Abstract
We examined the interaction between shared illness appraisal and self-efficacy among couples in which one partner was diagnosed with type 1 diabetes (n = 199). We hypothesized that the relation between self-efficacy and health would be weakened under conditions of shared rather than individual appraisal. Multiple regression analyses demonstrated that partner shared illness appraisal interacted with the self-efficacy of the person with type 1 diabetes to predict overall psychological distress and daily diabetes stressors in the predicted direction. Plots of the interactions suggest that partner appraisal of diabetes as shared buffers individuals with lower levels of self-efficacy from poorer health.
Keywords: adults, diabetes, distress, self-efficacy, social network
Individuals with type 1 diabetes (T1D) must engage in a complex self-care regimen including checking blood glucose multiple times throughout the day, measuring and adjusting insulin, and counting carbohydrates in order to maintain optimal health. An important factor that may affect how one manages daily self-care tasks and one’s overall psychological health involves an individual’s self-efficacy, that is, confidence in one’s ability to perform or engage in the behaviors necessary for successful management of diabetes (Bandura and Adams, 1977). Diabetes-related self-efficacy is associated with reports of better self-care and lower diabetes distress among individuals with both type 1 and type 2 diabetes who vary in race and ethnicity (King et al., 2010; Nouwen et al., 2009; Sarkar et al., 2006; Van Der Ven et al., 2003).
Individual self-efficacy has been widely studied in the context of chronic illness, but researchers have less often acknowledged that self-efficacy is embedded within a social context. A growing literature has shown that an individual’s chronic illness is shared frequently with other family members (Berg and Upchurch, 2007; Helgeson et al., 2018), which suggests that close relationships may influence an individual’s illness-specific self-efficacy. For example, interpersonal coping strategies, such as common dyadic coping, have been linked to higher self-efficacy in the context of type 2 diabetes (Johnson et al., 2013). In addition, collective efficacy, that is, a couple’s shared perception of efficacy to successfully engage in diabetes-relevant behaviors (Bandura, 2000), has been linked to more exercise among couples in which one partner had type 2 diabetes (Beverly and Wray, 2010). Thus, it is not surprising that romantic partners have been proposed as targets of interventions to increase an individual’s self-efficacy in the context of chronic illness (Wooldridge and Ranby, 2018). When another person’s resources are accessible to the individual, persons with diabetes may feel even more capable of engaging in appropriate self-care behaviors, which could reduce overall psychological distress, as well as diabetes-related distress, and improve diabetes outcomes.
One way to examine partner involvement in diabetes is by measuring whether the illness is considered to be an individual issue or a shared issue. A shared illness appraisal refers to an individual’s perception that a stressor is “our problem” as opposed to “your” or “my problem” (Helgeson et al., 2018; Lyons et al., 1998). People who hold shared illness appraisals report less psychological distress and more positive adjustment to chronic illness. A few studies have directly measured illness appraisals and shown links of an individual’s shared illness appraisal to good adjustment outcomes (e.g. Zajdel et al., 2018), but the majority of research in this area has inferred a shared illness appraisal from one person’s use of first-person pronoun usage, or “we-talk.”
Studies have provided evidence that first-person plural pronoun usage is related to good outcomes for individuals with a chronic illness, but these benefits are more likely to occur when it is the partner rather than the individual with the illness who uses this language. For example, in a study of couples in which one person had heart failure, partner we-talk, but not the person with the illness’ we-talk, was linked to increased illness self-efficacy and predicted positive changes in heart failure symptoms and general health over the next 6 months (Rohrbaugh et al., 2008). In addition, among women with breast cancer and their husbands, partner we-talk, but not we-talk of the women with breast cancer, was related to better dyadic adjustment and lower depressive symptoms among women (Robbins et al., 2013). Finally, among couples in which one person smoked, initial levels of partner we-talk predicted abstinence 12 months later, and increases in both the we-talk of the individual who smoked and the partner over an intervention period predicted cessation outcomes (Rohrbaugh et al., 2012). One reason that partner we-talk, which reflects a shared illness appraisal, might be particularly beneficial for the person with the illness is that it is partners’ perceptions of their involvement (as opposed to the perceptions of the person with the illness) that determines whether a partner is available and becomes involved in illness management.
