Summary
Poliovirus 2B protein is a well‐known viroporin implicated in plasma membrane permeabilization to ions and low‐molecular‐weight compounds during infection. Translation in mammalian cells expressing 2B protein is inhibited by hygromycin B (HB) but remains unaffected in mock cells, which are not permeable to the inhibitor. Here we describe a previously unreported bystander effect in which healthy baby hamster kidney (BHK) cells become sensitive to HB when co‐cultured with a low proportion of cells expressing poliovirus 2B. Viroporins E from mouse hepatitis virus, 6K from Sindbis virus and NS4A protein from hepatitis C virus were also able to permeabilize neighbouring cells to different extents. Expression of 2B induced permeabilization of neighbouring cell lines other than BHK. We found that gap junctions are responsible mediating the observed bystander permeabilization. Gap junctional communication was confirmed in 2B‐expressing co‐cultures by fluorescent dye transfer. Moreover, the presence of connexin 43 was confirmed in both mock and 2B‐transfected cells. Finally, inhibition of HB entry to neighbouring cells was observed with 18α‐glycyrrhethinic acid, an inhibitor of gap junctions. Taken together, these findings support a mechanism involving gap junctional intercellular communication in the bystander permeabilization effect observed in healthy cells co‐cultured with poliovirus 2B‐expressing cells.
Introduction
Poliovirus (PV) infection leads to plasma membrane permeabilization and proliferation of intracellular vesicles in which viral replication complexes are assembled (Bienz et al., 1992; Schlegel et al., 1996). PV 2B protein and its precursor 2BC are the main effectors of membrane leakiness, and together with 3A protein, they induce a significant rearrangement of internal cellular membranes (Aldabe and Carrasco, 1995; Suhy et al., 2000; Choe et al., 2005). PV 2B has been previously described as a genuine member of the viroporin family that is able to permeabilize bacteria, yeast and mammalian cells to ions and small molecules (Aldabe et al., 1996; van Kuppeveld et al., 1997; Agirre et al., 2002). Viroporins are small proteins encoded by animal viruses and contain at least one membrane‐spanning domain (Gonzalez and Carrasco, 1998; Ye and Hogue, 2007; Gan et al., 2008). The main function of these very hydrophobic proteins during the viral life cycle is to facilitate the release of viral particles from cells (Klimkait et al., 1990; van Kuppeveld et al., 1997; Sanz et al., 2003). Viroporins assemble in homopolymers to form ion channels in cellular membranes and they constitute a target for antiviral drug development (Pinto et al., 1992; Ewart et al., 1996; Melton et al., 2002; Pavlovic et al., 2003; Wilson et al., 2004; Madan et al., 2007; Griffin et al., 2008; Pielak et al., 2009). Recently, the three‐dimensional structure of a viroporin has been unravelled (Luik et al., 2009). Electron microscopy studies revealed that hexamers of the hepatitis C virus (HCV) p7 viroporin assemble to form a flower‐shaped structure with protruding petals oriented towards the ER lumen. These studies are not only of fundamental interest to understand viroporin architecture, but may also aid in the design of antiviral compounds against p7 protein. Indeed, HCV p7 is a key target to develop compounds that block HCV infection (Pavlovic et al., 2003; Griffin et al., 2008). Apart from HCV p7, picornavirus 2B is one of the best‐characterized viroporins (van Kuppeveld et al., 1997; Agirre et al., 2002). We recently reported that an amphipathic peptide spanning 2B residues 35–55 effectively allows diffusion of solutes with a molecular weight under 1 kDa both in mammalian cells and in boundary liposomes (Madan et al., 2007). In that study,addition of the peptide to patch‐clamped natural plasma membrane induced ion channel activity, indicating that this region of 2B is endowed with pore‐forming activity. In a comparative study of the ability of different viroporins to permeabilize the plasma membrane of baby hamster kidney (BHK) cells, PV 2B together with E protein from mouse hepatitis virus powerfully and rapidly induced permeabilization, whereas other viroporins such as HCV p7, influenza A virus M2 or 6K from Sindbis virus (SV) were less effective or required longer expression to achieve similar membrane alterations in BHK cells (Madan, 2008). To date, a combination of electrophysiological measurements and studies involving entry of unpermeant translation inhibitors, such as hygromycin B (HB), in cells expressing a specific viral product has allowed pore‐forming proteins to be distinguished from other viral proteins with cytotoxic properties, both in prokaryotic and in eukaryotic cells (Guinea and Carrasco, 1994; Wang et al., 1994; Ewart et al., 1996; Barco and Carrasco, 1998; Gonzalez and Carrasco, 1998; Wilson et al., 2004; Madan et al., 2005). In the last few years, certain viral proteins such as E and 3a from coronavirus and Vpr from HIV‐1 have been reported to be released from mammalian cells to the extracellular medium (Maeda et al., 1999; Huang et al., 2006; Xiao et al., 2008). However, their function either as soluble or integral membrane proteins in secreted lipid vesicles is still largely unknown. Extracellular Vpr has been shown to deregulate expression of various cytokines and inflammatory proteins, as well as to induce cell death in uninfected bystander cells (Huang et al., 2000; Moon and Yang, 2006; Xiao et al., 2008). Here, we investigate whether 2B protein is released from transfected cells to the extracellular medium and whether it permeabilizes non‐transfected neighbouring cells. Our findings reveal that 2B expression in a small proportion of BHK cells induces substantial permeabilization to HB in healthy neighbouring cells. We provide compelling evidence to support the hypothesis that 2B‐mediated bystander permeabilization of neighbouring cells takes place via HB passage through connexin gap junctions.
Results
Expression of PV 2B protein in BHK cells induces membrane permeabilization of neighbouring cells
Expression of PV 2B induces membrane permeabilization to ions and small molecules (e.g. nucleotides, Ca2+ ions or antibiotics such as HB) in mammalian cells to a similar extent as in the mid‐phase of virus infection (van Kuppeveld et al., 1997). In previous works we noted that even though a low percentage of BHK cells was transfected with SV‐derived replicons coding for 2B, the whole cell culture was almost entirely permeabilized. To assess membrane permeabilization to the translation inhibitor HB in cells neighbouring 2B‐expressing cells, decreasing proportions of 2B‐transfected cells were co‐cultured with non‐transfected cells. Membrane permeabilization was assayed by protein labelling with [35S] Met/Cys at different time points. In the presence of HB, protein synthesis is expected to occur only in non‐transfected neighbouring cells, while those that express 2B are permeable to the inhibitor and do not synthesize cellular proteins. We found that neighbouring cells were powerfully permeabilized to HB (∼50% translation inhibition) from 5 h post electroporation (hpe) when cultured with 2B‐expressing cells at ratios as low as 1:8 (Fig. 1A and C). By 8 hpe, translation of healthy cells was reduced to 20% compared with controls, indicating increased entry of HB (see right graph in Fig. 1C). Increasing proportions of viroporin‐expressing cells (from 1/4 or 25%) led to an almost complete permeabilization of neighbouring cells to HB from 5 hpe. Simultaneously, immunofluorescence assays using specific antibodies against 2B protein were performed in order to visualize the presence of the viral protein in these co‐cultures (Fig. 1B). PV 2B was not detected in the majority of cultured cells, with only an estimated 25% of cells displaying 2B protein specific fluorescence.
Figure 1.
