Summary
Our current understanding of virus‐specific T cell responses has been shaped by model systems with mice, where naive animals are infected with a single viral pathogen. Paradigms derived from such models, however, may not always be applicable to a natural setting, where a host is exposed to numerous pathogens over its lifetime. Accumulating data in animal models and with some human diseases indicate that a host's prior history of infections can impact the specificity of future CD8 T cell responses, even to unrelated viruses. This can have both beneficial and detrimental consequences for the host, including altered clearance of virus, distinct forms of immunopathology, and substantial changes in the pool of memory T cells. Here we will describe the characteristics of CD8 T cells and the dynamics of their response to heterologous viral infections in sequence.
Introduction
Disease severity during viral infections is a function of direct virus‐induced cytopathology and of the non‐specific inflammatory and antigen‐specific immune responses of the host. An immune host encountering a virus for a second time usually resists any disease associated with re‐infection, as the expanded pools of virus‐specific memory B and T cells effectively restrict the replication of the pathogen. Individuals exposed to a virus for the first time, however, can vary dramatically in their symptoms and viral load. This variation is sometimes attributed to the initial viral dose or to the genetics or physiological state of the host, but we suggest here that another major factor leading to this variation is the contribution of the pools of memory cells specific to previously encountered and sometimes completely unrelated pathogens.
It has been recognized for some time that B cell responses generated by prior viral infections will influence both the production of virus‐specific antibodies and the replication of virus during future infections with antigenically related but not identical viruses through phenomena such as ‘original antigenic sin’ (Francis, 1953; Fazekas de St and Webster, 1966) and antibody‐dependent immune enhancement (Tirado and Yoon, 2003). Because of the profound involvement of CD8 T cells in viral immunity and immunopathology and of their propensity for cross‐reactivity against a diverse array of epitopes, we have explored and documented their potential for being even more effective than cross‐reactive antibody in altering the pathogenesis of virus infections in sequence. Here we will describe the properties of CD8 T cells and examine their modulation and function under conditions of infections with heterologous viral pathogens in sequence (Fig. 1).
Figure 1.
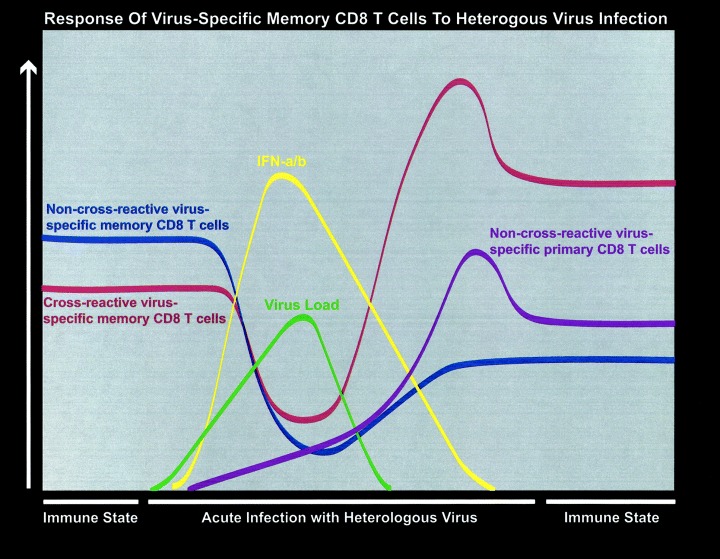
Response of both non‐cross‐reactive and cross‐reactive virus‐specific memory CD8 T cells to heterologous virus infections. This figure depicts the events occurring after a secondary infection of a virus‐immune mouse with a heterologous but cross‐reactive virus such as PV. Shown on the figure is the stability of memory CD8 T cells before re‐challenge, the virus‐induced lymphopenia, the peak of type 1 IFN, the peak of virus load, the expansion of T cells, the apoptotic decline, and the return to an immune state. It shows the loss of non‐cross‐reactive memory CD8 T cells and the maintenance of cross‐reactive memory.
Degeneracy in antigen recognition and cross‐reactivity
CD8 T cells recognize short, 8–11 amino acid residue peptide epitopes presented in the context of self‐MHC class I molecules (Townsend et al., 1986). The recognition of this peptide/MHC complex is mediated by the T cell receptor (TCR), which is a heterodimeric structure comprised of an α and β chain (Davis et al., 1998). Within each chain of the TCR are three variable sites, known as complementarity determining regions (CDRs) (van der Merwe and Davis, 2003), which protrude as loops from the TCR and directly contact sites on the peptide and the MHC molecule (Stewart‐Jones et al., 2003).
Theoretical considerations have suggested that the number of peptide‐MHC complexes that could be generated by environmental antigens exceeds the number of mature T cells within an individual (Regner, 2001). Thus, the breadth of the T cell repertoire would not be sufficient unless T cells had the capacity to recognize more than one antigen. Otherwise, non‐immunogenic pathogens would easily evolve to escape detection by T cells. T cells are, however, cross‐reactive against numerous epitopes, and a mathematical modelling analysis predicted that a single TCR may be able to interact with over one million different peptides (Mason, 1998). The CDR3 loops of the TCR undergo significant conformational changes in order to accommodate the 3‐dimensional structure of the peptide‐MHC surface (Garcia et al., 1998; Reiser et al., 2002; Wu et al., 2002; Kjer‐Nielsen et al., 2003). This flexibility imparted to the TCR may allow a single TCR to be promiscuous in the recognition of peptides and thereby be inherently cross‐reactive. This cross‐reactive nature of T cells may allow memory CD8 T cells generated by prior viral infections to play roles in subsequent infections with unrelated viruses.
Cross‐reactivity between virus‐specific CD8 T cells has been identified in a variety of different forms in both human and murine models. An expected form of cross‐reactivity would be against evolutionarily conserved sequences between very closely related viruses, where epitopes would either be identical or differ only slightly. Such cross‐reactivity has been observed in T cell responses between influenza virus A subtypes (Haanen et al., 1999; Belz et al., 2001), members of the Old World arenavirus family (Oldstone et al., 2001), the four serotypes of dengue virus (Spaulding et al., 1999; Mongkolsapaya et al., 2003), very closely related hantaviruses (including Hantaan and Seoul) (Van Epps et al., 1999), and serotypes of vesicular stomatitis virus (VSV‐Indiana and New Jersey) (Puddington et al., 1986; Yewdell et al., 1986). Cross‐reactivity does not have to be between homologous or highly conserved sequences, however. While mapping immunodominant epitopes cross‐reactivity was occasionally found between two different and non‐homologous sequences on the same protein or between different proteins derived from the same virus. Such cross‐reactivity has been reported for T cells specific for peptides derived from influenza virus (Kuwano et al., 1991; Anderson et al., 1992; Haanen et al., 1999), polyoma virus (Wilson et al., 1999), and respiratory syncytial virus (RSV) (Kulkarni et al., 1993).