In this article, we integrate research on illness appraisals with individual self-efficacy by examining how these two factors interact to influence the health outcomes of persons with T1D. Consistent with previous research, we hypothesize that self-efficacy will be related to positive adjustment outcomes, but we also hypothesize that this relation will be moderated by illness appraisals. Specifically, we hypothesize that self-efficacy will not be as strongly linked to adjustment outcomes when one person in the couple perceives the illness to be a shared rather than an individual issue. That is, individual self-efficacy may not be as predictive of health and well-being outcomes when partners are involved in illness management since persons with T1D’s efficacy may be combined with the partners’ efficacy. A shared illness appraisal signifies that involvement. Thus, shared illness appraisal may act as a buffer against the deleterious effects of low self-efficacy. Individuals with low self-efficacy in particular might benefit from shared illness appraisals because they do not feel sufficiently able to cope with the stressor at hand. That is, a shared illness appraisal may reflect partner involvement that may help individuals with low self-efficacy compensate for not feeling sufficiently able to cope with the stressor at hand. In addition, we hypothesize that the interaction between self-efficacy and illness appraisal will be more likely to occur in the context of the illness appraisals of partners rather than the persons with diabetes. This prediction is based on the “we-talk” literature, which shows stronger effects of partner language rather than the language of the person with the chronic illness.
In sum, the goal of this study is to determine whether partner illness appraisal interacts with individual self-efficacy to predict health outcomes, such that the link of self-efficacy to health is weakened under conditions of shared compared to individual illness appraisal. The health outcomes we examine in this article are indicators of psychological distress (e.g. depressive symptoms), diabetes distress, self-care behavior, and glycemic control. For diabetes distress, we distinguish between a generalized measure of distress that stems from the burden of managing the disease and its accompanying emotions and a measure of the daily stressors that are associated with diabetes (e.g. forgetting to check one’s blood sugar). Diabetes distress is distinct from other negative well-being outcomes (i.e. depressive symptoms), as it is perceptions of distress specifically associated with the difficulties of having and managing diabetes (Polonsky et al., 2005). We assess both diabetes distress and daily diabetes stressors because even for individuals who experience low levels of general psychological distress, there may be days in which they experience an accumulation of diabetes-related stressful events (Berg et al., 2013).
Method
Procedure
Participants were recruited from university-affiliated endocrinology clinics in Pittsburgh, Pennsylvania and Salt Lake City, Utah. Study procedures were approved by the Universities’ Institutional Review Boards. Individuals were eligible to participate if they were 25 years of age or older, had a diagnosis of T1D for at least one year, were taking insulin for T1D within 1 year of diagnosis, and were married or in a cohabitating relationship for at least 1 year.
At the Pittsburgh site, persons with T1D were approached in the clinic by their diabetes care provider who obtained permission to release their name to the project director. If persons with T1D agreed, the project director called to explain the study in detail. After persons with T1D agreed to participate, they provided contact information for their romantic partners. If partners agreed to participate, couples were enrolled in the study. Of the 206 persons approached in the clinic, 4 declined to have their contact information forwarded to the project director. Of the 202 contacted by the project director, 47 were ineligible (including 2 who were found to be ineligible after they had started study procedures), 57 declined participation, and 6 could not be reached to determine eligibility. Thus, 92 couples were scheduled and included in the study.
At the Salt Lake City site, a trained research assistant approached individuals in the clinic and provided information about the study. If persons with T1D agreed to participate, contact information was obtained for their partners, and partners were contacted by a research assistant about the study. Of the 319 persons with T1D approached and screened for eligibility, 66 were deemed ineligible and 118 declined participation. Of the remaining 135 couples, 107 were scheduled and included in the study.