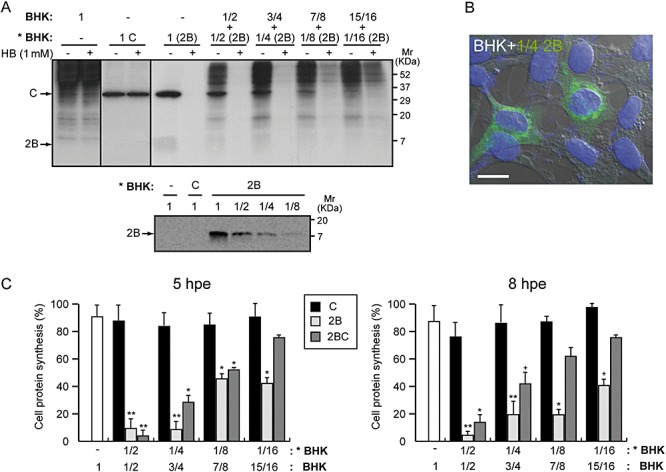
Expression of PV 2B protein in BHK cells induces permeabilization of neighbouring cells to HB. BHK cells were electroporated with in vitro synthesized RNA from the plasmids pT7 repC+2B or pT7 repC+2BC. Different proportions of electroporated cells (as indicate in the figure, * BHK) were mixed with mock BHK cells and seeded in 24‐well plates. Cell density (ρ) in each well was approximately 1.9 × 105 cells cm−2. At different times after transfection, proteins were labelled with [35S] Met/Cys in the absence (−) or presence (+) of 1 mM HB for 40 min. Samples were processed by SDS‐PAGE (17.5%) followed by fluorography and autoradiography. A. Membrane permeabilization of neighbouring cells (mock cells) assayed by the inhibition of translation as a result of HB entry induced by 2B protein at 8 h post electroporation (hpe) (a representative experiment). All of the cells expressing 2B are permeable to HB (proportion 1; cell ρ = 1.9 × 105 cells cm−2). A Western blot using polyclonal antibodies against 2B protein was performed to show 2B expression and its proportional decrease with dilutions of transfected cells (lower panel). B. Immunofluorescence staining at 8 hpe in a sample in which only 25% of BHK cells expressed 2B protein. Cells were fixed, permeabilized and double stained with anti‐2B antibodies (green) and DAPI (nuclei labelling, blue). The panel shows merged immunofluorescence and phase‐contrast images. Scale bar, 10 µM. C. Statistical analysis of membrane permeabilization of neighbouring cells caused by 2B and its precursor, 2BC, at 5 (left graph) and 8 hpe (right graph). Each bar represents the percentage of cellular protein synthesis in HB‐treated cells compared with untreated cells. Cellular proteins bands were quantified by densitometry. All data are shown as the mean ± SD of at least three independent experiments. (5 hpe; **P < 0.01, *P < 0.05, 8 hpe; **P < 0.001, *P < 0.01, + P < 0.05).
Similar results were obtained in permeabilization assays using untransfected cells and BHK cells expressing the 2B precursor 2BC (Fig. 1C, dark grey bars, and Fig. S1). These findings support the notion that healthy cells were permeable to HB when co‐cultured with cells that express PV 2B or 2BC. Moreover, the extent of permeabilization to HB in neighbouring cells was directly dependent on the proportion of 2B‐expressing cells that were co‐cultured as well as on the length of incubation. However, at 19 hpe, when signs of apoptosis are evident and cell death occurs in a high proportion of cells that express 2B (Madan et al., 2008), permeabilization of healthy counterparts was found to be significantly lower than that seen at earlier time points (data not shown). These differences strongly suggest that the permeabilization of healthy cells requires the 2B‐expressing cells to still be alive.
Several viroporins induce different degrees of permeabilization to HB in neighbouring cells
A comparative analysis was carried out using E protein from MHV‐A59 and 6K from SV to study whether other viroporins can permeabilize healthy neighbouring cells. PV 2B and coronavirus E proteins have been described to induce a rapid and efficient permeabilization of BHK cells, whereas SV 6K required a longer period of expression to permeabilize the plasma membrane extensively. In addition, a small and cytotoxic protein from HCV, NS4A, exhibited viroporin‐like activity several hours after its expression in BHK cells (Madan et al., 2008). To analyse membrane permeabilization of healthy cells to HB at different times by several viroporin‐expressing cells, we carried out assays using the co‐culture system. BHK cells were electroporated with the corresponding SV‐derived replicons (RNAs) coding for either SV C alone, 2B, E, NS4A or 6K. Equal proportions of transfected and non‐tranfected cells were mixed and seeded, and translation inhibition by HB was quantified at 8 hpe (C, 2B, E and NS4A) or 16 hpe (6K). Figure 2 shows a representative PAGE analysis in which C protein and viroporin synthesis was detected by metabolic protein labelling. The presence of NS4A was also confirmed by Western blotting, using a specific monoclonal antibody (data not shown). Expression of coronavirus E protein permeabilized transfected cells and also resulted in HB entry in neighbouring cells to a slightly lesser extent than observed in PV 2B co‐cultures. The permeabilization activity induced by HCV NS4A expression was delayed compared with that seen upon 2B or E protein expression (Madan et al., 2008). Consequently, HB entry in non‐transfected cells was notably impaired, as occurred in co‐cultures expressing the non‐permeabilizing C protein from SV. In contrast, permeabilization of healthy cells that were co‐cultured with 6K‐expressing cells for 16 h was unexpectedly lower than that seen in the 2B or E co‐cultures, even though 6K‐expressing cells were entirely permeabilized (Fig. 2, right panel). These findings reveal the different capabilities of the viroporins tested to promote permeabilization of neighbouring cells. Moreover, this may reflect the different viroporin activity of each viral protein.
Figure 2.
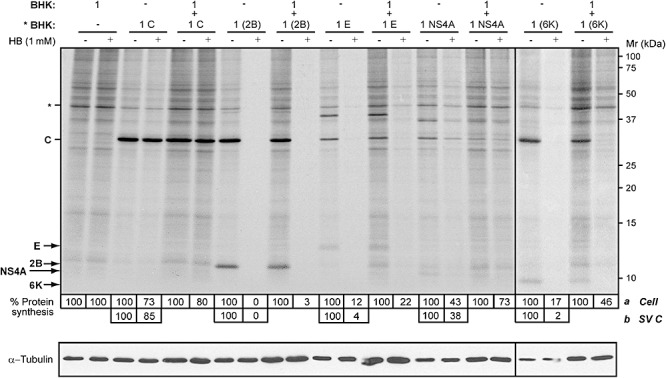
Expression of different viroporins induces permeabilization of neighbouring cells to HB to different extents. BHK cells were electroporated with in vitro synthesized RNA from the plasmids pT7 repC+2B, pT7 repC+E, pT7 repC+NS4A or pT7 repC+6K. Transfected cells (* BHK) were mixed with mock cells in equal proportions (ratio 1:1; total cell ρ = 1.9 × 105 cells cm−2) or seeded separately (proportion 1; total cell ρ = 8 × 104 cells cm−2) in 24‐well plates. As a negative control, cells were transfected with RNA from pT7 repC (encoding Sindbis virus capsid protein). At 8 h post electroporation (hpe) (cells expressing only C protein, 2B, E or NS4A protein co‐cultured with mock cells) or 16 hpe (cells expressing 6K co‐cultured with mock cells), proteins were metabolically labelled in the absence (−) or presence (+) of 1 mM HB for 40 min. Samples were processed by SDS‐PAGE (17.5%). To measure membrane permeabilization of neighbouring cells, protein synthesis in those cells was quantified by densitometry of bands corresponding to actin, * (a). Permeabilization of cells expressing either C protein or viroporins is represented as the decrease in protein synthesis which was quantified by densitometry of bands corresponding to C protein (b). Numbers below the gel indicate the percentage of cell protein synthesis in HB‐treated cells compared with untreated cells. Most cells expressing viroporins 2B, E or 6K were permeable to HB. A Western blot using monoclonal antibodies against α‐tubulin was performed as a control for protein load (lower panel).
Expression of 2B viroporin in BHK cells promotes translation inhibition by HB in other cell lines
To address the importance of this secondary permeabilization effect mediated by 2B on co‐cultured non‐transfected cells, two additional cell lines were assayed. Huh‐7 and HeLa tumour cell lines were mixed with 2B‐transfected BHK cells at different ratios (a total of 3 × 105 cells), and entry of HB was assayed at 8 hpe. Significant permeabilization of Huh‐7 cells (∼55% translation inhibition by HB) was accomplished when at least 75% of the mixed cell population were transfected BHK cells (Fig. 3 and Fig. S3). Assays using BHK/HeLa cell co‐cultures revealed that 50% 2B‐expressing BHK cells was sufficient to induce the same extent of permeabilization in HeLa cells as in Huh‐7 cells (Fig. S3‐1). In contrast to the results obtained with co‐cultures only composed of BHK cells (Fig. 1), co‐cultures containing less than 50% or 75% transfected BHK cells did not result in substantial permeabilization of neighbouring tumour cells. These results suggest that in addition to the viroporin activity of 2B, permeabilization to HB of non‐transfected cells is also influenced by the neighbouring cell line.
Figure 3.
