Summary
Entry of enveloped viruses into host cells depends on the interactions of viral surface proteins with cell surface receptors. Many enveloped viruses maximize the efficiency of receptor engagement by first binding to attachment‐promoting factors, which concentrate virions on target cells and thus increase the likelihood of subsequent receptor engagement. Cellular lectins can recognize glycans on viral surface proteins and mediate viral uptake into immune cells for subsequent antigen presentation. Paradoxically, many viral and non‐viral pathogens target lectins to attach to immune cells and to subvert cellular functions to promote their spread. Thus, it has been proposed that attachment of HIV to the dendritic cell lectin DC‐SIGN enables the virus to hijack cellular transport processes to ensure its transmission to adjacent T cells. However, recent studies show that the consequences of viral capture by immune cell lectins can be diverse, and can entail negative and positive regulation of viral spread. Here, we will describe key concepts proposed for the role of lectins in HIV attachment to host cells, and we will discuss recent findings in this rapidly evolving area of research.
Viruses need to access host cells to propagate and spread. The plasma membrane constitutes a physical barrier to infection, and enveloped viruses evolved specialized surface proteins (also termed envelope‐ or glycoproteins) to overcome this obstacle. These proteins mediate binding of viruses to host cells and subsequent fusion of the viral and a limiting host cell membrane, which allows the delivery of viral nucleic acid and protein into the host cell cytoplasm (Harrison, 2008). The first essential step in the entry cascade is the cognate binding of viral envelope proteins to components of the host cell surface, termed viral receptors (Harrison, 2008). Receptor binding can limit infection efficiency, for example when receptor expression levels and/or receptor affinity of the viral envelope protein are low (Bannert et al., 2000). To circumvent this limitation, viruses can also engage cellular attachment factors, which promote viral binding to the cell surface and thus increase the possibility of successful receptor engagement and infectious entry. For instance, HIV can incorporate cellular proteins into its envelope, which interact with binding partners on target cells and thereby augment viral attachment and entry (Cantin et al., 2005).
Dendritic cells are professional antigen presenting cells, which can initiate primary and stimulate memory immune responses. Immature dendritic cells are particularly adept in antigen capture, while mature dendritic cells efficiently present antigen to T cells. The major dendritic cell subsets in human blood are myeloid and plasmacytoid dendritic cells, which can produce high amounts of IL‐12 and IFN‐α respectively (Wu and KewalRamani, 2006). Dendritic cells of myeloid origin also line body surfaces and attachment of HIV to these cells in the anogenital mucosa might play a prominent role in the sexual transmission of HIV, the major route of viral spread, as discussed below. Initial evidence for an important role of myeloid dendritic cells in HIV transmission came from cell culture studies demonstrating that mature, myeloid dendritic cells isolated from blood boost HIV infection of cocultured T cells without becoming infected (trans‐infection) (Cameron et al., 1992). Subsequent studies extended these findings to monocyte‐derived immature and mature dendritic cells (reviewed by Wu and KewalRamani, 2006), model systems for myeloid dendritic cells in blood and tissues. A molecular basis for HIV trans‐infection by immature dendritic cells was provided by Geijtenbeek and colleagues, who showed that these cells express the lectin DC‐SIGN (for dendritic cell‐specific intercellular adhesion molecule 3‐grabbing nonintegrin), and that DC‐SIGN binds to glycans on HIVEnv and facilitates trans‐infection of adjacent T cells (Geijtenbeek et al., 2000). Based on these findings, and taking into account the natural ability of dendritic cells to migrate from the periphery into lymphoid tissue, it was proposed that sexually transmitted HIV hijacks submucosal dendritic cells via DC‐SIGN to promote its dissemination, a concept referred to as the Trojan horse model (Geijtenbeek et al., 2000). Reports that other viruses and non‐viral pathogens exploit DC‐SIGN on dendritic cells to augment their spread (Table 1) suggested that the Trojan horse model might be paradigmatic for the host invasion by a broad spectrum of pathogens. Recent work challenged important features of this model, but also demonstrated new mechanisms how pathogens can target DC‐SIGN to propagate.
Table 1.
Microbial ligands of DC‐SIGN.
In the present review, we will introduce potential consequences of HIV interactions with DC‐SIGN and other immune cell lectins and we will discuss recent developments in the field. Our focus will be on HIV, since important mechanisms underlying DC‐SIGN‐dependent augmentation of pathogen spread have been established with this virus, and apply to other viruses as well.
DC‐SIGN on dendritic cells and HIV trans‐infection: progress and pitfalls
DC‐SIGN is expressed by monocyte‐derived dendritic cells (MDDCs) and by dendritic cells in mucosal and lymphoid tissues, although concerns have been raised that DC‐SIGN‐positive cells found in some tissues might indeed be macrophages (Granelli‐Piperno et al., 2005; Gurney et al., 2005). In addition, expression of DC‐SIGN outside the macrophage/dendritic cell lineage has been noted; for instance a subset of B cells expresses DC‐SIGN (Rappocciolo et al., 2006a). DC‐SIGN is a type II transmembrane protein, in which the following domains have been identified: a cytoplasmic domain, a transmembrane domain, a neck‐region and a lectin domain. The cytoplasmic domain contains motifs involved in receptor internalization and signalling, while the neck region facilitates DC‐SIGN tetramerization, which is required for high‐affinity ligand binding (Serrano‐Gomez et al., 2008; Tabarani et al., 2009). Finally, the lectin domain, which requires calcium for its structural integrity (C‐type), binds to high‐mannose and fucose residues on pathogens and cellular proteins (Guo et al., 2004).
Trans‐infection was reported to depend on DC‐SIGN‐mediated binding and cellular uptake of HIV into dendritic cells (Geijtenbeek et al., 2000; Kwon et al., 2002), followed by intracellular transport of virions to sites of dendritic cell–T cell contact, termed infectious synapses (McDonald et al., 2003) (Fig. 1). At the T cell site of the infectious synapse CD4 and coreceptor are concentrated (McDonald et al., 2003), resulting in the establishment of a microenvironment ideally suited for HIV trans‐infection. Collectively, these results suggest that HIV exploits mechanisms normally used for antigen presentation by dendritic cells to increase its infectivity for adjacent T cells. In the following, we will review recent work examining key features of this concept.
