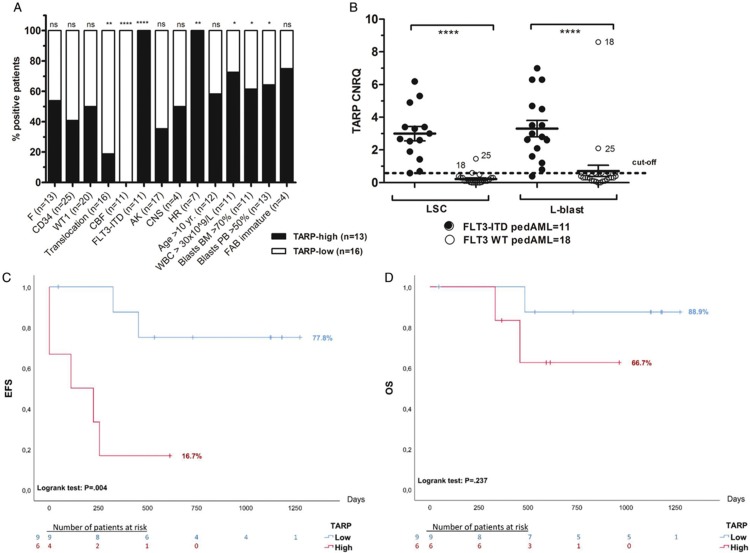
A significantly increased TARP expression (p < .0001) was demonstrated in LSC and L-blast compared to their healthy counterparts (Fig. S2A). PedAML patients were dichotomized as TARP-high (n = 13/29, 44.8%) and TARP-low (n = 16/29, 55.2%), using a cut-off based on the average expression measured in healthy controls plus 2 times the standard deviation (Table S2). The presence of translocations, including core-binding factor leukemia, was significantly inversely correlated to TARP expression (p < .01 and < .0001, respectively). FLT3-ITD mutations (p < .0001) and HR profiles (p < .01) were exclusively observed in TARP-high patients (Fig. 2A). Within the TARP-high group, a significantly higher proportion of patients showed WBC counts >30 × 109/L and blasts >70% in BM and >50% in PB (p < .05).
Figure 2.
TARP transcript expression in pedAML in relation to subgroups and outcome. Correlation between patient characteristics and outcome between pedAML patients dichotomized as TARP-low (n = 16) and TARP-high (n = 13). TARP expression was measured by qPCR, and CNRQ values were interpreted against a cut-off calculated based on the expression in healthy controls (see Supplemental Materials). p values <.05 were considered as significant. One, two, three or four asterisks are indicative for the level of significance (p < .05, p < .01, p < .001 and p < .0001, respectively). (A) Bars display the percentage of patients (%), harboring the characteristic shown in the x-axis, for TARP-high (black) and TARP-low (white) pedAML. The total number of patients positive for each characteristic is shown between parentheses. (B) Differential TARP expression between FLT3-ITD mutated and FLT3 WT pedAML patients measured in the LSC and L-blast compartment. FLT3-ITD mutated pedAML showed a significantly higher TARP expression in both LSC (p < .0001) and L-blast (p < .0001). Thirteen out of the 29 pedAML patients were classified as TARP-high, that is, 11/11 FLT3-ITD pedAML and 2/18 FLT3 WT pedAML (encoded by “18” and “25”). Horizontal bars indicate means, error bars indicate ±SEM, horizontal square brackets represent statistical comparisons and the dotted line represent the cut-off for elevated TARP expression. (C-D) Kaplan–Meier EFS and OS survival plots based on 15 pedAML treated in the NOPHO-DBH AML2012 protocol, dichotomized as TARP-high (n = 6, 4/6 FLT3 ITD and 2/6 FLT3 WT) or TARP-low (n = 9, 9/9 FLT3 WT). The number of days is shown on the x-axis, and the percentage as a ratio (100% equals 1.0) on the y-axis. Drop-outs of the patients are indicated at the bottom per block of 250 days. (C) EFS was significantly lower in TARP-high versus TARP-low patients (16.7% vs 77.8%, respectively, p < .01). (D) OS was lower in TARP-high vs TARP-low patients (66.7% versus 88.9%, respectively), though at a non-significant level (p > .05). AK = abnormal karyotype, BM = bone marrow, CEBPA = CCAAT/enhancer-binding protein alpha, CNRQ = calibrated normalized relative quantity, CNS = central nerve system, F = female, FAB = French-British-American, FLT3 = fms-like tyrosine kinase receptor-3, HR = high risk, ITD = internal tandem duplication, LSC = leukemic stem cell, L-blast = leukemic blast, M = male, MT = mutated, NK = normal karyotype, NPM1 = nucleophosmin, PB = peripheral blood, PedAML = pediatric acute myeloid leukemia, qPCR = quantitative polymerase chain reaction, SEM = standard error of the mean, SR = standard risk, TARP = T-cell receptor γ chain alternate reading frame protein, WT1 = Wilms’ tumor 1, WBC = white blood cell, WT = wild type, yr = years.
