Abstract
Aminopeptidase N (CD13) was recently identified as a molecular target of the cholesterol absorption inhibitor Ezetimib. Regarding that CD13 is expressed in lipid rafts of monocytic cells, we have investigated whether Ezetimib influences raft function in these cells. Expression of raft‐associated antigens (CD11b, CD13, CD14, CD16, CD36, and CD64) was followed by flow cytometry and/or immunoblot in human monocyte‐derived macrophages in response to in vitro administration of Ezetimib. Cellular redistribution of CD13 was assessed by confocal imaging. Ezetimib significantly decreased the surface expression of CD13, CD16, CD64, and CD36; furthermore, it induced a shift of CD13 from plasma membrane to intracellular vesicles, and thus it quite likely modulated monocytic raft‐assembly. © 2006 International Society for Analytical Cytology
Keywords: CD13, Ezetimib, detergent‐resistant microdomain
Aminopeptidase N (CD13) is a transmembrane ectoenzyme of zinc metalloproteases with a broad expression in cells/tissues, including myeloid cells, hepatocytes, various epithelial cells, nervous system, and placenta (1). CD13 is involved in certain biological processes, such as growth and invasion of tumors, cellular motility, regulation of angiogenesis, cellular internalization of human coronavirus 229E, and modulation of the effect of IL‐6 or IL‐8, neuropeptides, and chemotactic peptides [1, 2, 3 and references therein]. More recently, CD13 has been implicated as a molecular target of Ezetimib, an azetidinone‐type cholesterol absorption inhibitor and plasma cholesterol lowering drug, in enterocytic brush border membrane (4).
CD13 is a raft microdomain‐resident, type II membrane protein, and both of its homodimeric polypeptides have a very short cytoplasmic domain of eight amino acid residues with no described signaling motifs (2, 3). Thus, it is tempting to speculate that signal transduction through CD13 requires additional membrane proteins, quite likely localized in rafts. In a recent study, Mina‐Osorio and Ortega provide evidence that CD13 functionally interacts with the Fcγ receptor I (CD64) on monocytic cells (3). As characterized by fluorescence resonance energy transfer (FRET), human monocytes contain a CD14, CD32, CD64, CD47, and CD55‐containing, raft‐associated, constitutive receptor cluster (5). Activation of monocytes by lipopolysaccharide (LPS) or lipoteichoic acid leads to coassembly of CD14, CD32, CD64, CD55, CD11b/CD18, CD16, CD81, CD36, and the toll‐like receptor‐4 (TLR4), but not CD47 in these rafts (5).
Since Ezetimib binds to CD13, and CD13 quite likely interacts with other receptor(s) in raft microdomains, we have investigated whether Ezetimib influences the assembly of monocyte rafts or the expression of raft‐associated antigens or both.
MATERIALS AND METHODS
Isolation and In Vitro Differentiation of Human Monocytes
Monocytes of healthy volunteers were isolated by leukapheresis followed by counterflow elutriation after informed consent. Cells were cultured in serum‐free macrophage medium (SFM; Gibco/Invitrogen, Karlsruhe, Germany) containing 50 ng/ml recombinant human macrophage‐colony stimulating factor (M‐CSF; R&D Systems, Wiesbaden, Germany), with or without supplementation of 10 μM Ezetimib ((3R,4S)‐1‐(4‐fluorphenyl)‐3‐[(3S)‐3‐(4‐fluorphenyl)‐3‐hydroxypropyl]‐4‐(4‐hydroxyphenyl)azetidin‐2‐on (Aventis Pharma, Frankfurt am Main, Germany), up to 5 days. The percentage of damaged (i.e., apoptotic or necrotic) cells as determined by propidium iodide exclusion was <20% irrespective of treatment.
Flow Cytometry
Cultured monocyte‐derived macrophages were stained with saturating concentrations of fluorochrome‐conjugated, antihuman mAbs, against extracellular epitopes of CD11b‐PE (D12), CD14‐APC (MΦP9), CD36‐PE (CB38/NL07/), CD45‐PerCP (2D1), CD64‐FITC (BD Biosciences, Heidelberg, Germany), CD16‐FITC (3G8, Immunotech/Beckman‐Coulter, Krefeld, Germany), and CD13‐FITC (WM‐47, Sigma–Aldrich, Taufkirchen, Germany) according to standard protocols. Four‐parametric flow cytometry was carried out by a FACSCalibur flow cytometer (BD Biosciences).
Detergent Lysis and Sucrose Flotation Gradients, Immunoblotting
Detergent lysis with 1% Lubrol WX (Serva, Heidelberg, Germany) and sucrose gradient flotation was carried out as described previously (6). Immunoblotting of cellular fractions or whole cell lysates was performed as described earlier (5), using the CD13 mAb (3D8, Santa Cruz Biotechnology, Heidelberg, Germany).
