Abstract
These guidelines from the AST Infectious Diseases Community of Practice review the diagnosis and management of pneumonia in the post‐transplant period. Clinical presentations and differential diagnosis for pneumonia in the solid organ transplant recipient are reviewed. A two‐tier approach is proposed based on the net state of immunosuppression and the severity of presentation. With a lower risk of opportunistic, hospital‐acquired, or exposure‐specific pathogens and a non‐severe presentation, empirical therapy may be initiated under close clinical observation. In all other patients, or those not responding to the initial therapy, a more aggressive diagnostic approach including sampling of tissue for microbiological and pathological testing is warranted. Given the broad range of potential pathogens, a microbiological diagnosis is often key for optimal care. Given the limited literature comparatively evaluating diagnostic approaches to pneumonia in the solid organ transplant recipient, much of the proposed diagnostic algorithm reflects clinical experience rather than evidence‐based data. It should serve as a template which may be modified according to local needs. The same holds true for the suggested empiric therapies, which need to be adapted to the local resistance patterns. Further study is needed to comparatively evaluate diagnostic and empiric treatment strategies in SOT recipients.
Keywords: diagnosis, pneumonia, transplantation
1. INTRODUCTION
Pneumonia is a frequent infectious complication of solid organ transplantation (SOT). The occurrence of post‐transplant pneumonia adversely impacts both graft and recipient survival, as well as the cost of care for SOT recipients.1 Numerous micro‐organisms can cause pneumonia in the SOT recipient with some etiologies resulting in self‐limited infection and others causing significant morbidity and mortality. As a result of the varied clinical presentations and etiologies of pneumonia in SOT recipients, arriving at a specific microbiologic diagnosis can be challenging but is important for optimal care, especially with complicated or refractory pneumonias. In this section, the clinical presentation, differential diagnosis, diagnostic testing, and empiric antimicrobial treatment of pneumonia in SOT recipients are reviewed. Pathogen‐specific sections within the AST ID Guidelines are referenced for further information regarding the diagnosis and treatment of specific pathogens that cause pneumonia in this vulnerable population.
2. CLINICAL PRESENTATION
The clinical presentation of pneumonia in the SOT recipient is highly variable. The traditional presentation with cough, increased sputum production, and fever should always prompt evaluation for pneumonia. However, frequently more subtle symptoms predominate with the diagnosis of pneumonia being reached only after careful clinical evaluation. Clinical evolution can vary from very rapid, indicative of bacterial or viral pathogens, to the subacute or chronic presentation often seen with fungal or mycobacterial infections. A high index of suspicion for pneumonia is essential when any signs of infection are present in a solid organ transplant recipient. Lung involvement should be evaluated in any transplant patient presenting with an unexplained fever, even in the absence of other lung‐specific clinical findings.
3. DIFFERENTIAL DIAGNOSIS
3.1. Considerations impacting differential diagnosis
The differential diagnosis for pneumonia in SOT recipients includes infectious and non‐infectious etiologies (Table 1). An understanding of infectious etiologies in these patients is driven by epidemiologic investigation of both transplanted2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 and otherwise healthy adult and pediatric patients.13, 14 In earlier eras, studies of pneumonia in SOT recipients were focused on the high incidence of opportunistic infections such as invasive mold infections and cytomegalovirus (CMV).12 With improved infection prevention strategies, better diagnostic tools, and newer immunosuppressive strategies, the importance of community‐acquired bacterial and viral infection in SOT‐associated pneumonia has been increasingly appreciated.2
Table 1.
Differential diagnosis of pneumonia in the solid organ transplant recipient
| Viral | Respiratory viruses: Influenza, Parainfluenza, Respiratory Syncytial virus, Human metapneumovirus, Adenovirus, Rhinovirus, Coronavirus, |
| Herpesviruses: Herpes simplex virus, Varicella zoster virus, Cytomegalovirus | |
| Bacterial | Community‐acquired: Streptoccocus pneumonia, Haemphilus influenzae, Moraxella cattarhalis, Staphylococcus aureus |
| Healthcare‐associated: Klebsiella spp, Enterobacter spp, Escherichia coli, and other Enterobacteriaceae; Pseudomonas aeruginosa, Stenotrophomonas maltophila, Acinetobacter spp, others | |
| Atypical: Mycoplasma pneumoniae, Ureaplasma urealyticum, Chlamydia trachomatis, Legionella spp | |
| Mycobacterial: Mycobcaterium. tuberculosis and Nontuberculous Mycobacteria | |
| Zoonoses: Chlamydia psittaci, Francisella tularensis, Coxiella burnetti, Rhodococcus equi, Pasteurella multocida | |
| Other: Nocardia spp and Actinomyces spp | |
| Fungal | Endemic/Dimorphic Fungi: Histoplasma capsulatum (var capsulatum and var duboisii); Blastomyces dermatiditis; Coccidioides immitis; Penicillium. marneffei |
| Yeasts and Yeast‐like Fungi: Cryptococcus spp; Pneumocystis jirovecii | |
| Molds: Aspergillus spp; Mucormysosis; Fusariosis; Scedopsporium spp | |
| Parasitic | Protozoan: Toxoplasma gondii |
| Helminth: Strongyloides stercoralis | |
| Flatworm: Echinococcus spp | |
| Non‐infectious | mTORi‐induced pneumonitis; Pulmonary embolism; Pulmonary hemorrhage; Lung tumor (primary or metastasis); PTLD; Pulmonary edema; Hepatopulmonary syndrome |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
The differential diagnosis of pneumonia in SOT recipients depends upon a multitude of non‐specific factors including but not limited to age and timing post‐transplant, net state of immunosuppression, specific organ transplanted, environmental exposures, site of pneumonia acquisition (community vs. healthcare/hospital‐acquired), and radiographic infiltrate pattern. The risk of pneumonia in SOT recipients is determined by the degree/depth of immunosuppression.15 Healthcare and/or ventilator‐associated pneumonia are frequently diagnosed in the early post‐transplant period. In two series, early onset pneumonia post–liver transplant was dominated by bacterial etiologies, the majority of which were Gram‐negative bacilli.7, 8 The majority of pneumonia episodes occur later post‐transplant with 50% occurring >1 year post‐transplant9 in renal recipients and 70.4% of episodes occurring at >6 months post‐transplant in a multicenter point prevalence study.2
The type of organ transplanted influences the incidence, timing, and microbiologic spectrum of pneumonia in SOT recipients.16 Lung transplantation poses unique challenges given the potential for donor‐derived infection and recipient airway colonization to impact the occurrence and microbiology of pneumonia. In a prospective, multicenter study of pneumonia in lung transplant recipients, pneumonia incidence was 72 episodes per 100 lung transplants per year.3 Though bacterial pneumonia was the most frequent etiology, fungal and viral pneumonia together accounted for 24.4% of episodes. The majority of pneumonia episodes in this series occurred in the two months post‐transplant.3 In a large cohort of SOT recipients with invasive fungal infection, Aspergillus was the most common IFI in lung transplant recipients with presumably many of the invasive aspergillosis (IA) episodes presenting with primary lung involvement.17 In other studies, over half of IA episodes occurred over 1 year post‐transplant.18, 19
In a retrospective study of 40 adult small bowel/multivisceral transplant (SmB/MV) recipients, 17% of recipients developed infection involving the lung(s) in the 30‐day post‐transplant period. Most of these were due to Pseudomonas aeruginosa.20 In a prospective study of infections in SmB/MV recipients, 14% of patients developed pneumonia though no etiology‐specific pneumonia data were provided.21
Radiographic features can contribute to narrowing the differential diagnosis for pneumonia in SOT recipients though do not frequently lead to identification of a specific etiology. Diffuse, bilateral infiltrates suggest a range of etiologies including CMV, respiratory viruses, Mycoplasma, Legionella, and Pneumocystis jirovecii (PJP).22 Ground glass or mixed ground glass/micronodular infiltrate raises concern for PJP and CMV.23 Single or multinodular lung involvement indicates potential for invasive mold infection, Nocardia, tuberculosis (TB) or non‐tuberculous mycobacterial disease, endemic fungal infection, or malignancy.24 Association of lung infiltrates with mediastinal or hilar adenopathy raises concern for TB and endemic fungal infection. The presence of pleural effusion or empyema suggests a range of etiologies including community‐acquired and hospital‐acquired bacterial pneumonia, Cryptococcus and other fungal infections, or tuberculosis depending on the clinical presentation and exposure history.25, 26
Epidemiology, environmental exposures, and seasonality also significantly influence pneumonia etiologies. Geographic location of the donor and recipient may impart risk for endemic fungal infections (Histoplasma, Coccidioides, Blastomyces, Penicillium), TB, or Burkholderia pseudomallei.26, 27, 28, 29 Zoonotic pneumonia can occur secondary to an array of pathogens following exposure to birds (Chlamydia psittaci), cats/dogs (Pasteurella multocida), rabbits and other wildlife (Francisella tularensis), horses (Rhodococcus equi), and farm animals (Coxiella burnetti).30, 31, 32, 33 Environmental exposures to contaminated water (Legionella pneumophila and Pseudomonas aeruginosa) as well as soil (Cryptococcus, Nocardia, Penicillium, endemic fungi, molds) can also indicate potential etiology for pneumonia.34 Seasonality and community contacts contribute to relative risk of respiratory viral infections such as influenza, parainfluenza, enteroviruses, and rhinoviruses.35, 36
3.2. Potential pathogens
Potential pathogens causing pneumonia in the SOT recipient include bacteria, viruses, fungi, and parasites (Table 1). Though specific etiologies are discussed in greater detail in pathogen‐specific sections of this guideline series, broad categories of microbiologic etiologies that cause pneumonia in SOT recipients merit attention.