Figure 1.
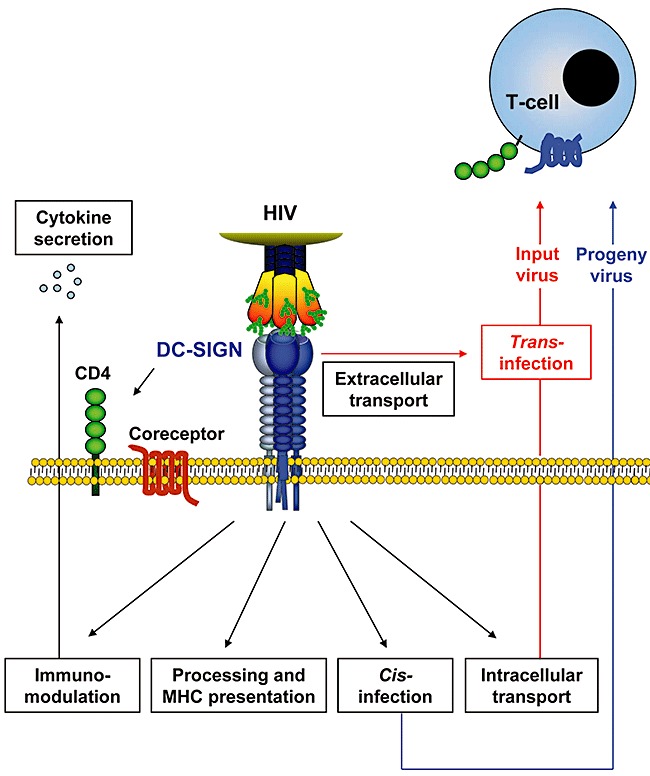
Consequences of HIV attachment to DC‐SIGN on dendritic cells. Engagement of DC‐SIGN on dendritic cells can promote infection of adjacent target cells in two ways. First, DC‐SIGN can augment productive infection of dendritic cells, a process termed cis‐infection, and progeny virions released from the dendritic cells can infect nearby target cells. Second, dendritic cells can transfer captured virus (input virus) to adjacent target cells without becoming productively infected, a pathway termed trans‐infection. Trans‐infection occurs at infectious synapses, contact points between dendritic cells and T cells, and HIV might traffic to infectious synapses via intracellular and extracellular routes. The latter trafficking pathway might involve transport of HIV in intracellular but surface connected compartments. In addition, viruses bound to DC‐SIGN can be internalized and processed for MHC presentation. Finally, DC‐SIGN engagement can trigger signal transduction, which modulates TLR‐dependent cytokine production.
Sticky and sweet: several lectins mediate HIV binding to dendritic cells
Initial work suggested that DC‐SIGN was required for efficient MDDC‐mediated HIV trans‐infection (Geijtenbeek et al., 2000). Albeit this finding is controversial (Gummuluru et al., 2003; Boggiano et al., 2007), a number of reports indicate that blockade of DC‐SIGN by either antibodies, carbohydrates (Baribaud et al., 2002; Wu et al., 2002; Wang et al., 2007a) or RNAi (2004a, 2004b) indeed diminishes HIV trans‐infection (Gurney et al., 2005). In addition, DC‐SIGN promotes HIV dissemination by migratory cells from cervical explants (Hu et al., 2004). However, the indicated role of DC‐SIGN in HIV trans‐infection by dendritic cells is not universal. Thus, different types of dendritic cells employ different C‐type lectins, DC‐SIGN, mannose receptor (MR) and langerin, as well as CD4 for binding to HIV Env, and dendritic cell maturation shifts Env capture from lectins to CD4 (Turville et al., 2002). Accordingly, DC‐SIGN is believed to contribute to HIV trans‐infection mediated by immature but not by mature dendritic cells (Izquierdo‐Useros et al., 2007; Wang et al., 2007a), although one study reached a different conclusion (Baribaud et al., 2002), and coexpression of CD4 was found to diminish DC‐SIGN‐mediated trans‐infection, possibly by facilitating uptake and transport of HIV into late endosomal compartments (Wang et al., 2007b). The list of lectins involved in HIV attachment to dendritic cells was recently expanded to include the C‐type lectin DCIR (dendritic cell immunoreceptor), but the relative contributions of DC‐SIGN and DCIR to HIV trans‐infection remain to be established (Lambert et al., 2008). Finally, HIV attachment to dendritic cells can also proceed in a lectin‐ and CD4‐independent fashion (de Witte et al., 2007a; Hatch et al., 2009; Izquierdo‐Useros et al., 2009). Thus, dendritic cells have multiple means to capture HIV, and the prevalent mode of viral binding depends on the origin and the maturation status of the cells.
DC‐SIGN‐dependent HIV uptake and trafficking in dendritic cells: degradation versus trans‐infection
Kwon and colleagues found that HIV transfer to T cells by DC‐SIGN‐positive cells was dependent on lectin‐mediated viral internalization into low pH compartments (Kwon et al., 2002), in which viral infectivity was preserved (Geijtenbeek et al., 2000; Kwon et al., 2002) (Fig. 1). An LL motif in the cytoplasmic tail of DC‐SIGN facilitates ligand endocytosis by DC‐SIGN (Engering et al., 2002). However, a contribution of the LL motif to HIV transmission by DC‐SIGN expressing cell lines was not observed by a subsequent study (Burleigh et al., 2006), and the role of a low pH compartment in HIV trans‐infection has been questioned (Nobile et al., 2005; Wang et al., 2007a). The former observation is in agreement with work demonstrating that DC‐SIGN contributes to HIV uptake into dendritic cells, but targets the majority of internalized virions for degradation and MHC presentation (2004, 2006) (Fig. 1). The identification of LSP1 as a cellular binding partner of the cytoplasmic tail of DC‐SIGN further supports such a scenario (Smith et al., 2007). Thus, normal expression of LSP1 diverts HIV into the proteasome of dendritic cells, while LSP1 knock‐down increases trans‐infection (Smith et al., 2007).