The final sample included 199 couples across both sites. More detail is provided on recruitment elsewhere (Tracy et al., 2018). Just over half of persons with T1D were female, and the majority were non-Hispanic White. Demographic information for persons with T1D and partners is shown in Table 1. Once couples were recruited for the study, they were emailed online surveys (that included consent) to complete at home prior to the in-lab visit. During the laboratory visit, couple members provided written consent for all study procedures and were then placed in separate rooms to complete an additional online questionnaire and a brief interview. Glycemic control was obtained from persons with T1D. Couples were compensated individually for study participation.
Table 1.
Demographics of sample.
| Persons with T1D | Partners | |
|---|---|---|
| Gender | 52% female | 47% female |
| Age | 46.81 | 46.40 |
| Ethnicity | 6% Hispanic or Latino | 3% Hispanic or Latino |
| Race | 92.5% White | 94% White |
| College educated | 72% | 67% |
| Income | 40% > US$100,000 | – |
| Marriage status | 92% Married | – |
| Marriage length | 19.39 years | – |
| Number of children | 2.21 | – |
| Age at diagnosis | I9.54 years | – |
| Length of diabetes | 26.97 years | – |
T1D: type 1 diabetes.
Measures
All measures were completed by the person with T1D with the exception of illness appraisals, which were completed by both the person with diabetes and the partner.
Illness appraisal.
Persons with T1D and partners completed a brief interview on coping separately. At the end of the interview, each was asked: “When you think about diabetes, choose one of the following phrases that best describes how you think about it.” Partners chose from the following response options: “It is my partner’s issue to deal with”; “It is my partner’s issue but I know it affects me”; “It is a shared issue”; or “It is my issue to deal with.” As no partners responded with “It is my issue to deal with,” responses were categorized as a shared appraisal (e.g. “it is a shared issue”) or an individual appraisal (e.g. “it is my partner’s issue” or “it is my partner’s issue but it affects me”). Persons with T1D chose from the following response options: “It is my issue to deal with”; “It is my issue, but I know it affects my partner”; “It is a shared issue”; or “It is my partner’s issue to deal with.” Responses were again categorized as a shared appraisal (e.g. “it is a shared issue”) or an individual appraisal (e.g. “it is my issue” or “it is my issue but I know it affects my partner”).
Self-efficacy.
The self-efficacy subscale of the Multidimensional Diabetes Questionnaire (Talbot et al., 1997) was used to assess diabetes-specific self-efficacy. This six-item measure asked participants to indicate on a scale from 0 to 100 how confident they are in managing various aspects of diabetes (e.g. “How confident are you in your ability to follow your diet?” “How confident are you in your ability to test your blood glucose regularly?”; α = .83).
Psychological distress.
Participants were asked a series of questions that assess psychological distress: depressive symptoms, life satisfaction, and perceived stress. Depressive symptoms were assessed through the Center for Epidemio-logic Studies Depression Scale (CES-D; Rad-loff, 1977). The CES-D assesses depressive symptoms with 20 items, each of which is rated on a 0 (rarely or none of the time) to 3 (all of the time, 5–7 days) scale. It had good reliability in this sample, α = .90. Life satisfaction was assessed via the Satisfaction with Life Scale (Diener et al., 1985) which consists of five items, each of which measure how much participants agree or disagree on a 7-point scale from 1 (strongly disagree) to 7 (strongly agree). It had good reliability in this sample, α = .87. Finally, perceived stress was assessed via a short version of the Perceived Stress Scale (Cohen et al., 1983), which measures the degree to which situations in one’s life over the past month are appraised as stressful. Four items designed to detect how unpredictable, uncontrollable, and overloaded respondents find their lives were rated on a scale from 0 (never) to 4 (very often) (α = .80).
Depressive symptoms, life satisfaction, and perceived stress were strongly correlated; rs ranged from .53 to .70. Thus, we reverse coded life satisfaction, standardized the three scores, and took the average to form a composite distress index.
Diabetes-specific distress.