Bacterial pneumonia in the solid organ transplant recipient can be caused by community‐ or hospital‐acquired organisms as well as by donor‐derived pathogens in the setting of lung transplantation. Hospital acquisition, including ventilator‐associated pneumonia, occurs predominantly early post‐transplantation while community‐acquired pneumonia becomes relatively more frequent later post‐transplant.2 Community‐acquired bacterial pneumonia pathogens include Streptococcus pneumoniae,37, 38 Haemophilus influenzae, Mycoplasma spp, Legionella spp, and Chlamydia spp Nosocomial/opportunistic bacterial etiologies include Pseudomonas spp, enteric Gram‐negative bacilli, as well as Stenotrophomonas spp, and others. Additionally, bacteria such as Nocardia, tuberculous, and non‐tuberculous mycobacteria can be significant pathogens in SOT recipients.26, 39, 40, 41
Fungal pulmonary infection in SOT recipients can be due to PJP, Aspergillus spp and other invasive molds, Cryptococcus, and endemic/dimorphic fungi such as Histoplasma, Blastomyces, and Coccidioides.42, 43 Though prophylaxis strategies have markedly decreased the occurrence of PJP in SOT recipients, late‐onset 44 and outbreak‐associated 45, 46 infections remain a problem. In Histoplasma infection in SOT recipients, 81% of cases have pulmonary involvement and 34% of infections occur in the first year post‐transplant.47 Cryptococcal infection may present with pneumonia alone, or with dissemination, including to the central nervous system, in SOT recipients.25, 48, 49, 50, 51 Notably, CMV infection can predispose to invasive aspergillosis in SOT recipients.19
Viral etiologies of pneumonia in SOT recipients include DNA viruses such as adenovirus,52, 53 CMV, and other herpesviruses, as well as community‐acquired respiratory RNA viruses including influenza,54 RSV,55, 56 HMPV,57, 58 and parainfluenza. Less data exist on the frequency of infection with rhinoviruses, coronaviruses, and respiratory enteroviruses as a cause of pneumonia in SOT recipients.55 Additionally, viral respiratory tract infections can predispose to secondary bacterial pneumonia.59, 60 Lung transplant recipients are at high risk for lower respiratory tract infection due to respiratory viruses61, 62 with potential for significant post‐infection increases in acute rejection rates and decline in lung function.63
Parasitic infection is a rare cause of pneumonia in SOT recipients. Toxoplasma infection or reactivation is infrequently reported as a cause of pneumonitis either alone64 or as part of a disseminated infection.65 Strongyloides hyperinfection syndrome occurring as a result of antecedent recipient infection or donor‐derived transmission can present with bilateral, multifocal, and/or interstitial pulmonary infiltrates with or without Gram‐negative bacterial sepsis.66, 67
3.3. Non‐infectious etiologies
An array of non‐infectious etiologies can mimic infectious pneumonia in the SOT recipient (Table 1). Post‐transplant lymphoproliferative disease (PTLD) may present with lung/thoracic involvement including pulmonary nodules and mediastinal adenopathy, especially in lung/heart‐lung transplant recipients.68, 69 Pulmonary PTLD can also radiographically mimic infectious etiologies of pneumonia.70 mTOR inhibitor‐induced pneumonitis is an infrequent though potentially severe medication side effect that generally resolves with discontinuation of mTOR inhibitor therapy and may co‐exist with infectious etiologies including PJP or community‐acquired respiratory viruses.71 Non‐PTLD primary or metastatic lung cancer can also occur in SOT recipients.72 Other complications such as pulmonary embolism, pulmonary hemorrhage, and pulmonary edema are also reported.73, 74
4. DIAGNOSTIC TESTING
No studies have prospectively compared diagnostic approaches to pneumonia in SOT recipients. While some specific diagnostic tests have been evaluated more systematically, individual test performance is often defined in healthy subjects or in groups of subjects with a variety of immunocompromising conditions. Therefore, the appropriate diagnostic evaluation for pneumonia in SOT recipients should be highly individualized based on local epidemiology, locally available tests, and practice parameters, as well as patient‐specific risk factors, exposures, and medical history. Guidelines for diagnosis of pneumonia by other groups can also be informative though do not always specifically apply to the SOT recipient.75, 76, 77, 78 A tiered approach to the diagnostic evaluation of pneumonia encompasses an initial approach, followed by a more extensive evaluation if the diagnosis remains unclear or if the patient is deteriorating. If the clinical situation is critical or if a more specific diagnosis is initially suspected, respective tests are applied and the order of diagnostic tests modified. One potential diagnostic approach to pneumonia in the SOT recipient is outlined in Figure 1.
The diagnostic evaluation of SOT recipients with suspected or confirmed pneumonia should be performed using a tiered approach with the pace and extent of evaluation informed by the severity and acuity of presentation, the degree of immunosuppression, and the patient's risk factor/exposure history (strong, low).
We recommend against using current pneumonia severity scores in SOT recipients with pneumonia (strong, very low). Pneumonia severity scores have been evaluated in non‐transplant patients with community‐acquired pneumonia and have variable ability to stratify for severity and clinical outcome.79 No studies of pneumonia severity scoring systems have been performed in SOT recipients though the PSI and CURB‐65 scores had poor performance characteristics in cancer patients presenting with pneumonia.80
-
Practitioners evaluating SOT recipients with symptoms or signs of pneumonia should assess the following at initial evaluation:
-
ͦ
Key features of the patient's medical, transplant, immunization, and social histories (strong, moderate).
