Abstract
Taro (Colocasia esculenta) corm is a rustic staple food, rich in small starch granules, fibers, and bioactive phytoconstituents such as flavonoids, alkaloids, sterols, tannins, phytates, micronutrients, and proteins, including tarin, a GNA‐related lectin. Tarin exhibits recognized biocide activities against viruses and insects, has antitumoral properties and is an immunomodulator molecule candidate. It has been isolated in highly purified form (>90%) from taro corms through low‐cost and single‐step affinity chromatography. It comprises 2‐domain 27 to 28 kDa protomer, posttranslational cleaved into 2 nonidentical monomers, 11.9 and 12.6 kDa, held by noncovalent binding. At least 10 tarin isoforms sharing over 70% similarity have been described. The monomers assume the β‐prism II fold, consisting of 3 antiparallel β‐sheets formed by 4 β‐strands each. Tarin exhibits an expanded‐binding site for complex and high‐mannose N‐glycan chains 49, 212, 213, 358, 465, and 477 found on cell surface antigens of viruses, insects, cancer, and hematopoietic cells, explaining its broad biological activities. Tarin may stimulate innate and adaptive immune responses, enabling hosts to recover from infections or immunosuppressed status inherent to several pathological conditions. In a murine model, tarin stimulates the in vitro and in vivo proliferation of total spleen and bone marrow cells, especially B lymphocytes. Granulocyte repopulation has also been demonstrated in long‐term mice bone marrow cell cultures. As a potential immunomodulator, tarin, administered to immunosuppressed mice, attenuated cyclophosphamide‐induced leukopenia. We propose a molecular model that unites the potential prophylactic and therapeutic action of tarin on hematopoietic and cancer cells, as a potential immunomodulator.
Keywords: Colocasia esculenta L. Schott, plant lectin, primary sequence homology, B lymphocytes, granulocyte repopulation
Introduction
A brief overview on plant lectins
Lectins are ubiquitous proteins found in many living organisms in the animal and plant kingdom, such as bacteria, fungi, viruses and algae (Lis & Sharon, 1998). These molecules include all “proteins and glycoproteins of nonimmune origin containing at least one noncatalytic domain that binds reversibly and specifically to mono or oligosaccharides”. Lectins can also be called agglutinins or hemagglutinins, since many can agglutinate cells and/or precipitate glycoconjugates through the binding of lectin–carbohydrate complexes, which involves, mainly, hydrogen ligations, although van der Waals and hydrophobic interactions can also occur (Carlini & Grossi‐de‐Sa, 2002; Goldstein, Hughes, Monsigny, Osawa, & Sharon, 1980; Peumans & Van Damme, 1995a; Sharon & Lis, 1995).
The plant kingdom is a rich source of lectins, the so‐called plant lectins or phytohemagglutinins. These lectins are a heterogeneous group of polypeptides with variable molecular mass, quaternary structure, molecular organization, and binding site (Lis & Sharon, 1998; Sharon, 2008). Although it was thought that plant lectins were confined to seeds, evidence indicates that they are found all over the plant body, mainly in storage organs, such as tubers, rhizomes, bulbs, and roots. They are detected in minor amounts in other vegetal tissues (leaves, flowers, fruits, and phloem), however, they are not necessarily identical, in structure or carbohydrate binding specificity, to the lectins present in the storage organs (Ingale & Hivrale, 2013; Kennedy, Palva, Corella, Cavalcanti, & Coelho, 1995; Rudiger, 1998; Van Damme, 2008; Vasconcelos & Oliveira, 2004).
Plant lectins primordially play an essential role in plant defense systems since they bind preferentially to complex and foreign glycans, typically found in animal cells (Lannoo & Van Damme, 2014; Van Damme, 2008). Except for some enzymes, such as chitinase, glucanase, and glycosidase, lectins are unique plant proteins capable of recognizing and binding glycoconjugates on the outer surface of microorganisms and on the membranes of gastrointestinal insect and herbivore mammal cells, in order to protect plants against phytopathogens, phytophagous insects, and herbivores (Michiels, Van Damme, & Smagghe, 2010; Peumans & Van Damme, 1995a, 1995b; Peumans & Van Damme, 1996; Van Damme, 2008). Some plant lectin characteristics contribute to their insecticidal property, such as resistance to proteolysis and stability in a wide range of pH, enough to guarantee their passage through the gastrointestinal tract without degradation. Hence, these proteins would be free to bind glycoconjugates on the outer surface of epithelial cells from the gastrointestinal system, causing changes in cellular morphology, leading to impairment of stomach and intestinal metabolism and absorption process (Vasconcelos & Oliveira, 2004). The consumption of raw or partially cooked Phaseolus vulgaris by humans can cause foodborne illness, due to its high lectin content, which is inactivated by adequate cooking (Carlini & Grossi‐de‐Sa, 2002; Ohno, Naganuma, Ogawa, & Muramoto, 2006; Varki, 1993).
Fortunately, most plant lectins in the human diet do not produce toxic or antinutritional effects, since dietary doses are not high enough. Moreover, not all lectins are toxic; some can contribute in improving nutrient absorption (Peumans & Van Damme, 1996; Rudiger et al., 2000). The lectins from Glycine max (soybean), Canavalia gladiate (sword bean), and Triticum vulgaris (wheat germ) enhance permeability to nutritional factors, such as isoflavone and calcium ions (Ohno et al., 2006).
The reversible and specific binding of lectins to a wide variety of carbohydrates in several kinds of cells can trigger various biological effects, such as antiviral, antifungal, antibacterial, antitumoral, and insecticidal properties, as well as mitogenic lymphocyte stimulation, resulting in benefits to humans (Ingale & Hivrale, 2013).
Based on this, plant lectins have been targets as tools for broad applications of several scientific interests (Pusztai, Bardocz, & Ewen, 2007). Since 1888, many plant lectins have been purified and described in detail (Stillmark, 1888). Over the last century, studies revealed a very heterogeneous group of proteins with diverse specificities, primary sequences and structural features. Accumulated information allowed the classification of plant lectins into 12 families, based on their evolutionary and sequence relationships, as follows: (i) Agaricus bisporus agglutinin homologs; (ii) Amaranthins; (iii) Class V chitinase homologs; (iv) Cyanovirin family; (v) Euonymus europaeus family; (vi) Galanthus nivalis (GNA)‐related family; (vii) Proteins with hevein domains; (viii) Jacalins; (ix) Proteins with legume‐lectin domains; (x) LysM domain; (xi) Nictaba family (formally Cucurbitaceae phloem lectins), and (xii) Ricin‐B family (Van Damme, 2014; Van Damme, Lannoo, & Peumans, 2008).
Tuberous plants are valuable matrices abundant in lectins, accounting for up to 50% of the total protein content (Shet & Madaiah, 1988; Van Damme, 2008). They are widely cultivated and consumed in tropical and subtropical countries as a staple food, supplying carbohydrate and proteins for human and animals. However, the importance of tuberous plants is not restricted to their nutritive value, as some of them exhibit remarkable pharmaceutical properties due to their lectin content (Chandrasekara & Josheph Kumar, 2016; de Castro, Carneiro, Neshich Dde, & de Paiva, 1992; Monte‐Neshich et al., 1995).
The taro plant [Colocasia esculenta (L.) Schott]
Scientifically named Colocasia esculenta, taro is a tuberous plant classified as monocotyledonous, belonging to the Liliatae class and Araceae family. Taro originated from Asia, but was distributed in humid tropics and subtropics, where it can grow even in climate and soil conditions considered unfavorable to traditional agriculture (Heredia Zárate, Vieira, & Silva, 1996; Lim, 2015; Mesquita, 2002). Currently, taro crops are widespread through Oceania, Central, and South America, Asia and Africa, the latter being the major producer (Alcantara, Hurtada, & Dizon, 2013). In Brazil, taro crops are more pronounced in the Central‐South region, with Rio de Janeiro, Minas Gerais, and Espírito Santo states leading the production (Mesquita, 2002). Genetic characterization and relationship studies among taro cultivars in Brazil have focused on the sequence analysis of exons of the plastid genes rbcL and matK loci and the pbsA‐trnH intergenic sequence used alone or in combination (Nunes, Del Aguila, Paschoalin, & da Silva, 2014). Polymorphisms among taro cultivars have been evaluated by using SSR (Simple sequence repeats) polymorphisms analysis in 7 loci (Xuqtem55, Xuqtem73, Xuqtem84, Xuqtem88, Xuqtem91, Xuqtem97, and Xuqtem110) (Nunes et al., 2012). Both microsatellite markers and matK locus and the noncoding sequence pbsA‐trnH contributed to establish the genetic diversity among taro cultivars grown in Brazil. The taro cultivar mostly consumed in Rio de Janeiro is the Chinese (Chinês or Chinezinho) variety (Nunes et al., 2012). A voucher specimen was deposited at the RFA Herbarium belonging to the Federal Univ. of Rio de Janeiro [Univ. Federal do Rio de Janeiro (UFRJ)], Centro de Ciências da Saúde, Departamento de Botânica (Rio de Janeiro, Brazil) under the designation RFA‐39.962 (Pereira, Silva, Verícimo, Paschoalin, & Teixeira, 2015).
Taro – edible parts and processing
Edible taro parts include stems and leaves, although the corms are traditionally the most consumed part of this plant worldwide. Some taro cultivars display high oxalate content, mainly in leaves, that can form nonabsorbable salts with divalent cations, such as Ca2+, Fe2+, or Mg2+, and these oxalate crystals can be deposited in the kidneys, leading to renal failure (Du Thanh et al., 2017). The insoluble oxalates, especially needle like calcium oxalate crystal—raphides—may cause irritation, and swelling of mouth and throat. Thermal treatments, such as boiling or cooking taro (corm, stems, or leaves) can remove oxalates, by converting the salts into soluble phases (Kumoro, Putri, Budiyati, & Retnowati, 2014).
Taro corm storage for long periods is directly affected by the high moisture content present in this plant portion. Postharvest processing represents an alternative to extend taro corm shelf‐life, improving food security and, hence, minimizing crop losses (Kaushal, Kumar, & Sharma, 2013). Corms can be steamed, fried, baked or processed into flour. Taro flour composition is comparable to other vegetable and grain flours, such as potato, corn and soybean, exhibiting high ash concentrations and crude fiber content in a low‐fat product, making taro flour an adequate substitute for currently available flours. Substitutions or combinations with wheat could be an economic alternative for developing countries that depend on wheat imports, but present appropriate climate and soil conditions for taro cultivation. Moreover, supplementation of wheat‐derived products with taro flour could improve nutritional food quality, since wheat is poor in essential amino acids. Taro flour may be used in many preparations, such as cookies, noodle, paste, bread and infant formulations, reaching not only regular consumers, but also those that display dietary restrictions, such as gluten intolerance or food allergenic disorders (Kaur, Kaushal, & Sandhu, 2013; Kaushal et al., 2013; Noorfarahzilah, Lee, Sharifudin, MohdFadzelly, & Hasmadi, 2014).