Of significance is that virus‐specific CD8 T cells have also been shown to recognize epitopes derived from heterologous or more distantly related viruses. CD8 T cell cross‐reactivity amongst heterologous viruses has been defined between the two distantly related Arenaviruses (lymphocytic choriomeningitis virus (LCMV) and Pichinde virus (PV)) (Brehm et al., 2002), between different flaviviruses (Murray Valley encephalitis virus, yellow fever virus, West Nile virus, and dengue virus) (Regner et al., 2001), between influenza virus and EBV (Welsh et al., 2004), between influenza virus and hepatitis‐C virus (Wedemeyer et al., 2001), between influenza virus and rotavirus (Shimojo et al., 1989), between human papillomavirus‐16 and a human coronavirus (Nilges et al., 2003), and between LCMV and vaccinia virus (VV) (Welsh et al., 2004). Cumulatively, these findings demonstrate the highly cross‐reactive nature of virus‐specific T cell responses, and it seems likely that with further investigation more examples will be discovered.
Impact of viral infections on memory T cells
The T cell repertoire of a host that has been previously infected with a virus will be significantly altered relative to a naïve individual because a robust and stable population of memory T cells will have been established. New viral infections can have a profound impact on these pre‐existing pools of memory cells as a consequence of inflammatory cytokines and chemokines and of antigen‐dependent TCR stimulation (Fig. 1).
Memory T cell apoptosis and compensatory division
Initial stages of infection often involve a profound depletion of memory CD8 T cells throughout the body in both lymphoid and peripheral tissue (Fig. 1). This is a manifestation of a global virus‐induced lymphopenia that occurs with many viral infections and that affects many cell types (Peacock et al., 2003). This lymphopenia is at least partially dependent on type 1 IFN, as it parallels the type 1 IFN response, can be induced by the IFN stimulator poly I:C, and does not occur in type 1 IFN receptor knock‐out mice (Binder et al., 1997; McNally et al., 2001). This cell loss is associated with high levels of apoptosis in the memory CD8 T cell subset and lower levels in the naïve CD8 T cell subset (McNally et al., 2001). Whether IFN works alone or through another mechanism is not clear at present, but it should be noted that IFN can induce other pro‐apoptotic proteins such as TRAIL (Barber, 2001;Chawla‐Sarkar et al., 2003) and that it can enhance the effects of other pro‐apoptotic molecules such as TNF‐α (Binder et al., 1997).
This early loss of T cells is followed by antigen‐specific and non‐specific homeostatic events to fill the available space. Treatment with agents such as poly(I:C), IFN‐γ, LPS, and IL‐15 can stimulate CD44hi memory‐phenotype CD8 T cells to divide, as determined by uptake of bromodeoxyuridine (BrdU) into DNA (Tough et al., 1996; 1997; Zhang et al., 1998). However, this bystander division does not result in an overall increase in the number of T cells, suggesting that the division is balanced by cell death in order to maintain homeostasis (McNally et al., 2001; Turner et al., 2001). A careful examination of this division after poly(I:C) treatment shows first a profound apoptosis‐driven loss of memory CD8 T cells (McNally et al., 2001), followed by an IL‐15‐dependent recovery to steady‐state levels (Zhang et al., 1998; Kim and Welsh, 2004). Studies examining the recovery of memory cell populations after various forms of experimental lymphopenia showed that bona fide virus‐specific memory CD8 T cells underwent fewer cell divisions than other homeostatically responding cells, such that there was a substantial permanent loss in virus‐specific T cells under those conditions (McNally et al., 2001; Peacock et al., 2003).
Viral infections resemble poly I:C in their induction of memory cell loss, but they also provide antigenic peptide epitopes to stimulate T cells. T cells stimulated by their cognate ligands are induced into many cycles of division and far out‐compete the homeostatically dividing T cells (Fig. 2) (Kim et al., 2002). Whether antigen ligation can prevent the initial apoptosis of memory T cells has been investigated. Studies using an adoptive transfer model to examine the attrition of memory T cells demonstrated that LCMV‐specific memory CD8 T cells were significantly reduced in number 2 days after infection with the heterologous but cross‐reactive PV, and T cells specific for either the cross‐reactive or non‐cross‐reactive peptides underwent similar levels of attrition (Peacock et al., 2003; Kim and Welsh, 2004). This suggests that the attrition of memory is a generalized phenomenon affecting all memory T cell specificities independent of TCR signalling. Cross‐reactive T cells, however, selectively and rapidly expand in number following the early attrition process (Fig. 1) (Brehm et al., 2002). A recent study examining the susceptibility of naïve T cells to attrition concluded that infection with Listeria monocytogenes selectively depleted naïve CD8 T cells that were not specific for antigens expressed by the bacteria by 3 days, while antigen‐specific naïve T cells were not lost (Jiang et al., 2003).
Figure 2.
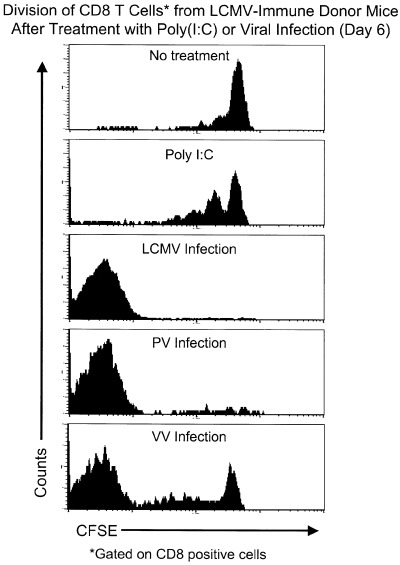
CD8 T cells from LCMV‐immune mice are stimulated to divide following infection with homologous and heterologous viruses. Splenocytes from LCMV‐immune mice (Thy1.1) were labelled with CFSE and adoptively transferred into congenic naïve mice (Thy1.2). One day following transfer, recipient mice were left untreated, injected with poly(I:C), or infected with LCMV, PV, or VV. Six days later, splenocytes from recipient mice were stained with antibodies to CD8 and the congenic marker (Thy1.1) and then analysed by flow cytometry. The histograms showing the loss of CFSE were analysed by gating on cells positive for the donor marker and CD8. This figure was courtesy of S.K. Kim and based on previous studies ( Kim et al., 2002 ).
Cytokine production.
