Abstract
Objective:
To examine the association between glycated hemoglobin (HbA1c) and severe hypoglycemia rates in patients with type 1 diabetes receiving usual care, by analysing data from the US Type 1 Diabetes Exchange (T1DX), German/Austrian Diabetes Patienten Verlaufsdokumenation (DPV), and Western Australian Children Diabetes Database (WACDD) diabetes registries.
Methods:
Data for patients with type 1 diabetes, aged <18 years with a minimum duration of diabetes of 2 years, were extracted from each registry for a 12-month observation period between 2011 and 2012 (7,102 T1DX, 18,887 DPV, and 865 WACDD). Rates of severe hypoglycemia (self-reported loss of consciousness/convulsion) were estimated per 100 patient-years and analyzed by HbA1c, source registry, treatment regimen, and age group.
Results:
Overall, the severe hypoglycemia rate per 100 patient years was 7.1, 3.3, and 6.7 in T1DX, DPV, and WACDD patients, respectively. Lower HbA1c was not associated with an increased rate of severe hypoglycemia when examined by source registry, treatment regimen, or age group.
Conclusion:
An inverse relationship between mean HbA1c and risk of severe hypoglycemia was not observed in this study of 3, independent cohorts of children and adolescents with type 1 diabetes. Investigation in other large, longitudinal cohorts is recommended to further characterize the contemporary relationship between glycemic control and risk of severe hypoglycemia rates in pediatric patients with type 1 diabetes.
Keywords: adolescent, child, diabetes mellitus, hypoglycemia, registries, type 1
1 |. INTRODUCTION
An ongoing challenge for clinicians and patients with type 1 diabetes is how to achieve optimal glycemic control, without increasing the risk of severe hypoglycemia.1 The landmark Diabetes Control and Complications Trial (DCCT) reported a significantly reduced risk of diabetes-related complications in patients with type 1 diabetes, aged between 13 and 39 years at trial entry, who received intensive (median HbA1c 7%; 54 mmol/mol) compared with conventional (median HbA1c 9%; 75 mmol/mol) diabetes treatment.2 However, the major adverse finding in this clinical trial was a 3-fold increased risk of severe hypoglycemia events in patients who were randomized to the intensive management arm of the study. In keeping with this, the DCCT reported a significantly higher risk of severe hypoglycemic events (when defined as requiring assistance or when defined as seizure or coma) associated with lower glycemic control.3 Consistent with this, a higher rate of severe hypoglycemia was associated with lower glycemic control and in non-trial cohorts in the 1990s, such as the population-based pediatric sample in Western Australia.4 The relationship between lower glycemic control and increased risk of severe hypoglycemia, together with the life-threatening nature of these events, has understandably contributed to a fear of hypoglycemia in patients and their care givers, and been a major barrier in the achievement of optimal glycemic control.5,6
In recent years, studies in large longitudinal cohorts of pediatric patients with type 1 diabetes suggest that the relationship between glycemic control and severe hypoglycemia may have weakened.7–10 For example, in youth aged <20 years in Germany and Austria, the severe hypoglycemia rate decreased significantly between 1995 and 2012 despite simultaneous improvements in glycemic control,8 and in Denmark, the national severe hypoglycemia rate halved in children with type 1 diabetes between 2008 and 2013 and no association was found with prevailing HbA1c.7
Considering advances made in diabetes therapies over the past decades in insulin analogues, insulin delivery systems, glucose-monitoring technologies, and patient education, a change in the relationship between HbA1c and risk of severe hypoglycemia is theoretically plausible, and would have important clinical implications for achieving glycemic targets in pediatric patients with type 1 diabetes. The establishment of well-standardized, longitudinal diabetes registers in many developed countries which span this time period provide an invaluable resource for monitoring changes in diabetes-related exposures and outcomes, and enable comparisons between countries and populations. Therefore, the aim of this study was to investigate the hypothesis that lower HbA1c is no longer associated with an increased risk of severe hypoglycemia in contemporary pediatric patients with type 1 diabetes, by examining the relationship between HbA1c and severe hypoglycemia in 3, independent cohorts of patients with type 1 diabetes.
2 |. METHODS
2.1 |. Study populations
Data were analyzed from the Type 1 Diabetes Exchange clinic registry (T1DX) in the United States, the DPV (Diabetes Patienten Verlaufsdokumenation) database in Germany and Austria and the Western Australian Children’s Diabetes Database (WACDD) in Western Australia. All are longitudinal, prospective diabetes registries that have previously been described in detail.11–13
The T1DX which was established in 2010, is a non-population based, multicentre clinic registry with patient data collected via annual questionnaires and from clinic visits.11 Approximately one-fourth of patients attending the participating centres are enrolled in the T1DX. The DPV, established in 1995, and the WACDD, established in 1987, are both population-based registries with patient data collected at quarterly clinic visits and have estimated case-ascertainment rates of 80% and >99%, respectively.12,13
2.2 |. Patient selection
Data were extracted from each registry, for all patients diagnosed with type 1 diabetes, with data available for a minimum of 12 months between 2011 and 2012. To be included in the analysis, patients had to be aged <18 years and have a minimum duration of diabetes of 2 years at their last clinic visit during the study period.