Persons with T1D completed the 17-item Diabetes Distress Scale (DDS; Polonsky et al., 2005) to assess distress associated in a number of domains. They were asked to “indicate the degree to which each item may be bothering you in your life, NOT merely whether the item is true for you” from 1 (not a problem) to 6 (a very serious problem). Items assess distress in four distinct domains: emotional burden (e.g. “Feeling that diabetes is taking up too much of my mental and physical energy every day”; α = .90), regimen distress (e.g. “Feeling that I am not testing my blood sugars frequently enough”; α = .85), interpersonal distress (e.g. “Feeling that friends or family are not supportive enough of my self-care efforts”; α = .84), and physician distress (e.g. “Feeling that my doctor doesn’t know enough about diabetes and diabetes care”; α = .79). The diabetes distress score is the average of these four subscales.
Daily diabetes stressors.
Persons with T1D completed a brief checklist (1 = yes, 0 = no) to indicate the occurrence of six common diabetes-specific stressors that individuals may encounter on a daily basis over the course of 2 weeks. The six stressors were: problems with high blood sugar, problems with low blood sugar, taking the wrong amount of insulin, problems with food management, feeling bad (upset, angry, and sad) because of diabetes, and forgetting or skipping a blood glucose check. This measure has been used previously in emerging adult and adult samples with T1D (Berg et al., 2013; Tracy et al., 2018). Daily counts of stressors (0–6) were averaged across 2 weeks to understand the unique role of experiencing more or less diabetes-related stressors in addition to the experience of overall diabetes distress. Persons with T1D reported .25 diabetes-specific stressors on average each day (standard deviation (SD) = .22).
Self-care behavior.
Persons with T1D completed the revised Self-Care Inventory (Lewin et al., 2009). Participants rated how often they completed each recommended behavior (e.g. glucose checking and administering correct insulin dose) in the past month from 1 (did not do) to 5 (always did without fail). We removed one item from the original measure (“ketone testing”) because of healthcare provider recommendations. The scale had acceptable reliability in this sample (α = .76).
Glycemic control.
Individuals provided a capillary blood sample during the in-lab visit. Glycemic control was then measured, via hemoglobin A1c (HbA1c) using the DCA Vantage analyzer.
Overview of analyses
Before proceeding with analyses, we examined the distribution of all variables. We noted that individual self-efficacy was non-normally distributed: M = 79.28, SD = 15.26; skew-ness = −1.87 (standard error (SE) = .05); kurtosis = 5.65 (SE = .09). The negative skew indicated that there were only a few persons with T1D who reported lower levels of self-efficacy. To improve the normality of the distribution, we transformed self-efficacy by first reflecting the self-efficacy score and then taking the square root. This resulted in a variable with a more normal distribution (skew-ness = .45; SE = .05 and kurtosis = .95; SE = .09). In order to increase interpretability of the results, we re-reflected the transformed variable so that higher numbers represent higher self-efficacy.
Because the distribution of self-efficacy was skewed, we took the opportunity to investigate the characteristics of the small group of individuals who reported low levels of self-efficacy. The average self-efficacy for the sample was quite high (M = 79.28; SD = 15.26), but there were nine individuals whose self-efficacy scores were more than 2SD below this mean. Exploratory analyses were undertaken to try to understand the profile of those individuals compared to the rest of the sample. Independent t-tests showed that these individuals with very low self-efficacy did not differ from the larger group on any of the demographic variables shown in Table 1 with the exception of relationship length, age, and ethnicity. Individuals with low self-efficacy tended to be married for a shorter period of time (11.56 vs 19.77 years; t = −3.86, p = .002), were younger in age (38.81 vs 47.06 years; t = −3.47, p = .01), and were more likely to be Hispanic or Latino (33% vs 5%; chi-square = 12.40, p < .001).