-
ͦ
Current and prior microbial colonization and current and prior antimicrobial prophylaxis regimens (strong, moderate).
-
ͦ
Social history for exposure(s) which would suggest risk of untreated latent infection such as latent TB infection, coccidioidomycosis, and strongyloidiasis (strong, moderate).
-
ͦ
The acuity of the patient's clinical presentation and use of this information in guiding the differential diagnosis, diagnostic evaluation, and empiric antimicrobial therapy (strong, low).
-
ͦ
The need for contact or respiratory isolation should be assessed and acted upon early in the patient evaluation to avoid exposure of other SOT recipients, patients, and/or staff to potentially contagious pathogens (strong, high).81
Local epidemiology of respiratory viruses, and in particular influenza virus, should be reviewed and considered in the evaluation (strong, high).54
Figure 1.
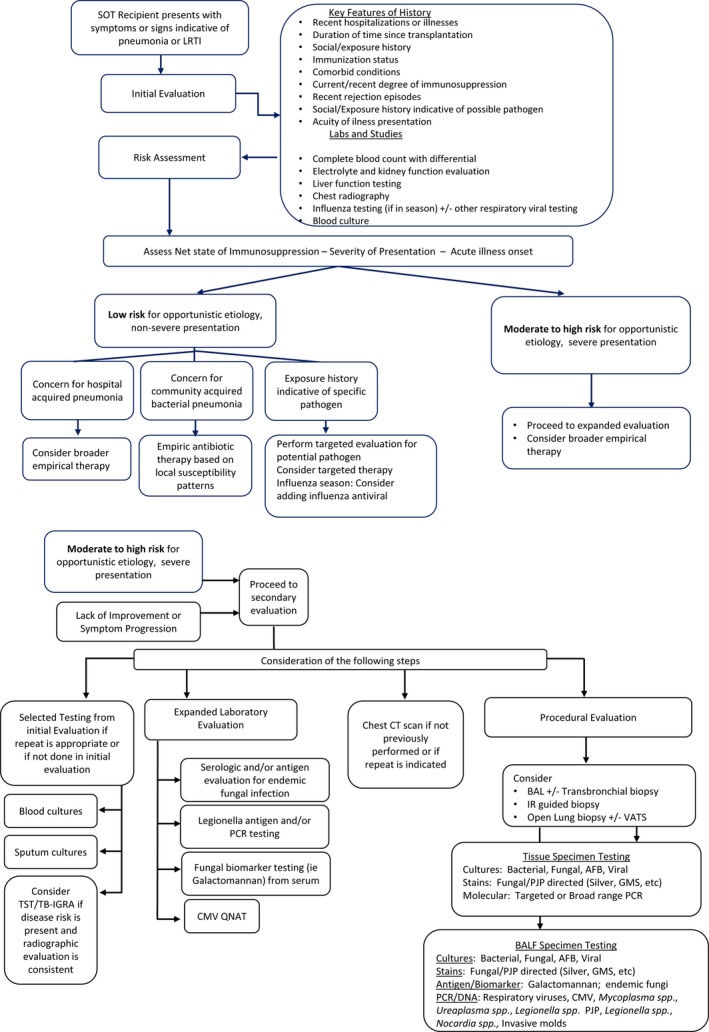
Evaluation of the SOT recipient with suspected pneumonia
The initial evaluation of a SOT recipient with pneumonia should include both blood and radiologic testing with consideration given to viral detection from the nasopharynx.
Clinicians should obtain complete blood count with differential, electrolyte chemistries, and liver function testing as part of the initial laboratory evaluation both for aiding in diagnosis and determining risk of toxicity associated with empiric or directed antimicrobial treatment (strong, low).
Blood cultures should be obtained at presentation of pneumonia and prior to initiation of antibiotic therapy, especially if the patient is febrile or requiring hospitalization (strong, low). While blood culture yields for etiologic organisms are low in both healthy and SOT recipients with pneumonia, a positive blood culture significantly impacts clinical care.2, 14
A chest X‐ray should be performed in all SOT patients with suspected pneumonia (strong, moderate).
Performance of a chest CT scan in the initial evaluation for pneumonia is recommended in settings of high acuity or high net state of immunosuppression (weak, low).
Risk for illness attributable to CMV should be assessed by considering CMV donor‐recipient serostatus, status of CMV‐active prophylaxis, duration of time post‐transplant, and depth of immunosuppression in all SOT patients presenting with symptoms indicative of pneumonia (strong, low).
Testing of urine for Legionella antigens is recommended (weak, very low, low). Though sensitivity is low and urine testing does not detect non‐Legionella pneumophila species, diagnosis of Legionella through this non‐invasive test can accelerate diagnosis and institution of appropriate antimicrobial therapy.82
Testing of the nasopharynx for influenza virus by PCR in seasonally appropriate times is recommended to clarify the need for antiviral treatment (strong, high).54, 83
Multiplex molecular respiratory virus testing is recommended in the evaluation of pneumonia in SOT recipients in seasons of high respiratory virus incidence (weak, moderate). Identifying a respiratory viral etiology can impact clinical decisions regarding further evaluation as well as the need for antimicrobial therapy though the impact has not been specifically evaluated in SOT recipients.84, 85
In older children or adults in whom sputum production is present, sputum culture is recommended for gram stain and bacterial culture (weak, very low).
The contribution of inflammatory markers (leukocyte count, C‐reactive protein, procalcitonin) has been extensively studied as a tool for the general practitioner to decide whether empiric antibiotic treatment may be necessary with mixed results.86 Several studies have evaluated the utility of procalcitonin measurement for detecting and differentiating bacterial infection from other solid organ transplant complications,87, 88, 89, 90, 91, 92, 93, 94 reviewed in.95 Importantly, procalcitonin levels are impacted by surgery as well as by ATG administration which may decrease the utility of this assay in first week post‐transplant.95 In general, though few studies have evaluated procalcitonin use in the setting of SOT recipients with pneumonia, an elevated procalcitonin level may indicate the presence of an infection. Elevated procalcitonin levels beyond the first week post‐transplantation may indicate the presence of a bacterial infection. However, data are limited in SOT recipients and the specific clinical in scenarios in which to apply procalcitonin testing are not clear.
With pneumonia established by imaging and selected laboratory evaluation underway, empiric therapy as discussed below should be started (see Empiric Initiation Treatment section). Clinicians should progress to the second tier of evaluation (Figure 1) if, despite empiric therapy, the clinical situation does not improve in the first 24 to 48 hours or deteriorates and no diagnosis was made following the first tier of diagnostic evaluation (Figure 1). Second tier testing should be performed with focus on each of four areas—repeating of selected tests from the first tier as appropriate; imaging studies to further define the location and nature of pulmonary involvement; expanded diagnostic evaluation as guided by exposures, radiographic results, and clinical course; and invasive testing with the goal of securing a microbiologic diagnosis for targeted treatment (Figure 1).
Laboratory tests not obtained as part of the initial evaluation should be performed (weak, low).
If not done yet, a CT scan of the chest should be performed to better delineate the radiologic pattern and location of infiltrate and to identity potential opportunity for invasive diagnostic procedures (strong, high).
Additional blood testing guided by radiographic pattern, exposures, and degree of immunosuppression should include antigen and/or serologic evaluation for endemic mycoses (strong, high)47 (see Endemic Fungi section of 4th edition of AST ID Guidelines).
Fungal biomarkers measured in the blood/serum, such as galactomannan and 1,3β‐d‐glucan, have poor performance characteristics in SOT recipients as compared with HSCT recipients.18, 96, 97 No studies have evaluated whether performance characteristics vary depending on post‐transplant timing of infection. Given the paucity of data evaluating utility of 1,3β‐d‐glucan, we do not recommend the use of this test currently in SOT recipients (strong, very low) 98, 99, 100 (see Aspergillus, Candida, and Pneumocystis sections of 4th edition of AST ID Guidelines).