The data extract consisted of 1 or more records per patient, depending on the number of clinic visits they attended during the study period. Variables available for analysis included age at diagnosis, sex, date of visit, current age, duration of diabetes, HbA1c, total daily insulin dose, insulin treatment regimen, and number of severe hypoglycemia events since last clinic visit/questionnaire.
2.3 |. Primary outcome
The primary outcome of this study was the occurrence of severe hypoglycemia, defined in accordance with International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines, as a hypoglycemic event resulting in loss of consciousness or seizures.14 In the T1DX, data on severe hypoglycemia events were collected via a questionnaire that asked how many times over the past 12 months the participant experienced a severe hypoglycemic event where seizure or loss of consciousness occurred.11 In the DPV and WACDD registries, the number of self-reported severe hypoglycemia events occurring since the last clinic visit was recorded at each visit, with most patients attending approximately every 3 months.12,13 Patients are asked by their clinician how many times since the last clinic visit they had experienced a hypoglycemic event. If any events are reported, further questions relating to whether or not assistance was required to administer glucose or glucagon and whether or not the event resulted in loss of consciousness/coma or a hospital visit are determined in order to classify the event as being either moderate or severe.
2.4 |. Covariates
The main exposure of interest in this study was the mean HbA1c calculated for each patient for the 12-month study observation period. Patients were categorized into 4 groups according to their mean HbA1c (<7.0% [<53.0 mmol/mol], 7.0% to <8.0% [53 to <64 mmol/ mol], 8.0% to <9.0% [64 to <75 mmol/mol], ≥9.0% [≥75 mmol/mol]), with the groups representing clinically relevant categories ranging from very good to poor glycemic control.
To enable adjustment for potential confounders or effect modifiers, data available for duration of diabetes, age and insulin treatment regimen were also included in the analyses. Patients were classified as having a duration of diabetes of 2-≤5 years or >5 years, into age groups according to their current age at the last visit (<6 years, 6 to <13 years, 13 to <18 years) and as being treated with either injections or Continuous Subcutaneous Insulin Infusion (CSII) according to the treatment regimen they were on for the majority of the observation period. The age group categories used in this study reflect preschool, pre-adolescent and adolescent ages and the social/psychosocial factors inherent to these life-stages.
2.5 |. Statistical analyses
For each patient included in the study, the total number of severe hypoglycemia events and total observation time (in years) over which these events were collected for were calculated. For individuals in the T1DX, data for an exact observation period of 12 months was extracted, resulting in each individual having 1 year of observation time. For the DPV and WACDD registries, data were extracted for all available observation time during the study period 2011–2012, during which the patient met the study inclusion criteria. Therefore, patients from these 2 registries contributed variable observation time. To calculate the severe hypoglycemia rate per category analyzed, the total number of severe hypoglycemia events of relevant patients was used as the numerator and their summed total observation time used as the denominator. The resulting rate was then multiplied by 100 to provide the severe hypoglycemia rate per 100 patient years. To determine the relative risk of severe hypoglycemia according to HbA1c, the incidence rate ratio was estimated for each HbA1c category, using the 7.0% to <8.0% (53 to <64 mmol/mol) group as the reference group.
Multivariable analysis was conducted using negative binomial regression modelling to account for over-dispersion of severe hypoglycemia events.15 The base model for severe hypoglycemia included HbA1c category as the only independent variable, providing unadjusted severe hypoglycemia rates for each HbA1c category.
Duration of diabetes, current age, sex, and treatment regimen categories were then added to the model to estimate severe hypoglycemia rates for each HbA1c category, after adjusting for these potential confounding factors. To examine duration of diabetes, age, and treatment regimen for effect modification on the relationship between HbA1c and severe hypoglycemia, cross-product terms between each HbA1c group and each of these variable groups were added in turn to the adjusted model and tested for significant interaction effects.
A two-tailed P-value <.05 was considered statistically significant, with the Bonferroni-Stepdown method for correction of P-values applied for multiple comparisons. All the analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North California).
2.6 |. Ethics
The study was performed according to the principles of the Declaration of Helsinki for medical research, and approved by the ethics committees of each registry centre, as well as the local institutional review boards of participating centres.