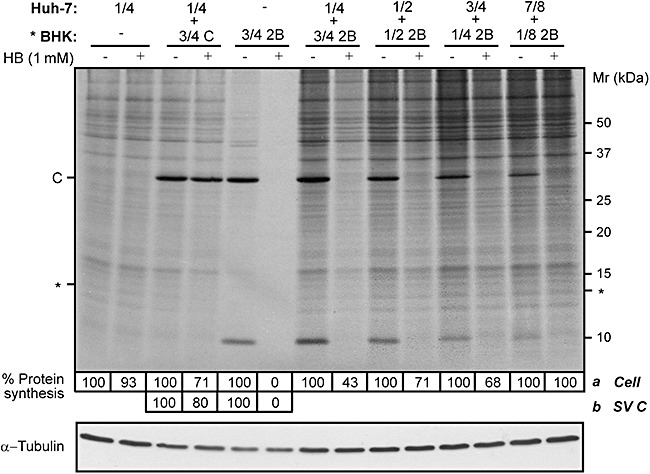
Expression of 2B viroporin in BHK cells promotes translation inhibition by HB in other cell lines. Different proportions of BHK cells, transfected with RNA from SV replicon encoding 2B protein or C (* BHK), and Huh‐7 cells (mock cells) were mixed (total cell ρ = 1.9 × 105 cells cm−2). Permeabilization of neighbouring Huh‐7 cells to HB was analysed at 8 h post electroporation (hpe) by metabolic labelling of proteins in the absence (−) or presence (+) of 1 mM HB for 40 min. Protein synthesis in Huh‐7 cells (a) was quantified by densitometry of bands corresponding to a specific protein of Huh‐7 cells (*). As a negative control, a high proportion of BHK cells expressing SV C protein were mixed with mock Huh‐7 cells (3 BHK:1 Huh‐7). Permeabilization of BHK cells expressing 2B was quantified by densitometry of the SV C protein band (b). Numbers below the gel indicate the percentage of protein synthesis in HB‐treated cells compared with untreated cells. A Western blot using monoclonal antibodies against α‐tubulin was performed as a control for protein load (lower panel).
Next, we explored the possibility that permeabilization of healthy cells was only increased when both transfected and non‐transfected cells belonged to the same cell line. To this end, Huh‐7 cells were electroporated with SV‐derived replicon coding for 2B (Rep C+2B) and co‐cultured with healthy Huh‐7 cells at ratios from 1:4 to 1:2. As shown in Fig. 4, although transfected Huh‐7 cells were powerfully permeabilized at 8 hpe, the neighbouring cells exhibited little permeabilization to HB. In this case, 2B protein synthesized from 50% transfected cells only promoted a weak permeabilization of the non‐transfected counterparts, leading to a translation inhibition of about 40%. However, in BHK‐cell co‐cultures, equal and even lower ratios of 2B‐expressing BHK cells gave rise to almost total permeabilization of neighbouring cells. Co‐cultures of HeLa cells cannot be assayed, since this cell line is not permissive for SV or its replicons. Taken together these observations strongly suggest that cellular factors might be involved both in the enhancement and resistance to HB permeabilization of non‐transfected cells.
Figure 4.
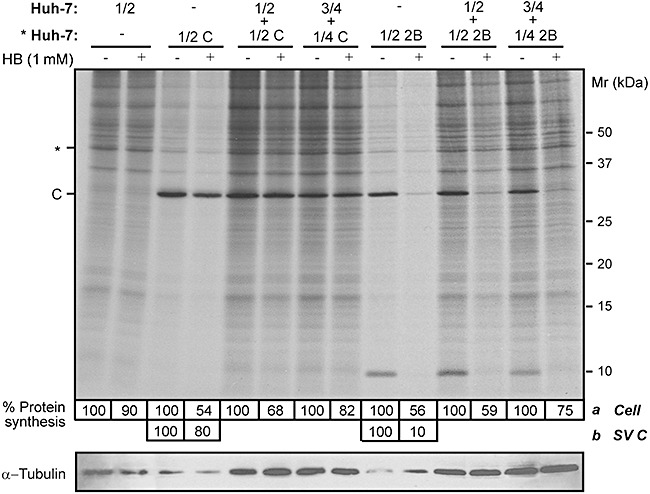
Permeabilization of neighbouring cells to HB depends on the cell line that expresses 2B. Huh‐7 cells were electroporated with SV‐derived replicons encoding C+2B or only C protein (negative control) as described in Experimental procedures. Different proportions of transfected cells (* Huh‐7) were mixed with mock Huh‐7 cells (total cell ρ = 1.9 × 105 cells cm−2) or seeded separately (proportion 1/2; total cell ρ = 8 × 104 cells cm−2). At 8 h post electroporation (hpe), permeabilization of neighbouring Huh‐7 cells was assayed by the inhibition of translation as a result of HB entry induced by 2B protein. Protein synthesis in Huh‐7 cells (a) was quantified by densitometry of bands corresponding to actin (*). Permeabilization of Huh‐7 cells expressing 2B was quantified by densitometry of SV C protein band (b). Numbers below the gel indicate the percentage of protein synthesis in HB‐treated cells compared with untreated cells. A Western blot using monoclonal antibodies against α‐tubulin was performed as a control for protein load (lower panel).
Permeabilization of neighbouring cells to HB is dependent on cell–cell contact
To examine whether cell contact is involved in mediating the transfer of HB from cells expressing 2B protein to bystander cells, we co‐cultured mixtures of BHK cells at various densities. Co‐cultures including a fixed proportion of 25% 2B‐expressing cells and 75% healthy cells were seeded on plates with different growth areas. As shown in Fig. 5A, the permeabilization of neighbouring cells by 2B at 8 hpe proved to be directly proportional to the cell density (confluency) of the co‐cultures. This finding suggests that contact between cells that express 2B and non‐transfected cells is important for the bystander permeabilization effect to occur. To further assess the significance of intercellular communication in cell permeabilization, we used similar co‐cultures in which there was no direct contact between the two cell populations (see schemes in Fig. 5B). Although both cell types shared the same culture medium, when HB entry was evaluated at 8 hpe only the cells expressing 2B protein were permeabilized (Fig. 5B). This finding makes it unlikely that permeabilization of cultured cells is mediated by release of 2B alone or 2B‐containing vesicles into the culture medium. Moreover, 2B was not detected in the supernatant obtained from the culture medium of transfected cells at 8 hpe (Fig. 5C). Under these experimental conditions, no cell lysis was observed after expression of viral proteins, as both C protein and α‐tubulin were absent in the supernatants. Detection of E protein from MHV in the culture medium served as a positive control to confirm that proteins can be released from E‐expressing cells (Maeda et al., 1999).
Figure 5.
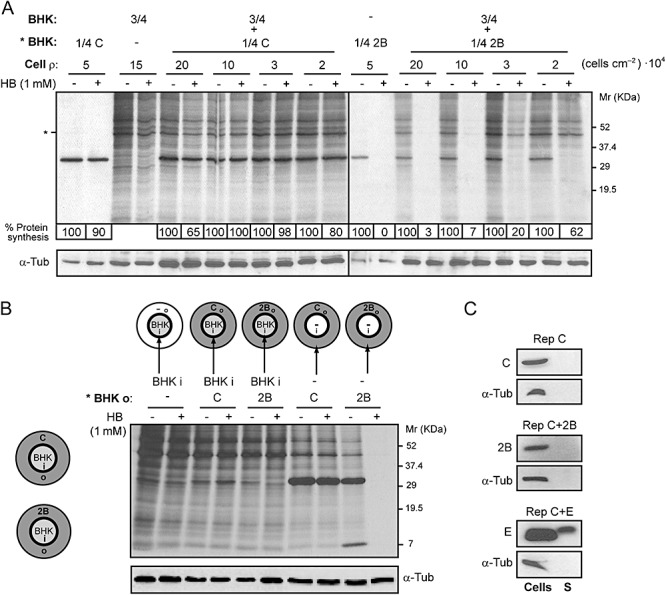
Permeabilization of neighbouring cells to HB is dependent on cell–cell contact. A. A fixed proportion of transfected (with replicons encoding C+2B or C alone; * BHK) and mock cells (*BHK : BHK, 1:3; total number of cells = 4 × 105; cell ρ at maximum confluence = 20 × 104 cells cm−2) was seeded on plates with different growth areas (2, 3.8, 11.8 and 19.5 cm2). At 8 h post electroporation (hpe), permeabilization of mock cells was assayed by the inhibition of translation as a result of HB entry induced by 2B protein. Protein synthesis in mock cells was quantified by densitometry of bands corresponding to actin (*). Numbers below the gel indicate the percentage of protein synthesis in HB‐treated cells compared with untreated cells. As a control for protein load, α‐tubulin was detected by Western blotting (lower panel). B. Permeabilization of BHK cells is not induced when cell contact with 2B‐expressing cells is not established. Schematic drawings of culture plates (growth area = 11.8 cm2) containing a central ring that divides the plate into an independent inner (i) chamber (growth area ≈ 2 cm2) and an outer (o) concentric region (left). Cells transfected with replicons encoding C+2B or C alone were seeded on the outer region of the plate (grey) and mock cells were independently seeded into the central chamber (white). Once cells were settled, the central ring was removed in such a way that all cells shared the same culture medium. At 8 hpe, cells were metabolically labelled for 40 min in the absence (−) or presence (+) of 1 mM HB. Transfected and mock cells were independently collected in loading buffer by first replacing the central ring the culture plate. Mock cells (from the central chamber) and transfected cells (outer region) expressing C+2B or C (negative control) were loaded separately, as indicated in the figure (right panel), and processed by SDS‐PAGE. It can be observed that only cells that express 2B protein are permeable to HB. As a protein loading control, α‐tubulin was detected by Western blotting (lower panel). C. 2B protein is not released into the culture medium. Cells expressing C alone, 2B or E protein from MHV‐A59 (positive control) and their respective culture media were collected separately at 8 hpe. Cells were resuspended in loading buffer while culture media were centrifuged and proteins from supernatants (S) were precipitated using trichloroacetic acid (see Experimental procedures) and resuspended in loading buffer. The presence of viral proteins in cells and supernatants was analysed by Western blotting using rabbit polyclonal antibodies directed against C, 2B and E. The absence of cellular proteins in supernatant was confirmed by Western blotting using monoclonal antibodies specific for α‐tubulin.
