ABSTRACT
Malignant esophageal strictures often require stent placement to alleviate dysphagia and improve quality of life. We present a novel application of a lumen-apposing metal stent to bypass a malignant esophageal stricture in the setting of altered gastric anatomy.
INTRODUCTION
Esophageal strictures may occur because of benign or malignant disease. Such strictures can cause severe dysphagia, weight loss, and decreased quality of life. The lumen-apposing metal stent (LAMS) was designed for the drainage of peripancreatic fluid collections, especially walled-off necrosis and pseudocysts after acute pancreatitis. The design of the LAMS includes a larger lumen, which allows for improved drainage of cyst contents.1 We present a case of malignant esophageal stricture due to adenocarcinoma arising from the gastroesophageal junction in a patient with altered gastric anatomy. The use of the LAMS was efficacious in bypassing the tumor and helped relieve the patient's dysphagia.
CASE REPORT
A 71-year-old woman with esophageal adenocarcinoma presented with severe dysphagia and weight loss. She also reported a remote history of partial gastrectomy (Billroth II) to treat a gastric ulcer. Esophagogastroduodenoscopy (EGD) revealed a circumferential, obstructing mass at the gastroesophageal junction (Figure 1). The patient was unable to meet her daily caloric requirements because of severe dysphagia. The placement of a gastrostomy tube was not feasible because of previous partial gastrectomy. Hence, the patient was referred to interventional gastroenterology for possible stent placement. EGD with endoscopic ultrasound (EUS) examination demonstrated an obstructing T2 lesion, which could only be traversed with an ultrathin gastroscope. Endoscopic and fluoroscopic visualization of the stomach revealed a small gastric pouch, consistent with the patient's history of partial gastrectomy (Figure 2).
Figure 1.
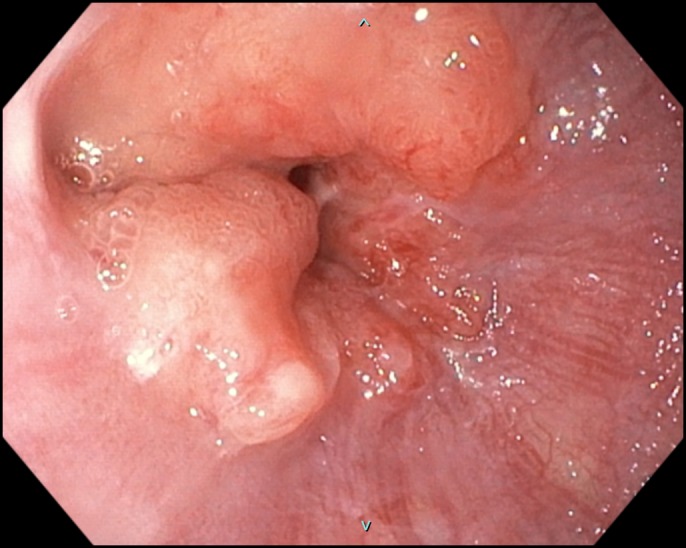
Esophagogastroduodenoscopy demonstrating mass at gastroesophageal junction.
Figure 2.
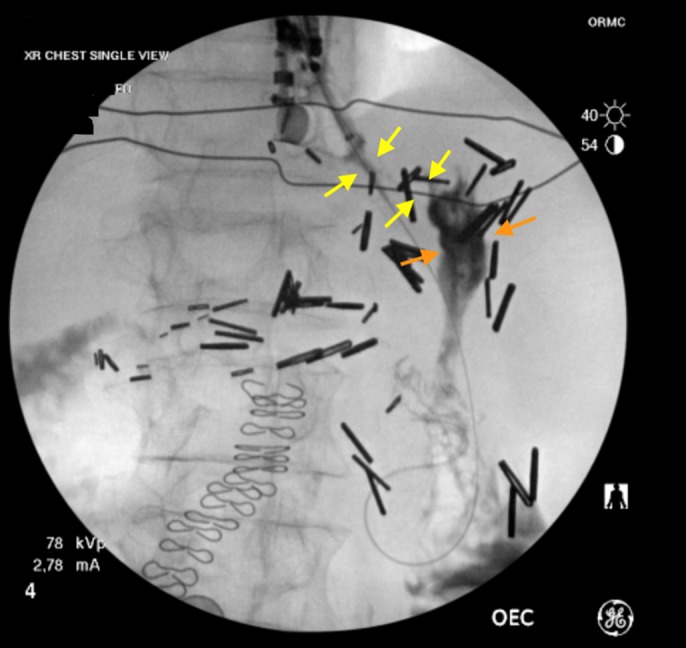
Fluoroscopy before stent placement (yellow arrows show stricture and orange arrows show gastric pouch).