3 |. RESULTS
In total, 7,102 patients were included from the T1DX registry, 18,787 from the DPV registry and 865 from the WACDD registry (Table 1). There were no clinically significant differences across the registries in the proportion of boys, mean age at diagnosis, or mean duration of diabetes (Table 1). Notably, 59% of patients in the T1DX registry were treated by CSII, compared with 41% of DPV and 35% of WACDD patients. The mean HbA1c was 8.6% (70 mmol/mol) in T1DX patients compared with 8.0% (64 mmol/mol) in DPV and 8.2% (66 mmol/mol) in WACDD patients (Table 1).
TABLE 1.
Patient characteristics and severe hypoglycemia rate by source registry
| T1DX | DPV | WACDD | |
|---|---|---|---|
| Total cases (N) | 7,102 | 18,787 | 865 |
| % Males | 50 | 52 | 52 |
| Mean age ± SD (years) | 12.7 ± 3.4 | 12.8 ± 3.6 | 13.4 ± 3.4 |
| Mean duration of diabetes ± SD (years) | 5.9 ± 3.3 | 5.8 ± 3.3 | 6.1 ± 3.4 |
| % Treated with CSII | 59 | 41 | 35 |
| Median HbA1c (Q1, Q3) | |||
| % | 8.3 (7.7, 9.2) | 7.7 (7.1, 8.6) | 7.9 (7.4, 8.8) |
| mmol/mol | 67 (60, 77) | 61 (54, 70) | 63 (57, 73) |
| Mean HbA1c ± SD | |||
| % | 8.6 ± 1.4 | 8.0 ± 1.4 | 8.2 ± 1.3 |
| mmol/mol | 70 ± 15 | 64 ± 15 | 66 ± 14 |
| ≥1 Severe hypoglycemia event (%) | 4.9 | 3.0 | 5.8 |
| Total patient years of observation | 7,102 | 21,484 | 978 |
| Severe hypoglycemia rate per 100 patient years (95%CI) | 7.1 (6.3–8.0) | 3.4 (3.1–3.7) | 6.8 (4.8–9.5) |
Abbreviations: DPV, Diabetes Patienten Verlaufsdokumenation; HbA1c, glycated hemoglobin; T1DX, Type 1 Diabetes Exchange; WACDD, Western Australian Children Diabetes Database.
3.1 |. Rate of severe hypoglycemia (loss of consciousness/seizure) by source registry
Overall, there was a severe hypoglycemia rate of 3.3 (95%CI: 3.0–3.6) per 100 patient-years in the DPV cohort, 7.1 (95%CI: 6.3–8.0) per 100 patient-years in the T1DX cohort, and 6.7 (95%CI: 4.8–9.5) per 100 patient-years in the WACDD cohorts (Table 1).
3.2 |. Rate of severe hypoglycemia by source registry and HbA1c category
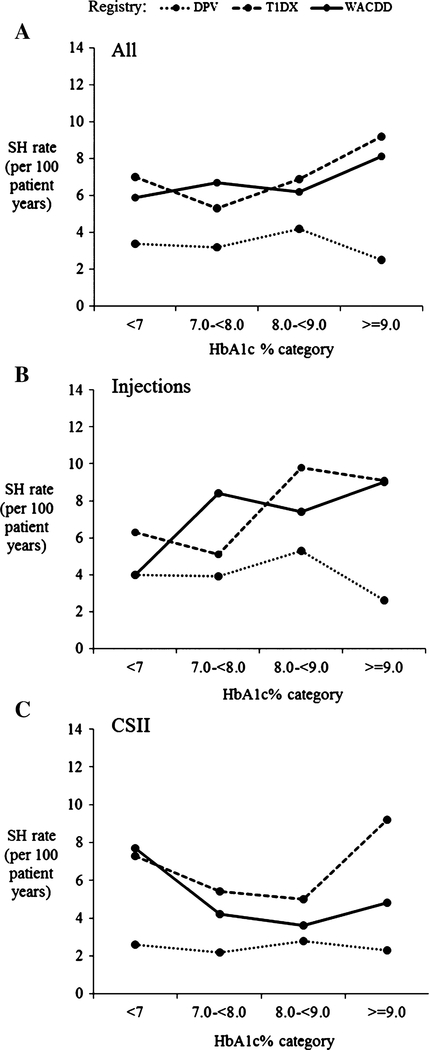
An inverse relationship between the severe hypoglycemia rate and glycemic control as measured by HbA1c, was not observed in any of the registry cohorts (Figure 1A, Table 2). In the T1DX cohort, the severe hypoglycemia rate in patients with a mean HbA1c ≥9.0% (≥75 mmol/mol) was significantly higher than that in patients with a mean HbA1c of 7.0% to <8.0% (53 to <64 mmol/mol) (P = .001, Table 2). In the DPV cohort, a low and relatively stable severe hypoglycemia rate was observed across all HbA1c categories (dotted line in Figure 1A, Table 2). The severe hypoglycemia rates observed in both the T1DX and WACDD cohorts were significantly higher than those observed in the DPV cohort (rate ratio T1DX vs DPV 2.14 [95%CI: 1.86–2.62], P < .001; WACDD vs DPV 2.03 [95%CI: 1.42–2.88], P < .001) but not significantly different from each other (rate ratio WACDD vs T1DX 0.95 [95%CI: 0.65–1.34], NS) (Figure 1A).