Some viral and bacterial infections can induce a high proportion of memory CD8 T cells to synthesize IFN‐γ early in infection (Chen et al., 2001; Lertmemongkolchai et al., 2001; Berg et al., 2003). This is an important observation relating to heterologous immunity, as many pathogens are controlled by IFN‐γ. Some work has suggested that cytokines alone may stimulate memory cells to produce IFN‐γ (Berg et al., 2002; Kambayashi et al., 2003). However, the mechanisms mediating this early production of IFN‐γ have not been satisfactorily elucidated, as viral and bacterial pathogens may encode numerous foreign epitopes having the capacity to trigger the TCR at low affinity, and the role of T cell cross‐reactivity in these studies was not excluded. Results from our laboratory highlight the difficulties in distinguishing between TCR‐dependent and TCR‐independent stimulations. LCMV‐immune CD8 T cells produced IFN‐γ 3 days after vaccinia virus (VV) infection (Chen et al., 2001). It could be argued that this was a non‐specific phenomenon because T cells of multiple specificities were activated. However, IFN‐γ was made by higher proportions of some LCMV epitope‐specific T cells than other epitope‐specific populations. This diversity in the proportions of epitope‐specific populations producing IFN‐γ suggested a level of selectivity that may be supported by the recent identification of some cross‐reactive epitopes between VV and LCMV (Welsh et al., 2004). Regardless of the mechanism for IFN‐γ production, the IFN‐γ inhibited the replication of VV.
Recruitment to sites of inflammation.
Memory CD8 T cells, surviving the early attrition process, can be non‐specifically recruited to peripheral sites of infection early following a viral challenge (Zhang and Mak, 1986). This has been shown in transgenic and non‐transgenic systems. Memory TCR‐transgenic CD8 T cells specific for OVA and the LCMV‐epitope GP33 migrate to the lung following intranasal infection with the unrelated influenza virus and RSV, respectively (Topham et al., 2001; Ostler et al., 2003). Migration of MHC‐tetramer‐defined memory T cell populations to peripheral sites of infection was also shown in studies demonstrating that memory CD8 T cells specific to influenza virus, LCMV, murine γ herpes virus 68, and Sendai virus were recruited into the lung following infection with heterologous viruses (Topham et al., 2001; Ely et al., 2003; Ostler et al., 2003). The mechanism mediating the recruitment of memory CD8 T cells to the lung following intranasal infection is not yet fully defined but would be consistent with a generalized migration to an inflammatory site driven by the production of chemokines (Stephens et al., 2002), as this influx of T cells is independent of cell division (Ely et al., 2003; Ostler et al., 2003). The generalized recruitment of virus‐specific CD8 T cells to the site of infection may be a normal characteristic of an inflammatory response that allows for efficient effector function in the periphery.
Sequential infections with homologous and heterologous viruses
Memory CD8 T cells generated by prior viral infections will be reactivated upon re‐challenge with the originally encountered virus and will contribute to the overall immunity against the second infection. Normally the presence of neutralizing antibody will mute a secondary T cell response by restricting viral replication and antigen presentation to T cells, but in the absence of neutralizing antibody sufficient antigen is expressed to induce an anamnestic T cell response. A well documented example is in the influenza system, where antigenic shift produces subtypes of influenza A virus that are not recognized by neutralizing antibodies generated by previous infections with different influenza A subtypes (Wright and Webster, 2001). In the absence of those protective antibodies, prior infection with one influenza subtype generally still provides substantial protection against a challenge with a different subtype. This is referred to as heterosubtypic immunity (Schulman and Kilbourne, 1965). The protection afforded against subsequent infections with heterologous influenza virus subtypes has been attributed, in some studies, to the massive cross‐reactive CD8 T cell response at the site of infection following rechallenge (Yewdell et al., 1985; Townsend et al., 1986; Belz et al., 2001). Similar types of responses have been seen between the New Jersey and Indiana strains of VSV, which do not share neutralizing antibody epitopes but have cross‐reactive T cell epitopes that differ in only two amino acids (Puddington et al., 1986; Yewdell et al., 1986). All of these systems indicate that if virus replication is not restricted by neutralizing antibody, the response of epitope‐stimulated T cells can be quite profound, and this feature could contribute to dramatic T cell responses under conditions of heterologous immunity.
The promiscuous nature of antigen recognition by the TCR allows memory CD8 T cells generated by prior viral infections to cross‐react with heterologous viruses and to impact the generation of immune responses against subsequently encountered pathogens. To characterize the response of virus‐specific memory CD8 T cells against subsequent infections with heterologous but cross‐reactive viruses, the proliferation of these memory cells was examined after a viral challenge using an adoptive transfer model that allows the division of CD8 T cells to be directly quantified (Lyons and Parish, 1994). Splenocytes from LCMV‐immune mice were labelled with a fluorescent dye, CFSE, which divides equally between daughter cells during division and then transferred into a naive host. Infection of a recipient with the homologous virus, LCMV, induced substantial division and accumulation of the donor CD8 T cells (Fig. 2), with virtually all detectable T cells specific for LCMV undergoing at least seven divisions, the limit of detection with CFSE (Kim et al., 2002). In contrast, treatment of recipient mice with poly (I:C) induced no increase in cell number of donor cells and stimulated only limited cell division, again demonstrating the requirement for TCR engagement for sustained proliferation. In this transfer model, infection with the heterologous but cross‐reactive viruses PV and VV stimulated significant proliferation of a subset of the donor cells (Fig. 2), which were presumably those cross‐reactive between the viruses. These findings show that cross‐reactivity results in the proliferation of selected populations of virus‐specific memory CD8 T cells and raises the question of what effect these expanded populations of memory T cells will have on the response against the current infection.
The stimulation of LCMV‐specific CTL activity and proliferation of populations of virus‐specific memory CD8 T cells by subsequent infections with heterologous viruses resemble the phenomenon of ‘original antigenic sin’ that has been described for B cell responses against influenza virus subtypes (Francis, 1953; Selin et al., 1994; Kim et al., 2002). In agreement, mice previously infected with a wild‐type strain of LCMV and then challenged with variant strains of LCMV that encode altered T cell epitopes preferentially generated CD8 T cells that were specific for the epitope encoded for by the previously encountered wild‐type virus (Klenerman and Zinkernagel, 1998). However, the recall T cell responses generated by secondary infection with the LCMV‐variants did not efficiently recognize the variant epitopes encoded for by the second virus and resulted in the impaired clearance of the variant viruses. This impairment in immunity during sequential infections suggests that in some instances memory T cells generated by prior infections can have detrimental consequences for a host's defence against pathogens.