Taro flour in combination with wheat flour has been reported for the preparation of breads with similar or better functional characteristics than breads prepared exclusively with wheat flour (Ammar, Hegazy, & Bedeir, 2009; Emmanuel, Osuchukwu, & Oshiele, 2010; Noorfarahzilah et al., 2014). Emmanuel et al. (2010) demonstrated that the incorporation of up to 10% of taro flour in the production of wheat/taro bread in its composition minimally affected the pasting properties responsible for conferring food characteristics and, hence, acceptability. Taro flour incorporation produced comparable values for bulk density and swelling capacity (unblanched flour), improvement of gelling ability and, although it caused a decrease in water and oil absorption capacity, this was not enough to interfere in the sensorial characteristics of the final product. Similarly, Ammar et al. (2009) reported that the incorporation of taro flour in wheat bread preserved its organoleptic and rheological properties when concentrations did not exceed 10% substitution. Moreover, the combination of taro/wheat flour in bread formulations increases the amounts of ash, crude fibers and carbohydrates of the final product (Ammar et al., 2009).
Poi
Another interesting formulation prepared from taro corms is called “poi,” a common probiotic dish in Hawaii, where taro is considered a sacred food Poi is a starchy paste prepared by cooking and crushing the corms, followed by the addition of water and allowing the wet mixture to spontaneously be fermented by yeast and bacteria (mainly Lactobacillus sp and Lactococcus sp) (Huang, Lam, Nakayama, & Lin, 1994). Poi can be allowed to reach a sour stage, where lactic acid and acetic acid accumulate, after the utilization of simple sugars by the microbial consortium. The nutritional composition of poi is quite similar to that of taro corms, but carbohydrate decreases and secondary metabolites comprise a probiotic food that could be used in medical nutrition therapy. During the early 1950s, poi was used in Hawaiian hospitals to successfully ameliorate infant food allergies and failure‐to‐thrive (Brown & Valiere, 2004). Studies on poi are scarce, but 1 study analyzed the effect of poi extracts in an in vitro assay concerning the proliferation of rat YYT colorectal cancer cells (Brown, Reitzenstein, Liu, & Jadus, 2005). Poi partially inhibited the proliferation of colorectal cancer cells in a dose‐dependent manner, reaching maximum inhibition at 25%. Further studies revealed that poi extract induced cancer cell apoptosis in vitro and was also able to stimulate hematopoietic cells from normal mouse spleen, especially T cells.
Taro nutrients
Taro corms provide multiple nutrients, such as carbohydrates, proteins, vitamins (thiamine, riboflavin, niacin, vitamins A and C), minerals (potassium, calcium, sodium, phosphorus, and iron) and fibers (Kaushal et al., 2013; Subhash, Sarla, & Jaybardhan, 2012; Temesgen & Retta, 2015). This staple crop offers mainly high quality, gluten‐free carbohydrates (29% on a fresh weight bases), composed of small and resistant starch granules, which accounts for a controlled glucose release and absorption, making taro carbohydrates highly digestible and an important adjuvant candidate to reduce the risk of obesity, diabetes, heart disease and certain types of cancers (Kaushal et al., 2013).
Taro leaves and stems provide the same nutrients found in the corms but in different amounts. Except for thiamine and carbohydrate content, taro leaves exhibit higher levels of all the other nutrients. On the other hand, the stems are poor in these nutrients compared to the corms, offering higher contents only for calcium, iron and vitamin A, while the other nutrients are mostly found in smaller amounts (Kaushal et al., 2013).
Corm proteins
As a gluten‐free food, the consumption of taro corms may be a healthier alternative carbohydrate source, avoiding food allergy reactions and related disorders. Taro corm also provides significant amount of soluble and nonsoluble dietary fibers, which may optimize intestinal transit and possibly reduce the risk of colorectal cancer (Alcantara et al., 2013; Lim, 2015; Prajapati, Kalariya, Umbarkar, Parmar, & Sheth, 2011). The lower protein content (1.4% to 3.0% on a fresh weight basis) of taro corms contributes to their hypoallergenicity. Moreover, taro corms are a low‐fat and high calorie crop (Maga, 1992; Temesgen & Retta, 2015).
The polypeptide composition of taro corm extracts can change slightly among distinct cultivars, as demonstrated by electrophoresis analyses (Carneiro, Rodrigues, De Castro, Da Silva, & Coutinho, 1990; Hirai, Sato, & Takayanagi, 1989). The aqueous extracts prepared from taro corms are mainly composed of four major proteins, classified as albumins (A1 and A2) and globulins (G1 and G2) (Carneiro et al., 1990; Hirai, Nakamura, Imai, & Sato, 1993; Monte‐Neshich et al., 1995). Globulins (G1 and G2) are the most abundant, accounting for 80% of total soluble proteins, and are well‐characterized in C. esculenta corms (Monte‐Neshich et al., 1995). On the other hand, little information is available about the identification of albumins or their biological properties. A1 Albumin is not always found in the aqueous extract, perhaps due to cultivar differences, extraction procedures or corm maturation stage (de Castro et al., 1992). The aqueous extract prepared by our research group did not show the presence of the A1 albumin in any of the two extraction procedures applied (Figure 1A and B) (Pereira et al., 2014, 2015). The A1 albumin has been purified and characterized, revealing that it accounts for 11% of total soluble proteins and that its expression decreases along corm development (Carneiro et al., 1990). This protein, termed colocasin, is composed of six 8.3 kDa homogeneous monomers, which are held together to form a 50 kDa hexamer with an isoelectric point (pI) ranging from 5.3 to 6.1. A2 albumin exhibits an apparent molecular mass of about 55 to 60 kDa,with pI varying from 5.5 to 6.0. This albumin accumulates in the first two stages of corm development, decreasing in the last 3 stages (de Castro et al., 1992).
Figure 1.
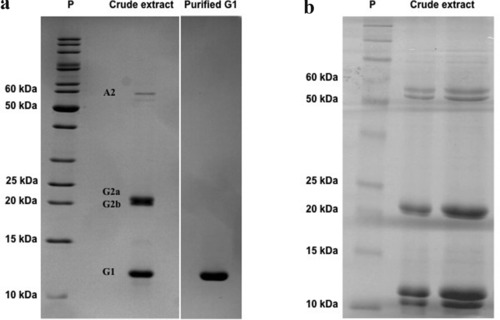
Electrophoretic polypeptide patterns of taro corm aqueous extracts. Protein extract from taro corms, prepared by 2 distinct procedures, described by Roy et al. (2002) (A left panel) or by Carneiro et al. (1990) (B panel), was fractionated on a 15% or 12.5% SDS‐PAGE, respectively. Proteins from taro corm extract are indicated as A1, G2a/G2b, and G1/tarin. Purified G1 protein corresponding to tarin (A – right‐hand panel). Figures are representative of multiple experiments.
G2 Globulins are composed of two polypeptide bands of about 24 kDa (G2a) and 22 kDa (G2b), with pI of 7.5. These globulins account for approximately 40% of total soluble proteins and have a native molecular mass of 50 kDa, indicating that they occur as a heterodimer (de Castro et al., 1992; Hirai et al., 1993; Monte‐Neshich et al., 1995; Shewry, 2003). Additionally, de Castro et al. (1992) found that there is no amino acid homology among the N‐terminal chains of the two globulin families (G1 and G2), indicating that they might be encoded by distinct genes. The amino acid sequencing of the G2 polypeptide indicates that it is homologous to the trypsin inhibitor family members found in soybeans, winged beans, sweet potato and barley (Hirai et al., 1993).
The G1 globulin, which accounts for 40% of total soluble proteins, was identified as a lectin, named tarin, from C. esculenta (Van Damme et al., 1995). This taro lectin exhibits remarkable biological properties, such as in vitro and in vivo antimetastatic and mitogenic activities and in vitro antitumoral, insecticidal and antiviral activities, making tarin a promising candidate as a future biopharmaceutical molecule (Table 1 and Figure 2, dashed green lines). Our research group has successfully purified and fully characterized tarin from C. esculenta using a simple, low‐cost and single‐step purification technique on an affinity chromatography column (Cibacron Blue 3GA) which released a high purity fraction (>90%) (Pereira et al., 2014, 2015) (Figure 1A).
Table 1.
Antimetastatic, antitumoral, antiviral, mitogenic and biocide activities of tarin purified by distinct chromatographic procedures
| Bioactivity | Tarin purification steps | Type of assay | Target | Effects | Reference |
|---|---|---|---|---|---|
| Antimetastatic | Ultrafiltration, Size exclusion chromatography (SEC), Anion exchange chromatography | in vivo | Murine mammary tumor cell line 66.1 | Inhibition of lung colonizing ability of 66.1 cells | Kundu et al., 2012 |
| Antitumoral | Ultrafiltration, Anion exchange chromatography, SEC and second anion exchange chromatography | in vitro | Murine mammary tumor cell line 66.1 | 35% inhibition of 66.1 cells proliferation | Kundu et al., 2012 |
| Anion exchange chromatography, Anion exchange and Gel Filtration | Hepatoma HepG2 cells | 22.3 μM tarin reduced 75% of cell proliferation in 48h | Chan et al., 2010 | ||
| Antiviral | Affinity chromatography | in vitro | SARS‐CoV | EC50>60 μg/mL | Keyaerts et al., 2007 |
| FIPV | EC50 >2.5 ± 0.6 μg/mL | ||||
| Mitogenic/ Potential immunomodulator | Anion exchange, Anion exchange and Gel filtration | in vitro | Total spleen cells | Cytokines expression IL‐2, IL‐1β, INF‐γ and TNF‐α | Chan et al., 2010 |
| Affinity chromatography | in vitro | Mice spleen cells mice bone marrow cells | Dose‐dependent proliferative response | Pereira et al., 2014 | |
| Anion exchange, Anion exchange and Gel Filtration | Chan et al., 2010 | ||||
| Affinity chromatography | in vivo | Mice spleen cells | 3.3‐fold proliferation increase on 5th day | Pereira et al., 2014 | |
| Mice spleen B lymphocytes | 4.1‐fold proliferation increase on 5th day | ||||
| Biocide | Affinity chromatography | in vitro | Lipaphis erysimi | LC50 = 11.87 μg/mL | Roy et al., 2014 |
| Gel filtration | Bemisia tabaci | LC50 = 5.17 μg/mL | |||
| Affinity chromatography | in vitro | Dysdercus koenigii/cingulatus | LC50 = 19.9 ± 0.98 μg/mL | Roy et al., 2002 | |
| Tarin 1 expressed in Nicotiana tabacum | in vitro | Spodoptera frugiperda larvae | Provoke higher mortality | Leal‐Bertioli et al., 2003 | |
| Pseudomonas syringae pv. tomato | Growth inhibition | ||||
| Affinity chromatography | in vitro | Bactrocera cucurbitae | LC50 = 51.6 μg/mL | Thakur et al., 2013 | |
| Affinity chromatography and anion exchange chromatography | in vitro | Aphis gossypii | LC50 = 9.98 ± 0.239 μg/mL | ||
| Dysdercus cingulatus | LC50 = 16.95 ± 0.279 μg/mL | ||||
| Aphis craccivora | LC50 = 15.21 ± 0.274 μg/mL |
This article is being made freely available through PubMed Central as part of the COVID-19 public health emergency response. It can be used for unrestricted research re-use and analysis in any form or by any means with acknowledgement of the original source, for the duration of the public health emergency.