In concordance with our previous work, TARP transcript expression was significantly increased in LSC and L-blast (p < .0001) from FLT3-ITD pedAML (n = 11) compared to FLT3 WT pedAML (n = 18) (Fig. 2B). Sixty-three percent of FLT3-ITD positive patients harbored a single ITD, 27% two ITDs and one patient presented four ITDs (Table S3). The length of the duplicated region ranged between 20 and 96 base pairs (bp) (median 33 bp), and allelic ratios (ARs) varied from 2.7 to 70.8% (median 17.7%). There was no significant association between the number of ITDs and the level of TARP expression. One patient presented only a single FLT3-ITD clone with an AR of 3.0%, but was still classified as TARP-high. Paired comparison of TARP expression measured in LSCs and L-blasts sorted from 9/11 FLT3-ITD mutated pedAML demonstrated a significantly higher expression in the latter compartment (p = .041, Fig. S2B). This finding is in agreement with our published micro-array data of paired LSC and L-blast couples (GSE 128103).
Two FLT3 WT pedAML patients showed elevated TARP expression in LSCs and L-blasts (pedAML18 and pedAML25). The presence of TRGC rearrangements, able to confound TARP expression due to common TRGC coding regions and sporadically observed in AML,12 was excluded. FLT3-ITD analysis was repeated on L-blast subpopulations to exclude a possible false negative result (not performed in LSC due to too low DNA concentration). One patient (pedAML18) harbored a rare KMT2A-SEPT9 fusion protein, presented central nerve system (CNS) invasion and was classified as HR. The other patient showed a normal karyotype and WT1 overexpression. Both TARP-high/FLT3 WT patients with and without KMT2A-SEPT9 relapsed after 8.4 months and presented with resistant disease, respectively. The first patient died 15.1 months after diagnosis.
Among NOPHO-DBH AML2012-treated patients (n = 15), TARP-high pedAML (n = 6) showed a significantly lower event-free survival (EFS) compared to TARP-low pedAML (n = 9) (16.7% vs 77.8%, respectively, logrank p < .01, Fig. 2C). Relapse occurred in 3/6 TARP-high patients and 2/6 showed resistant disease, with an estimated time to event of 6.6 months. Two of the nine TARP-low patients also relapsed, with an estimated time to event of 34.6 months. Univariate Cox regression analysis confirmed this finding, showing a significant hazard ratio of 8.41 (95% confidence interval (CI) 1.52 – 46.6, p = .015) for the occurrence of an event in TARP-high patients. Multivariate analysis did not show an association between TARP expression and EFS (Table S4). However, association with EFS did remain significant when including all diagnostic pedAML patients, irrespective of the treatment protocol (n = 27/29, hazard ratio 3.83 (95% CI 1.1 – 13.0), p = .032). No significant correlation was found between the level of TARP expression and OS (hazard ratio 3.90 (95% CI 0.35–43.9), p = .27, Fig. 2D).
In conclusion, we here confirm that both TRGC1- and TRGC2-encoded TARP transcripts co-exist in AML. We demonstrate that TARP presentation on leukemic cells may induce beneficial immune responses in pedAML patients. We consolidate our previous finding that all FLT3-ITD positive pedAML patients display TARP overexpression, though also conclude that TARP overexpression is not exclusive for FLT3-ITD mutated patients. Furthermore, investigation on the role of FLT3 inhibitors in TARP-high pedAML patients, and their impact on TARP expression levels, is warranted. TARP expression was significantly inversely correlated with EFS in a small cohort of NOPHO-DBH AML2012-treated patients. The hypothesis that TARP−high/FLT3-WT pedAML patients may define a (till now undetectable) poor prognosis group with HR genetic lesions (KMT2A-SEPT9) and poor outcome will require further evaluation. Although promising, these data need confirmation in larger, preferentially multicenter cohorts.