A similar phenomenon has been demonstrated for dengue virus‐specific CD8 T cell responses in humans (Mongkolsapaya et al., 2003). Dengue viruses are arthropod‐borne flaviviruses occurring as four serotypes defined in part by neutralizing antibody (Rothman and Ennis, 1999). Initial infection with one dengue serotype provides protective immunity against re‐infection with the identical virus, but subsequent infection with other serotypes can be associated with the development of life‐threatening disease such as dengue haemorrhagic fever and dengue shock syndrome. Interestingly, secondary infections with dengue virus elicited CD8 T cells with a higher affinity for different dengue serotypes than for the current infecting virus, suggesting the reactivation of memory CD8 T cells specific to previously encountered dengue virus serotypes.
T cell immunodominance and sequential viral infections
Epitope‐specific CD8 T cells generated by viral infections have defined hierarchies, with some dominating the response and others being weak to barely detectable (Mylin et al., 1995). These immunodominance hierarchies are extremely reproducible between individual hosts of the same haplotype and are maintained in the memory population (Belz et al., 2000). Multiple parameters, including the efficiency of peptide processing and presentation, the affinity of the peptide for the presenting MHC molecule, the quantity of MHC‐peptide complexes, the available TCR repertoire, and competition between T cells (immunodomination) are thought to contribute to these immunodominance profiles (Yewdell and Bennink, 1999).
The activation of virus‐specific memory CD8 T cells following infections with heterologous viruses is a newly described factor influencing the immunodominance of T cells specific for the challenge virus (Brehm et al., 2002). Cross‐reactive T cells are not always readily apparent following an infection with a single virus because the frequencies of cross‐reactive cells may be very low. Cross‐reactivity is more easily detectable following multiple infections, because it is those cross‐reactive T cells that are selectively amplified after infection (Selin et al., 1994;Haanen et al., 1999). The expansion of cross‐reactive CD8 T cell responses by sequential infection with natural variants of influenza virus has been elegantly demonstrated (Haanen et al., 1999). The two influenza variants differ by two amino acid residues in the dominant epitope NP366. Sequential infection with the two influenza variants preferentially stimulated the expansion of the cross‐reactive CD8 T cells that represent only a minor population during infection with a single variant. These findings show that the potential for cross‐reactivity between heterologous viruses may be completely obscured unless the cross‐reactive T cells are amplified by infections in sequence.
The immunodominance hierarchies for H‐2b‐restricted CD8 T cells specific for LCMV are dramatically altered in mice previously infected with heterologous but cross‐reactive viruses (Brehm et al., 2002). Following sequential infections with LCMV and PV in either order, CD8 T cells specific for the normally subdominant but cross‐reactive NP205 epitope become dominant, while CD8 T cells specific for the normally dominant epitopes are reduced in frequency (Fig. 3). Alterations in the immunodominance hierarchies of LCMV‐specific memory CD8 T cells have also been observed following infection with VV (Chen et al., 2001; Kim et al., 2002). These findings all lead to the conclusion that the nature of CD8 T cell immunodominance in a ‘real world’ scenario where a host is continuously exposed to viral pathogens may be very different when compared to a naive host.
Figure 3.
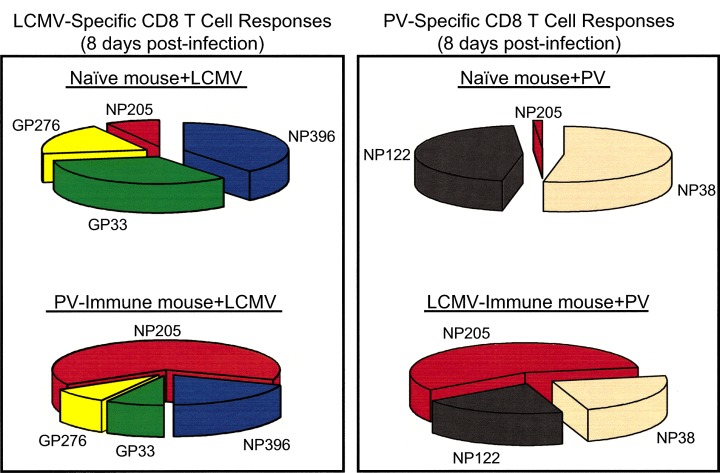
The immunodominance hierarchies of virus‐specific CD8 T cell responses are altered in mice that have been previously infected with a heterologous virus. The sections of the pie charts reflect the proportion of each epitope‐specific response relative to the T cell repertoire shown. The LCMV‐specific response is shown on the left and the PV‐specific response is shown on the right. The cross‐reactive T cells specific for the NP205 epitope are shown in red for both responses. The figure is based on previous studies ( Brehm et al., 2002 ).
Heterologous immunity and immunopathology
A host's history of viral infections may also influence how well the new infection is controlled, and this is a function of heterologous immunity. Mice previously infected with a panel of heterologous viruses (LCMV, PV, MCMV and VV) were often protected against challenges with the unrelated viruses (Selin et al., 1998). For example, LCMV‐immune, PV‐immune and MCMV‐immune mice were afforded a significant level of protection against challenge with VV (greater than a 100‐fold decrease in titres relative to naïve mice by four days post infection). Both CD4 and CD8 T cells from LCMV‐immune mice were necessary to provide protection against PV and VV, and immunity against VV was mediated at least in part by the production of IFN‐γ. Of note is that this immunity was not reciprocal, as VV‐immune mice were not protected against infection with any of the heterologous viruses. This lack of reciprocity for heterologous immunity in VV‐immune mice correlated with the functional activity of T cells, as VV‐specific memory T cells were not stimulated by LCMV infection to proliferate or to be cytolytic (Selin et al., 1994; Kim et al., 2002).
Heterologous immunity has been studied extensively with respiratory viral infections (Chen et al., 2001; 2003). LCMV‐immune mice were protected against a lethal dose of VV delivered intranasally, with significant reductions in VV titres in the lung compared to naïve mice. Mice previously infected with MCMV or influenza also were protected against intranasal challenge with VV. Following VV challenge of mice that were previously infected with either LCMV or influenza, a shift in cytokine production towards a Th1 phenotype (such as IFN‐γ production) was observed in the lungs 3 days after infection in comparison to the cytokines produced in VV‐infected naïve mice. Heterologous immunity in the lung was not always protective, however, as clearance of LCMV and MCMV was impaired in influenza‐immune mice, and a history of an LCMV infection impaired the ability of mice to clear RSV from the lung following an intranasal inoculation (Ostler et al., 2003). The mechanisms of diminished protective immunity in mice previously infected with heterologous virus are uncertain and may correlate with altered cytokine profiles. Alternatively, the reduced levels of protection may be attributed to the stimulation of memory T cells that are not effective at controlling the infection and to the suppressed development of T cells that would be effective, as shown in other studies on original antigenic sin (Klenerman and Zinkernagel, 1998; Mongkolsapaya et al., 2003). Overall these results indicate that prior viral infections can either augment or inhibit viral clearance during subsequent infections with unrelated viruses.