In some cases, more invasive testing is needed to obtain a diagnosis. Invasive, procedural diagnostic testing in the SOT recipient with pneumonia includes bronchoscopy with bronchoalveolar lavage (BAL), transbronchial biopsy, CT‐guided biopsy, video‐assisted thoracotomy (VATS) with lung biopsy, and open lung biopsy. These procedures are particularly important for the diagnosis of fungal disease (see the Aspergillus, Cryptococcus, Endemic Fungi, and Emerging Fungi sections of 4th edition of AST ID Guidelines) and to evaluate for non‐infectious causes such as malignancy. Though potential exists for achieving a diagnosis with one of these methods, the decision to pursue further procedural evaluation in an SOT recipient with pneumonia must balance both procedural risks and potential benefits.
Physicians caring for an SOT recipient with pneumonia should have a low threshold for procedural evaluation especially in settings in which patients are not improving, are highly immunocompromised, or the diagnosis remains uncertain (strong, moderate).
Bronchoalveolar lavage offers the opportunity to obtain microbiologic diagnosis either with or without transbronchial biopsy. The utility of BAL in SOT recipients with pneumonia, lung infiltrates, or lung nodules has been evaluated in several small series.5, 22, 68, 101, 102 Microbiologic yield for BAL in these studies ranges from 39% to 77%, with the highest yields in patients with nosocomial pneumonia,5 and those with onset of symptoms between 1 and 6 months post‐transplant.102 Importantly, microbiologic yield is clearly influenced by the pre‐test probability of infection and the relative incidence of infections in distinct populations.102, 103 BAL studies listed in Figure 1 are proposed as a complete list of diagnostic options. While extensive, performance of the complete list of these studies may not be needed in all SOT recipients with pneumonia.
We recommend the performance of BAL in the setting of diffuse or focal pulmonary infiltrate in patients with failure to improve on empiric antibacterial therapy in whom diagnosis has not been reached by non‐invasive testing (strong, moderate). The decision to proceed with BAL versus lung biopsy for initial invasive testing must be highly individualized for each patient and is dependent on the clinician's risk/benefit analysis.
-
We recommend the following studies as a complete BAL fluid evaluation with modifications as needed depending on an individual patient's clinical presentation, radiographic findings, and clinical course (Figure 1):
-
ͦ
Traditional bacterial, fungal, viral, and mycobacterial culture (see Aspergillus, Cryptococcus, Endemic Fungi, Emerging Fungi, CMV, HSV, VZV, Respiratory Viruses, Non‐tuberculous Mycobacterial, Tuberculosis, and Nocardia sections of 4th edition of AST ID Guidelines) (strong, moderate).
-
ͦ
Diagnostic testing including PJP‐directed stains or nucleic acid‐based testing for PJP from BAL fluid (strong, high), in particular in patients either no longer receiving prophylaxis or on non‐TMP‐SMX prophylaxis (see Pneumocystis section of 4th edition of AST ID Guidelines).
-
ͦ
Fungal‐directed stains in patients with radiographic findings suggestive of invasive fungal infection (strong, low).
-
ͦ
Galactomannan in patients with radiographic findings suggestive of invasive fungal infection (weak, low).97, 104
-
ͦ
Antigen‐based testing for endemic fungal infections such as Histoplasma, Blastomyces, Coccidioides, and Paracoccidioides for patients with a compatible clinical history and geographic exposure risk (strong, low).
-
ͦ
Nucleic acid‐based testing (PCR, QNAT, Film array) testing for the following pathogens depending on test availability and on the patient's clinical history, exposure risk, and radiographic findings (strong, low):
-
▪
Respiratory viruses
-
▪
CMV
-
▪
Mycoplasma spp, Ureaplasma spp, and/or Legionella spp
-
▪
Nocardia spp
-
▪
Invasive mold species
-
▪
Mycobacterium tuberculosis.
-
ͦ
Biopsy‐based evaluation offers the ability to directly identify infectious agents in tissue and also determine the histologic pattern of inflammation present. Open lung biopsy (OLB) was studied in a single‐center kidney transplant population with bilateral lung infiltrates.105 The overall OLB diagnostic yield was 85.1% with findings from 53% of OLBs resulting in a therapeutic management change. Complications were frequent (28.7% of OLBs) and associated with higher mortality rate.105 A similar study comparing SOT and non‐SOT patients with diffuse lung disease demonstrated a therapeutic management change frequency of 33%.106 Percutaneous CT‐guided lung biopsy has been evaluated in one series of SOT recipients with parenchymal lung nodule(s).107 Diagnostic yield in this series of 45 biopsies was 53% with the most frequent diagnoses being fungal disease and malignancy. Yield was highest with the use of both fine‐needle aspiration and core biopsy combined in the procedure. Complications occurred in 13% of patients.107 The decision to proceed with lung biopsy must be highly individualized for each patient and is dependent on the clinician's risk/benefit analysis.
We recommend lung biopsy (Open, VATS, or CT‐guided) in the setting of diffuse or focal pulmonary infiltrate in patients with failure to improve on empiric antibacterial therapy and in whom diagnosis has not been reached by non‐invasive testing or BAL (strong, moderate).
We recommend lung biopsy (transbronchial, Open, VATS, or CT‐guided) for patients with focal pulmonary nodule(s) where diagnosis has not already been established by other means due to the risk for malignancy (including PTLD) or invasive fungal infection (weak, low). Consideration can be given to close follow‐up with serial imaging prior to performance of invasive diagnostic procedures for cases in which the risk for malignancy and/or invasive fungal infection is felt to be low (weak, low).
In patients who live in geographic areas that impart risk for endemic fungal infection and who have exposure history suggestive of a moderate to high risk for endemic fungal infection, we recommend deferral of lung biopsy until after antigen/serologic assessment for endemic fungal infection is complete (weak, low).
Non‐hypothesis–driven tests such as broad‐spectrum bacterial PCR based on 16sRNA, panfungal, or mycobacterial genus PCR are not universally available and not systemically studied in SOT recipients. However, these assays may be helpful in patients where all other tests do not identify an etiology. Unbiased next‐generation sequencing may assist in defining a specific etiology if available, though more clinical studies are needed to define the utility of a sequencing‐based diagnostic approach to pneumonia.
5. EMPIRIC INITIAL TREATMENT
Given the complex and varied nature of specific treatment for individual pathogens that cause pneumonia in the SOT recipient, this section will address recommendations for empiric antimicrobial therapy in this setting. Various factors significantly impact empiric antimicrobial regimens for pneumonia in the SOT recipient. The degree of immunosuppression and pace of illness onset (acute, subacute, chronic) are significant determinants in the breadth of empiric antimicrobial therapy provided to an individual patient. Except during influenza season, where empiric therapy may include an agent directed against influenza, antibacterial therapy is the cornerstone of the initial empiric therapy for pneumonia in the SOT recipient.108 Empiric antifungal chemotherapy is rarely initiated early, except in instances where the clinical and radiological presentation strongly suggests a fungal origin. The choice of the initial therapy will depend on the following considerations:
Empiric treatment regimens for pneumonia in the SOT recipient should take into account each individual patient's known microbial colonization as well as prior antimicrobial resistance patterns of specific colonizing or pathogenic organisms with special emphasis on multi‐drug resistant bacteria colonizing the airway in lung transplant recipients (strong, low).