Regardless of the role of DC‐SIGN, it is undisputed that HIV is efficiently taken up into dendritic cells and important differences between immature and mature dendritic cells have been noted (Frank et al., 2002; Wang et al., 2007a). Thus, virions taken up into mature dendritic cells accumulate in large endocytic compartments, which resemble structures seen in macrophages upon HIV uptake by macropinocytosis (Wang et al., 2007a). In comparison, virions associated with immature dendritic cells are mostly found close to the cell surface, with only a few virions being present in intracellular vesicles, which exhibit a clathrin‐coat (Wang et al., 2007a). Notably, the ability to traffic HIV into deep intracellular compartments was found to correlate with protection of virus from proteases, and inhibitors of intracellular trafficking and cytoskeleton integrity were shown to inhibit trans‐infection (2007a, 2008b), in agreement with the proposal that HIV traffics intracellularly, via a tetraspanin‐sorting pathway, to infectious synapses (Garcia et al., 2005). In contrast, a separate study postulated that virions reach infectious synapses exclusively by transport on the cell surface instead of travelling along intracellular routes (Cavrois et al., 2007). In agreement with this scenario, it was reported that HIV is routed towards the infectious synapse in a surface accessible, intracellular compartment (Yu et al., 2008). Irrespective of the route of HIV trafficking, there is ample evidence that HIV capture does not preserve viral infectivity (Turville et al., 2004; Nobile et al., 2005; Burleigh et al., 2006; Wang et al., 2007a), with the initially postulated conservation of viral infectivity likely being due to productive infection of the transmitting cells (Nobile et al., 2005; Burleigh et al., 2006).
Collectively, HIV trans‐infection driven by dendritic cells is short lived (hours) and DC‐SIGN on immature dendritic cells contributes to this process in at least two ways: DC‐SIGN promotes capture and potentially uptake of virions subsequently transferred to T cells. In addition, DC‐SIGN seems to stimulate the formation of infectious synapses by a so far incompletely understood mechanism (Arrighi et al., 2004a).
DC‐SIGN signals dendritic cells to transmit HIV
Recent studies revealed a novel facet of HIV interactions with DC‐SIGN on dendritic cells, the modulation of the immune response. It was demonstrated that binding of HIV to MDDCs induces ERK‐dependent signal transduction, which correlates with production of the immunosuppressive cytokine IL‐10 and compromised maturation of dendritic cells (Shan et al., 2007). These effects were dependent on appropriate Env glycosylation and on C‐type lectin expression, and a role of DC‐SIGN was postulated (Shan et al., 2007). Removal of mannose‐rich glycans from Env improved immunogenicity of the protein (Banerjee et al., 2009), further pointing towards a role of mannose‐specific lectins in immune responses shaped by dendritic cells. In agreement with these findings, HIV binding to DC‐SIGN on dendritic cells was demonstrated to induce signalling via the Rho guanine nucleotide–exchange factor LARG and the small GTPase RhoA, which results in aberrant dendritic cell maturation (Hodges et al., 2007). Thus, the cells fail to upregulate CD86 and MHC II but readily form infectious synapses with T cells (Hodges et al., 2007), indicating that HIV signalling via DC‐SIGN compromises the immune function of dendritic cells and simultaneously primes the cells for trans‐infection. A separate study showed that binding of HIV to DC‐SIGN induces signals via a multi‐protein complex, including the kinase Raf‐1, which modulates TLR‐induced cytokine production by regulating acetylation of the NFκB subunit p65 (Gringhuis et al., 2007). Interestingly, activation of Raf‐1 was dependent on LARG and RhoA and on the carbohydrate profile of the pathogen bound to DC‐SIGN. Thus, HIV and Mycobacterium tuberculosis, which bound to DC‐SIGN via high‐mannose residues, activated Raf‐1 signalling and induced production of a cytokine profile different from that triggered by Helicobacter pylori, which was due to recognition of fucose containing structures and did not involve Raf‐1 activation (Gringhuis et al., 2009) (1, 2). Finally, signalling via TLR8 and DC‐SIGN was required for NFκB‐dependent recruitment of the transcription factor pTEF‐b to the viral promoter, and thus for the generation of full‐length HIV transcripts in dendritic cells – a prerequisite for productive infection (Gringhuis et al., 2010) (Fig. 2). However, HIV infection of dendritic cells is inefficient compared with T cells and macrophages (reviewed by Wu and KewalRamani, 2006), and a contribution of this mechanism to viral spread in vivo remains to be established. In sum, DC‐SIGN has signalling capacity, which can be exploited by pathogens to modulate immune responses and to establish productive infection of dendritic cells and adjacent target cells.
Figure 2.
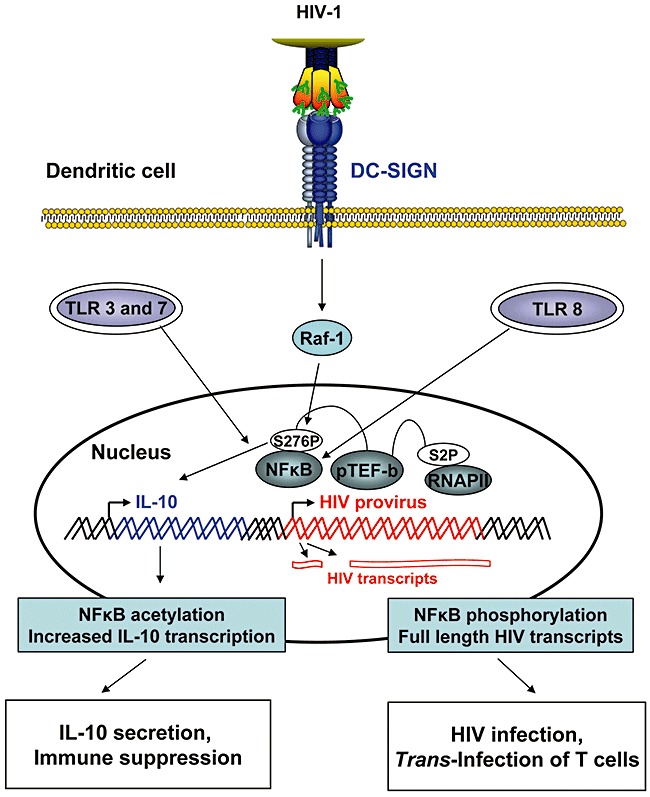
HIV induces signalling via DC‐SIGN, which promotes dendritic cell infection and release of immunosuppressive cytokines. Triggering TLR‐3 or TLR‐7 induces NFκB activation. Concomitant engagement of DC‐SIGN by HIV triggers Raf‐1‐dependent signalling, which results in phosphorylation of Ser276 of the NFκB subunit p65. Phosphorylation of Ser276 in turn induces acetylation of lysines in p65, which increases and prolongs transcription of the IL‐10 gene. IL‐10 inhibits the Th1‐mediated response and incapacitates the antigen presentation capabilities of dendritic cells. Induction of TLR‐8 signalling by HIV activates NFκB and allows synthesis of short HIV transcripts. Parallel triggering of DC‐SIGN signalling by HIV induces phosphorylation of the p65 subunit of NFκB at Ser276. This phosphorylation event allows recruitment of the transcription‐elongation factor pTEF‐b to the HIV promoter, which phosphorylates RNA polymerase II at Ser2. Phosphorylation increases processivity of the enzyme and thus allows synthesis of full‐length HIV transcripts.