FIGURE 1.
A-C, Unadjusted mean severe hypoglycemia (SH) rate per 100 patient years by glycated hemoglobin (HbA1c) category and registry for (A) all patients combined, (B) patients treated with injections, and (C) patients treated with CSII
TABLE 2.
Severe hypoglycemia (SH) rate per 100 patient years (95%CI) adjusted for gender, age group and duration of diabetes and incidence rate ratio (IRR) (95%CI) by registry and HbA1c category
| HbA1c (%) category | <7% | 7% to <8% | 8% to <9% | ≥9% | |
|---|---|---|---|---|---|
| DPV | No. cases | 4,335 | 6,734 | 4,477 | 3,241 |
| No. SH events | 167 (113, 54) | 242 | 215 | 94 | |
| SH rate | 3.3 (2.7–4.0) | 3.2 (2.7–3.7) | 4.2 (3.5–5.0) | 2.5 (1.9–3.1) | |
| IRR | 1.05 (0.82–1.34) | 1.00 | 1.32 (1.05–1.67)* | 0.78 (0.58–1.04) | |
| T1DX | No. cases | 474 | 2,126 | 2,363 | 162 |
| No. SH events | 33 | 113 | 162 | 195 | |
| SH rate | 6.6 (4.2–10.6) | 5.1 (4.0–6.5) | 6.9 (5.6–8.5) | 8.7 (7.1–10.7) | |
| IRR | 1.30 (0.77–2.18) | 1.00 | 1.34 (0.98–1.84) | 1.70 (1.24–2.32)† | |
| WACDD | No. cases | 90 | 357 | 231 | 187 |
| No. SH events | 6 | 27 | 16 | 17 | |
| SH rate | 4.7 (1.7–12.8) | 5.4 (3.3–8.9) | 5.8 (3.2–10.7) | 9.0 (4.8–16.8) | |
| IRR | 0.87 (0.29–2.60) | 1.00 | 1.08 (0.50–2.34) | 1.67 (0.74–3.78) | |
Abbreviations: DPV, Diabetes Patienten Verlaufsdokumenation; HbA1c, glycated hemoglobin; T1DX, Type 1 Diabetes Exchange; WACDD, Western Australian Children Diabetes Database.
P = .02,
P = .001.
3.3 |. Rate of severe hypoglycemia by registry, HbA1c category, and treatment regimen
As treatment regimen was found to have significant effect modification (P < .001) in the multivariable analyses for all 3 registries, the relationship between HbA1c category and severe hypoglycemia rates for each registry cohort was analyzed stratified by treatment regimen, and results are presented for all patients combined (Figure 1A, Table 2) as well as patients treated by injections (Figure 1B, Table 3) and CSII separately (Figure 1C, Table 3).
TABLE 3.
Severe hypoglycemia (SH) rate per 100 patient years (95%CI) adjusted for gender, age group and duration of diabetes and incidence rate ratio (IRR) (95%CI) by treatment regimen, registry, and HbA1c category
| PATIENTS TREATED BY CSII | |||||
|---|---|---|---|---|---|
| HbA1c (%) category | <7% | 7% to <8% | 8% to <9% | ≥9% | |
| DPV | No. cases | 2,512 | 3,676 | 2,647 | 2,197 |
| SH rate | 2.6 (1.9–3.6) | 2.3 (1.8–3.0) | 2.8 (2.1–3.8) | 2.3 (1.5–3.6) | |
| IRR | 1.15 (0.77–1.73) | 1.00 | 1.22 (0.82–1.83) | 1.01 (0.61–1.68) | |
| T1DX | No. cases | 143 | 624 | 897 | 1,214 |
| SH rate | 6.8 (4.0–11.8) | 5.2 (3.9–6.9) | 4.9 (3.2–7.7) | 8.4 (6.1–11.4) | |
| IRR | 1.31 (0.71–2.42) | 1.00 | 0.95 (0.64–1.42) | 1.61 (1.06–2.44)* | |
| WACDD | No. cases | 45 | 211 | 157 | 149 |
| SH rate | 6.9 (2.0–2.4) | 3.5 (1.4–8.4) | 3.6 (1.0–13.0) | 5.6 (0.1–28.3) | |
| IRR | 1.99 (0.43–9.13) | 1.00 | 1.03 (0.22–4.88) | 1.61 (0.25–10.24) | |
| PATIENTS TREATED BY INJECTIONS | |||||
| HbA1c (%) category | <7% | 7% to <8% | 8% to <9% | ≥9% | |
| DPV | No. cases | 1,823 | 3,058 | 1,830 | 1,044 |
| SH rate | 3.8 (3.0–4.9) | 3.9 (3.2–4.7) | 5.1 (4.1–6.3) | 2.5 (1.9–3.4) | |
| IRR | 0.99 (0.73–1.38) | 1.00 | 1.32 (0.99–1.76) | 0.66 (0.46–0.93)† | |
| T1DX | No. cases | 331 | 1,502 | 1,466 | 925 |
| SH rate | 6.1 (2.6–14.4) | 4.9 (3.9–6.9) | 10.1 (7.4–13.7) | 8.9 (6.8–11.6) | |
| IRR | 1.23 (0.47–3.25) | 1.00 | 2.05 (1.20–3.51) ‡ | 1.80 (1.08–3.02)* | |
| WACDD | No. cases | 45 | 146 | 74 | 38 |
| SH rate | 2.8 (0.6–14.3) | 0.7 (3.7–12.1) | 6.8 (3.4–13.6) | 1.0 (5.0–19.3) | |
| IRR | 0.42 (0.08–2.30) | 1.00 | 1.02 (0.42–2.50) | 1.47 (0.59–3.68) | |
Abbreviations: DPV, Diabetes Patienten Verlaufsdokumenation; HbA1c, glycated hemoglobin; T1DX, Type 1 Diabetes Exchange; WACDD, Western Australian Children Diabetes Database.