During influenza season, empiric administration of an antiviral drug active against influenza is recommended in SOT recipients with influenza‐like illness, including pneumonia, while awaiting results of influenza‐specific testing unless there is access to PCR‐based detection methods with rapid turnaround time (strong, moderate)54, 83 (See RNA Respiratory Viruses section of 4th edition of AST ID Guidelines).
In a stable patient considered suitable for outpatient therapy who is at lower risk for opportunistic or hospital‐acquired infection and in whom no specific pathogen is suspected, empiric therapy should cover community‐acquired pneumonia and should follow national/international guidelines influenced by local resistance and prevalence patterns (strong, low). Beta‐lactams or fluoroquinolones (FQ) with coverage of respiratory pathogens may be considered for empiric therapy in this setting (strong, low). Practitioners should consider the role of drug interactions, adverse reactions, and increasing antimicrobial resistance when considering risks and benefits for the FQ use in SOT recipients.
Consideration should be given to empiric coverage of intracellular pathogens (such as Mycoplasma pneumoniae, Chlamydia pneumonia, and Legionella spp) in SOT recipients with community‐acquired pneumonia (weak, low) with greater consideration given to empiric intracellular pathogen treatment of pediatric SOT recipients with community‐acquired pneumonia (strong, moderate).13, 14 One important consideration is the selection and use of empiric therapy in this setting in which drug‐drug interactions are present between macrolides and immunosuppressive agents. Respiratory fluoroquinolones may be considered in settings with a high incidence of macrolide resistance for Mycoplasma spp with consideration given to the above‐noted issues in considering risk and benefit of FQ use (weak, low).
With outpatient regimens discussed above, the inclusion of antibiotic therapy against methicillin‐resistant Staphylococcus aureus (MRSA) or Pseudomonas spp is dependent on local resistance patterns, prevalence, the individual patient's infection, and bacterial colonization history, as well as the features of the presenting illness (eg, radiographic evidence of lung abscess) (strong, low) (see MRSA and MDR GNR sections of 4th edition of AST ID Guidelines).
In SOT recipients who require hospitalization for pneumonia, combination therapy with a beta‐lactam agent (±MRSA and ±antipseudomonal activity) and an agent active against intracellular organisms (Mycoplasma spp in children and Legionella spp in adults) is recommended (strong, low). The breadth of coverage of the penicillin‐based agent used depends on local resistance patterns (especially for S pneumoniae) and whether a nosocomial approach is warranted to cover hospital‐acquired gram‐negative pathogens (eg, recent hospitalization, underlying chronic or bronchiectatic lung disease, or the presence prosthetic material). Fluoroquinolones are an alternative in this setting (strong, low). For hospitalized patients with nosocomial or ventilator‐associated pneumonia, international/national guidelines apply with attention to local guidelines, prevalence, and epidemiology (strong, moderate).
Any empiric therapy must be adapted to new clinical and microbiological findings. As appropriateness of initial empiric therapy is measured in large part by the patient's clinical response, close monitoring including repeated clinical evaluation is a prerequisite for any outpatient approach. If this is not feasible or if any doubts prevail, inpatient management should be favored. Furthermore, with any empiric therapy for pneumonia, key gaps in antimicrobial coverage must be kept in mind. Most empiric regimens will not optimally treat Nocardia spp or Pneumocystis jirovecii. Mycobacterial infections may be partially mitigated by the activity of drugs used for empiric pneumonia therapy (eg, imipenem, quinolones, and oxazolidinones). In addition, empiric antibacterial regimens will clearly not provide coverage against invasive, opportunistic, and/or endemic fungal infections. Clinicians caring for SOT recipients with subacute or chronic illness presentations which include pneumonia should consider pathogens such as Nocardia, tuberculosis, non‐tuberculous mycobacteria, endemic fungi, and invasive molds. These pathogens require bronchoalveolar fluid or tissue testing for diagnosis and their treatment entails prolonged courses of antimicrobial regimens. Empiric therapy is usually not instituted prior to diagnostic testing or procedure. However, in unique situations of high acuity or inability to perform diagnostic procedures, empiric therapy for these pathogens may be needed.
6. CONCLUSIONS
The diagnosis and treatment of pneumonia in the SOT recipient is challenging due to varied clinical presentations, numerous potential etiologies, and breadth of both empiric and targeted treatment options. Diagnostic strategies must consider numerous factors including post‐transplant timing, degree of immunosuppression, environmental/community/hospital exposures, and seasonal epidemiology. Empiric treatment is in large part determined by local epidemiology and resistance patterns. Novel diagnostic techniques and future prospective studies will continue to impact the diagnosis and treatment of pneumonia in SOT recipients.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
Dulek DE, Mueller NJ; on behalf of the AST Infectious Diseases Community of Practice . Pneumonia in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33:e13545 10.1111/ctr.13545
REFERENCES
- 1. Kutinova A, Woodward Rs, Ricci Jf, Brennan Dc. The incidence and costs of sepsis and pneumonia before and after renal transplantation in the United States. Am J Transplant. 2006;6(1):129‐139. [DOI] [PubMed] [Google Scholar]
- 2. Giannella M, Muñoz P, Alarcón Jm, Mularoni A, Grossi P, Bouza E. Pneumonia in solid organ transplant recipients: a prospective multicenter study. Transpl Infect Dis. 2014;16(2):232‐241. [DOI] [PubMed] [Google Scholar]
- 3. Aguilar‐Guisado M, Givaldá J, Ussetti P, et al. Pneumonia after lung transplantation in the RESITRA Cohort: a multicenter prospective study. Am J Transplant. 2007;7(8):1989‐1996. [DOI] [PubMed] [Google Scholar]
- 4. Cisneros Jm, Munoz P, Torre‐Cisneros J, et al. Pneumonia after heart transplantation: a multi‐institutional study. Clin Infect Dis. 1998;27(2):324‐331. [DOI] [PubMed] [Google Scholar]
- 5. Cervera C, Agustí C, Angeles Marcos M, et al. Microbiologic features and outcome of pneumonia in transplanted patients. Diagn Microbiol Infect Dis. 2006;55(1):47‐54. [DOI] [PubMed] [Google Scholar]
- 6. Torres A, Xaubet A, Mas A, et al. Etiology and microbial patterns of pulmonary infiltrates in patients with orthotopic liver transplantation. Chest. 2000;117(2):494‐502. [DOI] [PubMed] [Google Scholar]
- 7. Weiss E, Dahmani S, Bert F, et al. Early‐onset pneumonia after liver transplantation: microbiological findings and therapeutic consequences. Liver Transpl. 2010;16(10):1178‐1185. [DOI] [PubMed] [Google Scholar]
- 8. Ikegami T, Shirabe K, Matono R, et al. Etiologies, risk factors, and outcomes of bacterial pneumonia after living donor liver transplantation. Liver Transpl. 2012;18(9):1060‐1068. [DOI] [PubMed] [Google Scholar]