Langerin on langerhans cells: a roadblock to HIV transmission?
Langerhans cells are located in the top layer of the mucosa and are most likely the first cell type to come in contact with sexually transmitted HIV. Langerhans cells are DC‐SIGN‐negative but express CD4 and the C‐type lectin langerin (Soilleux and Coleman, 2001). Pioneer work by de Witte and colleagues provided evidence that langerin constitutes a defence mechanism against HIV invasion (de Witte et al., 2007b). Thus, langerin binds to Env and targets bound virions into Birbeck granules, an intracellular compartment specific to Langerhans cells, where the virus is degraded (de Witte et al., 2007b). Counter‐intuitively, however, C‐type lectins were reported to play a minor role in HIV entry into vaginal Langerhans cells (Hladik et al., 2007), and examination of skin explants inoculated with HIV indicated HIV infection of Langerhans cells, but not other types of dendritic cells, and transfer of virus from Langerhans cells to T cells (Kawamura et al., 2008). The latter observations suggest that infection of Langerhans cells might be an important early event in HIV transmission. Such a scenario can be reconciled with the findings of de Witte and colleagues, when taking into account that the barrier imposed by langerin can be overcome by high doses of virus (de Witte et al., 2007b) and that TNF‐α, produced upon genital coinfections, like candida albicans, might increase permissiveness of Langerhans cells to HIV infection (de Jong et al., 2008b) – hypotheses that should be tested in animal models.
DC‐SIGN: target for many viruses
DC‐SIGN is targeted by different viruses which all contain envelope proteins with an appropriate glycan signature. Three major consequences of viral engagement of DC‐SIGN have been described: first, DC‐SIGN can function as a bona fide entry receptor. In this case, expression of DC‐SIGN is sufficient to render cells susceptible to infection. Such a scenario has been suggested, e.g. for dendritic cell infection by human herpesvirus 8 (HHV‐8) (Rappocciolo et al., 2008), the causative agent of Karposi sarcoma and for Ebolavirus infection of T cells (Alvarez et al., 2002). However, it is technically challenging to discriminate between entry mediated solely by DC‐SIGN (receptor function) and entry augmented by DC‐SIGN but facilitated by a coexpressed receptor, a process termed cis‐infection (Lee et al., 2001). In fact, DC‐SIGN most likely functions as an attachment factor in the context of Ebolavirus entry into lymphoid cells (Marzi et al., 2007). Second, DC‐SIGN might augment cis‐infection, as described above. DC‐SIGN‐driven cis‐infection has been established for dengue virus infection of dendritic cells, which are important early targets of this pathogen (Lozach et al., 2005). Polymorphisms in the DC‐SIGN gene were shown to impact disease development, suggesting that cis‐infection might facilitate viral spread in patients (Sakuntabhai et al., 2005). Third, DC‐SIGN can promote trans‐infection of target cells. While this route has first been established for HIV, trans‐infection of several viruses by DC‐SIGN has been demonstrated, including Measles virus (de Witte et al., 2008) and Ebolavirus (Alvarez et al., 2002), and might promote transmission of these pathogens. Whether DC‐SIGN engagement by viruses other than HIV also modulates cytokine release and thus impacts the establishment of an immune response remains to be determined.
Conclusions
Detailed information on the processes ensuing exposure of anogenital mucosa to sexually transmitted HIV is indispensable to understand viral dissemination and pathogenesis and to develop effective antiviral strategies. There is continued and well founded belief that dendritic cells play an important role in the establishment of HIV infection. However, the idea of the role of DC‐SIGN in HIV interactions with dendritic cells has changed considerably (Fig. 1). It has become clear that dendritic cells have several means to bind and transfer HIV to cells, with C‐type lectins being one of them. In addition, an appreciable protection of HIV from antiviral agents upon attachment to dendritic cells is under discussion, and the concept of long‐term storage and subsequent regurgitation of infectious HIV by dendritic cells had to be abandoned. On the other hand, DC‐SIGN‐mediated HIV trans‐infection, although found to be a short‐lived process, and cis‐infection might promote viral amplification in the genital mucosal, which could be critical for the establishment of the primary infection. Conversely, langerin on Langerhans cells was discovered to target HIV for degradation, suggesting a barrier function of this lectin. Finally, intriguing new findings revealed a signalling capacity of DC‐SIGN (and other lectins), which is exploited by HIV to compromise immune defences and to promote its spread. Vaccine development should therefore encompass the optimization of the glycan profiles of candidate substances, and new strategies for immunotherapy can be envisioned. Ultimately, key concepts need to be evaluated in improved cell culture systems and animal models for sexual transmission of HIV. An important but often overlooked parameter determining outcome and significance of such experiments is the source of the virus. Glycosylation of HIV depends on the producer cell type, and viruses generated in macrophages are unlikely to be detected efficiently by C‐type lectins on mucosal dendritic cells (Lin et al., 2003). Consequently, these viruses might overcome the mucosal barrier with different efficiency and potentially by employing different strategies compared with viruses generated in T cells – and similar considerations apply to most if not all other DC‐SIGN ligands.
Acknowledgements
We thank TF Schulz for support and the BMBF (grant 01KI 0703) and DFG (SFB 900) for funding. We apologize to all colleagues whose work could not be cited because of space constraints.