Heterologous immunity may also be associated with the development of severe immunopathology. LCMV‐immune mice infected intraperitoneally with VV developed an IFN‐γ‐dependent acute fatty necrosis associated with a cellular infiltrate in the visceral fat pads (Selin et al., 1998). In an intranasal model, LCMV‐immune mice infected with VV developed a severe lung pathology that was characterized by a strong lymphocytic response, and the induction of bronchiolitis obliterans, which involves the obstruction of bronchioles by fibrin and inflammatory cells (Chen et al., 2001). This development of immunopathology during sequential viral infections correlates with the activation of pre‐existing memory LCMV‐specific CD8 T cells, but additional factors may also contribute to the pathology, including altered cytokine profiles. Other studies have shown that prior infections with MCMV, influenza, and Sendai can dramatically alter the histopathology of the lung during subsequent infections with unrelated viruses (Jakab, 1990; Chen et al., 2003). Some of the immunopathologies developed following sequential viral infections resemble forms of immune‐mediated human diseases of unknown aetiology, with acute fatty necrosis being similar to erythema nodosum, the most common form of human panniculitis, and the induction of bronchiolitis obliterans in humans being associated with viral and bacterial infections and transplant rejections (Bolognia and Braverman, 1992; Schlesinger et al., 1998).
Maintenance of T cell memory during sequential viral infections
Virus‐specific memory CD8 T cells generated by a single virus are maintained at relatively stable frequencies over time (Selin et al., 1996; Homann et al., 2001). However, following a subsequent viral infection these populations of memory CD8 T cells are dramatically altered, with the permanent loss of the non‐cross‐reactive T cells from the memory repertoire and an enrichment of T cells that are cross‐reactive with the challenge virus (Fig. 1) (Selin et al., 1996; 1999; Brehm et al., 2002). Challenge of virus‐immune mice with heterologous viruses that establish either acute sterilizing infections (LCMV strain Armstrong, PV, MCMV, and VV) or persistent infections (murine γherpes virus 68 and LCMV clone 13), can trigger the attrition of virus‐specific CD8 T cells from the memory repertoire (Liu et al., 2003; Kim and Welsh, 2004). Infection with the clone 13 variant of LCMV is associated with the continuous production of cytokines and T cell activation and resulted in an even more dramatic attrition of memory CD8 T cells relative to LCMV strain Armstrong, with greater than 10‐fold drops in CD8 memory T cell frequencies specific to VV, VSV, and PV‐encoded antigens (Kim and Welsh, 2004).
This depletion of CD8 T cell memory could be a consequence of competition between newly generated memory T cells and previously formed memory cells for protective niches in lymphoid tissue. Alternatively it could be a by‐product of the virus‐induced lymphopenia that leads to memory T cell apoptosis early in infection. Recent kinetic studies indicate that once memory cells are lost early in infection, they never return to their original frequencies, arguing for the importance of the virus‐induced lymphopenia in this process (Kim and Welsh, 2004). Thus, pre‐existing memory populations can play substantial roles in the host response to new virus infections, and those virus infections permanently alter the memory T cell pool that was present before infection.
Sequential viral infections and transplantation
Virus‐specific CD8 T cells can cross‐react with allogeneic MHC molecules presenting endogenous non‐viral peptides (Braciale et al., 1981; Yang and Welsh, 1986; Nahill and Welsh, 1993; Burrows et al., 1994; Brehm et al., 2003), and have the potential to hinder transplantation of allogeneic tissues. Acute infections with viruses such as LCMV and PV interfere with the induction of tolerance using costimulatory blockade against allogeneic skin grafts (Welsh et al., 2000). Recent studies have also shown that LCMV‐immune mice are refractory to the induction of tolerance against allogeneic skin resulting in graft rejection (Brehm et al., 2003), and mice that have been sequentially infected with heterologous viruses become even more difficult to tolerise to allo‐antigens (Adams et al., 2003). Sequential infections with heterologous viruses can increase the frequency of T cells specific for allo‐antigens, and create a larger pool of memory cells to tolerize before engraftment. In H‐2b mice that had previously been infected with PV and then infected with LCMV, the induction of allo‐antigen‐specific CD8 T cells was altered, with higher frequencies of T cells specific for H‐2k antigens and slightly reduced populations specific for H‐2d antigens in comparison to naïve mice infected with LCMV (Brehm et al., 2003). The alterations in the allo‐antigen‐specific T cell response generated in PV‐immune mice infected with LCMV may reflect the changes in the hierarchies of the LCMV epitope‐specific (Fig. 3) CD8 T cells, which cross‐react with allo‐antigens. These findings suggest that the generation of allo‐antigen‐specific memory CD8 T cells by viral infections presents a unique impediment for transplantation.
Concluding remarks
We demonstrated here that a host's history of viral infections, by changing the repertoire of the T cell memory pool, may influence T cell immunodominance, protective immunity, and immunopathology occurring in response to subsequent infections. Although parameters of this heterologous immunity have been studied most definitively in murine systems, the identification of T cells cross‐reactive between many common human viruses suggests that the imprint left on the human immune system by previous viral infections may alter the course of human diseases. Many viral infections present more severely in teenagers and young adults than in children (Weinstein and Meade, 1956; Simonsen et al., 1998), and one questions whether prior infections encountered earlier in life have altered the immune responses to these pathogens. Similarly, because of T cell cross‐reactivity, vaccination might unexpectedly impact responses to pathogens unrelated to the vaccine. The full impact of heterologous T cell‐dependent immunity in human infections should become clear as more is learned about the activation and fate of cross‐reactive T cells in human viral infections.
Acknowledgements
This work was supported by research grants AI17672, AI46578, AI46629, AI49320, AR 35506, and CA34461, by diabetes and endocrinology core grant DK32520, and by training grant AI07349, all from the United States National Institutes of Health. We thank Sung‐Kwon Kim, PhD, for helpful suggestions and for providing a figure.