P = .03,
P = .02,
P = .009.
In the DPV cohort, a low severe hypoglycemia rate was observed across all HbA1c categories, with no inverse relationship observed in patients combined (dotted line in Figure 1A, Table 2), patients treated by injections or patients treated with CSII (dotted line in Figure 1B,C, Table 3).
For patients treated with injections in the T1DX and WACDD cohorts, the severe hypoglycemia rate increased, rather than decreased, with higher HbA1c categories (solid and dashed lines in Figure 1B, Table 3). The relationship between HbA1c and severe hypoglycemia rate was less clear for patients treated by CSII in the WACDD cohort (dashed line in Figure 1C, Table 3), probably due to the smaller number of patients in this cohort. These observations remained unchanged after adjusting for age, sex, and duration of diabetes (Table 3).
With all registry cohorts combined, the severe hypoglycemia was significantly higher in patients treated with injections compared with those on CSII (rate ratio inj. vs CSII: 1.34 [95%CI: 1.16–1.56], P < .001). When analyzed separately, a significantly higher severe hypoglycemia rate was observed for patients treated with injections compared with CSII in the DPV (rate ratio Inj. vs CSII: 1.56 [95%CI: 1.28–1.90] P < .001) and T1DX (rate ratio inj. vs CSII: 1.38 [95%CI :1.09–1.76] P < .001) cohorts. A statistically significant difference was not found for the WACDD cohort, most likely due to the smaller number of cases in this cohort and reduced statistical power (rate ratio Inj. vs CSII: 1.67 [95%CI: 0.84–3.31] NS).
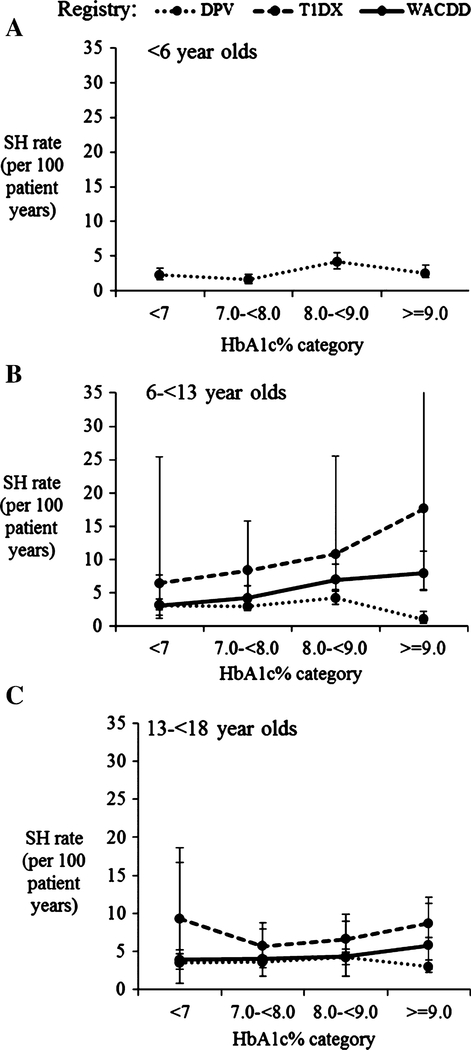
3.4 |. Rate of severe hypoglycemia by HbA1c category and age group
Due to inadequate cases numbers in the youngest age group for the WACDD and T1DX cohorts, the relationship between severe hypoglycemia rates and HbA1c in children aged <6 years was restricted to patients in the DPV registry, in whom no association between HbA1c and severe hypoglycemia rates was observed (Figure 2A). The mean severe hypoglycemia rate was stable across all HbA1c categories for all 3 age groups in the DPV registry (Figure 2A-C).