References
- Alvarez, C.P. , Lasala, F. , Carrillo, J. , Muniz, O. , Corbi, A.L. , and Delgado, R. (2002) C‐type lectins DC‐SIGN and L‐SIGN mediate cellular entry by Ebola virus in cis and in trans . J Virol 76: 6841–6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi, J.F. , Pion, M. , Garcia, E. , Escola, J.M. , van Kooyk, Y. , Geijtenbeek, T.B. , and Piguet, V. (2004a) DC‐SIGN‐mediated infectious synapse formation enhances X4 HIV‐1 transmission from dendritic cells to T cells. J Exp Med 200: 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrighi, J.F. , Pion, M. , Wiznerowicz, M. , Geijtenbeek, T.B. , Garcia, E. , Abraham, S. , et al (2004b) Lentivirus‐mediated RNA interference of DC‐SIGN expression inhibits human immunodeficiency virus transmission from dendritic cells to T cells. J Virol 78: 10848–10855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, K. , Andjelic, S. , Klasse, P.J. , Kang, Y. , Sanders, R.W. , Michael, E. , et al (2009) Enzymatic removal of mannose moieties can increase the immune response to HIV‐1 gp120 in vivo. Virology 389: 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannert, N. , Schenten, D. , Craig, S. , and Sodroski, J. (2000) The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T‐tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses. J Virol 74: 10984–10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribaud, F. , Pöhlmann, S. , Leslie, G. , Mortari, F. , and Doms, R.W. (2002) Quantitative expression and virus transmission analysis of DC‐SIGN on monocyte‐derived dendritic cells. J Virol 76: 9135–9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro, L.B. , Quach, H. , Krahenbuhl, J. , Khaliq, S. , Mohyuddin, A. , Mehdi, S.Q. , et al (2006) DC‐SIGN interacts with Mycobacterium leprae but sequence variation in this lectin is not associated with leprosy in the Pakistani population. Hum Immunol 67: 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman, M.P. , Engering, A. , Smits, H.H. , van Vliet, S.J. , van Bodegraven, A.A. , Wirth, H.P. , et al (2004) Helicobacter pylori modulates the T helper cell 1/T helper cell 2 balance through phase‐variable interaction between lipopolysaccharide and DC‐SIGN. J Exp Med 200: 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggiano, C. , Manel, N. , and Littman, D.R. (2007) Dendritic cell‐mediated trans‐enhancement of human immunodeficiency virus type 1 infectivity is independent of DC‐SIGN. J Virol 81: 2519–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh, L. , Lozach, P.Y. , Schiffer, C. , Staropoli, I. , Pezo, V. , Porrot, F. , et al (2006) Infection of dendritic cells (DCs), not DC‐SIGN‐mediated internalization of human immunodeficiency virus, is required for long‐term transfer of virus to T cells. J Virol 80: 2949–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambi, A. , Gijzen, K. , de Vries, J.M. , Torensma, R. , Joosten, B. , Adema, G.J. , et al (2003) The C‐type lectin DC‐SIGN (CD209) is an antigen‐uptake receptor for Candida albicans on dendritic cells. Eur J Immunol 33: 532–538. [DOI] [PubMed] [Google Scholar]
- Cameron, P.U. , Freudenthal, P.S. , Barker, J.M. , Gezelter, S. , Inaba, K. , and Steinman, R.M. (1992) Dendritic cells exposed to human immunodeficiency virus type‐1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257: 383–387. [DOI] [PubMed] [Google Scholar]
- Cantin, R. , Methot, S. , and Tremblay, M.J. (2005) Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J Virol 79: 6577–6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavrois, M. , Neidleman, J. , Kreisberg, J.F. , and Greene, W.C. (2007) In vitro derived dendritic cells trans‐infect CD4 T cells primarily with surface‐bound HIV‐1 virions. PLoS Pathog 3: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccaldi, P.E. , Delebecque, F. , Prevost, M.C. , Moris, A. , Abastado, J.P. , Gessain, A. , et al (2006) DC‐SIGN facilitates fusion of dendritic cells with human T‐cell leukemia virus type 1‐infected cells. J Virol 80: 4771–4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenares, M. , Puig‐Kroger, A. , Pello, O.M. , Corbi, A.L. , and Rivas, L. (2002) Dendritic cell (DC)‐specific intercellular adhesion molecule 3 (ICAM‐3)‐grabbing nonintegrin (DC‐SIGN, CD209), a C‐type surface lectin in human DCs, is a receptor for Leishmania amastigotes. J Biol Chem 277: 36766–36769. [DOI] [PubMed] [Google Scholar]
- Curtis, B.M. , Scharnowske, S. , and Watson, A.J. (1992) Sequence and expression of a membrane‐associated C‐type lectin that exhibits CD4‐independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc Natl Acad Sci USA 89: 8356–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, C.W. , Nguyen, H.Y. , Hanna, S.L. , Sánchez, M.D. , Doms, R.W. , Pierson, T.C. (2006) West Nile virus discriminates between DC‐SIGN and DC‐SIGNR for cellular attachment and infection. J Virol 80: 1290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Die, I. , van Vliet, S.J. , Nyame, A.K. , Cummings, R.D. , Bank, C.M. , Appelmelk, B. , et al (2003) The dendritic cell‐specific C‐type lectin DC‐SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology 13: 471–478. [DOI] [PubMed] [Google Scholar]
- Engering, A. , Geijtenbeek, T.B. , Van Vliet, S.J. , Wijers, M. , van Liempt, E. , Demaurex, N. , et al (2002) The dendritic cell‐specific adhesion receptor DC‐SIGN internalizes antigen for presentation to T cells. J Immunol 168: 2118–2126. [DOI] [PubMed] [Google Scholar]