Figure 2.
Bioactivities attributed to taro corm extracts and/or tarin. Representation of the biological activities reported for the set of bioactive molecules present in taro corms and indicated inside the gray circles. Those indicated by dashed green lines refer to the in vitro and/or in vivo biological activities exerted by tarin.
Bioactive compounds from taro
Apart from its nutritional quality, taro consumption goes beyond dietary purposes. Since ancient times, taro has been used for medicinal purposes by indigenous populations although some of its applications still require scientific support. The traditional use of taro comes from popular knowledge, and many parts of the plant (corm, leaf and petiole) are applied for several health disorders such as diabetes mellitus, internal hemorrhages, gastrointestinal diseases, alopecia, body ache, snakebite, anemia, as well as for the stimulation of the immune system in humans (Lim, 2015; Nwauzoma & Dappa, 2013). Taro leaf extracts have shown no in vitro toxicity against BHK‐21 fibroblast cells, which are found in the pulp, periodontal ligament and gingiva, enabling them to be included as a natural biocide in the formulation of a commercial mouthwash (L'Angelica collutorio – product registered in Italy under the number GTIN/EAN:8017331000069) (Meilena, 2017). Taro corm powder is marketed worldwide as a food supplement and ingredient.
The medicinal utilization of taro plant‐derivatives supports the presence of pharmacologically active molecules, which is in accordance with scientific reports. A wide variety of bioactive compounds can be extracted from taro, including organic acids, phenolic compounds, anthocyanins, tannins, sterols, phytocystatin, alkaloids, saponins, terpenes, and bioactive proteins (Ferreres et al., 2012; Lim, 2015; Reyad‐ul‐Ferdous et al., 2015). Such a set of bioactive molecules exhibits interesting pharmacological properties, based on in vitro and/or in vivo experiments in mice, consistent with some of their popular uses. Taro corm and leaf extracts have been reported as antitumoral/antimetastatic (Brown et al., 2005; Kundu et al., 2012), antihyperlipidemic/antihypercholesterolemic (Sakano et al., 2005), anxiolytic (Kalariya, Parmar, & Sheth, 2010), wound healing (Gonçalves et al., 2013), antimelanogenic (Kim, Moon, Kim, & Lee, 2010), anti‐inflammatory (Biren, Nayak, Bhatt, Jalalpure, & Seth, 2007), probiotic (Brown & Valiere, 2004), antihypertensive (Vasant et al., 2012), antidiabetic (Eleazu, Iroaganachi, & Eleazu, 2013; Kumawat, Chaudhari, Wani, Deshmukh, & Patil, 2010; H. M. Li, Hwang, Kang, Hong, & Lim, 2014), antioxidant (Lee, Wee, Yong, & Syamsumir, 2011), hepatoprotective (Chinonyelum, Uwadiegwu, Nwachukwu, & Emmanuel, 2015; Patil & Ageely, 2011), antimicrobial (Dhanraj, Kadam, Patil, & Mane, 2013), anthelmintic (Kubde, Khadabadi, Farooqui, & Deore, 2010), mitogenic (Pereira et al., 2014, 2015; Tulin & Ecleo, 2007), hypolipidemic (Boban, Nambisan, & Sudhakaran, 2006), insecticidal (Rajashekar, Tonsing, Shantibala, & Manjunath, 2016) and antiviral (Keyaerts et al., 2007). The biological properties attributed to taro corm extracts, the focus of this review, is displayed in Figure 2 (gray circles) alongside the activities reported for the lectin from taro corms, tarin (dashed green lines).
Tarin ‐ physicochemical and structural characterization
Due to its structural and functional similarities with Galanthus nivalis agglutinin, tarin was included within the GNA‐related lectins family, previously termed the monocot mannose‐binding family. The monomers that compose these lectins originate from single‐ or two‐domain protomers. In the former group, one protomer generates 1 monomer with exclusive specificity towards mannose, while each two‐domain protomer releases two monomers with complex glycan specificity. Each monomer originally possesses up to 3 functional binding sites (subdomains I, II, and III), characterized by the consensus sequence QxDxNxVxY, which exclusively binds mannose/oligomannose, in single‐domain lectins, and more complex glycans, including high‐mannose and complex N‐glycans, in two‐domain lectins. The amino acid composition within carbohydrate binding sites for mannose/oligomannosides in single‐domain lectins is highly conserved. On the other hand, two‐domain lectins exhibit a less conserved amino acid composition in the subdomain regions. This group evolved from their single‐domain counterparts by inter‐domain sequence divergence, generating nonidentical subdomains with a wider and dual specificity towards more complex and longer glycan chains (Van Damme et al., 2007).
Tarin, the two‐domain GNA‐related lectin from taro corms, is encoded by the tar1 gene, as a 27 to 28 kDa protomer containing the signal peptide and the linker sequence located between the monomer A and B sequences. The signal peptide and linker are removed after posttranslational processing, giving rise to monomer A, with 11.9 kDa, and monomer B, with 12.6 kDa. Both monomers are arranged as heterodimers, held together by noncovalent binding, where monomer A is at the N‐terminal and monomer B, at the C‐terminal (Bezerra et al., 1995; Hirai et al., 1993; Pereira et al., 2015). The heterodimers oligomerize as a heterotetramer, originating the functional structure of tarin, with 47 kDa (Figure 3). The tarin purification performed by our group produced a heterogeneous mixture of, at least, 10 tarin isoforms with similar apparent molecular mass (∼12 kDa), but distinct pI ranging from 5.5 to 9.5 and carbohydrate content of 2% to 3% (Pereira et al., 2015). de Castro et al. (1992) have sequenced the N‐terminal of the 4 major tarin isoforms, classifying them as G1a, G1b, G1c, and G1d. G1a and G1c share 88% identity in amino acid sequence while, G1b and G1d share 96%. The subgroups G1a/c and G1b/d are found at C‐terminal and N‐terminal, respectively, of the protomer and share 25% identity among the subgroups.
Figure 3.
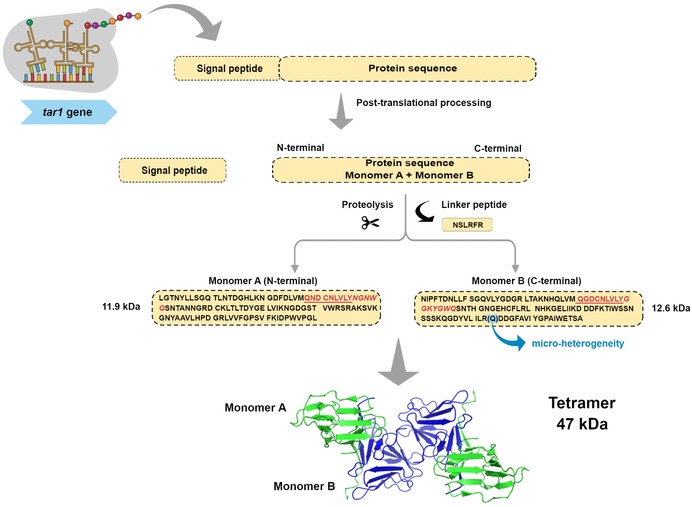
A schematic representation depicting tarin synthesis and folding. The tar1 gene encodes a protomer that is processed into 2 nonidentical monomers, with 11.9 and 12.6 kDa, which oligomerize as a heterotetramer with 47 kDa. The conserved binding sites (QxDxNxVxY) are underlined in red and their extended regions are represented in italics. The presence of microheterogeneity is represented by (Q) at position 203, where residues R and Q are found.
The heterogeneity in the lectin purified by our research group was evidenced during the deduction of primary sequence from tarin high‐resolution electron density data, where two amino acids, R and Q, share the position 203 at the monomer B, each with 50% occupancy (Figure 3) (Pereira et al., 2017). Additionally, a comparison between the 6 available primary sequences of C. esculenta lectin (A5HMM7, R9RL27, Q43418, Q39487, 5J76_A, and 5J76_B, 5D5G_A and 5D5G_B) revealed that, although they share many similarities, they are not identical, supporting the heterogeneous mixture data found in the purified tarin fraction by our group and other authors (Figure 4). Except for the last sequence identified by Q43418, the lectin purified by our group is highly identical to the other ones, reaching from 88% to 94% identity, filling the criteria to consider them as distinct isoforms of the same lectin. In fact, evidence indicates that tar1 is a multigene family, which also occurs within other members of the GNA‐related lectins as already discussed by our group (Pereira et al., 2015).
Figure 4.
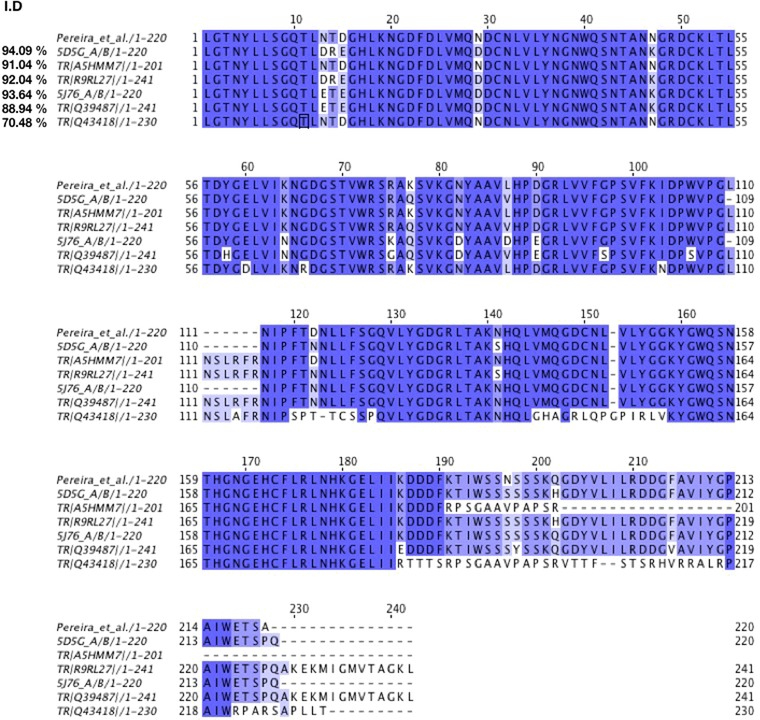
Tarin sequences alignment. Comparison between tarin sequences, available at Natural Center for Biotechnology Information (NCBI) protein data bank https://www.ncbi.nlm.nih.gov/protein/?term=Colocasia+esculenta+lectin, and tarin purified by our group (Pereira et al.). Alignments were performed at UniProt website https://www.uniprot.org/align/ and analyzed on Jalview version 2 software (Waterhouse, Procter, Martin, Clamp, & Barton, 2009). Color corresponds to percentage identity considering Pereira et al. as the 100% reference. Similarities in amino acid sequences are presented in color gradient as follows: dark blue = >80%, light blue >60%, lighter blue >40% and white <40%.The overall identity percentage is represented in the left side of the figure.