GAP junctional communication in BHK cells expressing PV 2B protein
Once it was established that permeabilization of neighbouring cells by 2B‐expressing cells was not mediated by the release of 2B to the medium and required cell–cell contact, we explored the possibility that HB transfer occurred through gap junctions. In order to study gap junctional communication, BHK cells were labelled with two fluorescent probes, DiI and calcein AM (acetomethyl ester). DiI is a hydrophobic dye that labels cell membranes and does not diffuse through gap junctions. Calcein AM is a colourless uncharged molecule that freely enters cells and is hydrolysed by endogenous esterases to generate calcein, the charged fluorescent form. Calcein cannot diffuse through the plasma membrane but is able to pass across gap junctions. Therefore, the transfer of calcein from cell to cell is a suitable indicator of gap junctional communication. First, BHK cells transfected with 2B replicon or control cells (transfection with C replicon) were double labelled and washed before being mixed with unlabelled cells (1:3). Non‐transfected co‐cultures established functional contacts as assessed by transfer of intracellular green fluorescent calcein to the cytoplasm of neighbouring DiI‐negative cells (Fig. 6A). Similarly, calcein transfer from 2B‐expressing cells to healthy cells was also observed at 8 hpe (Fig. 6C). Furthermore, we noted that the number of neighbouring cells which received calcein was comparable both in 2B and C protein co‐cultures, although it was slightly lower than that obtained for non‐transfected co‐cultures (Fig. 5B and C and data not shown). This difference could be accounted for by the shut off induced by the SV‐derived replicons (Sanz et al., 2007). To gain further insights into the significance of these results, connexion 43 (Cx 43) level was examined by immunoblotting in cells that express the viral products and in healthy cells. Cx 43 is widely expressed in different types of mammalian cells and assembles gap junction channels. Although Cx 43 expression in BHK cells has been previously reported, other investigators described them as communication‐deficient cells since they detected endogenous Cx 43 retained in the Golgi (Udawatte and Ripps, 2005). We found the expression of Cx 43 in BHK cells as well as two products which migrate above Cx 43, corresponding to phosphorylated forms of the protein (Asklund et al., 2003). Although the amount of Cx 43 was similar in both transfected and untransfected cells, a slight reduction in accumulated connexin was observed after synthesis of the viral proteins (Fig. 6D). In order to examine the subcellular localization of Cx 43 in BHK cells, an immunofluorescence assay was performed using specific antibodies. An intracellular Cx 43 staining pattern, probably resulting from the association of connexin with the Golgi complex, was observed, consistent with previous studies (Udawatte and Ripps, 2005). Unexpectedly, Cx 43 was also clearly observed at the plasma membrane. Moreover, punctate Cx 43 staining was concentrated at cell–cell contact areas corresponding to bona fide gap junctions (Fig. 6E). It is important to mention that this localization pattern of Cx 43 was not altered after expression of SV C or PV 2B proteins at 8 hpe and, furthermore, Cx 43 was detected at contacts between transfected and healthy non‐transfected BHK cells (Fig. 6F). Nevertheless, a very low proportion of 2B‐expressing cells exhibited a reduced level of Cx 43‐associated fluorescence. This observation is consistent with the slight reduction reported above. Taken together, these findings support the notion that gap junctions assembled by Cx 43 are functional both in healthy and in 2B‐expressing BHK cells and could mediate the transfer of HB from permeabilized 2B‐expressing cells to neighbouring cells, as occurs with the calcein dye‐coupling assay.
Figure 6.
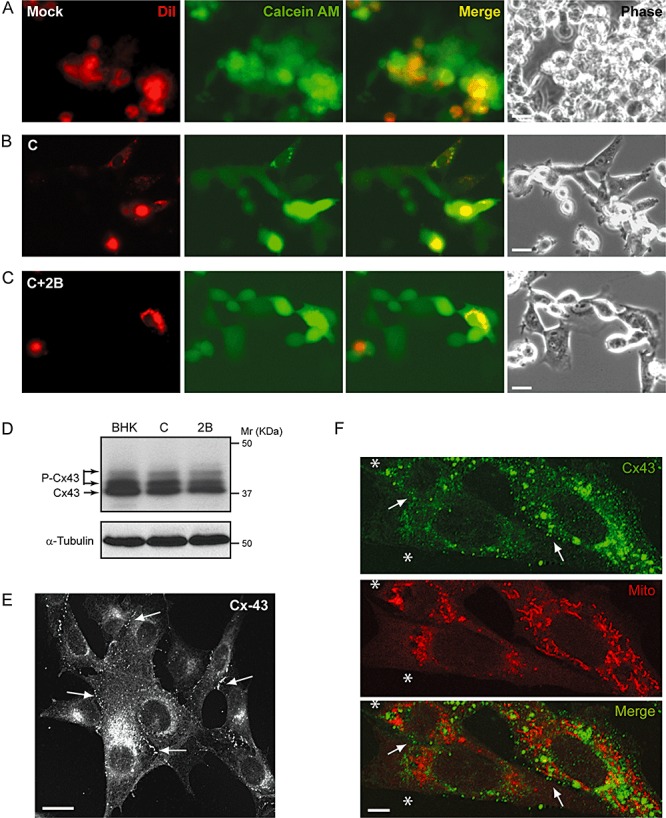
Gap junction‐mediated fluorescent dye transfer. A–C. Mock BHK cells (A) or cells electroporated with SV replicon encoding C (B) or C+2B proteins (C) were preloaded with DiI and calcein AM fluorescent probes and mixed with unlabelled non‐transfected cells as indicated in Experimental procedures. Diffusion of calcein was analysed at 8 h post electroporation (hpe) in living cells. Calcein spread to DiI‐negative cells, indicating the presence of intercellular coupling (A–C, merged panels), while DiI was retained in the preloaded cells (A–C, left panels). The right‐hand panels show phase‐contrast images of these cells. Bars, 10 µm. D. Cx 43 levels in transfected cells. Expression of Cx 43 in non‐transfected cells and cells expressing C or C+2B was analysed by Western blotting using a rabbit anti‐Cx 43 antibody. Detection of α‐tubulin served as a loading control. P‐Cx43, phosphorylated forms of Cx 43. E and F. Cx 43 distribution in BHK cells. Immunofluorescence of non‐transfected cells (E) using a rabbit anti‐Cx 43 antibody reveals Cx 43 staining of intracellular and plasma membranes (arrows). Bar, 10 µm. Immunofluorescence staining at 8 hpe in a sample in which only 25% of BHK cells expressed 2B protein (F). To detect the 2B‐expressing cells (labelled with a white asterisk, *), ‘mitotracker’, a vital marker of mitochondria, was used. 2B expression induces a perinuclear redistribution of mitochondria that allows us to discriminate the transfected from the non‐transfected cells, which show a normal mitochondrial pattern (Madan et al., 2008). Bar, 5 µm.
Blocking gap junctional intercellular communication inhibits permeabilization of neighbouring cells
To further investigate whether permeabilization of neighbouring cells to HB is mediated by gap junctional communication, we assessed the effect of 18α‐glycyrrhethinic acid (18‐α‐GA), a selective inhibitor of gap junctions. Co‐cultures composed of 25% 2B‐expressing cells and 75% healthy cells were employed. HB treatment was carried out in the presence or absence of 25 µM or 50 µM 18‐α‐GA, to analyse possible changes in protein synthesis occurring in healthy cells (Davidson et al., 1986). Figure 7A shows that blockade of gap junctions by 18‐α‐GA prevented the entry of HB into neighbouring cells. As a consequence, protein synthesis in these cells was not inhibited by the presence of HB.
Figure 7.
