Abstract
What effect does isoflurane have upon ciliary beat pattern: an in vivo study The effect of anaesthetic gases given via laryngeal mask on nasal ciliary beat pattern and frequency has not been studied. Anaesthetic gases such as isoflurane, halothane and enflurane are known to reduce ciliary beat frequency, but it is unknown whether they also cause cilia to beat in a dyskinetic fashion. Brush biopsies of nasal mucosa were taken pre‐ and post‐anaesthesia with isoflurane, given via a laryngeal mask, from patients undergoing nasal surgery. The samples were observed by light microscopy, and high‐speed digital video recordings were made to determine ciliary beat frequency. Using slow‐motion playback, the ciliary beat pattern was scored for dysmotility, and the proportion of immotile cilia in the sample was determined. We found that ciliary beat frequency decreased significantly (P < 0.01) after exposure to isoflurane (10.24 Hz compared to 9.20 Hz). However, isoflurane did not alter the ciliary beat pattern or the proportion of immotile cilia.
Keywords: anaesthetics volatile isoflurane, mucociliary clearance, nasal mucosa, anaesthesia inhalation, cilia
Effective mucociliary clearance plays a major role in the host defence of the respiratory tract, reducing the build‐up of mucus and the risk of secondary bacterial infection. It is particularly important in the postoperative period where patients may have a depressed respiratory drive, and abdominal wounds may inhibit clearance of secretions by coughing. Inhibition of effective mucociliary clearance can lead to postoperative complications such as basal atelectasis, respiratory infections 1933 , 1992 and sinusitis. 3
Certain anaesthetic agents have been shown to decrease mucociliary clearance, 1976 , 1975 most probably by affecting ciliary function. In vitro studies, using perfusion chambers, have shown that direct exposure to the inhalational anaesthetics halothane, enflurane and isoflurane decreases ciliary beat frequency. 1996 , 1996 , 1994 A recent study of patients undergoing elective surgery found that facemask anaesthesia, maintained with isoflurane, also caused a significant decrease in nasal ciliary beat frequency. 9 Other agents used in anaesthesia such as morphine, midazolam and propofol have been found not to affect ciliary beat frequency. 1997 , 1998 , 1996
Ex vivo measurement of ciliary beat frequency has been widely used to assess the toxicity and effect of various substances and drugs including anaesthetic agents. It has been assumed that if there is no change in beat frequency, the ability of the cilia to propel mucus is not affected. This can no longer be assumed as a dyskinetic beat pattern may be seen in patients with a normal ciliary beat frequency. Using a high‐speed video system, we have recently shown that nasal cilia, examined 3 days after infection of healthy volunteers with coronavirus, had a normal beat frequency but beat in a dyskinetic fashion. 12 In addition, we found that patients with primary ciliary dyskinesia, caused by a transposition defect of the cilia ultrastructure, had a normal ciliary beat frequency but with a circular beat pattern that is ineffective in propelling mucus. 13 It is unknown whether anaesthetic agents cause ciliary dyskinesia as well as a reduction in ciliary beat frequency. This is important to study, as a combination of reduced ciliary beat frequency and ciliary dyskinesia may have a significant effect on mucociliary clearance.
As patients may develop nasal symptoms, such as sinusitis, following general anaesthesia, we were interested to determine whether nasal cilia were affected when the anaesthetic was administered via a laryngeal mask. In this study, we used a high‐speed digital video system to determine the effects of isoflurane, given via a laryngeal mask, on the beat frequency and beat pattern of nasal cilia obtained from patients during elective nasal surgery. We used ciliary beat frequency, ciliary beat pattern and ciliary immotility scores as the outcome measures.
Materials and methods
Patient selection
Patients, over 16 years old, undergoing septoplasty and reduction of turbinates were invited to participate in the study.
Anaesthetic protocol
All patients had induction of anaesthesia with intravenous propofol 2 mg/kg. Maintenance of anaesthesia was with isoflurane gas (1–4% depending upon the individual patient's response) with air‐oxygen delivered via a laryngeal mask. Intra‐operative analgesia was also given in the form of intravenous opiode (morphine) and/or intravenous non‐steroidal anti‐inflammatory drugs (NSAIDs) (ketolorac). No preoperative sedation was given.