- 9. Hoyo I, Linares L, Cervera C, et al. Epidemiology of pneumonia in kidney transplantation. Transplant Proc. 2010;42(8):2938‐2940. [DOI] [PubMed] [Google Scholar]
- 10. Kupeli E, Ulubay G, Colak T, et al. Pulmonary complications in renal recipients after transplantation. Transplant Proc. 2011;43(2):551‐553. [DOI] [PubMed] [Google Scholar]
- 11. Bonatti H, Pruett Tl, Brandacher G, et al. Pneumonia in solid organ recipients: spectrum of pathogens in 217 episodes. Transplant Proc. 2009;41(1):371‐374. [DOI] [PubMed] [Google Scholar]
- 12. Singh N, Gayowski T, Wagener M, Marino IR, Yu VL. Pulmonary infections in liver transplant recipients receiving tacrolimus. Changing pattern of microbial etiologies. Transplantation. 1996;61(3):396‐401. [DOI] [PubMed] [Google Scholar]
- 13. Jain S, Williams DJ, Arnold SR, et al. Community‐acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835‐845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jain S, Self WH, Wunderink RG, et al. Community‐acquired pneumonia requiring hospitalization among U.S. Adults. N Engl J Med. 2015;373(5):415‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fishman JA. Infection in solid‐organ transplant recipients. N Engl J Med. 2007;357(25):2601‐2614. [DOI] [PubMed] [Google Scholar]
- 16. Dorschner P, McElroy LM, Ison MG. Nosocomial infections within the first month of solid organ transplantation. Transpl Infect Dis. 2014;16(2):171‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neofytos D, Fishman Ja, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis. 2010;12(3):220‐229. [DOI] [PubMed] [Google Scholar]
- 18. Neofytos D, Chatzis O, et al. Epidemiology, risk factors and outcomes of invasive aspergillosis in solid organ transplant recipients in the Swiss Transplant Cohort Study. Transpl Infect Dis. 2018;20(4):e12898. [DOI] [PubMed] [Google Scholar]
- 19. López‐Medrano F, Fernández‐Ruiz M, Silva Jt, et al. Multinational case‐control study of risk factors for the development of late invasive pulmonary aspergillosis following kidney transplantation. Clin Microbiol Infect. 2018;24(2):192‐198. [DOI] [PubMed] [Google Scholar]
- 20. Primeggia J, Matsumoto Cs, Fishbein Tm, Karacki Ps, Fredette Tm, Timpone Jg. Infection among adult small bowel and multivisceral transplant recipients in the 30‐day postoperative period. Transpl Infect Dis. 2013;15(5):441‐448. [DOI] [PubMed] [Google Scholar]
- 21. Guaraldi G, Cocchi S, Codeluppi M, et al. Outcome, incidence, and timing of infectious complications in small bowel and multivisceral organ transplantation patients. Transplantation. 2005;80(12):1742‐1748. [DOI] [PubMed] [Google Scholar]
- 22. Danes C, Gonzalez‐Martin J, Pumarola T, et al. Pulmonary infiltrates in immunosuppressed patients: analysis of a diagnostic protocol. J Clin Microbiol. 2002;40(6):2134‐2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Knollmann Fd, Mäurer J, Bechstein Wo, Vogl Tj, Neuhaus P, Felix R. Pulmonary disease in liver transplant recipients: Spectrum of CT features. Acta Radiol. 2000;41(3):230‐236. [DOI] [PubMed] [Google Scholar]
- 24. Lee P, Minai OA, Mehta AC, DeCamp MM, Murthy S. Pulmonary nodules in lung transplant recipients: etiology and outcome. Chest. 2004;125(1):165‐172. [DOI] [PubMed] [Google Scholar]
- 25. Singh N, Alexander BD, Lortholary O, et al. Pulmonary cryptococcosis in solid organ transplant recipients: clinical relevance of serum cryptococcal antigen. Clin Infect Dis. 2008;46(2):e12‐e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abad C, Razonable RR. Mycobacterium tuberculosis after solid organ transplantation: a review of more than 2000 cases. Clin Transplant. 2018;32(6):e13259. [DOI] [PubMed] [Google Scholar]
- 27. Abdala E, Miller R, Pasqualotto AC, et al. Endemic fungal infection recommendations for solid‐organ transplant recipients and donors. Transplantation. 2018;102(2S Suppl 2):S52–S59. [DOI] [PubMed] [Google Scholar]
- 28. Hart J, Dyer Jr, Clark Bm, McLellan Dg, Perera S, Ferrari P. Travel‐related disseminated Penicillium marneffei infection in a renal transplant patient. Transpl Infect Dis. 2012;14(4):434–439. [DOI] [PubMed] [Google Scholar]
- 29. Jabbar Z, Han TM, Gagan F. Expect the unexpected: pleuro‐pulmonary melioidosis in a renal transplant recipient. Transpl Infect Dis. 2013;15(1):E40–E43. [DOI] [PubMed] [Google Scholar]
- 30. Yamshchikov AV, Schuetz A, Lyon GM. Rhodococcus equi infection. Lancet Infect Dis. 2010;10(5):350–359. [DOI] [PubMed] [Google Scholar]
- 31. Khoury JA, Bohl DL, Hersh MJ, Argoudelis AC, Brennan DC. Tularemia in a kidney transplant recipient: an unsuspected case and literature review. Am J Kidney Dis. 2005;45(5):926–929. [DOI] [PubMed] [Google Scholar]
- 32. La Rocca E, Gesu G, Caldara R, et al. Pulmonary infection caused by Rhodococcus equi in a kidney and pancreas transplant recipient: a case report. Transplantation. 1998;65(11):1524–1525. [DOI] [PubMed] [Google Scholar]
- 33. Kotton CN. Zoonoses in solid‐organ and hematopoietic stem cell transplant recipients. Clin Infect Dis. 2007;44(6):857–866. [DOI] [PubMed] [Google Scholar]
- 34. Sahathevan M, Harvey FA, Forbes G, et al. Epidemiology, bacteriology and control of an outbreak of Nocardia asteroides infection on a liver unit. J Hosp Infect. 1991;18(Suppl A):473–880. [DOI] [PubMed] [Google Scholar]
- 35. Obando‐Pacheco P, Justicia‐Grande AJ, Rivero‐Calle I, et al. Respiratory syncytial virus seasonality: a global overview. J Infect Dis. 2018;217(9):1356–1364. [DOI] [PubMed] [Google Scholar]
- 36. Newman LP, Bhat N, Fleming LP, Neuzil KM. Global influenza seasonality to inform country‐level vaccine programs: an analysis of WHO FluNet influenza surveillance data between 2011 and 2016. PLoS ONE. 2018;13(2):e0193263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Olarte L, Lin PL, Barson WJ, et al. Invasive pneumococcal infections in children following transplantation in the pneumococcal conjugate vaccine era. Transpl Infect Dis. 2017;19(1). [DOI] [PubMed] [Google Scholar]
- 38. Kumar D, Humar A, Plevneshi A, et al. Invasive pneumococcal disease in solid organ transplant recipients–10‐year prospective population surveillance. Am J Transplant. 2007;7(5):1209–1214. [DOI] [PubMed] [Google Scholar]
- 39. Longworth Sa, Vinnard C, Lee I, Sims Kd, Barton Td, Blumberg Ea. Risk factors for nontuberculous mycobacterial infections in solid organ transplant recipients: a case‐control study. Transpl Infect Dis. 2014;16(1):76–83. [DOI] [PubMed] [Google Scholar]
- 40. Majeed A, Beatty A, Iftikhar A, et al. A 20‐year experience with nocardiosis in solid organ transplant (SOT) recipients in the Southwestern United States: a single‐center study. Transpl Infect Dis. 2018;20(4):e12904. [DOI] [PubMed] [Google Scholar]
- 41. Coussement J, Lebeaux D, van Delden C, et al. Nocardia infection in solid organ transplant recipients: a multicenter European Case‐Control Study. Clin Infect Dis. 2016;63(3):338–345. [DOI] [PubMed] [Google Scholar]
- 42. Hoyo I, Sanclemente G, Cervera C, et al. Opportunistic pulmonary infections in solid organ transplant recipients. Transplant Proc. 2012;44(9):2673–2675. [DOI] [PubMed] [Google Scholar]
- 43. Pappas P, Alexander B, Andes D, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant‐Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50(8):1101–1111. [DOI] [PubMed] [Google Scholar]