FIGURE 2.
A-C, Severe hypoglycemia rate (SH) adjusted for sex and diabetes duration by registry and glycated hemoglobin (HbA1c) category for patients aged <6 years (A); 6 to <13 years (B) and 13 to <18 years (C). Error bars represent upper and lower 95% confidence interval of estimated marginal mean SH rate. A, Includes patients from the Diabetes Patienten Verlaufsdokumenation (DPV) registry only due to small case numbers in this subgroup in the Type 1 Diabetes Exchange (T1DX), and Western Australian Children Diabetes Database (WACDD) registries
In 6 to <13 year olds, a higher mean severe hypoglycemia rate was estimated in those with an HbA1c ≥9.0% (≥75 mmol/mol) for patients in the T1DX and WACDD registries; however, the estimates had wide 95% CIs reflecting a low number of cases in this age group (Figure 2B). In the oldest age group of 13 to <18 years, no inverse association was observed between the mean severe hypoglycemia rate and HbA1c categories in all 3 registry cohorts (Figure 2C).
4 |. DISCUSSION
This international, collaborative study reports the lack of an inverse relationship between rates of severe hypoglycemia and glycemic control as measured by HbA1c, in 3, independent contemporary cohorts of youth with type 1 diabetes. Importantly, the lack of inverse relationship between severe hypoglycemia and HbA1c reported in this study, was observed in patients treated with both CSII and injection therapy.
The benefits of near normal glycemic control in partially preventing or delaying microvascular complications of diabetes and reducing the morbidity and mortality in patients with type 1 diabetes are well established.16,17 Similarly, the real risks of morbidity and mortality associated with hypoglycemia are also established,6 and it is known that hypoglycemia can be fatal.18 In young children with type 1 diabetes, the challenge of optimizing glycemic control whilst minimizing the risk of severe hypoglycemia is even greater because of their erratic and often unpredictable eating and physical activity levels and the potential increased risk of long-term neurocognitive deficits due to the effect of hypoglycemic episodes on the developing brain.19,20 Reassuringly, a recent study has shown that optimal glycemic control is achievable without increasing the risk of severe hypoglycemia even in young children with type 1 diabetes.9
A change in the association between higher HbA1c and rates of severe hypoglycemia during the past decades has been reported in several populations.7,8,10,21 For example, a significant reduction in severe hypoglycemia rates was observed in Germany and Austria between 1995 and 2012, despite the maintenance of or improvement in HbA1c over the same period8 and in Western Australia a halving of severe hypoglycemia rates was observed between 1999 and 2011 despite improvements in overall HbA1c over the same time period.22
The strengths of this study are its use of data from multiple, independent, prospective diabetes registries. The data are either population-based or in the case of the T1DX, considered to be generally representative of the total pediatric population attending the participating diabetes centres.11 In contrast to the relationships observed between HbA1c and severe hypoglycaemia rates in the DCCT, which were based on patients in a well-controlled clinical trial setting, the relationships reported in this study are based on registry data from patients receiving usual care in a variety of clinical settings, and reflect contemporary advances in clinical practice and application of diabetes therapies in multiple, international pediatric populations. For instance, patients with an HbA1c <7% in the cohorts examined in this study probably represent a self-selected group of patients achieving this high level of glycemic control whilst receiving their usual clinical care, in contrast to patients in the intensively managed arm of the DCCT who achieved lower HbA1c levels with additional, intensive oversight provided by health care providers. Availability of data on sex, age, duration of diabetes, and treatment regimen enabled adjustment of these known potential confounders on the rate of severe hypoglycemia in this study.23 As individual level indicators of socioeconomic status were not available for individuals from all registries included in this study, any influence of this measure could not be analyzed.
Limitations of the study include the use of HbA1c as a measure of glycemic control which reflects the average glycemic control over the previous 4 to 12 weeks, without any information on glycemic fluctuations or variability over that period. However, HbA1c is still recommended as the most useful measure of glycemic control in patients with diabetes, and remains the only measure for which good evidence is available in terms of later risk of complications of diabetes.17 Another limitation of this study is that severe hypoglycemia event data were retrospectively collected either at quarterly clinic visits in the DPV and WACCD, or via an annual questionnaire in the T1DX, resulting in the potential for recall bias and possible under or over-reporting. However, this is anticipated to be minimal as, due to their severity, it has been shown that recall of severe hypoglycemia events is relatively accurate 1 year after they occur.24 Although the T1DX is not population-based and may have selection bias with patients more likely to be of higher socioeconomic status and treated at academic treatment centres, this is unlikely to have biased the association of HbA1c to severe hypoglycemia rates, but may impact the generalizability of the findings to the whole type 1 diabetes population in the United States.