As a typical GNA‐related lectin, tarin monomers A and B assume the β‐prism II fold of three antiparallel β‐sheets formed by 4 β‐strands each. Both monomers exhibit a hydrophobic core and a disulfide bond contributing to the β‐prism stability. Monomers A (11.9 kDa) and B (12.6 kDa) are held together as a heterodimer by a C‐terminal exchange. At the C‐terminal region, the fourth β‐strand in monomer A molds the β‐sheet in monomer B and vice‐versa, characterizing the C‐terminal exchanges and stabilizing the heterodimer 2 [A+B]. Four monomers (2 heterodimers or 2 [A+B]) oligomerize, giving rise to the tarin native structure, a stable 47 kDa heterotetramer capable of recovering or maintaining its functionality after being exposed to a wide range of pH, from 1 to 11, and high temperatures (Pereira et al., 2015, 2017; Vajravijayan, Pletnev, Pletnev, Nandhagopal, & Gunasekaran, 2016). Tarin exhibits one functional binding site per monomer and, consequently, a total of four binding sites in each tetramer. Since both binding sites (A and B) are not identical, tarin can bind a wide variety of ligands, including high‐mannose and complex N‐glycans, which are commonly found in animals. A closer analysis of tarin binding sites able to complexed to trimannose [Manα(1,3)Manα(1,6)Man] revealed that tarin can exhibit different affinities to the same ligand (Pereira et al., 2017). Moreover, the interaction with the ligand is not restricted to the conserved domain QxDxNxVxY shared by GNA lectins (Figure 4). Very close to the consensus sequence, comes a sequence of three (NGN) and five (GGKYG) amino acids in monomers A and B, respectively, which forms a loop that connects the β‐strands three and four of two β‐sheets (Figure 4). The loop sequence takes part in the trimannose interaction, particularly 1,6‐terminal mannose, creating an extended binding site area, allowing the protein to accommodate more complex and longer glycan chains (Pereira et al., 2017). In fact, tarin binds preferentially complex N‐glycans over high mannoses (Pereira et al., 2017). With a longer loop, monomer B allows a closer binding with the ligand, establishing a superior number of interactions with the trimannose compared to monomer A. A careful comparison of the loop sequence in tarin with the 10 β‐prism II lectins showed that these amino acids are limited by a conserved tyrosine (Y) and tryptophan (W), preserved in all the 11 lectins. The number and amino acid composition in the region between Y and W is highly variable, ranging from three to six amino acids, suggesting an important role of these residues on lectins‐fine specificity. The loop conformation also contributes to tarin specificity, since it assumes a flat conformation expanding the surface area contact, which is two amino acids longer in the monomer B. In classical mannose‐binding lectins like concanavalin A and the GNA family, the loop does not assume a flat conformation but it folds like a pocket, sandwiching the ligand and limiting their binding to less complex glycans with short chains, such as trimannose (Pereira et al., 2017).
Some particularities exclusively found in tarin structure were evidenced by our research group (Pereira et al., 2017). Tarin crystallized as a unique octamer due to the dimerization of monomers A from two tetramers, forming a circle of eight monomers into the asymmetric unit. Four hydrogen bonds and two salt bridges were found in the interface, which we believe to be a crystallographic artifact. Similarly, tarin structure bound to trimannose also oligomerizes as an octamer, but now, two octamers were found into the asymmetric unit. Although not relevant to the biological activity of tarin, the octamer formation contributes to trimannose binding to monomer A, sandwiching the ligand with the participation of amino acids from the adjacent monomer A and giving rise to a highly stable octamer organization (unpublished data).
Tarin‐ biological and therapeutic properties
It is well known that GNA‐related lectins are important molecules with broad therapeutic applications. The lectins belonging to the GNA family are especially known for their antitumoral, antiviral and biocide activities, among others, no less important, biological activities (C.‐y. Li, Meng, Liu, & Bao, 2009; Van Damme, 2008; Wu & Bao, 2013). The bioactivities of lectins are strictly related to their glycan specificity, which were evidenced by the glycan array screening for tarin ligand profiling (Pereira et al., 2015). Tarin exhibits specificity towards glycan chains that make part of many cell surface antigens including cancer cells, viruses, insect cells and also hematopoietic cells, providing the molecular basis that might explain its broad bioactivities (Table 1). Distinct protein purification procedures adopted by researchers have evidenced tarin's multiple bioactivities (Table 1).
Biocide activity
Most plant lectins bind foreign glycans, typical constituents of insect, microorganism and vertebrate membranes, making these molecules an essential part of the defense mechanism of plants (Van Damme, 2008). N‐glycans classified as paucimannose (trimannosides) and high‐mannose representatives of over 90% of these glycan group to which tarin is able to bind (Pereira et al., 2015; Walski, De Schutter, Van Damme, & Smagghe, 2017). This binding specificity can be extended to the other lectins from the GNA‐related family, which exhibit high insecticidal activity due to their high specificity towards complex and high‐mannose N‐glycans, the main ligands abundantly found along the intestinal tract of insects and superior animals. The insecticidal effects exerted by lectin molecules are of great importance in the development of tools to promote crop protection against pests (Macedo, Oliveira, & Oliveira, 2015). The bio pesticide activity of tarin has been extensively reported in the literature (Table 1) and endorsed by our group (Pereira et al., 2015). We have demonstrated that tarin displays ligand‐specificity toward high‐mannose N‐glycans number 477 [Manα1‐6(Manα1‐3)Manβ1‐4GlcNAcβ1‐4(Fucα1‐6)GlcNAcβ] and number 49 [Manα1‐3(Manα1‐6)Manβ1‐4GlcNAcβ1‐4GlcNAcβ], found in insect and nematode cell membranes (Pereira et al., 2015; Walski et al., 2017). Further studies are required to confirm the participation of such glycans on tarin insecticidal properties.
Das, Roy, Hess, and Das (2013) and Roy, Gupta, Hess, Das, and Das (2014), Roy, Banerjee, Majumder, and Das (2002) described the insecticidal activity of tarin against the hemipteran sucking pest red cotton bug (Dysdercus koenigii/cingulatus), cowpea aphid (Aphis craccivora), cotton aphid (Aphis gossypii), white fly (Bemisia tabaci), and mustard aphid (Liphaphis erysimi). Tarin, when included in insect diets, can affect insect pupation and emergence and, when in high concentrations, can be lethal to several agricultural pests (Table 1). Interestingly, the toxic effects of tarin on Dysdercus koenigii/cingulatus were shown to be transferred to the next generation, where nymphs presented almost 50% reduced size/weight and different colors (Roy et al., 2002). Studies on tarin‐treated B. cucurbitae larvae have demonstrated that lectin was able to induce the insect detoxification system, by increasing esterase and phosphatase activities, as well as the anti‐oxidant defense system by increasing superoxide dismutase (SOD), catalase (CAT) and glutathione‐S‐transferase (GST) activities(Thakur et al., 2013).
The insecticidal activity of tarin has been elucidated (Roy & Das, 2015; Roy et al., 2014). Tarin is able to bind to the glycan chains on the surface of midgut epithelial cells of Dysdercus koenigii/cingulatus, reaching the hemolymph and ovary, resulting in the aforementioned entomotoxic effects (Roy & Das, 2015). The putative binding partners have been identified: ATP synthase, which is directly involved in the insect growth, loss of fecundity, development and midgut morphology. Another target for tarin was the cytochrome P450, involved in the detoxification of insecticide molecules causing failure in the insecticide resistance mechanism and making insects vulnerable when its activity decreases in the presence of lectin. Additional findings came from studies with Bemisia tabaci and Liphaphis erysimi where some specific tarin binding partners were confirmed and others were revealed in each species (Roy et al., 2014). In B. tabaci, tarin binds to ATP synthase and sarcoplasmic endoplasmic reticulum type Ca2+ ATPase while in L. erysimi, tarin partners are the previously mentioned ATP synthase, but heat shock protein 70 and clathrin heavy chain assemble protein were also included, explaining insect growth and reproduction impairment and mortality enhancement.
Leal‐Bertioli et al. (2003) demonstrated the potential application of tarin for pest management using plant engineering techniques. Genetically modified tobacco able to express tarin promoted plant protection against Spodoptera frugiperda larvae and Pseudomonas syringae pv. tomato, which cause severe crop losses. S. frugiperda is a natural predator of a variety of crops, including maize, rice, sorghum, cotton, peanut, soybean and pastures, while P. syringae pv. tomato affects tomato, crucifers and tobacco plantations. Tarin‐engineered plants challenged by these insect infections were able to reduce the survival, average weight, accumulated mass and pupation of S. frugiperda larvae and inhibit the growth of P. syringae pv. tomato.
Antiviral effects
Although antiviral activity is widespread among plant lectins, the majority displaying this bioactivity are found within the GNA‐related family members, due to their ability to bind complex and high mannose N‐glycans, usually found on the viral envelope (Akkouh et al., 2015; Wu & Bao, 2013). Keyaerts et al. (2007) screened the in vitro antiviral activity of 33 plant lectins, including tarin, against severe acute respiratory syndrome (SARS CoV, Frankfurt 1 strain) and feline infectious peritonitis virus (FIPV, strain 79–1146). SARS‐CoV affects humans, causing a severe infection in the lower respiratory tract, and its discovery boosted the finding of other human coronaviruses (HCoV‐NL63 and HCoV‐HKU1). FIPV is responsible for chronic peritonitis in cats, a rare and fatal disease with no clinically effective treatment.
The in vitro screening results revealed that the antiviral activity was dependent on lectin specificity. A high correlation between the antiviral activity against both viruses and lectins from the GNA family was evidenced by the study, confirming the statement above. Tarin reached 50% effectiveness against SARS‐CoV at a concentration >60 μg/mL, and against FIPV at 2.5 ± 0.6 μg/mL (Table 1). To study the virucidal mechanism, the GNA‐related lectin Hippeastrum hybrid agglutinin (HHA) was chosen as a representative agent to perform virus adsorption and entry assays. Virus entry and release from the target cells were inhibited by HHA, which may interact with specific surface glycoproteins that are directly involved in the mechanism of infection. SRAS‐CoV entry into cells is mediated by the ACE2 receptor, a protein glycosylated by the addition of high‐mannose and complex N‐glycans to some of the 12 candidate sites (Krokhin et al., 2003).