The placement of an esophageal stent was not considered safe because of concern for distal stent obstruction secondary to gastric pouch anatomy. A 15 × 10 mm LAMS (AXIOS, Boston Scientific, Marlborough, MA) was hence successfully deployed across the stricture (Figure 3). Of note, EUS imaging was not necessary for stent deployment because the stent was successfully released under endoscopic and fluoroscopic guidance. Satisfactory stent position was confirmed via fluoroscopy and by injection of contrast through stent lumen after deployment (Figure 4).
Figure 3.
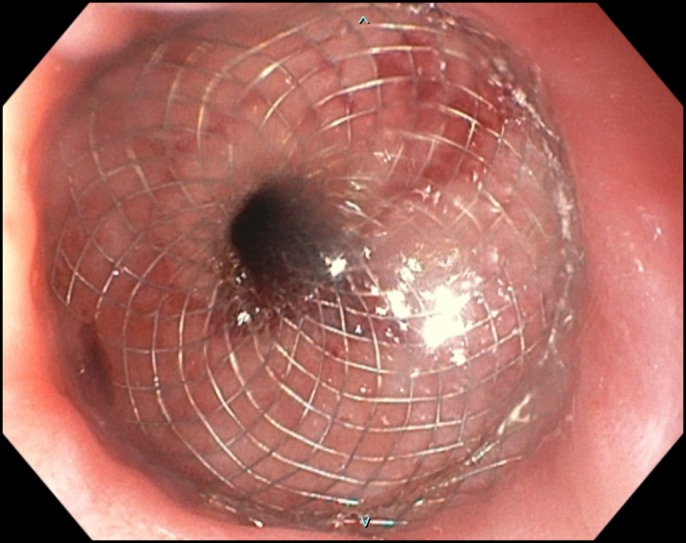
Esophagogastroduodenoscopy demonstrating gastroesophageal junction with stent.
Figure 4.
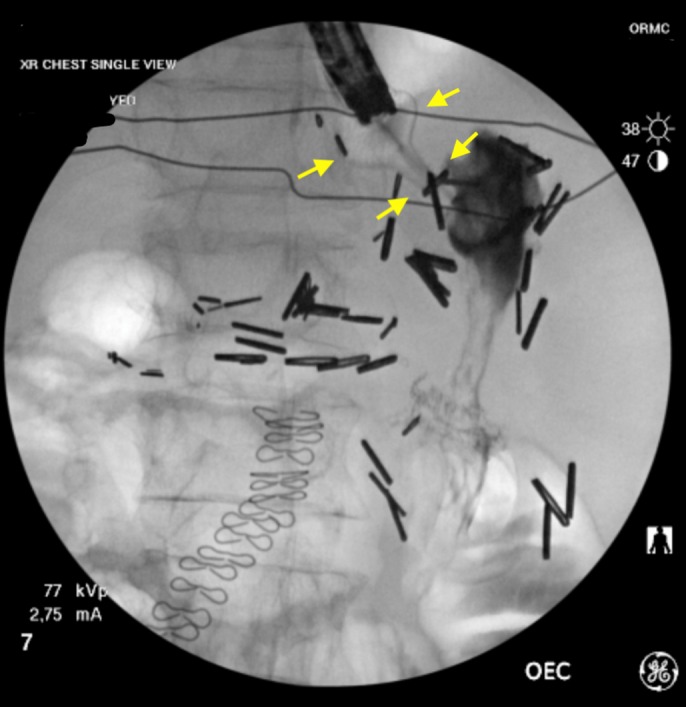
Fluoroscopy after stent placement (yellow arrows show stent).
The patient reported significant improvement in dysphagia postintervention (confirmed with the patient on day 1 and day 7 after stent placement). Repeat EGD performed 2 weeks later to evaluate stent position demonstrated patent stent lumen (Figure 5).
Figure 5.
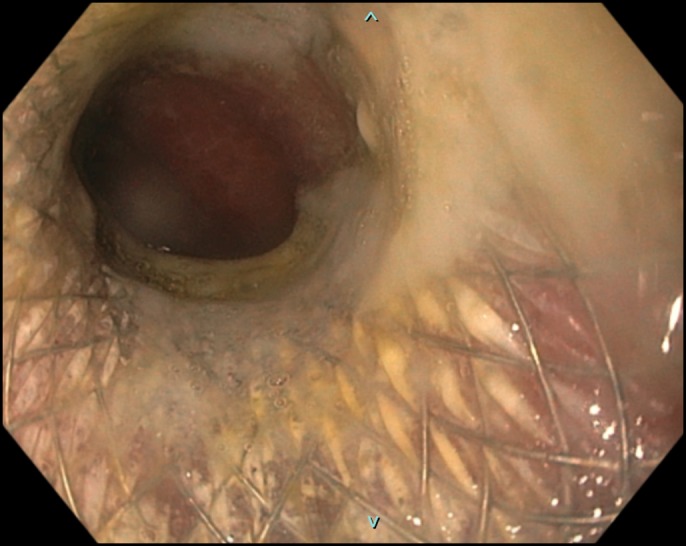
Esophagogastroduodenoscopy (performed 2 weeks after initial exam) demonstrating patent stent.