In interpreting the study findings, it is important to note that this study was unable to account for the temporary relationship between severe hypoglycemic events and concomitant changes in HbA1c. Therefore, possible explanations for the lack of inverse association observed between HbA1c and severe hypoglycemia rates resulting from variation in HbA1c due to changes in clinical targets, or lack of adherence to medical management cannot be excluded. For instance, it is possible that in patients who experienced a severe hypoglycemic event, the severity of the event may have resulted in a post-event increase in HbA1c due to increased fear of hypoglycemia and either patient or clinician-led loosening of glycemic control. Conversely, a tightening of glycemic control could precede the occurrence of a severe hypoglycemic event in patients with a high HbA1c resulting in an apparent increased risk of severe hypoglycemia being observed in patients with a higher mean HbA1c.
Keeping these limitations in mind, it is possible that advances in diabetes therapies and technologies over the past decades could be enabling better glycemic control without a corresponding increase in the risk of severe hypoglycemia. Such advances could include the introduction, and increased use, of insulin analogues, CSII therapy, increased frequency of SMBG, continuous glucose monitoring (CGM), use of carbohydrate counting and calculators for insulin dose adjustments and improvements in patient education and management of hypoglycemia.8,25,26 Irrespective of such advances in modern diabetes therapies and technologies, the application of clinical recommendations and patient education remain critical in minimizing the risk of hypoglycemia in patients with type 1 diabetes.20,27 This includes effective patient/caregiver education regarding hypoglycemia awareness and management, strategies for appropriate dietary intake and exercise-related insulin adjustments and the importance of self-blood glucose monitoring.20 Given the real risks associated with severe hypoglycemia, glycemic targets need to be individualized, taking into account the patient’s age, comorbidities, lifestyle, personal/caregiver preferences, and the potential impact of hypoglycemia.1,28
In conclusion, this study reports that an inverse relationship between HbA1c and severe hypoglycemia was not observed in 3 contemporary cohorts of youth with type 1 diabetes. Investigation in other large, longitudinal cohorts is recommended to further characterize the contemporary relationship between glycemic control and risk of severe hypoglycemia rates in pediatric type 1 diabetes.
ACKNOWLEDGMENTS
The Type 1 Diabetes Exchange Clinic Registry is supported by the Leona M. and Harry B. Helmsley Charitable Trust and the DPV registry is supported by German Center for Diabetes Research (DZD), German Diabetes Association (DDG) and European Foundation for the Study of Diabetes (EFSD).
Funding information
No specific funding was obtained for this study
Abbreviations:
- DCCT
Diabetes Control and Complications Trial
- DPV
German/Austrian Diabetes Patienten Verlaufsdokumenation database
- HbA1c
glycated hemoglobin
- T1DX
Type 1 diabetes Clinical Exchange Registry
- WACDD
Western Australian Children Diabetes Database
Footnotes
Conflict of interest
A.H., D.M.M, J.M.H., K.M.M., E.A.D., S.E.H., T.W.J., R.W.B., R.W.H. have no conflicts of interest related to this publication to declare. D.M.M. is on the advisory board for Insulet, consults for Abbott, and his institution has received research funding from Medtronic, Dexcom, and Roche. T.W.J. has received honoraria for speaking and advisory board membership from Eli-Lilly, Novo-Nordisk, Sanofi, and Medtronic. R.W.B. institution receives consulting and/or research funding from Dexcom, Bigfoot, Tandem, and Animas.