Next, we examined whether there were any demographic or disease variables that needed to be statistically controlled in the analyses. Gender, marital status, race, education, age of diagnosis, length of diagnosis, and use of insulin pump were unrelated to self-efficacy. Age was moderately correlated with self-efficacy (r = .14, p = .05) and was related to daily diabetes stressors (r = −.28, p < .001) and self-care (r = .23, p < .001), such that older participants reported higher levels of self-efficacy, fewer diabetes stressors, and better self-care. Therefore, age was statistically controlled in all analyses.
The primary hypothesis was tested with multiple regression analysis. We entered the age of the person with T1D, partner illness appraisal, and self-efficacy on the first step of the equation, and the interaction between partner illness appraisal and self-efficacy on the second step of the equation. Self-efficacy was centered before the interaction term was computed. When the interaction is significant, we show the results for the second and final step of the equation and report the simple slopes in the text. When the interaction is not significant, we show the results for the first step of the equation (i.e. main effects). Next, we repeated these analyses with the illness appraisals of those persons with T1D. Finally, we examined whether the congruence in illness appraisal between persons with T1D and partners interacted with self-efficacy to determine outcomes. We created a variable that indicated whether persons with T1D and their partners were congruent in their illness appraisals. This variable, together with the partner appraisal variable, tests whether congruence adds anything additional to the prediction of distress.
Results
Descriptive statistics
Over two-thirds of partners (69%) reported a shared appraisal of diabetes; 31 percent reported an individual appraisal of diabetes. For persons with T1D, 24 percent reported a shared appraisal of diabetes; 76 percent reported an individual appraisal. Appraisal of the person with T1D and partner were significantly correlated, but the correlation was quite low, r = .16, p < .05. In 21 percent of couples, both the person with T1D and the partner reported a shared illness appraisal; in 28 percent of couples, both the person with T1D and the partner reported an individual illness appraisal. Discrepancies occurred in the remaining cases.
Relations of partner appraisal and self-efficacy to health outcomes
These results are shown in Table 2.
Table 2.
Multiple regression analyses to predict outcomes.
| Psychological distress | Diabetes distress | Daily diabetes stressors | Self-care | HbA1c | |
|---|---|---|---|---|---|
| Age | .09 | .14* | −.25*** | .15** | .11 |
| Partner appraisal | −.04 | −.08 | −.13 + | .02 | .01 |
| Self-efficacy | −.33*** | − 37*** | −.25*** | .61*** | −.25** |
| Appraisal * Efficacy | .88** | .60* |
HbA1c: hemoglobin A1c.
p < .10,
p < .05,
p < .01,
p < .001.
Distress.
We examined whether partner appraisal and self-efficacy were related to two different measures of distress: (1) psychological distress and (2) diabetes-related distress. Self-efficacy was related to less distress and interacted with partner illness appraisal to predict psychological distress. As shown in Figure 1, the relation of self-efficacy to reduced psychological distress was weakened under conditions of shared illness appraisal (β = −.11, SE = .04, p = .02) compared to individual illness appraisal (β = −.36, SE = .07, p < .001). Figure 1 shows that individuals with lower levels of self-efficacy appear to be buffered from higher levels of psychological distress in the presence of partner-shared rather than individual illness appraisal.
Figure 1.