References
- Adams, A.B. , Williams, M.A. , Jones, T.R. , Shirasugi, N. , Durham, M.M. , Kaech, S.M. et al. (2003) Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest 111: 1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, R.W. , Bennink, J.R. , Yewdell, J.W. , Maloy, W.L. , and Coligan, J.E. (1992) Influenza basic polymerase 2 peptides are recognized by influenza nucleoprotein‐specific cytotoxic T lymphocytes. Mol Immunol 29: 1089–1096. [DOI] [PubMed] [Google Scholar]
- Barber, G.N. (2001) Host defense, viruses and apoptosis. Cell Death Differ 8: 113–126. [DOI] [PubMed] [Google Scholar]
- Belz, G.T. , Stevenson, P.G. , and Doherty, P.C. (2000) Contemporary analysis of MHC‐related immunodominance hierarchies in the CD8+ T cell response to influenza A viruses. J Immunol 165: 2404–2409. [DOI] [PubMed] [Google Scholar]
- Belz, G.T. , Xie, W. , and Doherty, P.C. (2001) Diversity of epitope and cytokine profiles for primary and secondary influenza a virus‐specific CD8+ T cell responses. J Immunol 166: 4627–4633. [DOI] [PubMed] [Google Scholar]
- Berg, R.E. , Cordes, C.J. , and Forman, J. (2002) Contribution of CD8+ T cells to innate immunity: IFN‐gamma secretion induced by IL‐12 and IL‐18. Eur J Immunol 32: 2807–2816. [DOI] [PubMed] [Google Scholar]
- Berg, R.E. , Crossley, E. , Murray, S. , and Forman, J. (2003) Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med 198: 1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, D. , Fehr, J. , Hengartner, H. , and Zinkernagel, R.M. (1997) Virus‐induced transient bone marrow aplasia: major role of interferon‐alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus. J Exp Med 185: 517–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognia, J.L. , and Braverman, I. (1992) Skin manifestations of internal disease In Harrison's Principles of Internal Medicine. Isselbacher K.J., Braunwald E., Wilson J., Martin J.B., Fauci A.S. and Kasper D.L, (eds). New York: McGraw‐Hill, pp. 290–307. [Google Scholar]
- Braciale, T.J. , Andrew, M.E. , and Braciale, V.L. (1981) Simultaneous expression of H‐2‐restricted and alloreactive recognition by a cloned line of influenza virus‐specific cytotoxic T lymphocytes. J Exp Med 153: 1371–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm, M.A. , Pinto, A.K. , Daniels, K.A. , Schneck, J.P. , Welsh, R.M. , and Selin, L.K. (2002) T cell immunodominance and maintenance of memory regulated by unexpectedly cross‐reactive pathogens. Nat Immunol 3: 627–634. [DOI] [PubMed] [Google Scholar]
- Brehm, M.A. , Markees, T.G. , Daniels, K.A. , Greiner, D.L. , Rossini, A.A. , and Welsh, R.M. (2003) Direct visualization of cross‐reactive effector and memory allo‐specific CD8 T cells generated in response to viral infections. J Immunol 170: 4077–4086. [DOI] [PubMed] [Google Scholar]
- Burrows, S.R. , Khanna, R. , Burrows, J.M. , and Moss, D.J. (1994) An alloresponse in humans is dominated by cytotoxic T lymphocytes (CTL) cross‐reactive with a single Epstein‐Barr virus CTL epitope: implications for graft‐versus‐host disease. J Exp Med 179: 1155–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla‐Sarkar, M. , Lindner, D.J. , Liu, Y.F. , Williams, B.R. , Sen, G.C. , Silverman, R.H. , and Borden, E.C. (2003) Apoptosis and interferons: role of interferon‐stimulated genes as mediators of apoptosis. Apoptosis 8: 237–249. [DOI] [PubMed] [Google Scholar]
- Chen, H.D. , Fraire, A.E. , Joris, I. , Brehm, M.A. , Welsh, R.M. , and Selin, L.K. (2001) Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol 2: 1067–1076. [DOI] [PubMed] [Google Scholar]
- Chen, H.D. , Fraire, A.E. , Joris, I. , Welsh, R.M. , and Selin, L.K. (2003) Specific history of heterologous virus infections determines anti‐viral immunity and immunopathology in the lung. Am J Pathol 163: 1341–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, M.M. , Boniface, J.J. , Reich, Z. , Lyons, D. , Hampl, J. , Arden, B. , and Chien, Y. (1998) Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol 16: 523–544. [DOI] [PubMed] [Google Scholar]
- Ely, K.H. , Cauley, L.S. , Roberts, A.D. , Brennan, J.W. , Cookenham, T. , and Woodland, D.L. (2003) Nonspecific recruitment of memory CD8+ T cells to the lung airways during respiratory virus infections. J Immunol 170: 1423–1429. [DOI] [PubMed] [Google Scholar]
- Fazekas de St, G. , and Webster, R.G. (1966) Disquisitions of original antigenic sin. I. Evidence in man. J Exp Med 124: 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, T. Jr (1953) Influenza: the new acquaintance. Ann Intern Med 39: 203–221. [DOI] [PubMed] [Google Scholar]
- Garcia, K.C. , Degano, M. , Pease, L.R. , Huang, M. , Peterson, P.A. , Teyton, L. , and Wilson, I.A. (1998) Structural basis of plasticity in T cell receptor recognition of a self peptide‐MHC antigen. Science 279: 1166–1172. [DOI] [PubMed] [Google Scholar]
- Haanen, J.B. , Wolkers, M.C. , Kruisbeek, A.M. , and Schumacher, T.N. (1999) Selective expansion of cross‐reactive CD8 (+) memory T cells by viral variants. J Exp Med 190: 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homann, D. , Teyton, L. , and Oldstone, M.B. (2001) Differential regulation of antiviral T‐cell immunity results in stable CD8+ but declining CD4+ T‐cell memory. Nat Med 7: 913–919. [DOI] [PubMed] [Google Scholar]
- Jakab, G.J. (1990) Sequential virus infections, bacterial superinfections, and fibrogenesis. Am Rev Respir Dis 142: 374–379. [DOI] [PubMed] [Google Scholar]
- Jiang, J. , Lau, L.L. , and Shen, H. (2003) Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J Immunol 171: 4352–4358. [DOI] [PubMed] [Google Scholar]
- Kambayashi, T. , Assarsson, E. , Lukacher, A.E. , Ljunggren, H.G. , and Jensen, P.E. (2003) Memory CD8+ T cells provide an early source of IFN‐gamma. J Immunol 170: 2399–2408. [DOI] [PubMed] [Google Scholar]
- Kim, S.K. , and Welsh, R.M. (2004) Comprehensive early and lasting loss of memory CD8 T cells and functional memory during acute and persistent viral infections. J Immunol 172: 3139–3150. [DOI] [PubMed] [Google Scholar]