Specimen collection
Brush biopsies were taken using bronchoscopy biopsy brushes (Diagmed Ltd., UK). Pre‐anaesthesia brushings were taken from the nasal cavity, whilst the patient was still in the anaesthetic room, after anaesthetic induction with propofol but before the use of volatile gases (isoflurane). After approximately 30 min of general anaesthesia time, the inferior turbinates were excised and collected for examination. Post‐anaesthesia samples were obtained by brushing the turbinates immediately after excision. The samples were transported and stored in media 199 (ICN Biochemicals, USA), chilled on ice and taken immediately to the laboratory for examination.
Ciliary beat frequency, ciliary beat pattern and immotility index
Ciliated strips of epithelium were suspended in a chamber created by the separation of a cover slip and a glass slide by two adjacent cover slips. The slide was placed on a heated stage (37 °C) of a Leitz, Diaplan microscope mounted on an antivibration table (Wentworth Laboratories Ltd.). Specimens were examined using a ×100 interference contrast lens. Only undisrupted strips greater than 50 µm in length were studied. Beating edges were recorded using a digital high‐speed camera (Kodak Motioncorder 1000) at a rate of 400 frames per second, using a shutter speed of 1 in 4000. The camera allowed video sequences to be played at reduced frame rates or frame by frame. This allowed the precise movement of individual cilia to be observed during their beat cycle.
The epithelial strips were divided on the video screen into 10‐µm zones, and a measurement of ciliary beat frequency was made from each zone. Groups of beating cilia were identified, and the number of frames to complete five cycles were recorded. This was converted to ciliary beat frequency by a simple calculation (ciliary beat frequency = (400/number of frames for five beats) × 5).
To assess the ciliary beat pattern, each edge was given a score based on a previously validated system. 14 Normal co‐ordinated ciliary beating in a forward backward motion was scored 0. Cilia that appeared to beat dyskinetically were scored from 1 to 3 depending on the extent of abnormal beating along the edge. The dysmotility score was determined by calculating the mean score of the epithelial edges.
The ciliary immotility index was calculated by recording the number of 10‐µm segments, whose cilia were immotile, and then converting this to an overall percentage of segments. 14 The equation used for this was (number of immotile segments/number of motile segments) × 100.
Ethics approval
The study was approved by the Leicestershire Ethical Committee.
Statistical analysis
The ciliary beat frequency, dysmotility score and ciliary immotility index results were tested for statistical differences using a paired Student's t‐test.
Results
A total of 30 patients were recruited for the study, of which 1 had no cilia on examination and 2 were not examined because of technical error. Twenty‐seven patients were successfully studied. The mean age of the patients was 38.2 years (range of 17–65 years). Four patients were women, and 24 were men.
The ciliary beat frequency for the preoperative specimens (mean 10.24 Hz, sd 1.50) was significantly (P < 0.01) higher than that for the postoperative specimens (mean 9.20 Hz, sd 1.76). The results for pre‐ and post‐anaesthesia ciliary beat frequency are shown in Fig. 1.
Figure 1.
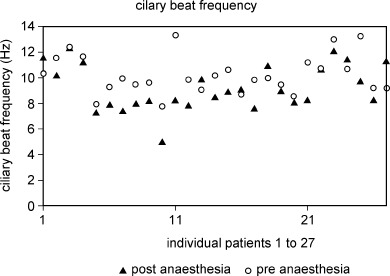
Ciliary beat frequency pre‐ and post‐anaesthesia.
The dysmotility score for the preoperative samples (mean 0.37, sd 0.34) was not statistically different (P = 0.95) from the postoperative samples (mean 0.37, sd 0.41).
The mean ciliary immotility index (2.46%, sd 3.67) for the preoperative samples was not significantly different (P = 0.33) from the postoperative samples (mean 4.01%: sd 8.10).