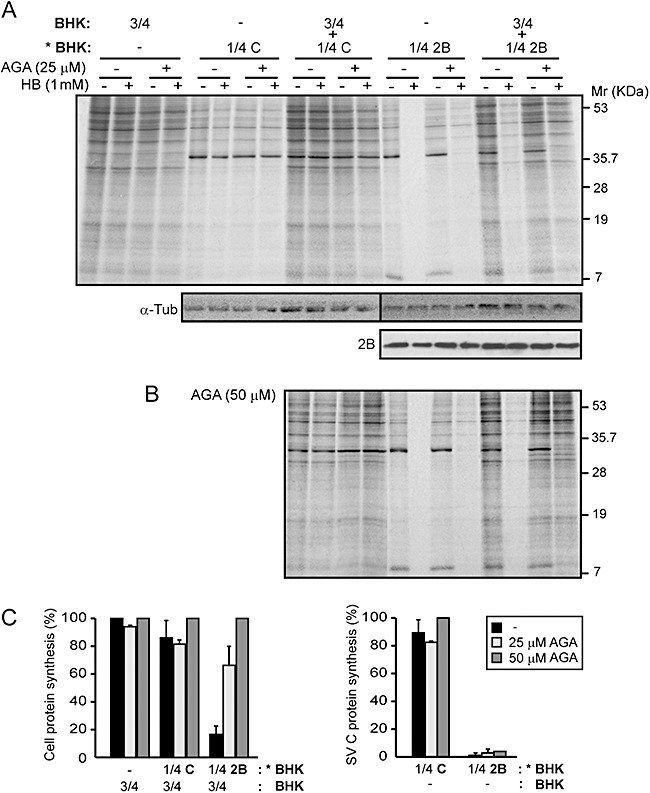
Permeabilization of neighbouring cells to HB is inhibited by 18α‐glycyrrhetinic acid. A and B. A fixed proportion of transfected cells (with replicons encoding C+2B or C alone; * BHK) and mock cells (BHK) was mixed (*BHK : BHK, 1:3; total cell ρ = 1.95 × 105 cells cm−2) or seeded separately (proportion 3/4 BHK; total cell ρ = 1.46 × 105 cells cm−2 and 1/4 *BHK; total cell ρ = 4.8 × 104 cells cm−2) in 24‐well plates in the absence or presence of 25 µM (A) or 50 µM (B) 18α‐glycyrrhetinic acid (18‐α‐GA). At 7 h post electroporation (hpe), proteins were metabolically labelled in the absence (−) or presence (+) of 1 mM HB and 18‐α‐GA for 40 min. As protein loading controls, α‐tubulin and 2B protein from 2B‐expressing cells or co‐cultured cells were detected by Western blotting (A, lower panel). C. Membrane permeabilization inhibition of neighbouring cells (BHK) by 18‐α‐GA at 8 hpe (left graph). Each bar represents the percentage of protein synthesis in HB‐treated cells compared with untreated cells. Cellular protein bands were quantified by densitometry. The effect of 18‐α‐GA on membrane permeabilization in 2B‐expressing BHK cells is shown in the right‐hand graph. C protein bands were quantified by densitometry. All data are represented as the mean ± SD of at least three independent experiments.
Notably, the inhibition of bystander permeabilization mediated by 18‐α‐GA was dose dependent and the maximum effect was observed at a concentration of 50 µM (Fig. 7B and C). Moreover, 18‐α‐GA had no apparent effect on permeabilization of 2B‐expressing cells to HB. Indeed, the weak signal of protein synthesis detected in the presence of both HB and 18‐α‐GA is likely to have come from a small proportion of cells that were not transfected during the electroporation process. These results are consistent with the hypothesis that permeabilization of healthy cells in co‐culture with 2B‐expressing cells is mediated exclusively by Cx 43 gap junctional intercellular communication.
Discussion
Viroporins are integral membrane proteins that permeabilize the cells in which they are synthesized to ions and small molecules (Gonzalez and Carrasco, 1998). To date, the permeabilizing activity of PV 2B protein to small molecules and ions had only been described to occur cell autonomously (de Jong et al., 2006; Madan et al., 2007). In this study, we have provided evidence that permeabilization of cells to the translation inhibitor HB by PV 2B viroporin promotes a bystander inhibition of protein synthesis in non‐transfected cells. This is the first report of a bystander permeabilization effect triggered by a viroporin. Permeabilization assays carried out using co‐cultures of 2B‐expressing cells with non‐transfected cells revealed that the entry of HB takes place efficiently in both healthy and transfected BHK cells at early time points after transfection, when the 2B permeabilizing activity is maximal. Moreover, the observation of a reduced bystander effect at later time points, when most 2B‐expressing cells exhibit clear signs of apoptosis, suggests that this phenomenon is primarily triggered by the synthesis of 2B protein.
The ability of other viroporins (MHV E and SV 6K) and the cytotoxic HCV NS4A protein to permeabilize healthy neighbouring cells was tested in co‐cultures containing equal proportions of transfected and non‐transfected BHK cells. Our present findings indicate that MHV E and SV 6K proteins also induce the entry of HB into surrounding non‐transfected cells. However, the extent of this effect depended on the viroporin tested and its permeabilization kinetics. The coronavirus E protein induced a rapid and powerful permeabilization of the plasma membrane soon after its synthesis, as observed in cells expressing 2B (Madan et al., 2005; 2008). The bystander effect in co‐cultures expressing E was also comparable to that promoted by PV 2B. In contrast, SV 6K protein requires longer times of expression to efficiently permeabilize cells. Although 6K‐expressing cells were entirely permeabilized at 16 hpe in this case, the entry of HB in healthy neighbouring cells was only moderate. As a consequence of the slow permeabilization kinetics of 6K, the shut‐off induced by the SV replicon for a longer period of time might have interfered with the function of other essential cellular factors required for the permeabilization of healthy cells. The expression of NS4A caused cytotoxicity in BHK cells but did not permeabilize them efficiently. Therefore, protein synthesis of non‐transfected cells remained unaltered. Previously, we reported that the viroporins included in this work induce caspase‐dependent apoptosis (Madan et al., 2008). However, since permeabilization already occurs soon after transfection, when no signs of cell death are apparent, it is unlikely that permeabilization of healthy cells to HB occurs as a result of cytotoxicity arising from the presence of apoptotic transfected cells. Moreover, in the case of NS4A, apoptosis can be detected in transfected cells at early time points after its expression but no bystander permeabilization was found (data not shown) (Madan et al., 2008).
The co‐culture experiments described here allowed us to establish that at least two parameters are also essential for powerful bystander permeabilization to occur: (i) an adequate proportion of BHK cells that express 2B protein (Fig. 1) and (ii) cell–cell contact between healthy and 2B‐expressing cells (Fig. 5A and B). Possible mechanisms underlying this phenomenon include endocytosis of toxic cell debris arising from viroporin‐expressing cells, exposure to secreted soluble viroporins or cell–cell transfer of HB (through gap junctions). We can rule out the two first possibilities, as healthy cells incubated for different periods of time with the medium obtained from cultures of 2B‐expressing cells were not permeabilized (data not shown). Furthermore, no 2B protein was found in the protein precipitate from the extracellular medium (Fig. 5C). The E protein from MHV‐A59 is released in vesicles from transfected cells to the extracellular medium (Maeda et al., 1999). Here, we have shown that E protein is able to induce a bystander permeabilization to HB in neighbouring cells similar to that exerted by 2B, but only the E protein is detected in the culture medium of transfected cells (2, 5). Further studies will be needed to define a possible role of extracellular E viroporin in mediating the bystander effect in neighbouring non‐transfected cells.
Our work provides evidence to support the hypothesis that 2B permeabilizes neighbouring cells through gap junctions. The observation that entry of HB into neighbouring cells is directly proportional to the cell density and requires contact between healthy and 2B‐expressing cells supports a requirement for additional cellular factors besides 2B synthesis for the bystander effect to occur (Fig. 5A and B). Our findings strongly support a role for gap junctions in the bystander permeabilization effect. Gap junctions mediate communication by means of cell coupling between neighbouring cells, allowing ions and water‐soluble substances to pass from one cell to another (Kumar and Gilula, 1996; Rose and Ransom, 1997). Gap junctional intercellular communication mediates a bystander effect and it is a natural amplifier of the therapeutic effect in herpes simplex virus‐thymidine kinase/ganciclovir (HSV‐tk/GCV) gene therapy (Dilber et al., 1997; Asklund et al., 2003). Gap junctional intercellular communication is often downregulated in cancer cells (Yamasaki, 1990; Zhang et al., 2007). Consistent with these observations, the bystander permeabilization to HB in two tumour cell lines, Huh‐7 and HeLa cells, that were co‐cultured with BHK or Huh‐7 cells expressing 2B protein, was significantly reduced in comparison with that reported for co‐cultures of transfected and healthy BHK cells. This observation is consistent with a recent study implicating Cx 43 in the reduction of gap junctional intercellular communication in Huh‐7 cells via downregulation of Cx 32 expression (Zhang et al., 2007). We have confirmed the presence of Cx 43 protein in non‐transfected and 2B‐expressing BHK cells and its distribution at the plasma membrane in areas of contact between adjacent cells (Fig. 6D–F). Cx 43 was also detected in Huh‐7 cells, although as a consequence of transfection of replicons encoding either C or C+2B protein, its amount was even more reduced than that observed in non‐transfected cells (Fig. S6). In our experiments, we still detected a bystander effect when co‐cultures include a high proportion of 2B‐expressing Huh‐7 cells. Moreover, we confirmed the functional status of gap junction channels in BHK cells with the ability to establish new cell–cell contacts and to mediate fluorescent dye transfer between healthy and 2B‐expressing cells (Fig. 6A–C).