There is no experimental evidence for tarin anti‐HIV activity, although the anti‐HIV effectiveness of many GNA‐related lectins has been well‐documented (Wu & Bao, 2013). However, tarin has been reported to specifically bind 2 glycan chains (numbers 212 and 213) present in the gp120 glycoprotein found on the surface of HIV‐1, which could impair its attachment to the target cells (Pereira et al., 2015).
Immunomodulator potential
According to Kumar, Manjunath, Thaminzhmani, Kiran, and Brahmaiah (2012), tarin seems to fill the requisites to consider this lectin an immunomodulatory candidate, since it exhibits the ability to act on immunological components of both innate or adaptive immunity, promoting stimulation, suppression or modulation. In general, plant lectins can interact with hematopoietic cells by binding to their surface carbohydrates (Daoudi, Abdel‐Satter, & Aarab, 2013; Shanmugham et al., 2006). Tarin specifically binds with high affinity to glycan chains, found in hematopoietic progenitor cells and peripheral neutrophils, as revealed by studies on tarin binding specificity (Pereira et al., 2015). Tarin binds to glycans 358 and 465, also known as the CD173/H2 and CD174/Lewis Y antigens, which are highly expressed in CD34+ progenitor cells and peripheral neutrophils, suggesting their participation in tarin activity (Cao, Merling, Karsten, & Schwartz‐Albiez, 2001; Dettke, Pálfi, & Loibner, 2000; Pereira et al., 2015).
Two research groups independently reported the effects of tarin on immune system components (Chan, Wong, & Ng, 2010; Pereira et al., 2014). Tarin is able to stimulate splenocyte proliferation in vitro with a maximum response at 0.698 μM (Chan et al., 2010) (Table 1). In a further investigation of the RNAm expression of these cells, the transcriptional activation of pro‐inflammatory cytokine genes, such as interleukin‐1β (IL‐1β), interleukin‐2 (IL‐2), interferon‐γ (INF‐γ), and tumor necrosis factor‐α (TNF‐ α) was observed. Cytokines are essential molecules in cancer treatments. Animal and clinical trial studies should to be conducted to investigate if tarin may trigger cytokine release, bringing health benefits. The expression of the aforementioned cytokines may be useful to help hosts fight against infection by stimulating innate or adaptive responses. Interleukin‐2 is involved in the proliferation and activation of T and B lymphocytes and NK cells in humans. Interferon‐γ activates macrophages and B‐lymphocytes, promotes the differentiation of T cells to TH1 and enhances the expression of MHC‐I and ‐II. Interleukin‐1β and TNF‐α participate in inflammatory responses through the activation of endothelial cells. Additionally, TNF‐α can also activate neutrophils, the main phagocytic cells involved in the first line of defense against infection (Abbas, Lichtman, & Pillai, 2014).
Our research group evaluated the effects of tarin on hematopoietic cells (Corrêa et al., 2017; Pereira et al., 2014). Mouse splenocyte cells were cultivated in the presence of increasing tarin concentrations, revealing a dose‐dependent proliferation curve with a maximum proliferation rate at 2.5 μg/mL. Additionally, the intraperitoneal administration of 100 μg of tarin promoted a 3.3‐fold increase in the number of total splenocytes and B lymphocytes 5 days after treatment (Table 1). Previous studies with taro corm extracts have indicated that tarin may also interfere in the B cell population in the bone marrow, causing a decrease in the number of mature (B220+ IgM+) and immature (B220+ IgM−) B lymphocytes, while at the same time increasing B220+ cell numbers in the spleen (Pereira et al., 2014, 2015). These results suggest that hematopoietic cells may be directly stimulated in the spleen or stimulated to migrate from the bone marrow to peripheral organs to fill the demand caused by tarin administration, as indicated in Figure 5.
Figure 5.
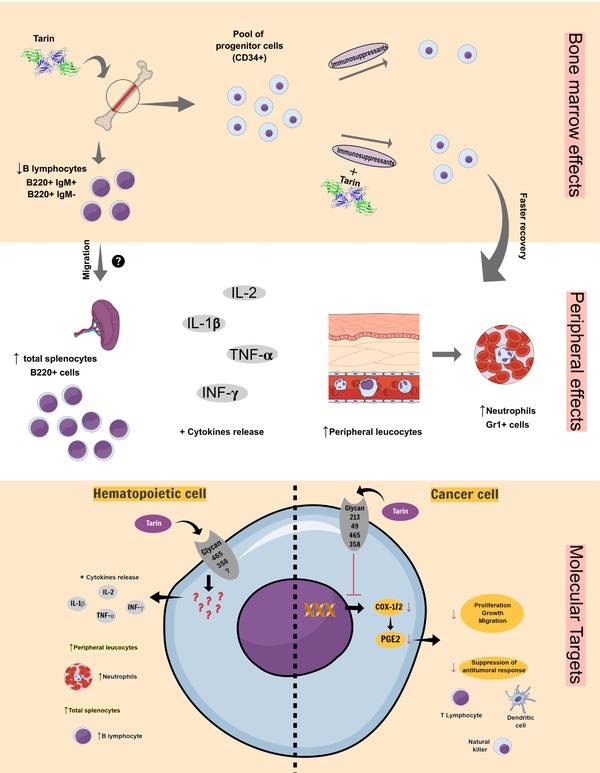
Tarin putative targets in hematopoietic or cancer cells. The in vitro and in vivo mitogenic and cytokine release effects previously reported for tarin are summarized in the panel bone marrow effects and peripheral effects, while antitumoral/antimetastatic effects are represented in the panel molecular targets (cancer cell). The panel molecular targets rises the possibility of tarin binding to cell surface carbohydrates, present in hematopoietic or cancer cells. As a result, hematopoietic cells could be activated (left‐hand panel) and inflammatory signaling from cancer cells could be down‐regulated favoring the antitumoral responses (right‐hand panel). This is a representative figure. Cells and structures are not scaled.
In a recent study by our group, long‐term mouse bone‐marrow cell in culture was stimulated by tarin, promoting repopulation with Gr‐1+ granulocytes (Corrêa et al., 2017). Since tarin binds with high affinity to the CD173/H2 and CD174/LewisY antigens present in CD34+ progenitor cells, these results strongly suggest that tarin could be capable of maintaining progenitor cells in vitro. In vivo studies indicated that the intraperitoneal administration of 200 μg of tarin in immunossupressed mice significantly minimized leukopenia induced by cyclophosphamide (CY) at 300 mg/kg. Moreover, tarin administration also protected erythrocyte lineage cells from death by reducing the number of micronucleated cells, an indicative of CY cytotoxic effects.
Tarin activity may possibly be explored as a potential immunomodulator to boost the immunological system in order to reverse immunosuppressive effects. By stimulating the host‐immune system, the organism could not only be able to reestablish its immunological status, but may be prepared to fight against microbial infections.
Anticancer activity
The study carried out by Chan et al. (2010) was the first to evaluate the in vitro antitumoral effects of purified tarin against hepatoma HepG2 cells, demonstrating that, although in a weak manner, tarin inhibited the proliferation of these tumoral cells. When cells were cultivated in 22.3 μM tarin, 64.5% of initial cells were still viable after 24 hr and 74.8% after 48 hr. As mentioned previously, tarin was able to induce in vitro cytokine release, including interleukin‐1β (IL‐1β), IL‐2, tumor necrosis factor‐α (TNF‐α), and interferon‐γ (INF‐γ), which are currently being used for cancer treatments (except for IL‐1β), to stimulate the immune response against tumor cells (Dranoff, 2004; Vacchelli et al., 2016). However, further studies need to be conducted to investigate if tarin would be able to induce cytokine release in vivo and if those molecules could interfere in the antitumoral and antimetastatic responses (Figure 5).
Kundu et al. (2012) described the isolation of a protein, later identified as tarin, which was suggested to be the antimetastatic active principle of taro corm extracts. A murine cell breast cancer lineage 66.1, highly metastatic and easily able to colonize lung and heart tissue was used in that study. According to the aforementioned study, tarin exhibited a modest antiproliferative effect, being able to reduce up to 35% of the 66.1 cell lineage proliferation in vitro. On the other hand, when syngeneic BALB/cByJ mice were treated with tarin prior to and after the injection of 66.1 cells, lung colonization was significantly reduced and no heart colonization was observed. Studies on taro corm extract mechanisms indicated that the antimetastatic activity could be induced by the down‐regulation of cyclooxygenase (COX)‐1 and ‐2 and subsequent inhibition of prostaglandins E2 (PGE2) synthesis, which are associated to tumor aggressiveness (Kundu et al., 2012). COX inhibitors, especially COX‐2 is highly expressed in certain pathological conditions and has been extensively used in clinical trials to treat breast, lung, prostate and colon cancer cells, since it is strongly associated to decreases in tumor proliferation, growth and migration and blockage of the inhibition of antitumoral responses as a result of the suppression of dendritic cells, natural killer cells and T cells by PGE2 (Liu, Qu, & Yan, 2015).
Our research group demonstrated that tarin binding specificity to cell membrane glycans could explain its anticancer properties. Tarin specifically binds the high mannose N‐glycan 49 [Manα1‐3(Manα1‐6)Manβ1‐4GlcNAcβ1‐4GlcNAcβ], which is commonly found in human cancer tissue but not in healthy ones (Zipser, Bello‐DeOcampo, Diestel, Tai, & Schmitz, 2012). Additionally, the complex N‐glycan 465 exhibits the terminal equivalent to the LeY antigen (Lewis Y/CD174 ‐Fucα1‐2Galβ1‐4[Fucα1‐3]GlcNAcβ1‐), a cell marker specifically associated to cancer. The LeY antigen is up‐regulated in a variety of cancer cells derived from colon, stomach, ovary, breast, pancreas, prostate, and lung (Dube & Bertozzi, 2005). Although it was not proved to be an effect caused exclusively by tarin, taro corm extract exhibits partial antiproliferative activity against rat YYT colon cancer cells, breast cancer cells (MCF‐7, MDA‐MB‐231, T47D, and MDA‐MB‐435) and prostate cancer cells (DU145, LNCaP, and PC3) (Brown et al., 2005; Kundu et al., 2012). Moreover, tarin is able to bind H2 antigen (CD173 ‐ Fucα1‐2Galβ1‐4GlcNAcβ1‐), present at the glycan 358 expressed in the leukemia cells lineages KG1 and KG1a (pro‐myeloid cells) and TF1 (pre‐erithroblastoid cells) (Cao et al., 2001). The CA‐125 antigen, which characterizes ovary cancer cells, is also a target for tarin binding (Wong et al., 2003). Taken together, these data are indicative of the possible broad spectrum of action of tarin anticancer effects. The aqueous extract from taro corms and the active principle, tarin, have been patented as a natural product for controlling cancer metastasis, to be administered in combination with chemotherapy (Fulton & Kundu, 2014).