- 44. Werbel WA, Ison MG, Angarone MP, Yang A, Stosor V. Lymphopenia is associated with late onset Pneumocystis jirovecii pneumonia in solid organ transplantation. Transpl Infect Dis. 2018;20(3):e12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mulpuru S, Knoll G, Weir C, et al. Pneumocystis pneumonia outbreak among renal transplant recipients at a North American transplant center: risk factors and implications for infection control. Am J Infect Control. 2016;44(4):425–431. [DOI] [PubMed] [Google Scholar]
- 46. Veronese G, Ammirati E, Moioli MC, et al. Single‐center outbreak of Pneumocystis jirovecii pneumonia in heart transplant recipients. Transpl Infect Dis. 2018;20(3):e12880. [DOI] [PubMed] [Google Scholar]
- 47. Assi M, Martin S, Wheat Lj, et al. Histoplasmosis after solid organ transplant. Clin Infect Dis. 2013;57(11):1542–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gassiep I, McDougall D, Douglas J, Francis R, Playford EG. Cryptococcal infections in solid organ transplant recipients over a 15‐year period at a state transplant center. Transpl Infect Dis. 2017;19(1). [DOI] [PubMed] [Google Scholar]
- 49. Forrest GN, Bhalla P, DeBess EE, et al. Cryptococcus gattii infection in solid organ transplant recipients: description of Oregon outbreak cases. Transpl Infect Dis. 2015;17(3):467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Camargo JF, Simkins J, Schain DC, et al. A cluster of donor‐derived Cryptococcus neoformans infection affecting lung, liver, and kidney transplant recipients: Case report and review of literature. Transpl Infect Dis. 2018;20(2):e12836. [DOI] [PubMed] [Google Scholar]
- 51. George IA, Santos C, Olsen MA, Powderly WG. Epidemiology of cryptococcosis and cryptococcal meningitis in a large retrospective cohort of patients after solid organ transplantation. Open Forum Infect Dis. 2017;4(1):ofx004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Humar A, Kumar D, Mazzulli T, et al. A surveillance study of adenovirus infection in adult solid organ transplant recipients. Am J Transplant. 2005;5(10):2555–2559. [DOI] [PubMed] [Google Scholar]
- 53. Watcharananan Sp, Avery R, Ingsathit A, et al. Adenovirus disease after kidney transplantation: course of infection and outcome in relation to blood viral load and immune recovery. Am J Transplant. 2011;11(6):1308–1314. [DOI] [PubMed] [Google Scholar]
- 54. Kumar D, Ferreira VH, Blumberg E, et al. A five‐year prospective multi‐center evaluation of influenza infection in transplant recipients. Clin Infect Dis. 2018;67(9):1322-1329 [DOI] [PubMed] [Google Scholar]
- 55. Danziger‐Isakov L, Steinbach WJ, Paulsen G, et al. A multicenter consortium to define the epidemiology and outcomes of pediatric solid organ transplant recipients with inpatient respiratory virus infection. J Pediatric Infect Dis Soc. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pilie P, Werbel Wa, Riddell J, Shu X, Schaubel D, Gregg Ks. Adult patients with respiratory syncytial virus infection: impact of solid organ and hematopoietic stem cell transplantation on outcomes. Transpl Infect Dis. 2015;17(4):551–557. [DOI] [PubMed] [Google Scholar]
- 57. Koo HJ, Lee HN, Choi SH, et al. Human metapneumovirus infection: pneumonia risk factors in patients with solid organ transplantation and computed tomography findings. Transplantation. 2018;102(4):699–706. [DOI] [PubMed] [Google Scholar]
- 58. Chu Hy, Renaud C, Ficken E, Thomson B, Kuypers J, Englund Ja. Respiratory tract infections due to human metapneumovirus in immunocompromised children. J Pediatric Infect Dis Soc. 2014;3(4):286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vilchez RA, McCurry K, Dauber J, et al. Influenza virus infection in adult solid organ transplant recipients. Am J Transplant. 2002;2(3):287–291. [DOI] [PubMed] [Google Scholar]
- 60. Morris DE, Cleary DW, Clarke SC. Secondary bacterial infections associated with influenza pandemics. Front Microbiol. 2017;8:1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bridevaux P‐O, Aubert J‐d, Soccal Pm, et al. Incidence and outcomes of respiratory viral infections in lung transplant recipients: a prospective study. Thorax. 2014;69(1):32–38. [DOI] [PubMed] [Google Scholar]
- 62. Liu M, Worley S, Arrigain S, et al. Respiratory viral infections within one year after pediatric lung transplant. Transpl Infect Dis. 2009;11(4):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Peghin M, Hirsch Hh, Len Ó, et al. Epidemiology and immediate indirect effects of respiratory viruses in lung transplant recipients: a 5‐year prospective study. Am J Transplant. 2017;17(5):1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barcan La, Dallurzo Ml, Clara Lo, et al. Toxoplasma gondii pneumonia in liver transplantation: survival after a severe case of reactivation. Transpl Infect Dis. 2002;4(2):93–96. [DOI] [PubMed] [Google Scholar]
- 65. Patrat‐Delon S, Gangneux J‐p, Lavoue S, et al. Correlation of parasite load determined by quantitative PCR to clinical outcome in a heart transplant patient with disseminated toxoplasmosis. J Clin Microbiol. 2010;48(7):2541–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rodriguez‐Hernandez MJ, Ruiz‐Perez‐Pipaon M, Cañas E, Bernal C, Gavilan F. Strongyloides stercoralis hyperinfection transmitted by liver allograft in a transplant recipient. Am J Transplant. 2009;9(11):2637–2640. [DOI] [PubMed] [Google Scholar]
- 67. Le M, Ravin K, Hasan A, et al. Single donor‐derived strongyloidiasis in three solid organ transplant recipients: case series and review of the literature. Am J Transplant. 2014;14(5):1199–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kumarasinghe G, Lavee O, Parker A, et al. Post‐transplant lymphoproliferative disease in heart and lung transplantation: defining risk and prognostic factors. J Heart Lung Transplant. 2015;34(11):1406–1414. [DOI] [PubMed] [Google Scholar]
- 69. Parrish A, Fenchel M, Storch GA, et al. Epstein‐Barr viral loads do not predict post‐transplant lymphoproliferative disorder in pediatric lung transplant recipients: A multicenter prospective cohort study. Pediatr Transplant. 2017;21(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mucha K, Foroncewicz B, Palczewski P, et al. Pulmonary post‐transplant lymphoproliferative disorder with a CT halo sign. Ann Transplant. 2013;18:482–487. [DOI] [PubMed] [Google Scholar]
- 71. Lopez P, Kohler S, Dimri S. Interstitial lung disease associated with mTOR inhibitors in solid organ transplant recipients: results from a large phase III clinical trial program of everolimus and review of the literature. J Transplant. 2014;2014:305931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sigel K, Veluswamy R, Krauskopf K, et al. Lung cancer prognosis in elderly solid organ transplant recipients. Transplantation. 2015;99(10):2181–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Gadre S, Kotloff RM. Noninfectious pulmonary complications of liver, heart, and kidney transplantation: an update. Clin Chest Med. 2017;38(4):741–749. [DOI] [PubMed] [Google Scholar]
- 74. Canet E, Osman D, Lambert J, et al. Acute respiratory failure in kidney transplant recipients: a multicenter study. Crit Care. 2011;15(2):R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bradley JS, Byington CL, Shah SS, et al. The management of community‐acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kalil AC, et al. Management of adults with hospital‐acquired and ventilator‐associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis. 2016;63(5):575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Miller JM, Binnicker MJ, Campbell S, et al. A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the infectious diseases society of America and the American society for microbiology. Clin Infect Dis. 2018;67(6):813‐816. [DOI] [PubMed] [Google Scholar]