Finally, the inhibition of Cx 43‐mediated gap junctional communication by 18‐α‐GA abolished the bystander entry of HB in healthy BHK cells co‐cultured with 2B‐expressing cells. This finding supports the hypothesis that the bystander phenomenon involves cell–cell transfer of HB via gap junctions (Fig. 7). We propose a model in which HB first enters 2B‐permeable cells passing through viral (a) or cellular pores at the plasma membrane (b) or via an as yet undefined pathway (c). Once inside cells, HB inhibits translation in 2B‐expressing cells and is simultaneously transferred to neighbouring cells through gap junctions (Fig. 8), leading to inhibition of protein synthesis in those healthy cells. Thus, the effect of HB on neighbouring cells would depend on the gap junctional intercellular communication and the proportion of cells that express 2B protein, which would act as the trigger for the bystander effect. In addition, the present findings suggest that cytotoxic drugs, having membrane permeability properties similar to HB, can enter viroporin‐expressing cells and subsequently may kill adjacent tumour cells.
Figure 8.
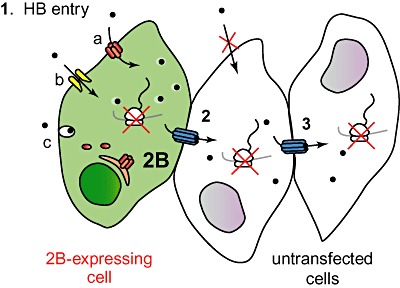
Model of bystander permeabilization to HB. Model illustrating that 2B‐expressing cells trigger HB entry in untransfected cells. 1. First, impermeable HB enters cells permeabilized by 2B protein and inhibits translation (see Discussion). 2. HB diffuses through gap junctions to non‐transfected cells in close contact with 2B‐expressing cells, resulting in inhibition of protein synthesis of these cells. 3. HB is transferred to a larger number of neighbouring cells.
Experimental procedures
Cell culture
BHK‐21 and Huh‐7 cells were routinely cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% and 10% fetal calf serum (FCS), respectively, non‐essential amino acids, antibiotics and antimycotics. Imaging was performed in glass‐bottomed dishes (MatTek) in DMEM lacking phenol red, FCS and antibiotics but containing l‐glutamine and non‐essential amino acids.
SV replicons
Sindbis virus‐derived replicons containing sequences encoding 2B (pT7 repC+2B) and 2BC (pT7 repC+2BC) proteins from PV (strain Mahoney‐1), 6K protein (pT7 repC+6K) from SV, E protein (pT7 repC+E) from MHV‐A59, NS4A protein (pT7 repC+NS4A) from HCV type b or only SV C (pT7 repC) protein have been described elsewhere (Sanz et al., 2003; Madan et al., 2008).
Transfection of BHK‐21 cells
BHK‐21 and Huh‐7 cells were electroporated with in vitro synthesized mRNAs. Transcription reactions were carried out with T7 RNA polymerase (Promega) and the corresponding plasmids as templates, according to the manufacturer's instructions. Subconfluent BHK‐21 and Huh‐7 cells were harvested, washed with ice‐cold phosphate‐buffered saline (PBS) and cytomix buffer (van den Hoff et al., 1992), respectively, and resuspended in PBS or cytomix at a density of approximately 2.5 × 106 cells ml−1. To electroporate BHK‐21 cells, an aliquot (50 ml) of the transcription mixture containing 15 mg of RNA from each of the different DNA replicons was added to 0.4 ml of cell suspension and transferred to a 2 mm electroporation cuvette (Bio‐Rad). In the case of Huh‐7 cells, two aliquots of the transcription mixture were added to 0.8 ml of cell suspension and transferred to a 4 mm electroporation cuvette (Bio‐Rad). Electroporation of BHK‐21 cells was performed at room temperature (RT) by generating two consecutive 1.5 kV, 25 mF pulses using a Gene Pulser apparatus (Bio‐Rad), as previously described (Liljestrom and Garoff, 1991). Electroporation of Huh‐7 cells was performed at RT by generating one 270 V, 960 mF pulse using a Gene Pulser II apparatus (Bio‐Rad). Finally, cells were diluted in DMEM supplemented with 10% FCS and seeded onto culture plates.
Permeabilization of neighbouring cells to HB
BHK‐21 or Huh‐7 cells were electroporated with the corresponding RNA synthesized in vitro from the different constructs andcounted using a Neubauer chamber. Different proportions of electroporated cells were mixed with non‐electroporated BHK‐21 or Huh‐7 cells (mock cells) and then seeded in wells of L‐24 plates (95–100% confluence). At different time points, cells were pre‐treated with 1 mM HB (Clontech) for 15 min at 37°C, or left untreated. Next, proteins were radiolabelled for 40 min with 10 mCi [35S] Met/Cys (Promix; Amersham Pharmacia) in methionine/cysteine‐free DMEM in the presence or absence of 1 mM HB. Finally, cells were collected in sample buffer, boiled for 4 min and analysed by SDS‐PAGE (17.5%) and fluorography. Protein synthesis was quantified by densitometry using a GS‐710 calibrated Imaging Densitometer (Bio‐Rad) and calculated by dividing the values obtained for samples treated with HB by the corresponding values obtained from untreated cells. Viral and cellular protein synthesis was quantified by densitometry of the C protein band or a cellular protein band (actin) respectively.
To study the permeabilization of non‐transfected cells co‐cultured with cells expressing 2B protein in the absence of cell–cell contact, cells were seeded separately in a p35 culture plate containing an independent central chamber. To create an independent central well (growth area ≈ 2 cm2), a methacrylate ring was reversibly fixed on the centre of the culture plate with 1.8% agarose. Non‐transfected cells were seeded into the central well and transfected cells were seeded on the outer region of the plate. After cell attachment, culture medium was carefully removed from the central area before removing the ring separating the regions. Cells were then cultured at 37°C in the medium initially added to the outer region. At the indicated time, cells were pre‐treated with HB and radiolabelled in the absence or presence of this translation inhibitor. The ring was placed back in the plate in order to collect the non‐transfected and transfected cells independently.
To study the effect of the gap junctional communication inhibitor 18‐α‐GA (Sigma) on permeabilization of neighbouring cells to HB, transfected and mock cell mixtures were seeded in the absence or presence of 25 µM or 50 µM 18‐α‐GA. Cells were radiolabelled at 7 hpe before pre‐treatment, in the absence or presence of HB and 18‐α‐GA, and processed as indicated above.
Precipitation of proteins with trichloroacetic acid
The culture medium from transfected cells was centrifuged at 2000 r.p.m. for 4 min to remove detached cells. Trichloroacetic acid was added to supernatants at a final concentration of 3% (at RT). Then supernatants were incubated at 65°C for 5 min, on ice for 5 min and centrifuged at 8000 r.p.m. for 10 min. The protein pellets were washed twice with cold acetone (−20°C), dried and resuspended in loading buffer. Samples were boiled and processed by SDS‐PAGE.
Western blotting
Electroporated cells expressing the different viral proteins, mock cells or co‐cultured cells (as indicated in the figures) were collected in sample buffer, boiled and processed by SDS‐PAGE. After electrophoresis, proteins were transferred to a nitrocellulose membrane as described previously (Barco and Carrasco, 1995). Mouse monoclonal anti‐α‐tubulin antibodies (Sigma) were used at a 1:5000 dilution to evaluate protein loading. To detect PV 2B and SV C proteins, specific rabbit polyclonal antibodies (Barco and Carrasco, 1995; Madan et al., 2005) were used at dilutions of 1:1000 and 1:10 000 respectively. Polyclonal rabbit antibodies against E protein from MHV‐A59 were generously provided by S. Makino (University of Texas Medical Branch at Galveston, Texas) and used at a 1:1000 dilution. Polyclonal anti‐connexin 43 (Cx 43) antibodies (Sigma) were used at a 1:1000 dilution. Incubation with primary antibodies was performed for 2 h at RT, and then the membrane was washed three times with PBS containing 0.2% Tween‐20 and incubated for 1 h with horseradish peroxidase‐conjugated anti‐mouse (Promega) or anti‐rabbit IgG antibodies (Amersham) at a 1:10 000 dilution. After washing three times, protein bands were visualized with the ECL detection system (Amersham).