Based on the in vitro and in vivo characteristics of tarin, we suggest a molecular model that unites its possible prophylactic and therapeutic actions on hematopoietic or cancer cells, which could boost murine responses (Figure 5). When administered to mice, tarin may act directly on hematopoietic progenitor cells (possibly CD34+) in the bone marrow, protecting them against immunosuppressant cytotoxicity. This protection could allow tarin‐treated immunosuppressed mice to maintain a higher pool of progenitor cells, which could aid mice in recovering faster from immunosuppression. The effects of tarin on the bone marrow may be reflected by increases in the number and activation of total peripheral leukocytes, especially neutrophils (Gr1+). Additionally, tarin inoculation in mice enhances the number of total B lymphocytes (B220+ cells) in the spleen, concomitantly with the decrease in the number of both mature (B220+IgM+) and immature (B220+IgM−) B lymphocytes in the bone marrow. In this case, we suggest that the number of total B cells may be enhanced in the spleen as a result of direct tarin action on splenocytes, activating and stimulating their proliferation. Another alternative would be the migration of mature and immature B cells from the bone marrow to the spleen. Cytokine release (IL‐2, IL1‐β, TNF‐α, and INF‐γ), which could stimulate innate or adaptive responses, may also be triggered by tarin inoculation in mice through leukocyte activation. All the effects observed in the bone marrow, peripheral cells and spleen, could be explained by the tarin ability to specifically bind to carbohydrates found on hematopoietic cell surfaces, including glycans 465 and 358. Cytokine release was reported only in vitro, while mature and immature B lymphocyte effects were reported in vivo following taro corm extract administration. Further studies are required to confirm in vivo cytokine release, to attribute the effects on B lymphocytes to tarin and to study the benefits they could bring to mice responses (Figure 5, left‐hand panel).
The antitumoral and antimetastatic effects exerted in vivo by tarin could also be explained by its binding to a variety of cell ligands, including glycans 213, 49, 465, and 358, which could be responsible for down‐regulation of COX‐1 and ‐2 mRNA expression and the subsequent decrease in prostaglandin E2 (PGE2) levels. As a result, tumor proliferation, growth, and migration may be reduced. Additionally, the inhibition of antitumoral immune responses, as a result of the suppression of dendritic cells (DCs), T cells and natural killer cells (NKs) by PGE2, may also be reduced (Figure 5, right‐hand panel).
Further studies should be performed to investigate the participation of the aforementioned glycans, as well as in vivo tarin effectiveness.
Conclusion
Taro (Colocasia esculenta), a tuberous medicinal plant is a rich source of tarin, a GNA‐related lectin. Highly pure (>90%) and stable tarin samples (200 to 300 mg) can be obtained from 100 g of taro corms through a simple, low‐cost and single‐step purification procedure. Many of the traditional uses of taro as a medicinal plant have been scientifically corroborated. Tarin applications for pest management using plant engineering techniques, as well as a functional additive in food should be considered. Similar to the members of the GNA‐related family, tarin is a noteworthy molecule due to its promising antiviral, antitumoral and insecticidal activities and potential as an immunomodulator molecule. Natural or manufactured food fortified by tarin could contribute to health maintenance and human well‐being. Tarin activity can be explored as a putative immunomodulatory by bringing new evidence regarding potential boosting of the immunological system in order to partially reverse immunosuppressive effects. Immunosupressive states may take advantage of tarin effects on hematopoietic and progenitor cells. Tarin may also be part of future cancer therapeutics, since it may interact directly with cancer cells to inhibit tumor proliferation and the migration of malignant cells. Tarin, in its purified form described herein, could be a promising adjuvant in chemotherapeutic treatment if animal and clinical trials support its effectiveness with no serious side effects to human cells associated with the protein preparation.
Author Contributions
Pereira, P. R. collected the data, interpreted the results and drafted the manuscript. Paschoalin V. M. F. designed the study, helped draft the manuscript and interpreted results. Correa, A. C. N. T. F. and Verícimo, M. A. helped draft the manuscript.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thank the financial support received from the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) during the development of this research.
References
- Abbas, A. K. , Lichtman, A. H. , & Pillai, S. (2014). Cellular and Molecular Immunology E‐Book. Elsevier Health Sciences. [Google Scholar]
- Akkouh, O. , Ng, T. , Singh, S. , Yin, C. , Dan, X. , Chan, Y. , … Cheung, R. (2015). Lectins with anti‐HIV activity: A review. Molecules, 20(1), 648–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara, R. M. , Hurtada, W. A. , & Dizon, E. I. (2013). The nutritional value and phytochemical components of taro [Colocasia esculenta (L.) Schott] powder and its selected processed foods. Journal of Nutrition and Food Sciences, 3(3), 1–7. [Google Scholar]
- Ammar, M. , Hegazy, A. , & Bedeir, S. (2009). Using of taro flour as partial substitute of wheat flour in bread making. World Journal of Dairy Food Sciences, 4(2), 94–99. [Google Scholar]
- Bezerra, I. C. , Castro, L. A. , Neshich, G. , de Almeida, E. R. , de Sa, M. F. , Mello, L. V. , & Monte‐Neshich, D. C. (1995). A corm‐specific gene encodes tarin, a major globulin of taro (Colocasia esculenta L. Schott). Plant Molecular Biology, 28(1), 137–144. [DOI] [PubMed] [Google Scholar]
- Biren, N. S. , Nayak, B. , Bhatt, S. , Jalalpure, S. , & Seth, A. (2007). The anti‐inflammatory activity of the leaves of Colocasia esculenta . Saudi Pharmaceutical Journal, 15(3/4), 228–232. [Google Scholar]
- Boban, P. T. , Nambisan, B. , & Sudhakaran, P. R. (2006). Hypolipidaemic effect of chemically different mucilages in rats: A comparative study. British Journal of Nutrition, 96(06), 1021–1029. [DOI] [PubMed] [Google Scholar]
- Brown, A. C. , Reitzenstein, J. E. , Liu, J. , & Jadus, M. R. (2005). The anti‐cancer effects of poi (Colocasia esculenta) on colonic adenocarcinoma cells in vitro . Phytotherapy Research, 19(9), 767–771. [DOI] [PubMed] [Google Scholar]
- Brown, A. C. , & Valiere, A. (2004). The medicinal uses of poi. Nutrition in Clinical Care, 7(2), 69–74. [PMC free article] [PubMed] [Google Scholar]
- Cao, Y. , Merling, A. , Karsten, U. , & Schwartz‐Albiez, R. (2001). The fucosylated histo‐blood group antigens H type 2 (blood group O, CD173) and Lewis Y (CD174) are expressed on CD34+ hematopoietic progenitors but absent on mature lymphocytes. Glycobiology, 11(8), 677–683. [DOI] [PubMed] [Google Scholar]
- Carlini, C. R. , & Grossi‐de‐Sa, M. F. (2002). Plant toxic proteins with insecticidal properties. A review on their potentialities as bioinsecticides. Toxicon, 40(11), 1515–1539. [DOI] [PubMed] [Google Scholar]
- Carneiro, M. , Rodrigues, C. A. , De Castro, L. A. B. , Da Silva, M. C. , & Coutinho, M. V. (1990). Isolation and characterization of the major albumin from Colocasia esculenta corms. Plant Science, 67(1), 39–46. [Google Scholar]
- Chan, Y. S. , Wong, J. H. , & Ng, T. B. (2010). Cytokine‐inducing hemagglutinin from small taros. Protein & Peptide Letters, 17, 823–830. [DOI] [PubMed] [Google Scholar]
- Chandrasekara, A. , & Josheph Kumar, T. (2016). Roots and tuber crops as functional foods: A review on phytochemical constituents and their potential health benefits. International Journal of Food Science, 2016, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinonyelum, A. N. , Uwadiegwu, A. P. , Nwachukwu, O. C. , & Emmanuel, O. (2015). Evaluation of hepatoprotective activity of Colocasia esculenta (L. Schott) leaves on thioacetamide‐induced hepatotoxicity in rats. Pakistan Journal of Pharmaceutical Sciences, 28(6 Suppl), 2237–2241. [PubMed] [Google Scholar]
- Corrêa, A. C. N. T. F. , Mérida, L. A. D. , Mattos, É. B. A. , Pereira, P. R. , Paschoalin, V. M. F. , Pinho, M. F. B. , & Vericimo, M. A. (2017). Tarin, the lectin from Colocasia esculenta, reduces cyto and genotoxic effects of cyclophosphamide in mice . Paper presented at the 21st International Conference and Expo on Functional Foods, San Diego, USA.
- Daoudi, A. , Abdel‐Satter, E. , & Aarab, L. (2013). The relationship between lectin compounds and immunomodulatory activities of protein extracted from plants. Journal of Plant Studies, 3(1), 56–64. [Google Scholar]
- Das, A. , Roy, A. , Hess, D. , & Das, S. (2013). Characterization of a highly potent insecticidal lectin from Colocasia esculenta tuber and cloning of its coding sequence. American Journal of Plant Sciences, 4(2A), 408–416. [Google Scholar]
- de Castro, L. A. , Carneiro, M. , Neshich Dde, C. , & de Paiva, G. R. (1992). Spatial and temporal gene expression patterns occur during corm development. Plant Cell, 4(12), 1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettke, M. , Pálfi, G. , & Loibner, H. (2000). Activation‐dependent expression of the blood group‐related Lewis Y antigen on peripheral blood granulocytes. Journal of Leukocyte Biology, 68(4), 511–514. [PubMed] [Google Scholar]
- Dhanraj, N. , Kadam, M. , Patil, K. , & Mane, V. (2013). Phytochemical screening and antibacterial activity of western region wild leaf Colocasia esculenta . International Journal of Biological Sciences, 2(10), 18–23. [Google Scholar]
- Dranoff, G. (2004). Cytokines in cancer pathogenesis and cancer therapy. Nature Reviews Cancer, 4(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Du Thanh, H. , Phan Vu, H. , Vu Van, H. , Le Duc, N. , Le Minh, T. , & Savage, G. (2017). Oxalate content of taro leaves grown in Central Vietnam. Foods, 6(2), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube, D. H. , & Bertozzi, C. R. (2005). Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nature Reviews Drug discovery, 4(6), 477–488. [DOI] [PubMed] [Google Scholar]
- Eleazu, C. O. , Iroaganachi, M. , & Eleazu, K. C. (2013). Ameliorative potentials of cocoyam (Colocasia esculenta L.) and unripe plantain (Musa paradisiaca L.) on the relative tissue weights of streptozotocin‐induced diabetic rats. Journal of Diabetes Research, 2013, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanuel, C. I. , Osuchukwu, N. , & Oshiele, L. (2010). Functional and sensory properties of wheat (Aestium triticium) and taro flour (Colocasia esculenta) composite bread. African Journal of Food Science, 4(5), 248–253. [Google Scholar]
- Ferreres, F. , Gonçalves, R. F. , Gil‐Izquierdo, A. , Valentão, P. , Silva, A. M. S. , Silva, J. B. , … Andrade, P. B. (2012). Further knowledge on the phenolic profile of Colocasia esculenta (L.) Schott. Journal of Agricultural and Food Chemistry, 60(28), 7005–7015. [DOI] [PubMed] [Google Scholar]
- Fulton, A. , & Kundu, N. (2014). USA Patent No. US 8,865,642 B2.