- Frank, I. , Piatak, M., Jr , Stoessel, H. , Romani, N. , Bonnyay, D. , Lifson, J.D. , and Pope, M. (2002) Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): differential intracellular fate of virions in mature and immature DCs. J Virol 76: 2936–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, E. , Pion, M. , Pelchen‐Matthews, A. , Collinson, L. , Arrighi, J.F. , Blot, G. , et al (2005) HIV‐1 trafficking to the dendritic cell‐T‐cell infectious synapse uses a pathway of tetraspanin sorting to the immunological synapse. Traffic 6: 488–501. [DOI] [PubMed] [Google Scholar]
- Gardner, J.P. , Durso, R.J. , Arrigale, R.R. , Donovan, G.P. , Maddon, P.J. , Dragic, T. , and Olson, W.C. (2003) L‐SIGN (CD 209L) is a liver‐specific capture receptor for hepatitis C virus. Proc Natl Acad Sci USA 100: 4498–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geijtenbeek, T.B. , Kwon, D.S. , Torensma, R. , Van Vliet, S.J. , van Duijnhoven, G.C. , Middel, J. , et al (2000) DC‐SIGN, a dendritic cell‐specific HIV‐1‐binding protein that enhances trans‐infection of T cells. Cell 100: 587–597. [DOI] [PubMed] [Google Scholar]
- Geijtenbeek, T.B. , van Vliet, S.J. , Koppel, E.A. , Sanchez‐Hernandez, M. , Vandenbroucke‐Grauls, C.M. , Appelmelk, B. , et al (2003) Mycobacteria target DC‐SIGN to suppress dendritic cell function. J Exp Med 197: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli‐Piperno, A. , Pritsker, A. , Pack, M. , Shimeliovich, I. , Arrighi, J.F. , Park, C.G. , et al (2005) Dendritic cell‐specific intercellular adhesion molecule 3‐grabbing nonintegrin/CD209 is abundant on macrophages in the normal human lymph node and is not required for dendritic cell stimulation of the mixed leukocyte reaction. J Immunol 175: 4265–4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gringhuis, S.I. , den Dunnen, J. , Litjens, M. , van het Hof, B. , van Kooyk, Y. , and Geijtenbeek, T.B. (2007) C‐type lectin DC‐SIGN modulates Toll‐like receptor signaling via Raf‐1 kinase‐dependent acetylation of transcription factor NF‐kappaB. Immunity 26: 605–616. [DOI] [PubMed] [Google Scholar]
- Gringhuis, S.I. , Dunnen, J. , Litjens, M. , van, der, V. , and Geijtenbeek, T.B. (2009) Carbohydrate‐specific signaling through the DC‐SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV‐1 and Helicobacter pylori. Nat Immunol 10: 1081–1088. [DOI] [PubMed] [Google Scholar]
- Gringhuis, S.I. , van, der, V. , van den Berg, L.M. , den Dunnen, J. , Litjens, M. , and Geijtenbeek, T.B. (2010) HIV‐1 exploits innate signaling by TLR8 and DC‐SIGN for productive infection of dendritic cells. Nat Immunol 11: 419–426. [DOI] [PubMed] [Google Scholar]
- Gummuluru, S. , Rogel, M. , Stamatatos, L. , and Emerman, M. (2003) Binding of human immunodeficiency virus type 1 to immature dendritic cells can occur independently of DC‐SIGN and mannose binding C‐type lectin receptors via a cholesterol‐dependent pathway. J Virol 77: 12865–12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y. , Feinberg, H. , Conroy, E. , Mitchell, D.A. , Alvarez, R. , Blixt, O. , et al (2004) Structural basis for distinct ligand‐binding and targeting properties of the receptors DC‐SIGN and DC‐SIGNR. Nat Struct Mol Biol 11: 591–598. [DOI] [PubMed] [Google Scholar]
- Gurney, K.B. , Elliott, J. , Nassanian, H. , Song, C. , Soilleux, E. , McGowan, I. , et al (2005) Binding and transfer of human immunodeficiency virus by DC‐SIGN+ cells in human rectal mucosa. J Virol 79: 5762–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halary, F. , Amara, A. , Lortat‐Jacob, H. , Messerle, M. , Delaunay, T. , Houles, C. , et al (2002) Human cytomegalovirus binding to DC‐SIGN is required for dendritic cell infection and target cell trans‐infection. Immunity 17: 653–664. [DOI] [PubMed] [Google Scholar]
- Harrison, S.C. (2008) Viral membrane fusion. Nat Struct Mol Biol 15: 690–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch, S.C. , Archer, J. , and Gummuluru, S. (2009) Glycosphingolipid composition of human immunodeficiency virus type 1 (HIV‐1) particles is a crucial determinant for dendritic cell‐mediated HIV‐1 trans‐infection. J Virol 83: 3496–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hladik, F. , Sakchalathorn, P. , Ballweber, L. , Lentz, G. , Fialkow, M. , Eschenbach, D. , and McElrath, M.J. (2007) Initial events in establishing vaginal entry and infection by human immunodeficiency virus type‐1. Immunity 26: 257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges, A. , Sharrocks, K. , Edelmann, M. , Baban, D. , Moris, A. , Schwartz, O. , et al (2007) Activation of the lectin DC‐SIGN induces an immature dendritic cell phenotype triggering Rho‐GTPase activity required for HIV‐1 replication. Nat Immunol 8: 569–577. [DOI] [PubMed] [Google Scholar]
- Hu, Q. , Frank, I. , Williams, V. , Santos, J.J. , Watts, P. , Griffin, G.E. , et al (2004) Blockade of attachment and fusion receptors inhibits HIV‐1 infection of human cervical tissue. J Exp Med 199: 1065–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo‐Useros, N. , Blanco, J. , Erkizia, I. , Fernandez‐Figueras, M.T. , Borras, F.E. , Naranjo‐Gomez, M. , et al (2007) Maturation of blood‐derived dendritic cells enhances human immunodeficiency virus type 1 capture and transmission. J Virol 81: 7559–7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo‐Useros, N. , Naranjo‐Gomez, M. , Archer, J. , Hatch, S.C. , Erkizia, I. , Blanco, J. , et al (2009) Capture and transfer of HIV‐1 particles by mature dendritic cells converges with the exosome‐dissemination pathway. Blood 113: 2732–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong, M.A.W.P. , de Witte, L. , Bolmstedt, A. , van Kooyk, Y. , and Geijtenbeek, T.B. (2008a) Dendritic cells mediate herpes simplex virus infection and transmission through the C‐type lectin DC‐SIGN. J Gen Virol 89: 2398–2409. [DOI] [PubMed] [Google Scholar]