Disclosures
All authors declare to have no possible conflicts of interest in the manuscript, including financial, consultant, institutional and other relationships that might lead to bias or a conflict of interest.
Acknowledgements
Our gratitude goes to prof. dr. F. Plasschaert of the orthopedic surgery, C. Matthys of the Cord Blood Bank, and the staff of the Department of Pediatric Hematology and Oncology of the Ghent University Hospital (Ghent, Belgium) for providing samples. We acknowledge the NIH Tetramer Core Facility for providing tetramers. We acknowledge the Nucleomics Core for performing mRNA sequencing and data analysis (www.nucleomics.be).
Supplementary Material
Footnotes
Citation: Depreter B, De Moerloose B, Vandepoele K, Uyttebroeck A, Van Damme A, Denys B, Dedeken L, Dresse MF, Van der Werff Ten Bosch J, Hofmans M, Kerre T, Vandekerckhove B, Philippé J, Lammens T. Clinical significance of TARP expression in pediatric acute myeloid leukemia. HemaSphere, 2020;4:2(e346). http://dx.doi.org/10.1097/HS9.0000000000000346
Jan Philippé and Tim Lammens possess Senior last authorship.
This research was supported by the Belgian Foundation against Cancer (grant 2014–265), FOD-KankerPlan (Actie29, grant to JP), vzw Kinderkankerfonds (grant to TL) and the Research Foundation - Flanders (Fonds voor Wetenschappelijk Onderzoek Vlaanderen, FWO, grant 1113117 to BD and grant 1831312N to TK).This work is submitted in partial fulfilment of the requirement for the PhD of candidate BD at Ghent University.
The authors have no conflicts of interest to disclose.
References
- 1.Rasche M, Zimmermann M, Borschel L, et al. Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012. Leukemia. 2018;32:2167–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Moerloose B, Reedijk A, de Bock GH, et al. Response-guided chemotherapy for pediatric acute myeloid leukemia without hematopoietic stem cell transplantation in first complete remission: Results from protocol DB AML-01. Pediatr Blood Cancer. 2019;66:e27605. [DOI] [PubMed] [Google Scholar]
- 3.Zwaan CM, Kolb EA, Reinhardt D, et al. Collaborative efforts driving progress in pediatric acute myeloid leukemia. J Clin Oncol. 2015;33:2949–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Annesley CE, Brown P. The Biology and Targeting of FLT3 in Pediatric Leukemia. Front Oncol. 2014;4:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levis M. FLT3 mutations in acute myeloid leukemia: what is the best approach in 2013? Hematology Am Soc Hematol Educ Program. 2013;2013:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaspers GJ. Pediatric acute myeloid leukemia. Expert Rev Anticancer Ther. 2012;12:405–413. [DOI] [PubMed] [Google Scholar]
- 7.Depreter B, Weening KE, Vandepoele K, et al. TARP is an immunotherapeutic target in acute myeloid leukemia expressed in the leukemic stem cell compartment. Haematologica. 2019;Doi: 10.3324/haematol.2019.222612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Essand M, Vasmatzis G, Brinkmann U, et al. High expression of a specific T-cell receptor gamma transcript in epithelial cells of the prostate. Proc Natl Acad Sci U S A. 1999;96:9287–9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefrancois L, Obar JJ. Once a killer, always a killer: from cytotoxic T cell to memory cell. Immunol Rev. 2010;235:206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gattinoni L, Speiser DE, Lichterfeld M, et al. T memory stem cells in health and disease. Nat Med. 2017;23:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coulie PG, Connerotte T. Human tumor-specific T lymphocytes: does function matter more than number? Curr Opin Immunol. 2005;17:320–325. [DOI] [PubMed] [Google Scholar]
- 12.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nature Immunology. 2013;14:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.