- Goldstein, I. J. , Hughes, R. C. , Monsigny, M. , Osawa, T. , & Sharon, N. (1980). What should be called a lectin? Nature, 285(5760), 66–66. [Google Scholar]
- Gonçalves, R. F. , Silva, A. M. , Silva, A. M. , Valentão, P. , Ferreres, F. , Gil‐Izquierdo, A. , … Andrade, P. B. (2013). Influence of taro (Colocasia esculenta L. Schott) growth conditions on the phenolic composition and biological properties. Food Chemistry, 141(4), 3480–3485. [DOI] [PubMed] [Google Scholar]
- Heredia Zárate, N. , Vieira, M. , & Silva, R. (1996). Produção de cinco clones de inhame em cinco épocas de plantio. Dourados–MS. SOBInforma, Rio de Janeiro, 15(2), 18–19. [Google Scholar]
- Hirai, M. , Nakamura, K. , Imai, T. , & Sato, T. (1993). cDNAs encoding for storage proteins in the tubers of taro (Colocasia esculenta Schott). Japanese Journal of Genetics, 68(3), 229–236. [DOI] [PubMed] [Google Scholar]
- Hirai, M. , Sato, T. , & Takayanagi, K. (1989). Classification of Japanese cultivars of taro (Colocasia esculenta (L.) Schott) based on electrophoretic pattern of the tuber proteins and morphological characters. Japanese Journal of Breeding, 39(3), 307–317. [Google Scholar]
- Huang, A. S. , Lam, S. Y. , Nakayama, T. M. , & Lin, H. (1994). Microbiological and chemical changes in poi stored at 20 °C. Journal of Agricultural and Food Chemistry, 42(1), 45–48. [Google Scholar]
- Ingale, A. G. , & Hivrale, A. U. (2013). Plant as a plenteous reserve of lectin. Plant Signaling & Behavior, 8(12), e26595–1‐e26595‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalariya, M. , Parmar, S. , & Sheth, N. (2010). Neuropharmacological activity of hydroalcoholic extract of leaves of Colocasia esculenta . Pharmaceutical Biology, 48(11), 1207–1212. [DOI] [PubMed] [Google Scholar]
- Kaur, M. , Kaushal, P. , & Sandhu, K. S. (2013). Studies on physicochemical and pasting properties of taro (Colocasia esculenta L.) flour in comparison with a cereal, tuber and legume flour. Journal of Food Science and Technology, 50(1), 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal, P. , Kumar, V. , & Sharma, H. K. (2013). Utilization of taro (Colocasia esculenta): A review. Journal of Food Science and Technology, 52(1), 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, J. F. , Palva, P. M. G. , Corella, M. T. S. , Cavalcanti, M. S. M. , & Coelho, L. C. B. B. (1995). Lectins, versatile proteins of recognition: A review. Carbohydrate Polymers, 26(3), 219–230. [Google Scholar]
- Keyaerts, E. , Vijgen, L. , Pannecouque, C. , Van Damme, E. , Peumans, W. , Egberink, H. , … Van Ranst, M. (2007). Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Research, 75(3), 179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. H. , Moon, E. , Kim, S. Y. , & Lee, K. R. (2010). Anti‐melanogenic fatty acid derivatives from the tuber‐barks of Colocasia antiquorum var. esculenta. Bulletin of the Korean Chemical Society, 31(7), 2051–2053. [Google Scholar]
- Krokhin, O. , Li, Y. , Andonov, A. , Feldmann, H. , Flick, R. , Jones, S. , … Standing, K. G. (2003). Mass spectrometric characterization of proteins from the SARS virus: A preliminary report. Molecular & Cellular Proteomics, 2(5), 346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubde, M. S. , Khadabadi, S. , Farooqui, I. , & Deore, S. (2010). In vitro antihelmintic activity of Colocasia esculenta . Der Pharmacia Lettre, 2(2), 82–85. [Google Scholar]
- Kumar, U. A. , Manjunath, C. , Thaminzhmani, T. , Kiran, Y. R. , & Brahmaiah, Y. (2012). A review on immunomodulatory activity of plants. Indian Journal of Novel Drug Delivery, 4(2), 93–103. [Google Scholar]
- Kumawat, N. , Chaudhari, S. , Wani, N. , Deshmukh, T. , & Patil, V. (2010). Antidiabetic activity of ethanol extract of Colocasia esculenta leaves in alloxan induced diabetic rats. International Journal of PharmTech Research, 2(2), 1246–1249. [Google Scholar]
- Kumoro, A. C. , Putri, R. D. A. , Budiyati, C. S. , & Retnowati, D. S. (2014). Kinetics of calcium oxalate reduction in taro (Colocasia esculenta) corm chips during treatments using baking soda solution. Procedia Chemistry, 9, 102–112. [Google Scholar]
- Kundu, N. , Campbell, P. , Hampton, B. , Lin, C. Y. , Ma, X. , Ambulos, N. , … Fulton, A. M. (2012). Antimetastatic activity isolated from Colocasia esculenta (taro). Anticancer Drugs, 23(2), 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannoo, N. , & Van Damme, E. J. (2014). Lectin domains at the frontiers of plant defense. Frontiers of Plant Science, 5, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal‐Bertioli, S. , Pascoal, A. V. , Guimarães, P. M. , SÁ, M. F. G. , Guimarães, R. L. , Monte, D. C. , & Bertioli, D. J. (2003). Transgenic tobacco plants expressing Tarin 1 inhibit the growth of Pseudomonas syringae pv. tomato and the development of Spodoptera frugiperda . Annals of Applied Biology, 143(3), 349–357. [Google Scholar]
- Lee, S. , Wee, W. , Yong, J. , & Syamsumir, D. (2011). Antimicrobial, antioxidant, anticancer property and chemical composition of different parts (corm, stem and leave) of Colocasia esculenta extract. Annales Universitatis Mariae Curie‐Sklodowska, Sectio DDD: Pharmacia, 24(3), 9–16. [Google Scholar]
- Li, C.‐y. , Meng, L. , Liu, B. , & Bao, J.‐k. (2009). Galanthus nivalis agglutinin (GNA)‐related lectins: Traditional proteins, burgeoning drugs? Current Chemical Biology, 3(3), 323–333. [Google Scholar]
- Li, H. M. , Hwang, S. H. , Kang, B. G. , Hong, J. S. , & Lim, S. S. (2014). Inhibitory effects of Colocasia esculenta (L.) Schott constituents on aldose reductase. Molecules, 19(9), 13212–13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, T. K. (2015). Colocasia esculenta In Edible medicinal and non medicinal plants (Vol. 9, pp. 454–492). Dordrecht, NL: Springer. [Google Scholar]
- Lis, H. , & Sharon, N. (1998). Lectins: Carbohydrate‐specific proteins that mediate cellular recognition. Chemical Reviews, 98(2), 637–674. [DOI] [PubMed] [Google Scholar]
- Liu, B. , Qu, L. , & Yan, S. (2015). Cyclooxygenase‐2 promotes tumor growth and suppresses tumor immunity. Cancer Cell International, 15(106), 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo, M. L. R. , Oliveira, C. F. , & Oliveira, C. T. (2015). Insecticidal activity of plant lectins and potential application in crop protection. Molecules, 20(2), 2014–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga, J. A. (1992). Taro: Composition and food uses. Food Reviews International, 8(3), 443–473. [Google Scholar]
- Meilena, T. (2017). Toxicity test on taro leaf extract (Colocasia esculenta L. Schott) as mouthwash on BHK‐21 fibroblast cell culture for denture users. (PhD thesis), Universitas Airlanggas, Surabaia, Indonésia.