- de Jong, M.A.W.P. , de Witte, L. , Oudhoff, M.J. , Gringhuis, S.I. , Gallay, P. , and Geijtenbeek, T.B.H. (2008b) TNF‐alpha and TLR agonists increase susceptibility to HIV‐1 transmission by human Langerhans cells ex vivo. J Clin Invest 118: 3440–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura, T. , Koyanagi, Y. , Nakamura, Y. , Ogawa, Y. , Yamashita, A. , Iwamoto, T. , et al (2008) Significant virus replication in Langerhans cells following application of HIV to abraded skin: relevance to occupational transmission of HIV. J Immunol 180: 3297–3304. [DOI] [PubMed] [Google Scholar]
- Klimstra, W.B. , Nangle, E.M. , Smith, M.S. , Yurochko, A.D. , and Ryman, K.D. (2003) DC‐SIGN and L‐SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell‐ and mammalian cell‐derived viruses. J Virol 77: 12022–12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel, E.A. , Saeland, E. , de Cooker, D.J. , van, K.Y. , and Geijtenbeek, T.B. (2005) DC‐SIGN specifically recognizes Streptococcus pneumoniae serotypes 3 and 14. Immunobiology 210: 203–210. [DOI] [PubMed] [Google Scholar]
- Kwon, D.S. , Gregorio, G. , Bitton, N. , Hendrickson, W.A. , and Littman, D.R. (2002) DC‐SIGN‐mediated internalization of HIV is required for trans‐enhancement of T cell infection. Immunity 16: 135–144. [DOI] [PubMed] [Google Scholar]
- Lambert, A.A. , Gilbert, C. , Richard, M. , Beaulieu, A.D. , and Tremblay, M.J. (2008) The C‐type lectin surface receptor DCIR acts as a new attachment factor for HIV‐1 in dendritic cells and contributes to trans‐ and cis‐infection pathways. Blood 112: 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, B. , Leslie, G. , Soilleux, E. , O'Doherty, U. , Baik, S. , Levroney, E. , et al (2001) cis Expression of DC‐SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J Virol 75: 12028–12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, G. , Simmons, G. , Pöhlmann, S. , Baribaud, F. , Ni, H. , Leslie, G.J. , et al (2003) Differential N‐linked glycosylation of human immunodeficiency virus and Ebola virus envelope glycoproteins modulates interactions with DC‐SIGN and DC‐SIGNR. J Virol 77: 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozach, P.Y. , Lortat‐Jacob, H. , de Lacroix de Lavalette, A. , Staropoli, I. , Foung, S. , Amara, A. , et al (2003) DC‐SIGN and L‐SIGN are high affinity binding receptors for hepatitis C virus glycoprotein E2. J Biol Chem 278: 20358–20366. [DOI] [PubMed] [Google Scholar]
- Lozach, P.Y. , Burleigh, L. , Staropoli, I. , Navarro‐Sanchez, E. , Harriague, J. , Virelizier, J.L. , et al (2005) Dendritic cell‐specific intercellular adhesion molecule 3‐grabbing non‐integrin (DC‐SIGN)‐mediated enhancement of dengue virus infection is independent of DC‐SIGN internalization signals. J Biol Chem 280: 23698–23708. [DOI] [PubMed] [Google Scholar]
- McDonald, D. , Wu, L. , Bohks, S.M. , KewalRamani, V.N. , Unutmaz, D. , and Hope, T.J. (2003) Recruitment of HIV and its receptors to dendritic cell‐T cell junctions. Science 300: 1295–1297. [DOI] [PubMed] [Google Scholar]
- Martina, B.E. , Koraka, P. , van den Doel, P. , Rimmelzwaan, G.F. , Haagmans, B.L. , and Osterhaus, A.D. (2008) DC‐SIGN enhances infection of cells with glycosylated West Nile virus in vitro and virus replication in human dendritic cells induces production of IFN‐alpha and TNF‐alpha. Virus Res 135: 64–71. [DOI] [PubMed] [Google Scholar]
- Marzi, A. , Gramberg, T. , Simmons, G. , Moller, P. , Rennekamp, A.J. , Krumbiegel, M. , et al (2004) DC‐SIGN and DC‐SIGNR interact with the glycoprotein of Marburg virus and the S protein of severe acute respiratory syndrome coronavirus. J Virol 78: 12090–12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzi, A. , Moller, P. , Hanna, S.L. , Harrer, T. , Eisemann, J. , Steinkasserer, A. , et al (2007) Analysis of the interaction of Ebola virus glycoprotein with DC‐SIGN (dendritic cell‐specific intercellular adhesion molecule 3‐grabbing nonintegrin) and its homologue DC‐SIGNR. J Infect Dis 196 (Suppl. 2): S237–S246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moris, A. , Nobile, C. , Buseyne, F. , Porrot, F. , Abastado, J.P. , and Schwartz, O. (2004) DC‐SIGN promotes exogenous MHC‐I‐restricted HIV‐1 antigen presentation. Blood 103: 2648–2654. [DOI] [PubMed] [Google Scholar]
- Moris, A. , Pajot, A. , Blanchet, F. , Guivel‐Benhassine, F. , Salcedo, M. , and Schwartz, O. (2006) Dendritic cells and HIV‐specific CD4+ T cells: HIV antigen presentation, T‐cell activation, and viral transfer. Blood 108: 1643–1651. [DOI] [PubMed] [Google Scholar]
- Navarro‐Sanchez, E. , Altmeyer, R. , Amara, A. , Schwartz, O. , Fieschi, F. , Virelizier, J.L. , et al (2003) Dendritic‐cell‐specific ICAM3‐grabbing non‐integrin is essential for the productive infection of human dendritic cells by mosquito‐cell‐derived dengue viruses. EMBO Rep 4: 723–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile, C. , Petit, C. , Moris, A. , Skrabal, K. , Abastado, J.P. , Mammano, F. , and Schwartz, O. (2005) Covert human immunodeficiency virus replication in dendritic cells and in DC‐SIGN‐expressing cells promotes long‐term transmission to lymphocytes. J Virol 79: 5386–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöhlmann, S. , Zhang, J. , Baribaud, F. , Chen, Z. , Leslie, G.J. , Lin, G. , et al (2003) Hepatitis C virus glycoproteins interact with DC‐SIGN and DC‐SIGNR. J Virol 77: 4070–4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappocciolo, G. , Piazza, P. , Fuller, C.L. , Reinhart, T.A. , Watkins, S.C. , Rowe, D.T. , et al (2006a) DC‐SIGN on B lymphocytes is required for transmission of HIV‐1 to T lymphocytes. PLoS Pathog 2: e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappocciolo, G. , Jenkins, F.J. , Hensler, H.R. , Piazza, P. , Jais, M. , Borowski, L. , et al (2006b) DC‐SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J Immunol 176: 1741–1749. [DOI] [PubMed] [Google Scholar]