REFERENCES
- 1.Cryer PE. Glycaemic goals in diabetes: trade-off between glycaemic control and iatrogenic hypoglycemia. Diabetes. 2014;63:2188–2195. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the diabetes control and complications trial. Diabetes. 1997;46:271–286. [PubMed] [Google Scholar]
- 4.Davis E, Keating B, Byrne G, Russell M, Jones T. Hypoglycemia: incidence and clinical predictors in a large population-based sample of children and adolescents with IDDM. Diabetes Care. 1997;20:22–25. [DOI] [PubMed] [Google Scholar]
- 5.Johnson SR, Cooper MN, Davis EA, Jones TW. Hypoglycemia, fear of hypoglycemia and quality of life in children with type 1 diabetes and their parents. Diabet Med. 2013;30:1126–1131. [DOI] [PubMed] [Google Scholar]
- 6.Frier BM. Hypoglycemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10:711–722. [DOI] [PubMed] [Google Scholar]
- 7.Fredheim S, Johansen A, Thorsen SU, et al. Nationwide reduction in the frequency of severe hypoglycemia by half. Acta Diabetol. 2015;52:591–599. [DOI] [PubMed] [Google Scholar]
- 8.Karges B, Rosenbauer J, Kapellen T, et al. Hemoglobin A1c levels and risk of severe hypoglycemia in children and young adults with type 1 diabetes from Germany and Austria: a trend analysis in a cohort of 37,539 patients between 1995 and 2012. PLoS Med. 2014;11: e1001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maahs D, Hermann J, DuBose S, et al. Contrasting the clinical care and outcomes of 2,622 children with type 1 diabetes less than 6 years of age in the United States T1D Exchange and German/Austrian DPV registries. Diabetologia. 2014;57:1578–1585. [DOI] [PubMed] [Google Scholar]
- 10.Cooper M, O’Connell S, Davis E, Jones T. A population-based study of risk factors for severe hypoglycemia in a contemporary cohort of childhood-onset type 1 diabetes. Diabetologia. 2013;56:2164–2170. [DOI] [PubMed] [Google Scholar]
- 11.Beck RW, Tamborlane WV, Bergenstal RM, et al. The T1D exchange clinic registry. J Clin Endocrinol Metab. 2012;97:4383–4389. [DOI] [PubMed] [Google Scholar]
- 12.Gerstl EM, Rabl W, Rosenbauer J, et al. Metabolic control as reflected by HbA1c in children, adolescents and young adults with type-1 diabetes mellitus: combined longitudinal analysis including 27,035 patients from 207 centers in Germany and Austria during the last decade. Eur J Pediatr. 2008;167:447–453. [DOI] [PubMed] [Google Scholar]
- 13.Haynes A, Bower C, Bulsara MK, Jones TW, Davis EA. Continued increase in the incidence of childhood type 1 diabetes in a population-based Australian sample (1985–2002). Diabetologia. 2004;47:866–870. [DOI] [PubMed] [Google Scholar]
- 14.Ly TT, Maahs DM, Rewers A, Dunger D, Oduwole A, Jones TW. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2014;15:180–192. [DOI] [PubMed] [Google Scholar]
- 15.Bulsara MK, Holman CDJ, Davis EA, Jones TW. Evaluating risk factors associated with severe hypoglycemia in epidemiology studies—what method should we use? Diabet Med. 2004;21:914–919. [DOI] [PubMed] [Google Scholar]
- 16.Nathan DM, for the DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rewers MJ, Pillay K, de Beaufort C, et al. Assessment and monitoring of glycaemic control in children and adolescents with diabetes. Pediatr Diabetes. 2014;15:102–114. [DOI] [PubMed] [Google Scholar]
- 18.Cryer PE. Death during intensive glycaemic therapy of diabetes: mechanisms and implications. Am J Med. 2011;124:993–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cato MA, Mauras N, Ambrosino J, et al. Cognitive functioning in young children with type 1 diabetes. J Int Neuropsychol Sci. 2014;20:238–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perantie DC, Koller JM, Weaver PM, et al. Prospectively determined impact of type 1 diabetes on brain volume during development. Diabetes. 2011;60:3006–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbauer J, Dost A, Karges B, et al. Improved metabolic control in children and adolescents with type 1 diabetes: a trend analysis using prospective multicenter data from Germany and Austria. Diabetes Care. 2012;35:80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson S, Cooper M, Jones T, Davis E. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case–control study. Diabetologia. 2013;56:23922400. [DOI] [PubMed] [Google Scholar]
- 23.Cariou B, Fontaine P, Eschwege E, et al. Frequency and predictors of confirmed hypoglycemia in type 1 and insulin-treated type 2 diabetes mellitus patients in a real-life setting: Results from the DIALOG study. Diabetes Metab. 2015;41:116–125. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen-Bjergaard U, Pramming S, Thorsteinsson B. Recall of severe hypoglycemia and self-estimated state of awareness in type 1 diabetes. Diabetes Metab Res Rev. 2003;19:232–240. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen-Bjergaard U, Kristensen P, Beck-Nielsen H, et al. Effect of insulin analogues on risk of severe hypoglycemia in patients with type 1 diabetes prone to recurrent severe hypoglycemia (HypoAna trial): a prospective, randomized, open-label, blinded-endpoint crossover trial. Lancet Diabetes Endocrinol. 2014;2:553–561. [DOI] [PubMed] [Google Scholar]
- 26.Blackman SM, Raghinaru D, Adi S, et al. Insulin pump use in young children in the T1D Exchange clinic registry is associated with lower hemoglobin A1c levels than injection therapy. Pediatr Diabetes. 2014;15:564–572. [DOI] [PubMed] [Google Scholar]
- 27.Blasetti A, Di Giulio C, Tocco AM, et al. Variables associated with severe hypoglycemia in children and adolescents with type 1 diabetes: a population-based study. Pediatr Diabetes. 2011;12:4–10. [DOI] [PubMed] [Google Scholar]
- 28.Fullerton B, Jeitler K, Seitz M, Horvath K, Berghold A, Siebenhofer A. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2014;2:CD009122. doi: 10.1002/14651858.CD009122.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]