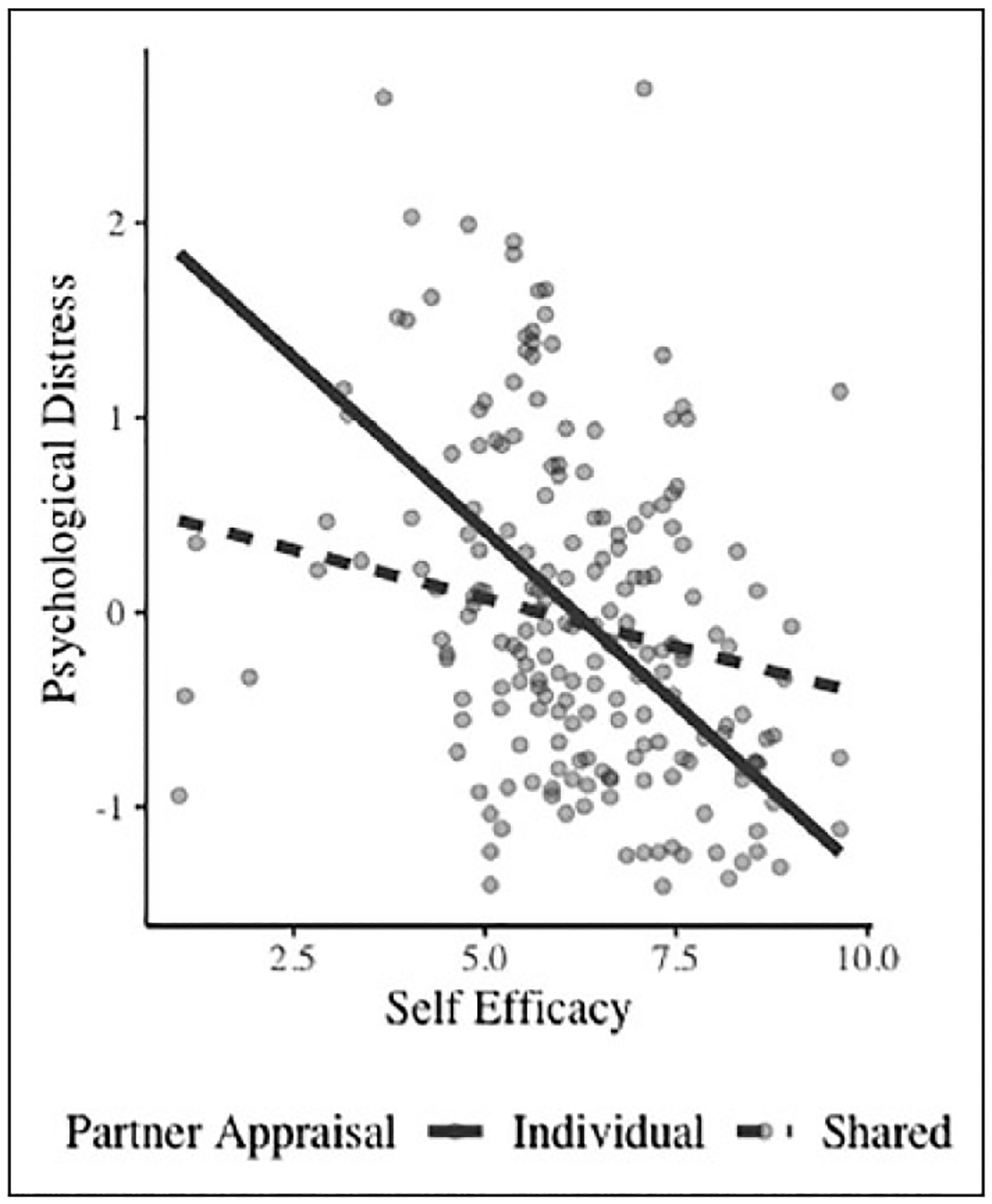
Interaction of self-efficacy and partner appraisal to psychological distress.
For diabetes-related distress, there was a main effect of self-efficacy (β = −.37, p < .001). Self-efficacy was related to lower diabetes-specific distress, but the interaction between partner appraisal and self-efficacy was not significant.
Diabetes outcomes.
In terms of daily diabetes stressors, self-efficacy was related to fewer diabetes stressors and interacted with partner illness appraisal. As shown in Figure 2, self-efficacy was less strongly linked to number of diabetes stressors when partners held a shared illness appraisal compared (β = −.01, SE = .01, p < .07) to an individual illness appraisal (β = −.04, SE = −.01, p < .001). Again, the figure shows that persons with T1D who had lower levels of self-efficacy appear to be buffered from a high number of diabetes stressors under conditions of shared illness appraisal.
Figure 2.