- Mesquita, A. (2002). Inhame e taro: Cenários dos mercados internacional, brasileiro e baiano. Bahia Agrícola, 5(2), 54–64. [Google Scholar]
- Michiels, K. , Van Damme, E. J. , & Smagghe, G. (2010). Plant‐insect interactions: What can we learn from plant lectins? Archives of Insect Biochemistry and Physiology, 73(4), 193–212. [DOI] [PubMed] [Google Scholar]
- Monte‐Neshich, D. C. , Rocha, T. L. , Guimarães, R. L. , Santana, E. F. , Loureiro, M. E. , Valle, M. , & de Sá, M. F. G. (1995). Characterization and spatial localization of the major globulin families of taro (Colocasia esculenta L. Schott) tubers. Plant Science, 112(2), 149–159. [Google Scholar]
- Noorfarahzilah, M. , Lee, J. S. , Sharifudin, M. , MohdFadzelly, A. , & Hasmadi, M. (2014). Applications of composite flour in development of food products. International Food Research Journal, 21(6), 2061–2074. [Google Scholar]
- Nunes, R. S. , Del Aguila, E. , Paschoalin, V. , & da Silva, J. (2014). DNA barcoding assessment of the genetic diversity of varieties of taro, Colocasia esculenta (L.) Schott in Brazil Breeding and genetic engineering: The biology and biotechnology research (1st ed.). Hong Kong: IConcept Press. [Google Scholar]
- Nunes, R. S. , Pinhati, F. R. , Golinelli, L. P. , Rebouças, T. N. H. , Paschoalin, V. M. F. , & Silva, J. T. d. (2012). Polymorphic microsatellites of analysis in cultivars of taro. Horticultura Brasileira, 30, 106–111. [Google Scholar]
- Nwauzoma, A. B. , & Dappa, M. S. (2013). Ethnobotanical studies of Port Harcourt Metropolis, Nigeria. ISRN Botany, 2013, 1–11. [Google Scholar]
- Ohno, Y. , Naganuma, T. , Ogawa, T. , & Muramoto, K. (2006). Effect of lectins on the transport of food factors in Caco‐2 cell monolayers. Journal of Agricultural and Food Chemistry, 54(2), 548–553. [DOI] [PubMed] [Google Scholar]
- Patil, B. R. , & Ageely, H. M. (2011). Antihepatotoxic activity of Colocasia esculenta leaf juice. International Journal of Advanced Biotechnology and Research, 2, 296–304. [Google Scholar]
- Pereira, P. R. , Del Aguila, E. M. , Vericimo, M. A. , Zingali, R. B. , Paschoalin, V. M. , & Silva, J. T. (2014). Purification and characterization of the lectin from taro (Colocasia esculenta) and its effect on mouse splenocyte proliferation in vitro and in vivo . Protein Journal, 33(1), 92–99. [DOI] [PubMed] [Google Scholar]
- Pereira, P. R. , Meagher, J. L. , Winter, H. C. , Goldstein, I. J. , Paschoalin, V. M. , Silva, J. T. , & Stuckey, J. A. (2017). High‐resolution crystal structures of Colocasia esculenta tarin lectin. Glycobiology, 27(1), 50–56. [DOI] [PubMed] [Google Scholar]
- Pereira, P. R. , Silva, J. T. , Verícimo, M. A. , Paschoalin, V. M. F. , & Teixeira, G. A. P. B. (2015). Crude extract from taro (Colocasia esculenta) as a natural source of bioactive proteins able to stimulate haematopoietic cells in two murine models. Journal of Functional Foods, 18(Part A), 333–343. [Google Scholar]
- Pereira, P. R. , Winter, H. C. , Vericimo, M. A. , Meagher, J. L. , Stuckey, J. A. , Goldstein, I. J. , … Silva, J. T. (2015). Structural analysis and binding properties of isoforms of tarin, the GNA‐related lectin from Colocasia esculenta . Biochimica et Biophysica Acta, Proteins and Proteomics, 1854(1), 20–30. [DOI] [PubMed] [Google Scholar]
- Peumans, W. J. , & Van Damme, E. J. (1995a). Lectins as plant defense proteins. Plant Physiology, 109(2), 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peumans, W. J. , & Van Damme, E. J. (1995b). The role of lectins in plant defense. Histochemical Journal, 27(4), 253–271. [DOI] [PubMed] [Google Scholar]
- Peumans, W. J. , & Van Damme, E. J. M. (1996). Prevalence, biological activity and genetic manipulation of lectins in foods. Trends in Food Science & Technology, 7(4), 132–138. [Google Scholar]
- Prajapati, R. , Kalariya, M. , Umbarkar, R. , Parmar, S. , & Sheth, N. (2011). Colocasia esculenta: A potent indigenous plant. International Journal of Nutrition, Pharmacology, Neurological Diseases, 1(2), 90–96. [Google Scholar]
- Pusztai, A. , Bardocz, S. , & Ewen, S. (2007). Uses of plant lectins in bioscience and biomedicine. Frontiers in Bioscience, 13, 1130–1140. [DOI] [PubMed] [Google Scholar]
- Rajashekar, Y. , Tonsing, N. , Shantibala, T. , & Manjunath, J. R. (2016). 2, 3‐dimethylmaleic anhydride (3, 4‐dimethyl‐2, 5‐furandione): A plant derived insecticidal molecule from Colocasia esculenta var. esculenta (L.) Schott. Scientific Reports, 6, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyad‐ul‐Ferdous, M. , Arman, M. S. I. , Tanvir, M. M. I. , Sumi, S. , Siddique, K. M. M. R. , Billah, M. M. , & Islam, M. S. (2015). Biologically potential for pharmacologicals and phytochemicals of medicinal plants of Colocasia esculenta: A comprehensive review. American Journal of Clinical and Experimental Medicine, 3(5‐1), 7–11. [Google Scholar]
- Roy, A. , Banerjee, S. , Majumder, P. , & Das, S. (2002). Efficiency of mannose‐binding plant lectins in controlling a homopteran insect, the red cotton bug. Journal of Agricultural and Food Chemistry, 50(23), 6775–6779. [DOI] [PubMed] [Google Scholar]
- Roy, A. , & Das, S. (2015). Molecular mechanism underlying the entomotoxic effect of Colocasia esculenta tuber agglutinin against Dysdercus cingulatus . Insects, 6(4), 827–846. [Google Scholar]
- Roy, A. , Gupta, S. , Hess, D. , Das, K. P. , & Das, S. (2014). Binding of insecticidal lectin Colocasia esculenta tuber agglutinin (CEA) to midgut receptors of Bemisia tabaci and Lipaphis erysimi provides clues to its insecticidal potential. Proteomics, 14(13‐14), 1646–1659. [DOI] [PubMed] [Google Scholar]
- Rudiger, H. (1998). Plant lectins ‐ more than just tools for glycoscientists: Occurrence, structure, and possible functions of plant lectins. Acta Anatomica (Basel), 161(1‐4), 130–152. [DOI] [PubMed] [Google Scholar]
- Rudiger, H. , Siebert, H. C. , Solis, D. , Jimenez‐Barbero, J. , Romero, A. , von der Lieth, C. W. , … Gabius, H. J. (2000). Medicinal chemistry based on the sugar code: Fundamentals of lectinology and experimental strategies with lectins as targets. Current Medicinal Chemistry, 7(4), 389–416. [DOI] [PubMed] [Google Scholar]
- Sakano, Y. , Mutsuga, M. , Tanaka, R. , Suganuma, H. , Inakuma, T. , Toyoda, M. , … Ebizuka, Y. (2005). Inhibition of human lanosterol synthase by the constituents of Colocasia esculenta (taro). Biological & Pharmaceutical Bulletin, 28(2), 299–304. [DOI] [PubMed] [Google Scholar]
- Shanmugham, L. N. , Castellani, M. L. , Salini, V. , Falasca, K. , Vecchiet, J. , Conti, P. , & Petrarca, C. (2006). Relevance of plant lectins in human cell biology and immunology . Paper presented at the Biology Forum/Rivista di Biologia. [PubMed]
- Sharon, N. (2008). Lectins: Past, present and future. Biochemical Society Transactions, 36(Pt 6), 1457–1460. [DOI] [PubMed] [Google Scholar]
- Sharon, N. , & Lis, H. (1995). Lectins‐proteins with a sweet tooth: Functions in cell recognition. Essays in Biochemistry, 30, 59–75. [PubMed] [Google Scholar]
- Shet, M. , & Madaiah, M. (1988). Lectin activity in different plant tubers, rhizomes and bulbs. Current Science, 57(20), 1107–1110. [Google Scholar]
- Shewry, P. R. (2003). Tuber storage proteins. Annals of Botany, 91(7), 755–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillmark, H. (1888). Ueber Ricin, eingiftiges Ferment aus den Samen von Ricinus comm. L. und einigenanderenEuphorbiaceen. (Doctorate), Universität Dorpat, Estonia.
- Subhash, C. , Sarla, S. , & Jaybardhan, S. (2012). Phytochemical screening of Garhwal Himalaya wild edible tuber Colocasia esculenta . International Research Journal of Pharmacy, 3(3), 181–186. [Google Scholar]
- Temesgen, M. , & Retta, N. (2015). Nutritional potential, health and food security benefits of taro Colocasia esculenta (L.): A review. Food Science and Quality Management, 36, 23–30. [Google Scholar]
- Thakur, K. , Kaur, M. , Kaur, S. , Kaur, A. , Kamboj, S. S. , & Singh, J. (2013). Purification of Colocasia esculenta lectin and determination of its anti‐insect potential towards Bactrocera cucurbitae . Journal of Environmental Biology, 34(1), 31–36. [PubMed] [Google Scholar]
- Tulin, E. E. , & Ecleo, Z. T. (2007). Cytokine‐mimetic properties of some Philippine food and medicinal plants. Journal of Medicinal Food, 10(2), 290–299. [DOI] [PubMed] [Google Scholar]
- Vacchelli, E. , Aranda, F. , Bloy, N. , Buqué, A. , Cremer, I. , Eggermont, A. , … Spisek, R. (2016). Trial watch—Immunostimulation with cytokines in cancer therapy. OncoImmunology, 5(2), e1115942–1–e1115942–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajravijayan, S. , Pletnev, S. , Pletnev, V. Z. , Nandhagopal, N. , & Gunasekaran, K. (2016). Structural analysis of β‐prism lectin from Colocasia esculenta (L.) Schott. International Journal of Biological Macromolecules, 91, 518–523. [DOI] [PubMed] [Google Scholar]
- Van Damme, E. J. M. (2008). Plant lectins as part of the plant defense system against insects In Schaller A. (Ed.), Induced plant resistance to herbivory (pp. 285–307). Dordrecht: Springer. [Google Scholar]
- Van Damme, E. J. M. (2014). History of plant lectin research In Hirabayashi J. (Ed.), Lectins: Methods and protocols (Vol. 1200, pp. 3–13). New York, USA: Springer. [DOI] [PubMed] [Google Scholar]
- Van Damme, E. J. M. , Goossens, K. , Smeets, K. , Van Leuven, F. , Verhaert, P. , & Peumans, W. J. (1995). The major tuber storage protein of araceae species is a lectin. Characterization and molecular cloning of the lectin from Arum maculatum L. Plant Physiology, 107(4), 1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme, E. J. M. , Lannoo, N. , & Peumans, W. J. (2008). Plant Lectins. Advances in Botanical Research, 48, 107–209. [Google Scholar]
- Van Damme, E. J. M. , Nakamura‐Tsuruta, S. , Smith, D. F. , Ongenaert, M. , Winter, H. C. , Rougé, P. , … Culerrier, R. (2007). Phylogenetic and specificity studies of two‐domain GNA‐related lectins: Generation of multispecificity through domain duplication and divergent evolution. Biochemistry Journal, 404(1), 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki, A. (1993). Biological roles of oligosaccharides: All of the theories are correct. Glycobiology, 3(2), 97–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasant, O. K. , Vijay, B. G. , Virbhadrappa, S. R. , Dilip, N. T. , Ramahari, M. V. , & Laxamanrao, B. S. (2012). Antihypertensive and diuretic effects of the aqueous extract of Colocasia esculenta Linn. Leaves in Experimental Paradigms. Iranian Journal of Pharmaceutical Research, 11(2), 621–634. [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos, I. M. , & Oliveira, J. T. A. (2004). Antinutritional properties of plant lectins. Toxicon, 44(4), 385–403. [DOI] [PubMed] [Google Scholar]
- Walski, T. , De Schutter, K. , Van Damme, E. J. , & Smagghe, G. (2017). Diversity and functions of protein glycosylation in insects. Insect Biochemistry and Molecular Biology, 83, 21–34, [DOI] [PubMed] [Google Scholar]
- Waterhouse, A. M. , Procter, J. B. , Martin, D. M. , Clamp, M. , & Barton, G. J. (2009). Jalview Version 2‐a multiple sequence alignment editor and analysis workbench. Bioinformatics, 25(9), 1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, N. K. , Easton, R. L. , Panico, M. , Sutton‐Smith, M. , Morrison, J. C. , Lattanzio, F. A. , … Patankar, M. S. (2003). Characterization of the oligosaccharides associated with the human ovarian tumor marker CA125. Journal of Biological Chemistry, 278(31), 28619–28634. [DOI] [PubMed] [Google Scholar]
- Wu, L. , & Bao, J.‐k. (2013). Anti‐tumor and anti‐viral activities of Galanthus nivalis agglutinin (GNA)‐related lectins. Glycoconjugate Journal, 30(3), 269–279. [DOI] [PubMed] [Google Scholar]
- Zipser, B. , Bello‐DeOcampo, D. , Diestel, S. , Tai, M.‐H. , & Schmitz, B. (2012). Mannitou monoclonal antibody uniquely recognizes paucimannose, a marker for human cancer, stemness, and inflammation. Journal of Carbohydrate Chemistry, 31(4‐6), 504–518. [Google Scholar]