- 79. Loke Yk, Kwok Cs, Niruban A, Myint Pk. Value of severity scales in predicting mortality from community‐acquired pneumonia: systematic review and meta‐analysis. Thorax. 2010;65(10):884–890. [DOI] [PubMed] [Google Scholar]
- 80. Gonzalez C, Johnson T, Rolston K, Merriman K, Warneke C, Evans S. Predicting pneumonia mortality using CURB‐65, PSI, and patient characteristics in patients presenting to the emergency department of a comprehensive cancer center. Cancer Med. 2014;3(4):962–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Siegel JD, Rhinehart E, Jackson M, et al. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 Suppl 2):S65–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sivagnanam S, Podczervinski S, Butler‐Wu SM, et al. Legionnaires' disease in transplant recipients: a 15‐year retrospective study in a tertiary referral center. Transpl Infect Dis. 2017;19(5). [DOI] [PubMed] [Google Scholar]
- 83. Kumar D, Michaels MG, Morris MI, et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid‐organ transplants: a multicentre cohort study. Lancet Infect Dis. 2010;10(8):521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Doan QH, Kissoon N, Dobson S, et al. A randomized, controlled trial of the impact of early and rapid diagnosis of viral infections in children brought to an emergency department with febrile respiratory tract illnesses. J Pediatr. 2009;154(1):91–95. [DOI] [PubMed] [Google Scholar]
- 85. Byington CL, Castillo H, Gerber K, et al. The effect of rapid respiratory viral diagnostic testing on antibiotic use in a children's hospital. Arch Pediatr Adolesc Med. 2002;156(12):1230–1234. [DOI] [PubMed] [Google Scholar]
- 86. Huang DT, Yealy DM, Filbin MR, et al. Procalcitonin‐guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018;379(3):236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Yu X‐y, Wang Y, Zhong H, Dou Q‐l, Song Y‐l, Wen H. Diagnostic value of serum procalcitonin in solid organ transplant recipients: a systematic review and meta‐analysis. Transplant Proc. 2014;46(1):26–32. [DOI] [PubMed] [Google Scholar]
- 88. Cooper D, Sharples L, Cornelissen J, et al. Comparison between procalcitonin, serum amyloid A, and C‐reactive protein as markers of serious bacterial and fungal infections after solid organ transplantation. Transplant Proc. 2001;33(1–2):1808–1810. [DOI] [PubMed] [Google Scholar]
- 89. Cousin VL, Lambert K, Trabelsi S, et al. Procalcitonin for infections in the first week after pediatric liver transplantation. BMC Infect Dis. 2017;17(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Madershahian N, Wittwer T, Strauch J, et al. Kinetic of procalcitonin in the early postoperative course following heart transplantation. J Card Surg. 2008;23(5):468–473. [DOI] [PubMed] [Google Scholar]
- 91. Suberviola B, Rellan L, Riera J, et al. Role of biomarkers in early infectious complications after lung transplantation. PLoS ONE. 2017;12(7):e0180202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Suberviola B, Castellanos‐Ortega A, Ballesteros Ma, Zurbano F, Naranjo S, Miñambres E. Early identification of infectious complications in lung transplant recipients using procalcitonin. Transpl Infect Dis. 2012;14(5):461–467. [DOI] [PubMed] [Google Scholar]
- 93. Desmard M, Benbara A, Boudinet S, et al. Post‐operative kinetics of procalcitonin after lung transplantation. J Heart Lung Transplant. 2015;34(2):189–194. [DOI] [PubMed] [Google Scholar]
- 94. Kaido T, Ogawa K, Fujimoto Y, et al. Perioperative changes of procalcitonin levels in patients undergoing liver transplantation. Transpl Infect Dis. 2014;16(5):790–796. [DOI] [PubMed] [Google Scholar]
- 95. Sandkovsky U, Kalil AC, Florescu DF. The use and value of procalcitonin in solid organ transplantation. Clin Transplant. 2015;29(8):689–696. [DOI] [PubMed] [Google Scholar]
- 96. Tabarsi P, Soraghi A, Marjani M, et al. Comparison of serum and bronchoalveolar lavage galactomannan in diagnosing invasive aspergillosis in solid‐organ transplant recipients. Exp Clin Transplant. 2012;10(3):278–281. [DOI] [PubMed] [Google Scholar]
- 97. Clancy Cj, Jaber Ra, Leather Hl, et al. Bronchoalveolar lavage galactomannan in diagnosis of invasive pulmonary aspergillosis among solid‐organ transplant recipients. J Clin Microbiol. 2007;45(6):1759–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Alexander BD, Smith PB, Davis RD, et al. The (1,3){beta}‐D‐glucan test as an aid to early diagnosis of invasive fungal infections following lung transplantation. J Clin Microbiol. 2010;48(11):4083–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mutschlechner W, Risslegger B, Willinger B, et al. Bronchoalveolar lavage fluid (1,3)beta‐D‐glucan for the diagnosis of invasive fungal infections in solid organ transplantation: a Prospective Multicenter Study. Transplantation. 2015;99(9):e140–e144. [DOI] [PubMed] [Google Scholar]
- 100. Levesque E, El Anbassi S, Sitterle E, Foulet F, Merle Jc, Botterel F. Contribution of (1,3)‐beta‐D‐glucan to diagnosis of invasive candidiasis after liver transplantation. J Clin Microbiol. 2015;53(3):771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vélez L, Correa LT, Maya MA, et al. Diagnostic accuracy of bronchoalveolar lavage samples in immunosuppressed patients with suspected pneumonia: analysis of a protocol. Respir Med. 2007;101(10):2160–2167. [DOI] [PubMed] [Google Scholar]
- 102. Lehto JT, Anttila V‐J, Lommi J, et al. Clinical usefulness of bronchoalveolar lavage in heart transplant recipients with suspected lower respiratory tract infection. J Heart Lung Transplant. 2004;23(5):570–576. [DOI] [PubMed] [Google Scholar]
- 103. Kupeli E, Akcay S, Ulubay G, Ozyurek Ba, Ozdemirel Ts, Haberal M. Diagnostic utility of flexible bronchoscopy in recipients of solid organ transplants. Transplant Proc. 2011;43(2):543–546. [DOI] [PubMed] [Google Scholar]
- 104. Husain S, Paterson DL, Studer SM, et al. Aspergillus galactomannan antigen in the bronchoalveolar lavage fluid for the diagnosis of invasive aspergillosis in lung transplant recipients. Transplantation. 2007;83(10):1330–1336. [DOI] [PubMed] [Google Scholar]
- 105. Tomotani D, Bafi AT, Pacheco ES, et al. The diagnostic yield and complications of open lung biopsies in kidney transplant patients with pulmonary disease. J Thorac Dis. 2017;9(1):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Defranchi S, Bertolotti AM, Vigliano CA, et al. Open lung biopsy for diffuse disease in patients with and without previously transplanted solid organs. Ann Thorac Surg. 2010;90(3):965–971; discussion 971–2. [DOI] [PubMed] [Google Scholar]
- 107. Hsu JL, Kuschner WG, Paik J, Bower N, Vazquez Guillamet MC, Kothary N. The diagnostic yield of CT‐guided percutaneous lung biopsy in solid organ transplant recipients. Clin Transplant. 2012;26(4):615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hamandi B, Holbrook AM, Humar A, et al. Delay of adequate empiric antibiotic therapy is associated with increased mortality among solid‐organ transplant patients. Am J Transplant. 2009;9(7):1657–1665. [DOI] [PubMed] [Google Scholar]