- Kim, S.K. , Brehm, M.A. , Welsh, R.M. , and Selin, L.K. (2002) Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation. J Immunol 169: 90–98. [DOI] [PubMed] [Google Scholar]
- Kjer‐Nielsen, L. , Clements, C.S. , Purcell, A.W. , Brooks, A.G. , Whisstock, J.C. , Burrows, S.R. et al. (2003) A structural basis for the selection of dominant alphabeta T cell receptors in antiviral immunity. Immunity 18: 53–64. [DOI] [PubMed] [Google Scholar]
- Klenerman, P. , and Zinkernagel, R.M. (1998) Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature 394: 482–485. [DOI] [PubMed] [Google Scholar]
- Kulkarni, A.B. , Morse, H.C. , 3rd, Bennink, J.R. , Yewdell, J.W. , and Murphy, B.R. (1993) Immunization of mice with vaccinia virus‐M2 recombinant induces epitope‐specific and cross‐reactive Kd‐restricted CD8+ cytotoxic T cells. J Virol 67: 4086–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano, K. , Reyes, V.E. , Humphreys, R.E. , and Ennis, F.A. (1991) Recognition of disparate HA and NS1 peptides by an H‐2Kd‐restricted, influenza specific CTL clone. Mol Immunol 28: 1–7. [DOI] [PubMed] [Google Scholar]
- Lertmemongkolchai, G. , Cai, G. , Hunter, C.A. , and Bancroft, G.J. (2001) Bystander activation of CD8+ T cells contributes to the rapid production of IFN‐gamma in response to bacterial pathogens. J Immunol 166: 1097–1105. [DOI] [PubMed] [Google Scholar]
- Liu, H. , Andreansky, S. , Diaz, G. , Turner, S.J. , Wodarz, D. , and Doherty, P.C. (2003) Quantitative analysis of long‐term virus‐specific CD8+‐T‐cell memory in mice challenged with unrelated pathogens. J Virol 77: 7756–7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons, A.B. , and Parish, C.R. (1994) Determination of lymphocyte division by flow cytometry. J Immunol Meth 171: 131–137. [DOI] [PubMed] [Google Scholar]
- Mason, D. (1998) A very high level of crossreactivity is an essential feature of the T‐ cell receptor. Immunol Today 19: 395–404. [DOI] [PubMed] [Google Scholar]
- McNally, J.M. , Zarozinski, C.C. , Lin, M.Y. , Brehm, M.A. , Chen, H.D. , and Welsh, R.M. (2001) Attrition of bystander CD8 T cells during virus‐induced T‐cell and interferon responses. J Virol 75: 5965–5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Merwe, P.A. , and Davis, S.J. (2003) Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol 21: 659–684. [DOI] [PubMed] [Google Scholar]
- Mongkolsapaya, J. , Dejnirattisai, W. , Xu, X.N. , Vasanawathana, S. , Tangthawornchaikul, N. , Chairunsri, A. et al. (2003) Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 9: 921–927. [DOI] [PubMed] [Google Scholar]
- Mylin, L.M. , Bonneau, R.H. , Lippolis, J.D. , and Tevethia, S.S. (1995) Hierarchy among multiple H‐2b‐restricted cytotoxic T‐lymphocyte epitopes within simian virus 40 T antigen. J Virol 69: 6665–6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahill, S.R. , and Welsh, R.M. (1993) High frequency of cross‐reactive cytotoxic T lymphocytes elicited during the virus‐induced polyclonal cytotoxic T lymphocyte response. J Exp Med 177: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilges, K. , Hohn, H. , Pilch, H. , Neukirch, C. , Freitag, K. , Talbot, P.J. , and Maeurer, M.J. (2003) Human papillomavirus type 16, E7 peptide‐directed CD8+ T cells from patients with cervical cancer are cross‐reactive with the coronavirus NS2 protein. J Virol 77: 5464–5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone, M.B. , Lewicki, H. , Homann, D. , Nguyen, C. , Julien, S. , and Gairin, J.E. (2001) Common antiviral cytotoxic T‐lymphocyte epitope for diverse arenaviruses. J Virol 75: 6273–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostler, T. , Pircher, H. , and Ehl, S. (2003) ‘Bystander’ recruitment of systemic memory T cells delays the immune response to respiratory virus infection. Eur J Immunol 33: 1839–1848. [DOI] [PubMed] [Google Scholar]
- Peacock, C.D. , Kim, S.K. , and Welsh, R.M. (2003) Attrition of virus‐specific memory CD8+ T cells during reconstitution of lymphopenic environments. J Immunol 171: 655–663. [DOI] [PubMed] [Google Scholar]
- Puddington, L. , Bevan, M.J. , Rose, J.K. , and Lefrancois, L. (1986) N protein is the predominant antigen recognized by vesicular stomatitis virus‐specific cytotoxic T cells. J Virol 60: 708–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regner, M. (2001) Cross‐reactivity in T‐cell antigen recognition. Immunol Cell Biol 79: 91–100. [DOI] [PubMed] [Google Scholar]
- Regner, M. , Lobigs, M. , Blanden, R.V. , Milburn, P. , and Mullbacher, A. (2001) Antiviral cytotoxic T cells cross‐reactively recognize disparate peptide determinants from related viruses but ignore more similar self‐ and foreign determinants. J Immunol 166: 3820–3828. [DOI] [PubMed] [Google Scholar]
- Reiser, J.B. , Gregoire, C. , Darnault, C. , Mosser, T. , Guimezanes, A. , Schmitt‐Verhulst, A.M. et al. (2002) A T cell receptor CDR3beta loop undergoes conformational changes of unprecedented magnitude upon binding to a peptide/MHC class I complex. Immunity 16: 345–354. [DOI] [PubMed] [Google Scholar]
- Rothman, A.L. , and Ennis, F.A. (1999) Immunopathogenesis of Dengue hemorrhagic fever. Virology 257: 1–6. [DOI] [PubMed] [Google Scholar]
- Schlesinger, C. , Meyer, C.A. , Veeraraghavan, S. , and Koss, M.N. (1998) Constrictive (obliterative) bronchiolitis: diagnosis, etiology, and a critical review of the literature. Ann Diagn Pathol 2: 321–334. [DOI] [PubMed] [Google Scholar]
- Schulman, J.L. , and Kilbourne, E.D. (1965) Induction of partial specific heterotypic immunity in mice by a single infection with influenza A virus. J Bacteriol 89: 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin, L.K. , Nahill, S.R. , and Welsh, R.M. (1994) Cross‐reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med 179: 1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin, L.K. , Vergilis, K. , Welsh, R.M. , and Nahill, S.R. (1996) Reduction of otherwise remarkably stable virus‐specific cytotoxic T lymphocyte memory by heterologous viral infections. J Exp Med 183: 2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin, L.K. , Varga, S.M. , Wong, I.C. , and Welsh, R.M. (1998) Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med 188: 1705–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin, L.K. , Lin, M.Y. , Kraemer, K.A. , Pardoll, D.M. , Schneck, J.P. , Varga, S.M. et al. (1999) Attrition of T cell memory: selective loss of LCMV epitope‐specific memory CD8 T cells following infections with heterologous viruses. Immunity 11: 733–742. [DOI] [PubMed] [Google Scholar]