Confocal Imaging
Cells were cultured on sodium borosilicate coverglasses (Nunc, Wiesbaden, Germany), fixed with 4% paraformaldehyde (Polysciences, Eppelheim, Germany), permeabilized by 0.25% Triton X‐100 (Sigma–Aldrich) and labeled according to standard procedures. In addition to the CD13‐FITC staining, plasma membrane was also visualized by mouse antihuman annexin II IgG1 (clone 5, BD Biosciences), and Texas Red‐conjugated horse anti‐mouse IgG (Vector Laboratories/Alexis, Gruenberg, Germany). Confocal images were taken by a TCS 4D inverted confocal laser scanning microscope (Leica Lasertechnik, Bensheim, Germany), and analyzed by the MetaMorph 6.2 software package (Universal Imaging, Glendale, WI).
RESULTS
Ezetimib Decreases the Expression of Raft‐Associated Antigens in Macrophage Plasma Membranes
Ezetimib significantly decreases the surface expression of CD13, CD16, CD64, and CD36 of monocyte‐derived macrophages, but has no marked effect on the surface expression of CD14 and CD11b (Fig. 1A).
Figure 1.
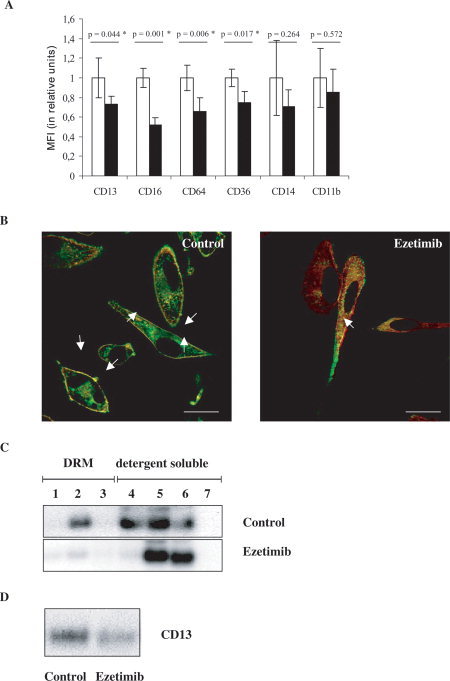
(A) Surface expression of raft‐associated antigens on monocyte‐derived macrophages in response to 10 μM Ezetimib. Relative mean fluorescence intensities (MFI) of control cells (treated with DMSO as solvent of Ezetimib, open bars) and Ezetimib‐treated cells (solid bars) were compared for each antigen (CD13, CD16, CD64, CD36, CD14, and CD11b, as indicated). MFI of control cells was taken as 1 unit, and the effect of Ezetimib was expressed as compared to control. Data are presented as mean ± S.D., P < 0.05 was considered as statistically significant (*) by using a paired t‐test for n = 3 repeats. (B) Cellular redistribution of CD13 (FITC, green) and annexin II (Texas Red, red) in control (left) and Ezetimib‐treated (right) human monocyte‐derived macrophages. Merged, representative confocal images are presented, showing an extensive shift of both proteins, from the plasma membrane to cytoplasmic vesicles, in response to 10 μM Ezetimib. Quantification of co‐localization (yellow) in the plasma membrane (arrows) was 96% in control (treated with DMSO as solvent of Ezetimib) and 11% in Ezetimib‐treated cells, respectively. Scale bars: 10 μm. (C) Shift of CD13 from detergent resistant (i.e. raft) to detergent soluble (i.e. non‐raft) fractions in response to Ezetimib. Sucrose density gradient fractions of Lubrol WX‐lysates were analyzed by immunoblot. Equal amounts of proteins were loaded on each lane. 1–3: detergent resistant membrane (DRM, i.e. raft) fractions, 4–7: detergent soluble (i.e. non‐raft) cellular fractions. In control cell (treated with DMSO as solvent of Ezetimib) lysates CD13 is clearly detectable in DRM and detergent soluble fractions. By contrast, in Ezetimib‐treated cell lysates CD13 is present only in detergent soluble fractions. (D) Decrease of the total cellular content of CD13 in monocyte‐derived macrophages in response to ezetimib. Immunoblot of whole cell lysates from control (left) and Ezetimib‐treated (right) cells. Equal amounts of protein were loaded on both lanes.
Ezetimib Induces a Shift of CD13 From the Plasma Membrane to Intracellular Vesicles and Decreases Cellular CD13 Content
To explore whether the decreased surface expression of CD13 is related to a cellular internalization of CD13, the cellular distribution of CD13 in Ezetimib‐treated cells was investigated by confocal imaging. In control cells, a substantial part of CD13‐fluorescence is detected at the cell surface showing extensive colocalization with the plasma membrane marker annexin II. By contrast, in Ezetimib‐treated cells, the major colocalization of CD13 and annexin II occurs in cytoplasmic vesicles, and thus Ezetimib quite likely induces a redistribution of CD13 or annexin II or both from the plasma membrane to intracellular vesicles (Fig. 1B).