Immunofluorescence microscopy
BHK cells electroporated with RepC+2B and non‐transfected cells were mixed and seeded on coverslips, fixed in 4% paraformaldehyde for 15 min, washed twice in PBS, and then permeabilized for 10 min with 0.2% Triton X‐100 in PBS. Cells were incubated with specific rabbit polyclonal antibodies against the PV 2B protein for 1 h in PBS containing 0.1% FCS and 0.1% Triton X‐100. Polyclonal anti‐Cx 43 antibodies (Sigma) were used at a 1:400 dilution to detect gap junctions. Coverslips were washed three times with PBS and then incubated with a mix of Alexa 488‐conjugated anti‐rabbit IgG (Molecular Probes) and To‐Pro‐3 (Invitrogen), both at a dilution of 1:500. Coverslips were mounted in ProLong Gold antifade reagent (Invitrogen) and examined with a Radiance 2000 (Bio‐Rad/Zeiss) confocal laser scanning microscope. For mitochondria staining, cells were incubated with 2 µM Mitotracker Red CMH2Ros (Molecular Probes) for 45 min before fixation.
Fluorescent dye transfer
BHK‐21 cells (1 × 106 cells) electroporated with SV replicon encoding C or C+2B proteins were labelled with 5 µM calcein‐AM (acetomethyl ester) and 10 µM DiI (Molecular Probes) diluted in serum‐free medium for 20 min at 37°C. Cells were washed twice in PBS and twice in serum‐free DMEM. Cells were resuspended in an adequate volume of fresh serum‐free medium. Next, ∼2 × 105 labelled cells were mixed with unlabelled non‐transfected cells (1:3) and transferred to a glass‐bottomed dish, allowing the cells to settle. Diffusion of calcein‐AM from transfected cells to non‐transfected cells was monitored under an Axiovert 200 (Zeiss) inverted microscope (20× objective). Images were recorded with a digital CCD camera (Hamamatsu).
Statistical analysis
Data are presented as mean values ± SD. Differences were tested for significance by Student's t‐test. The effect of viroporins on neighbouring cells was compared with controls (transfection of repC). The cut‐off for statistical significance was set at P < 0.05.
Supporting information
Fig. S1. Expression of poliovirus 2 BC protein in BHK cells induces permeabilization of neighbouring cells to HB. Membrane permeabilization of neighbouring cells (mock cells) assayed by the inhibition of translation as a result of HB entry induced by 2 BC protein at 8 h post electroporation (a representative experiment). It can be observed that all cells expressing 2 BC are permeable to HB (proportion 1:1; total cell ρ = 1.9 × 105 cells cm−2).
Fig. S3. Protein expression in Huh‐7 cells. Different proportions of Huh‐7 mock cells (input of experiment in Fig. 3) were seeded and protein expression was analysed by metabolic labelling of proteins in the absence (−) or presence (+) of 1 mM HB for 40 min. Protein synthesis in Huh‐7 cells was quantified by densitometry of bands corresponding to actin (*). Numbers below the gel indicate the percentage of protein synthesis in HB‐treated cells compared with untreated cells.
Fig. S3‐1. Expression of 2B viroporin in BHK cells induces HB entry in HeLa cells. Different proportions of BHK cells, transfected with RNA from SV replicon encoding 2B protein, and HeLa cells (mock cells) were mixed (total cell ρ = 1.9 × 105 cells cm−2). Permeabilization of HeLa cells to HB was analysed at 8 h post electroporation by metabolic labelling of proteins in the absence (−) or presence (+) of 1 mM HB for 40 min. Protein synthesis in HeLa cells (a) was quantified by densitometry of bands corresponding to cellular proteins. As a negative control, BHK cells expressing SV C protein and mock HeLa cells were mixed in equal proportions. Permeabilization of BHK cells expressing 2B was quantified by densitometry of the SV C protein band (b). Numbers below the gel indicate the percentage of protein synthesis in HB‐treated cells compared with untreated cells.
Fig. S6. Cx 43 levels in Huh‐7 cells. Expression of Cx 43 in non‐transfected cells and cells expressing C or C+2B was analysed by Western blotting using a rabbit anti‐Cx 43 antibody. Detection of α‐tubulin served as a loading control. P‐Cx43, phosphorylated form of Cx 43.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Acknowledgements
This study was supported by a DGICYT Grant (BFU 2006‐02182) and an Institutional Grant awarded to the Centro de Biología Molecular ‘Severo Ochoa’ by the Fundación Ramón Areces.
References
- Agirre, A. , Barco, A. , Carrasco, L. , and Nieva, J.L. (2002) Viroporin‐mediated membrane permeabilization. Pore formation by nonstructural poliovirus 2B protein. J Biol Chem 277: 40434–40441. [DOI] [PubMed] [Google Scholar]
- Aldabe, R. , and Carrasco, L. (1995) Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem Biophys Res Commun 206: 64–76. [DOI] [PubMed] [Google Scholar]
- Aldabe, R. , Barco, A. , and Carrasco, L. (1996) Membrane permeabilization by poliovirus proteins 2B and 2 BC. J Biol Chem 271: 23134–23137. [DOI] [PubMed] [Google Scholar]
- Asklund, T. , Appelskog, I.B. , Ammerpohl, O. , Langmoen, I.A. , Dilber, M.S. , Aints, A. , et al (2003) Gap junction‐mediated bystander effect in primary cultures of human malignant gliomas with recombinant expression of the HSVtk gene. Exp Cell Res 284: 185–195. [DOI] [PubMed] [Google Scholar]
- Barco, A. , and Carrasco, L. (1995) A human virus protein, poliovirus protein 2 BC, induces membrane proliferation and blocks the exocytic pathway in the yeast Saccharomyces cerevisiae . EMBO J 14: 3349–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barco, A. , and Carrasco, L. (1998) Identification of regions of poliovirus 2 BC protein that are involved in cytotoxicity. J Virol 72: 3560–3570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz, K. , Egger, D. , Pfister, T. , and Troxler, M. (1992) Structural and functional characterization of the poliovirus replication complex. J Virol 66: 2740–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe, S.S. , Dodd, D.A. , and Kirkegaard, K. (2005) Inhibition of cellular protein secretion by picornaviral 3A proteins. Virology 337: 18–29. [DOI] [PubMed] [Google Scholar]
- Davidson, J.S. , Baumgarten, I.M. , and Harley, E.H. (1986) Reversible inhibition of intercellular junctional communication by glycyrrhetinic acid. Biochem Biophys Res Commun 134: 29–36. [DOI] [PubMed] [Google Scholar]
- Dilber, M.S. , Abedi, M.R. , Christensson, B. , Bjorkstrand, B. , Kidder, G.M. , Naus, C.C. , et al (1997) Gap junctions promote the bystander effect of herpes simplex virus thymidine kinase in vivo . Cancer Res 57: 1523–1528. [PubMed] [Google Scholar]
- Ewart, G.D. , Sutherland, T. , Gage, P.W. , and Cox, G.B. (1996) The Vpu protein of human immunodeficiency virus type 1 forms cation‐selective ion channels. J Virol 70: 7108–7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, S.W. , Ng, L. , Lin, X. , Gong, X. , and Torres, J. (2008) Structure and ion channel activity of the human respiratory syncytial virus (hRSV) small hydrophobic protein transmembrane domain. Protein Sci 17: 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, M.E. , and Carrasco, L. (1998) The human immunodeficiency virus type 1 Vpu protein enhances membrane permeability. Biochemistry 37: 13710–13719. [DOI] [PubMed] [Google Scholar]
- Griffin, S. , Stgelais, C. , Owsianka, A.M. , Patel, A.H. , Rowlands, D. , and Harris, M. (2008) Genotype‐dependent sensitivity of hepatitis C virus to inhibitors of the p7 ion channel. Hepatology 48: 1779–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinea, R. , and Carrasco, L. (1994) Influenza virus M2 protein modifies membrane permeability in E. coli cells. FEBS Lett 343: 242–246. [DOI] [PubMed] [Google Scholar]
- van den Hoff, M.J. , Moorman, A.F. , and Lamers, W.H. (1992) Electroporation in ‘intracellular’ buffer increases cell survival. Nucleic Acids Res 20: 2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, C. , Narayanan, K. , Ito, N. , Peters, C.J. , and Makino, S. (2006) Severe acute respiratory syndrome coronavirus 3a protein is released in membranous structures from 3a protein‐expressing cells and infected cells. J Virol 80: 210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, M.B. , Weeks, O. , Zhao, L.J. , Saltarelli, M. , and Bond, V.C. (2000) Effects of extracellular human immunodeficiency virus type 1 vpr protein in primary rat cortical cell cultures. J Neurovirol 6: 202–220. [DOI] [PubMed] [Google Scholar]