- Shimojo, N. , Maloy, W.L. , Anderson, R.W. , Biddison, W.E. , and Coligan, J.E. (1989) Specificity of peptide binding by the HLA‐A2.1 molecule. J Immunol 143: 2939–2947. [PubMed] [Google Scholar]
- Simonsen, L. , Clarke, M.J. , Schonberger, L.B. , Arden, N.H. , Cox, N.J. , and Fukuda, K. (1998) Pandemic versus epidemic influenza mortality: a pattern of changing age distribution. J Infect Dis 178: 53–60. [DOI] [PubMed] [Google Scholar]
- Spaulding, A.C. , Kurane, I. , Ennis, F.A. , and Rothman, A.L. (1999) Analysis of murine CD8 (+) T‐cell clones specific for the Dengue virus NS3 protein: flavivirus cross‐reactivity and influence of infecting serotype. J Virol 73: 398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens, R. , Randolph, D.A. , Huang, G. , Holtzman, M.J. , and Chaplin, D.D. (2002) Antigen‐nonspecific recruitment of Th2 cells to the lung as a mechanism for viral infection‐induced allergic asthma. J Immunol 169: 5458–5467. [DOI] [PubMed] [Google Scholar]
- Stewart‐Jones, G.B. , McMichael, A.J. , Bell, J.I. , Stuart, D.I. , and Jones, E.Y. (2003) A structural basis for immunodominant human T cell receptor recognition. Nat Immunol 4: 657–663. [DOI] [PubMed] [Google Scholar]
- Tirado, S.M. , and Yoon, K.J. (2003) Antibody‐dependent enhancement of virus infection and disease. Viral Immunol 16: 69–86. [DOI] [PubMed] [Google Scholar]
- Topham, D.J. , Castrucci, M.R. , Wingo, F.S. , Belz, G.T. , and Doherty, P.C. (2001) The role of antigen in the localization of naive, acutely activated, and memory CD8 (+) T cells to the lung during influenza pneumonia. J Immunol 167: 6983–6990. [DOI] [PubMed] [Google Scholar]
- Tough, D.F. , Borrow, P. , and Sprent, J. (1996) Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272: 1947–1950. [DOI] [PubMed] [Google Scholar]
- Tough, D.F. , Sun, S. , and Sprent, J. (1997) T cell stimulation in vivo by lipopolysaccharide (LPS). J Exp Med 185: 2089–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend, A.R. , Rothbard, J. , Gotch, F.M. , Bahadur, G. , Wraith, D. , and McMichael, A.J. (1986) The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell 44: 959–968. [DOI] [PubMed] [Google Scholar]
- Turner, S.J. , Cross, R. , Xie, W. , and Doherty, P.C. (2001) Concurrent naive and memory CD8 (+) T cell responses to an influenza A virus. J Immunol 167: 2753–2758. [DOI] [PubMed] [Google Scholar]
- Van Epps, H.L. , Schmaljohn, C.S. , and Ennis, F.A. (1999) Human memory cytotoxic T‐lymphocyte (CTL) responses to Hantaan virus infection: identification of virus‐specific and cross‐reactive CD8 (+) CTL epitopes on nucleocapsid protein. J Virol 73: 5301–5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedemeyer, H. , Mizukoshi, E. , Davis, A.R. , Bennink, J.R. , and Rehermann, B. (2001) Cross‐reactivity between hepatitis C virus and Influenza A virus determinant‐specific cytotoxic T cells. J Virol 75: 11392–11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein, L. , and Meade, R.H. (1956) Respiratory manifestations of chickenpox. Arch Intern Med 98: 91–99. [DOI] [PubMed] [Google Scholar]
- Welsh, R.M. , Markees, T.G. , Woda, B.A. , Daniels, K.A. , Brehm, M.A. , Mordes, J.P. et al. (2000) Virus‐induced abrogation of transplantation tolerance induced by donor‐ specific transfusion and anti‐CD154 antibody. J Virol 74: 2210–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh, R.M. , Selin, L.K. , and Szomolanyi‐Tsuda, E. (2004) Immunological memory to viral infections. Annu Rev Immunol (in press). [DOI] [PubMed] [Google Scholar]
- Wilson, C.S. , Moser, J.M. , Altman, J.D. , Jensen, P.E. , and Lukacher, A.E. (1999) Cross‐recognition of two middle T protein epitopes by immunodominant polyoma virus‐specific CTL. J Immunol 162: 3933–3941. [PubMed] [Google Scholar]
- Wright, P.F. , and Webster, R.G. (2001) Orthomyxoviridae In Fields Virology. Knipe D.M. and Howley P.M., (eds). Philadelphia: Lippincott Williams & Wilkins, pp. 1533–1579. [Google Scholar]
- Wu, L.C. , Tuot, D.S. , Lyons, D.S. , Garcia, K.C. , and Davis, M.M. (2002) Two‐step binding mechanism for T‐cell receptor recognition of peptide MHC. Nature 418: 552–556. [DOI] [PubMed] [Google Scholar]
- Yang, H. , and Welsh, R.M. (1986) Induction of alloreactive cytotoxic T cells by acute virus infection of mice. J Immunol 136: 1186–1193. [PubMed] [Google Scholar]
- Yewdell, J.W. , and Bennink, J.R. (1999) Immunodominance in major histocompatibility complex class I‐restricted T lymphocyte responses. Annu Rev Immunol 17: 51–88. [DOI] [PubMed] [Google Scholar]
- Yewdell, J.W. , Bennink, J.R. , Smith, G.L. , and Moss, B. (1985) Influenza A virus nucleoprotein is a major target antigen for cross‐reactive anti‐influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci USA 82: 1785–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell, J.W. , Bennink, J.R. , Mackett, M. , Lefrancois, L. , Lyles, D.S. , and Moss, B. (1986) Recognition of cloned vesicular stomatitis virus internal and external gene products by cytotoxic T lymphocytes. J Exp Med 163: 1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y.H. , and Mak, N.K. (1986) Nonspecific influx of cytotoxic T cells into influenza virus‐infected lungs of mice. Inflammation 10: 9–14. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Sun, S. , Hwang, I. , Tough, D.F. , and Sprent, J. (1998) Potent and selective stimulation of memory‐phenotype CD8+ T cells in vivo by IL‐15. Immunity 8: 591–599. [DOI] [PubMed] [Google Scholar]