DISCUSSION
Benign esophageal strictures are usually treated effectively by dilation therapy.2 If refractory, these strictures may require rare stent placement.3 Malignant esophageal strictures, on the other hand, are typically managed with chemoradiation therapy or surgery. However, esophageal stent placement is often necessary for more immediate and definitive symptom relief.4 Covered self-expandable metal stents are typically used in the esophagus to prevent tumor ingrowth. Covered stents, however, do not embed into the tissue and thus carry some risk of stent migration.1
The LAMS has a reduced rate of migration because of its “dumbbell” design. Although the LAMS was originally designed to manage peripancreatic fluid collections, several reports have documented the use of LAMS in other gastrointestinal pathologies. The LAMS has been used to treat postoperative anastomotic strictures5 and recurrent malignant small bowel obstruction.6 The LAMS has also been used in the creation of de novo gastrointestinal anastomoses in patients with gastric outlet obstruction, afferent limb syndrome, and biliary obstruction in patients with altered anatomy as an alternative to surgical intervention.7 The use of LAMS has now been well established to access excluded stomach for biliary interventions in patients after Roux-en-Y gastric bypass surgery (EUS-directed trans-gastric ERCP or EDGE).8 The LAMS has additionally been used in the treatment of biliary tract pathologies, including choledochoduodenostomy,9 as well as to perform cholecystostomy in patients with acute cholecystitis who are poor candidates for surgery.10 As demonstrated, the LAMS may be safely deployed in some malignant strictures, and the use of the LAMS should be considered, especially when dealing with such strictures in the setting of altered postsurgical anatomy.
DISCLOSURES
Author contributions: RB Mirchin wrote the manuscript, reviewed the literature, and is the article guarantor. SK Mahmood edited the manuscript.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
REFERENCES
- 1.DeSimone ML, Asombang AW, Berzin TM. Lumen apposing metal stents for pancreatic fluid collections: Recognition and management of complications. World J Gastrointest Endosc. 2017;9(9):456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Egan JV. Esophageal dilation. Gastrointest Endosc. 2006;63(6):755–60. [DOI] [PubMed] [Google Scholar]
- 3.Fuccio L. Clinical outcomes following stent placement in refractory benign esophageal stricture: A systematic review and meta-analysis. Endoscopy. 2016;48(2):141–8. [DOI] [PubMed] [Google Scholar]
- 4.Evans JA, Early DS, Chandraskhara V, et al. The role of endoscopy in the assessment and treatment of esophageal cancer. Gastrointest Endosc. 2013;77(3):328–34. [DOI] [PubMed] [Google Scholar]
- 5.Alcalá FM, García FMA, Sánchez-Yague A, García AMA, Avila JC, Pozo JP. Treatment of a benign, anastomotic refractory rectal stricture with an AXIOS stent. Endoscopy. 2015;47(Suppl 1):E413–4. [DOI] [PubMed] [Google Scholar]
- 6.Mir A, Parekh PJ, Shakhatreh M, Yeaton P. Endoscopic ultrasound-guided creation of an enterocolostomy to relieve malignant bowel obstruction. Endosc Int Open. 2019;07(08):E1034–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain D, Chhoda A, Sharma A, Singhal S. De-novo gastrointestinal anastomosis with lumen apposing metal stent. Clin Endosc. 2018;51(5):439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngamruengphong S, Nieto J, Kunda R, et al. Endoscopic ultrasound-guided creation of a transgastric fistula for the management of hepatobiliary disease in patients with Roux-en-Y gastric bypass. Endoscopy. 2017;49(6):549–52. [DOI] [PubMed] [Google Scholar]
- 9.Sportes A, Airinei G, Kamel R, Raynaud J, Benamouzig R. Endoscopic ultrasound-guided choledochoduodenostomy with a lumen-apposing metal stent through an uncovered metal duodenal stent. Endosc Int Open. 2018;06(12):E1395–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbera C, Grande G, Alberghina N, Manno M, Conigliaro R. Single-step endoscopic ultrasound-guided fluoroless gallbladder drainage using the Axios lumen-apposing metal stent. Endoscopy. 2016;48(Suppl 1):E254. [DOI] [PubMed] [Google Scholar]