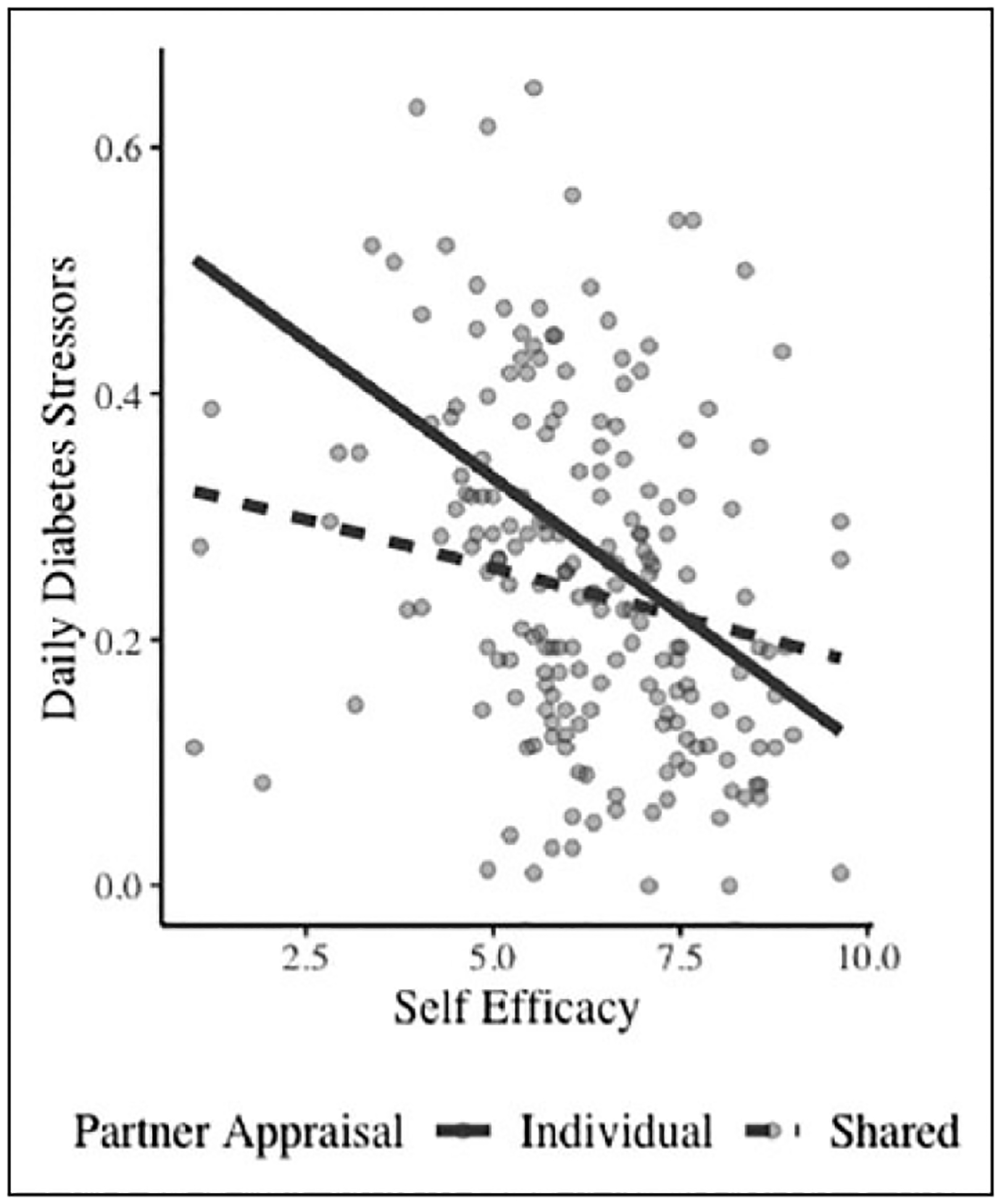
Interaction of self-efficacy and partner appraisal to daily diabetes stressors.
There were main effects of self-efficacy on self-care behavior and HbA1c, such that higher self-efficacy was linked to better self-care behavior and better glycemic control. However, partner illness appraisal did not interact with self-efficacy to predict either outcome.