- de Jong, A.S. , Visch, H.J. , de Mattia, F. , van Dommelen, M.M. , Swarts, H.G. , Luyten, T. , et al (2006) The coxsackievirus 2B protein increases efflux of ions from the endoplasmic reticulum and Golgi, thereby inhibiting protein trafficking through the Golgi. J Biol Chem 281: 14144–14150. [DOI] [PubMed] [Google Scholar]
- Klimkait, T. , Strebel, K. , Hoggan, M.D. , Martin, M.A. , and Orenstein, J.M. (1990) The human immunodeficiency virus type 1‐specific protein vpu is required for efficient virus maturation and release. J Virol 64: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, N.M. , and Gilula, N.B. (1996) The gap junction communication channel. Cell 84: 381–388. [DOI] [PubMed] [Google Scholar]
- van Kuppeveld, F.J. , Hoenderop, J.G. , Smeets, R.L. , Willems, P.H. , Dijkman, H.B. , Galama, J.M. , and Melchers, W.J. (1997) Coxsackievirus protein 2B modifies endoplasmic reticulum membrane and plasma membrane permeability and facilitates virus release. EMBO J 16: 3519–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljestrom, P. , and Garoff, H. (1991) Internally located cleavable signal sequences direct the formation of Semliki Forest virus membrane proteins from a polyprotein precursor. J Virol 65: 147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik, P. , Chew, C. , Aittoniemi, J. , Chang, J. , Wentworth, P., Jr , Dwek, R.A. , et al (2009) The 3‐dimensional structure of a hepatitis C virus p7 ion channel by electron microscopy. Proc Natl Acad Sci USA 106: 12712–12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan, V. , Garcia Mde, J. , Sanz, M.A. , and Carrasco, L. (2005) Viroporin activity of murine hepatitis virus E protein. FEBS Lett 579: 3607–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan, V. , Sanchez‐Martinez, S. , Vedovato, N. , Rispoli, G. , Carrasco, L. , and Nieva, J.L. (2007) Plasma membrane‐porating domain in poliovirus 2B protein. A short peptide mimics viroporin activity. J Mol Biol 374: 951–964. [DOI] [PubMed] [Google Scholar]
- Madan, V. , Castello, A. , and Carrasco, L. (2008) Viroporins from RNA viruses induce caspase‐dependent apoptosis. Cell Microbiol 10: 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda, J. , Maeda, A. , and Makino, S. (1999) Release of coronavirus E protein in membrane vesicles from virus‐infected cells and E protein‐expressing cells. Virology 263: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton, J.V. , Ewart, G.D. , Weir, R.C. , Board, P.G. , Lee, E. , and Gage, P.W. (2002) Alphavirus 6K proteins form ion channels. J Biol Chem 277: 46923–46931. [DOI] [PubMed] [Google Scholar]
- Moon, H. , and Yang, J. (2006) Role of HIV Vpr as a regulator of apoptosis and an effector on bystander cells. Mol Cells 21: 7–20. [PubMed] [Google Scholar]
- Pavlovic, D. , Neville, D.C. , Argaud, O. , Blumberg, B. , Dwek, R.A. , Fischer, W.B. , and Zitzmann, N. (2003) The hepatitis C virus p7 protein forms an ion channel that is inhibited by long‐alkyl‐chain iminosugar derivatives. Proc Natl Acad Sci USA 100: 6104–6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielak, R.M. , Schnell, J.R. , and Chou, J.J. (2009) Mechanism of drug inhibition and drug resistance of influenza A M2 channel. Proc Natl Acad Sci USA 106: 7379–7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto, L.H. , Holsinger, L.J. , and Lamb, R.A. (1992) Influenza virus M2 protein has ion channel activity. Cell 69: 517–528. [DOI] [PubMed] [Google Scholar]
- Rose, C.R. , and Ransom, B.R. (1997) Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia 20: 299–307. [DOI] [PubMed] [Google Scholar]
- Sanz, M.A. , Madan, V. , Carrasco, L. , and Nieva, J.L. (2003) Interfacial domains in Sindbis virus 6K protein. Detection and functional characterization. J Biol Chem 278: 2051–2057. [DOI] [PubMed] [Google Scholar]
- Sanz, M.A. , Castello, A. , and Carrasco, L. (2007) Viral translation is coupled to transcription in Sindbis virus‐infected cells. J Virol 81: 7061–7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel, A. , Giddings, T.H., Jr , Ladinsky, M.S. , and Kirkegaard, K. (1996) Cellular origin and ultrastructure of membranes induced during poliovirus infection. J Virol 70: 6576–6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhy, D.A. , Giddings, T.H., Jr , and Kirkegaard, K. (2000) Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy‐like origin for virus‐induced vesicles. J Virol 74: 8953–8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udawatte, C. , and Ripps, H. (2005) The spread of apoptosis through gap‐junctional channels in BHK cells transfected with Cx32. Apoptosis 10: 1019–1029. [DOI] [PubMed] [Google Scholar]
- Wang, C. , Lamb, R.A. , and Pinto, L.H. (1994) Direct measurement of the influenza A virus M2 protein ion channel activity in mammalian cells. Virology 205: 133–140. [DOI] [PubMed] [Google Scholar]
- Wilson, L. , McKinlay, C. , Gage, P. , and Ewart, G. (2004) SARS coronavirus E protein forms cation‐selective ion channels. Virology 330: 322–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, Y. , Chen, G. , Richard, J. , Rougeau, N. , Li, H. , Seidah, N.G. , and Cohen, E.A. (2008) Cell‐surface processing of extracellular human immunodeficiency virus type 1 Vpr by proprotein convertases. Virology 372: 384–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki, H. (1990) Gap junctional intercellular communication and carcinogenesis. Carcinogenesis 11: 1051–1058. [DOI] [PubMed] [Google Scholar]
- Ye, Y. , and Hogue, B.G. (2007) Role of the coronavirus E viroporin protein transmembrane domain in virus assembly. J Virol 81: 3597–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. , Kaneda, M. , Nakahama, K. , Arii, S. , and Morita, I. (2007) Connexin 43 expression promotes malignancy of HuH7 hepatocellular carcinoma cells via the inhibition of cell–cell communication. Cancer Lett 252: 208–215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Expression of poliovirus 2 BC protein in BHK cells induces permeabilization of neighbouring cells to HB. Membrane permeabilization of neighbouring cells (mock cells) assayed by the inhibition of translation as a result of HB entry induced by 2 BC protein at 8 h post electroporation (a representative experiment). It can be observed that all cells expressing 2 BC are permeable to HB (proportion 1:1; total cell ρ = 1.9 × 105 cells cm−2).
Fig. S3. Protein expression in Huh‐7 cells. Different proportions of Huh‐7 mock cells (input of experiment in Fig. 3) were seeded and protein expression was analysed by metabolic labelling of proteins in the absence (−) or presence (+) of 1 mM HB for 40 min. Protein synthesis in Huh‐7 cells was quantified by densitometry of bands corresponding to actin (*). Numbers below the gel indicate the percentage of protein synthesis in HB‐treated cells compared with untreated cells.
Fig. S3‐1. Expression of 2B viroporin in BHK cells induces HB entry in HeLa cells. Different proportions of BHK cells, transfected with RNA from SV replicon encoding 2B protein, and HeLa cells (mock cells) were mixed (total cell ρ = 1.9 × 105 cells cm−2). Permeabilization of HeLa cells to HB was analysed at 8 h post electroporation by metabolic labelling of proteins in the absence (−) or presence (+) of 1 mM HB for 40 min. Protein synthesis in HeLa cells (a) was quantified by densitometry of bands corresponding to cellular proteins. As a negative control, BHK cells expressing SV C protein and mock HeLa cells were mixed in equal proportions. Permeabilization of BHK cells expressing 2B was quantified by densitometry of the SV C protein band (b). Numbers below the gel indicate the percentage of protein synthesis in HB‐treated cells compared with untreated cells.
Fig. S6. Cx 43 levels in Huh‐7 cells. Expression of Cx 43 in non‐transfected cells and cells expressing C or C+2B was analysed by Western blotting using a rabbit anti‐Cx 43 antibody. Detection of α‐tubulin served as a loading control. P‐Cx43, phosphorylated form of Cx 43.
Supporting info item
Supporting info item
Supporting info item
Supporting info item