- Rappocciolo, G. , Hensler, H.R. , Jais, M. , Reinhart, T.A. , Pegu, A. , Jenkins, F.J. , and Rinaldo, C.R. (2008) Human herpesvirus 8 infects and replicates in primary cultures of activated B lymphocytes through DC‐SIGN. J Virol 82: 4793–4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuntabhai, A. , Turbpaiboon, C. , Casademont, I. , Chuansumrit, A. , Lowhnoo, T. , Kajaste‐Rudnitski, A. , et al (2005) A variant in the CD209 promoter is associated with severity of dengue disease. Nat Genet 37: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano‐Gomez, D. , Sierra‐Filardi, E. , Martinez‐Nunez, R.T. , Caparros, E. , Delgado, R. , Munoz‐Fernandez, M.A. , et al (2008) Structural requirements for multimerization of the pathogen receptor dendritic cell‐specific ICAM3‐grabbing non‐integrin (CD209) on the cell surface. J Biol Chem 283: 3889–3903. [DOI] [PubMed] [Google Scholar]
- Shan, M. , Klasse, P.J. , Banerjee, K. , Dey, A.K. , Iyer, S.P. , Dionisio, R. , et al (2007) HIV‐1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog 3: e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, A.L. , Ganesh, L. , Leung, K. , Jongstra‐Bilen, J. , Jongstra, J. , and Nabel, G.J. (2007) Leukocyte‐specific protein 1 interacts with DC‐SIGN and mediates transport of HIV to the proteasome in dendritic cells. J Exp Med 204: 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soilleux, E.J. , and Coleman, N. (2001) Langerhans cells and the cells of Langerhans cell histiocytosis do not express DC‐SIGN. Blood 98: 1987–1988. [DOI] [PubMed] [Google Scholar]
- Tabarani, G. , Thepaut, M. , Stroebel, D. , Ebel, C. , Vives, C. , Vachette, P. , et al (2009) DC‐SIGN neck domain is a pH‐sensor controlling oligomerization: SAXS and hydrodynamic studies of extracellular domain. J Biol Chem 284: 21229–21240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailleux, L. , Schwartz, O. , Herrmann, J.L. , Pivert, E. , Jackson. M. , Amara, A. , et al (2003) DC‐SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med 197: 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turville, S.G. , Cameron, P.U. , Handley, A. , Lin, G. , Pöhlmann, S. , Doms, R.W. , and Cunningham, A.L. (2002) Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol 3: 975–983. [DOI] [PubMed] [Google Scholar]
- Turville, S.G. , Santos, J.J. , Frank, I. , Cameron, P.U. , Wilkinson, J. , Miranda‐Saksena, M. , et al (2004) Immunodeficiency virus uptake, turnover, and 2‐phase transfer in human dendritic cells. Blood 103: 2170–2179. [DOI] [PubMed] [Google Scholar]
- Wang, J.H. , Janas, A.M. , Olson, W.J. , and Wu, L. (2007a) Functionally distinct transmission of human immunodeficiency virus type 1 mediated by immature and mature dendritic cells. J Virol 81: 8933–8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.H. , Janas, A.M. , Olson, W.J. , KewalRamani, V.N. , and Wu, L. (2007b) CD4 coexpression regulates DC‐SIGN‐mediated transmission of human immunodeficiency virus type 1. J Virol 81: 2497–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S.F. , Huang, J.C. , Lee, Y.M. , Liu, S.J. , Chan, Y.J. , Chau, Y.P. , et al (2008a) DC‐SIGN mediates avian H5N1 influenza virus infection in cis and in trans. Biochem Biophys Res Commun 373: 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J.H. , Wells, C. , and Wu, L. (2008b)Macropinocytosis and cytoskeleton contribute to dendritic cell‐mediated HIV‐1 transmission to CD4+ T cells. Virology 381: 143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte, L. , Abt, M. , Schneider‐Schaulies, S. , van, K.Y. , and Geijtenbeek, T.B. (2006) Measles virus targets DC‐SIGN to enhance dendritic cell infection. J Virol 80: 3477–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte, L. , Bobardt, M. , Chatterji, U. , Degeest, G. , David, G. , Geijtenbeek, T.B. , and Gallay, P. (2007a) Syndecan‐3 is a dendritic cell‐specific attachment receptor for HIV‐1. Proc Natl Acad Sci USA 104: 19464–19469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Witte, L. , Nabatov, A. , Pion, M. , Fluitsma, D. , de Jong, M.A. , de Gruijl, T. , et al (2007b) Langerin is a natural barrier to HIV‐1 transmission by Langerhans cells. Nat Med 13: 367–371. [DOI] [PubMed] [Google Scholar]
- de Witte, L. , de Vries, R.D. , van der, V. , Yuksel, S. , Litjens, M. , de Swart, R.L. , and Geijtenbeek, T.B. (2008) DC‐SIGN and CD150 have distinct roles in transmission of measles virus from dendritic cells to T‐lymphocytes. PLoS Pathog 4: e1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. , and KewalRamani, V.N. (2006) Dendritic‐cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol 6: 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, L. , Martin, T.D. , Vazeux, R. , Unutmaz, D. , and KewalRamani, V.N. (2002) Functional evaluation of DC‐SIGN monoclonal antibodies reveals DC‐SIGN interactions with ICAM‐3 do not promote human immunodeficiency virus type 1 transmission. J Virol 76: 5905–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z.Y. , Huang, Y. , Ganesh, L. , Leung, K. , Kong, W.P. , Schwartz, O. , et al (2004) pH‐dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC‐SIGN. J Virol 78: 5642–5650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, H.J. , Reuter, M.A. , and McDonald, D. (2008) HIV traffics through a specialized, surface‐accessible intracellular compartment during trans‐infection of T cells by mature dendritic cells. PLoS Pathog 4: e1000134. [DOI] [PMC free article] [PubMed] [Google Scholar]