Discussion
The ciliary beat frequencies found in the preoperative nasal samples (10.24 Hz) in this study are in good agreement with data from our laboratory and elsewhere. 1997 , 1998 , 1984 , 1982 , 1985 We found that the reduction in ciliary beat frequency after anaesthesia was in the region of 10%. This reduction is similar to the decreases in ciliary beat frequency found in previous in vitro studies on nasal epithelium. 1996 , 1996 , 1994 Small reductions in ciliary beat frequency have significant effects on mucociliary clearance because the relationship between ciliary beat frequency and mucus transport rate is thought to be logarithmic. 14 The 10% reduction in nasal ciliary beat frequency found in this study would therefore significantly affect the nasal mucociliary clearance and consequently decrease the protective function of nasal mucociliary transport.
Unlike the respiratory epithelium of the lower airways, the nasal epithelium was not intended to be directly exposed to isoflurane. Although laryngeal masks provide a good seal around the larynx, it is likely that leakage into the oropharynx and nasopharynx occurs. This exposure, coupled with systemic absorption and distribution of isoflurane, is likely to be responsible for the significant decrease in nasal ciliary beat frequency seen.
Despite a decrease in ciliary beat frequency, the ciliary dysmotility score of the pre‐ and postoperative samples were not significantly different from each other and were within normal limits (0.2–0.4). 12 Likewise, ciliary immotility was not significantly affected by isoflurane. Previous work has found that up to 10% of cilia are immotile in healthy respiratory epithelium, 12 which is in keeping with our results. Thus, although isoflurane reduced ciliary beat frequency, it did not appear to affect nasal ciliary beat pattern when administered via a laryngeal mask.
The anaesthetic induction in this study was standardized. An initial 2 mg/kg bolus of propofol was used, with further boluses given depending on clinical response. Propofol is very unlikely to have been responsible for the decrease in ciliary beat frequency, as it has not been shown to affect ciliary beat frequency when given either as a bolus or as an infusion. 1997 , 1996 , 2001 The concentration of isoflurane (1–4%) administered was determined by clinical response; however, all patients in this study were given isoflurane for at least 30 min. Previous studies have shown that there are no opiode receptors on the nasal mucosa, and that morphine does not alter ciliary beat frequency. 11 Therefore, the analgesia given is also unlikely to have affected ciliary beat frequency.
The biopsies were taken from the inferior turbinates by using a bronchoscopy brush. The samples obtained in this manner are very small strips of epithelium about 100 µm long. Such small samples have a high surface area to mass ratio. Anaesthetic agents are known to diffuse out of tissue into the culture medium, and the higher the surface area to mass ratio, the quicker the diffusion of volatile agents. To avoid the loss of isoflurane by diffusion, we took excised turbinates immediately to the laboratory where they were brushed and the ciliated epithelium was examined. It has been shown that it takes 60 min for the isoflurane to wash out of turbinates, 7 and our samples were all examined as soon as possible after collection, and in all cases within 30 min.
Our results suggest that isoflurane given via a laryngeal mask may significantly decrease the ciliary beat frequency but not the beat pattern of nasal cilia. For the first time we have shown that the decrease in ciliary beat frequency following isoflurane is not accompanied by significant ciliary dyskinesia. It is possible that anaesthetic agents, such as isoflurane, may play a part in nasal symptoms following general anaesthesia by depressing ciliary function. Whether direct exposure of the nasal epithelium or lower respiratory tract to higher concentrations of isoflurane would result in a greater degree of ciliary slowing, or whether this would then have an effect on ciliary beat pattern, requires further investigation.
Acknowledgements
The authors would like to acknowledge the support and assistance of the Ear, Nose and Throat and Anaesthesia Departments at Leicester Royal Infirmary. The authors would also like to thank the cilia laboratory at Leicester University for the use of their facilities.