Lysis of cells in 1% Lubrol WX followed by sucrose flotation gradient‐separation confirms the presence of CD13 in detergent resistant membranes (DRMs) (i.e. rafts) in control cells, whereas CD13 is elicited in detergent soluble (i.e. non‐raft) fractions in response to Ezetimib (Fig. 1C).
Parallel to the marked changes in cellular CD13 redistribution, Ezetimib also decreases the total cellular content of CD13 (Fig. 1D).
DISCUSSION
Ezetimib is a lipid‐lowering drug, which inhibits intestinal cholesterol absorption by an unknown cellular mechanism that requires binding of Ezetimib to CD13 in the enterocytic brush border (4). The binding site for Ezetimib on CD13 differs from the aminopeptidase activity of this antigen (4). Since CD13 is constitutively expressed in lipid rafts of monocytes/macrophages, it is tempting to speculate that Ezetimib influences structure and/or function of rafts in these cells. Indeed, the present study provides evidence that Ezetimib modulates the cellular redistribution and expression of CD13 in monocyte‐derived macrophages. Moreover, the expression of further raft‐associated antigens such as the Fcγ receptors CD16 and CD64, and the scavenger receptor CD36, extensively implicated in cellular uptake of modified lipoproteins or phagocytosis, is also down‐regulated in response to Ezetimib. The decreased expression of these receptors upon Ezetimib administration may lead to an impaired macrophage‐differentiation and lipid‐uptake, and thus Ezetimib quite likely exerts an in vitro antiatherogenic effect in monocyte‐derived macrophages, which involves rafts.
CD13 requires “co‐receptor(s)” for subsequent signaling, and CD64, CD16, or CD36 are demonstrated or are putative CD13‐interacting antigens (3). Ezetimib may exert its cellular effect through disruption of the coassembly of CD13 with CD64/CD16 or CD36, and in this scenario, the missing coassociation of receptors may lead to their disappearance from rafts. The finding that CD13 disappears from DRMs/rafts in response to Ezetimib (Fig. 1C) may support this hypothesis. Binding of Ezetimib to CD13 quite likely induces a conformational change in the extracellular domain of CD13, masking the specific sequence required for coassociation of CD13 with other receptors. Such a mechanism has recently been proposed for Ezetimib in enterocytes (4). Alternatively, shedding of CD13 in response to Ezetimib may also explain the loss of its expression.
In summary, Ezetimib likely modifies raft assembly in monocyte‐derived macrophages through a CD13‐dependent dissociation of preclustered receptor complexes.
Part of this work was presented at the 10th Leipziger Workshop on Systems Biology and Clinical Cytomics, April 7–9, 2005, Leipzig, Germany.
LITERATURE CITED
- 1. Olsen J, Kokholm K, Noren O, Sjöstrom H. Structure and expression of aminopeptidase N. Adv Exp Med Biol 1997; 421: 47–57. [DOI] [PubMed] [Google Scholar]
- 2. Navarrete Santos A, Roentsch J, Danielsen EM, Langer J, Riemann D. Aminopeptidase N/CD13 is associated with raft membrane microdomains in monocytes. Biochem Biophys Res Commun 2000; 269: 143–148. [DOI] [PubMed] [Google Scholar]
- 3. Mina‐Osorio P, Ortega E. Aminopeptidase N (CD13) functionally interacts with FcγRs in human monocytes. J Leukoc Biol 2005; 77: 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kramer W, Girbig F, Corsiero D, Pfenninger A, Frick W, Jähne G, Rhein M, Wendler W, Lottspeich F, Hochleitner EO, Orsó E, Schmitz G. Aminopeptidase N (CD13) is a molecular target of the cholesterol absorption inhibitor Ezetimibe in the enterocyte brush border membrane. J Biol Chem 2005; 280: 1306–1320. [DOI] [PubMed] [Google Scholar]
- 5. Pfeiffer A, Boettcher A, Orsó E, Kapinsky M, Nagy P, Bodnár A, Spreitzer I, Liebisch G, Drobnik W, Gempel K, Horn M, Holmer S, Hartung T, Multhoff G, Schütz G, Schindler H, Ulmer AJ, Heine H, Stelter F, Schütt C, Rothe G, Szölld́osi J, Damjanovich S, Schmitz G. Lipopolysaccharide and ceramide docking to CD14 provokes ligand‐specific receptor clustering in rafts. Eur J Immunol 2001; 31: 3153–3164. [DOI] [PubMed] [Google Scholar]
- 6. Drobnik W, Borsukova H, Boettcher A, Pfeiffer A, Liebisch G, Schütz GJ, Schindler H, Schmitz G. Apo AI/ABCA1‐dependent and HDL3‐mediated lipid efflux from compositionally distinct cholesterol‐based microdomains. Traffic 2002; 3: 268–278. [DOI] [PubMed] [Google Scholar]