References
- 1. King D.S. (1933) Postoperative pulmonary complications. Surg. Obstetr. Gynaecol. 56, 43–50 [Google Scholar]
- 2. Dilworth J.P. & White R.J. (1992) Postoperative chest infections after upper abdominal surgery: an important problem for smokers. Respir. Med. 86, 205–210 [DOI] [PubMed] [Google Scholar]
- 3. Gleckman R.A. & Roth R.M., (1986) Fever following abdominal surgery. Unusual infectious causes. Postgrad. Med. 79, 287–294 [DOI] [PubMed] [Google Scholar]
- 4. Gamsu G., Singer M.M., Vincent H.H. et al. (1976) Post‐operative impairment of mucus transport in the lung. Am. Rev. Respir. Dis. 114, 673–679 [DOI] [PubMed] [Google Scholar]
- 5. Lichtiger M., Landa J.F. & Hirsch J.A. (1975) Velocity of tracheal mucus in anaesthetised women undergoing gynaecological surgery. Anaesthesiology 42, 753–755 [DOI] [PubMed] [Google Scholar]
- 6. Raphael J.H., Selwyn D.A., Mottram S.D. et al. (1996) Effects of 3 MAC of halothane enflurane and isoflurane on ciliary beat frequency on human nasal epithelium in vitro . Br. J. Anaesth. 76, 116–121 [DOI] [PubMed] [Google Scholar]
- 7. Raphael J.H., Strupish J., Selwyn D. et al. (1996) Recovery of respiratory ciliary function after depression by inhalational anaesthetic agents: an in vitro study using turbinate explants. Br. J. Anaesth. 76, 854–859 [DOI] [PubMed] [Google Scholar]
- 8. O'Callaghan C., Atherton M., Karim K. et al. (1994) The effect of halothane on neonatal ciliary beat frequency. J. Paediatr. Child Health 30, 429–431 [DOI] [PubMed] [Google Scholar]
- 9. Raphael J.H. & Butt M.W. (1997) Comparison of isoflurane with propofol on respiratory cilia. Br. J. Anaesth. 79, 473–465 [DOI] [PubMed] [Google Scholar]
- 10. Hann H.C.L., Hall A.P., Raphael J.H. et al. (1998) An investigation into the effects of midazolam and propofol on human cilia beat frequency in vitro . Intensive Care Med. 24, 791–794 [DOI] [PubMed] [Google Scholar]
- 11. Selwyn D.A., Raphael J.H., Lambert D.G. et al. (1996) Effects of morphine on human nasal ciliary beat frequency in vitro . Br. J. Anaesth. 76, 274–277 [DOI] [PubMed] [Google Scholar]
- 12. Chilvers M.A., McKean M., Rutman A. et al. (2001) The effects of coronavirus on human nasal ciliated respiratory epithelium. Eur. Respir. J. 18, 965–970 [DOI] [PubMed] [Google Scholar]
- 13. Chilvers M.A., Rutman A. & O' Callaghan C. (2003) Ciliary beat pattern is associated with specific ultrastructural defects in primary ciliary dyskinesia. J. Allergy Clin. Immunol. 112, 518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chilvers M.A. & O Callaghan C. (2002) Analysis of ciliary beat pattern and beat frequency using digital high‐speed imaging: comparison with the photomultiplier and photodiode methods. Thorax 55, 314–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Low P., Luk C.K., Dulfano M.J. et al. (1984) Ciliary beat frequencies of human respiratory tract by different sampling techniques. Am. Rev. Respir. Dis. 130, 497–498 [DOI] [PubMed] [Google Scholar]
- 16. Baum G.L., Roth Y., Teichthl H. et al. (1982) Ciliary beat frequency of respiratory mucosal cells: comparison of nasal and tracheal sampling sites. Am. Rev. Respir. Dis. 125 (Suppl.), 244 7065530 [Google Scholar]
- 17. Hee J. & Guillerm R. (1985) Discussion on smoke and mucociliary transport. Eur. Respir. Dis. 66 (Suppl. 139), 86–88 [PubMed] [Google Scholar]
- 18. Selwyn D.A., Gyri A., Raphael J.H. et al. (1996) A perfusion system for the in vitro measurement of human cilia beat frequency. Br. J. Anaesth. 76, 111–115 [DOI] [PubMed] [Google Scholar]
- 19. Padda G.S., Kishioka C. & Rubin B.K. (2001) Propofol and methohexital have no significant effect on mucus secretion or clearance in the anaesthetised dog. Crit Care Med. 29, 1045–1048 [DOI] [PubMed] [Google Scholar]