Relations of person with T1D appraisal and self-efficacy to health outcomes
We repeated these analyses using the illness appraisals of persons with T1D. The effects for self-efficacy remained as self-efficacy was related to lower psychological distress (β = −.32, p < .001), lower diabetes-specific distress (β = −.37, p < .001), fewer daily diabetes stressors (β = −.25, p < .001), better self-care (β = .61, p < .001), and lower HbA1c (β = −.24, p < .001). In addition, there were main effects of persons with T1D shared illness appraisal on outcomes (reported elsewhere), but there were no interactions of person with T1D illness appraisal with self-efficacy in predicting psychological distress, diabetes-specific distress, daily diabetes stressors, self-care, or HbA1c (all ps > .05).
Relations of shared appraisal congruence and self-efficacy to health outcomes
We tested whether a variable reflecting congruence of the person with T1D appraisal and partner appraisal predicted outcomes over and above partner appraisal. There were no main effects of congruence in predicting outcomes. The interaction between appraisal discrepancies and the self-efficacy of the person with T1D did not predict any of the outcomes of interest.
Discussion
This study investigated whether shared illness appraisal would buffer individuals against the potentially harmful effects of low individual self-efficacy. There is a substantial body of work documenting the benefits of self-efficacy (King et al., 2010; Nouwen et al., 2009), but researchers have not examined the extent to which the interpersonal environment can ameliorate or compensate for the negative effects of low individual efficacy. We hypothesized that activating the resources of a partner would help to offset the deficits associated with low self-efficacy, as low self-efficacy has been strongly implicated in poor adjustment to chronic illness (Sarkar et al., 2006). Findings supported this hypothesis in terms of general psychological distress and in terms of the number of daily diabetes stressors reported. These findings suggest that partner resources help to buffer persons with T1D from the experience of general distress and specific diabetes stressors when one’s own individual resources may be lacking.
Interestingly, results indicated that it was the partner’s shared illness appraisal rather than the shared illness appraisal of the person with T1D that was important for those with low self-efficacy. As suggested in interpersonal coping theories (i.e. Helgeson et al., 2018), partner-shared appraisals may be more important than the shared appraisals of persons with T1D for outcomes because partner perceptions likely drive their actions to stay involved in diabetes management.
Future research should more fully explore the extent to which the combined appraisals of both persons with diabetes and partners influence outcomes. Although we did not find an added benefit of congruence in illness appraisals, our appraisal assessment was limited by use of single item measures of appraisal with restricted ranges. A more detailed assessment of illness appraisals in couple members would allow a better test of this hypothesis.
These results point to the importance of understanding how diabetes is appraised by partners when understanding the meaning and role of self-efficacy in psychological distress and diabetes stressors. The collective efficacy of the couple may be a resource that the person with diabetes uses to deal with daily stressors. Support for this idea comes from Johnson et al. (2013), who found that dyadic coping was associated with better diet among those with type 2 diabetes through higher efficacy of both the person with diabetes and the partner. Future research would benefit from assessing the efficacy of the partner in dealing with daily management tasks and understanding how that efficacy may compensate for lower self-efficacy of the person with diabetes. It may be that for those persons with diabetes whose partners view the diabetes as shared, it is the combined efficacy of the person without diabetes together with the person with T1D that is most predictive of successful psychosocial and diabetes outcomes. Future research would benefit from the measurement of partner self-efficacy to test this idea.
The findings also highlight the importance of distinguishing between diabetes distress and the experience of daily diabetes-related stressors. Although self-efficacy was related to less diabetes distress and fewer diabetes-related stressors, shared illness appraisal only played a role in the relation of self-efficacy to diabetes-related stressors. Although diabetes-related stressors and diabetes distress are associated with one another, they are distinct constructs conceptually. When partners hold a view that diabetes is shared, they may be able to more effectively assist individuals in their day-to-day management so that they avoid diabetes-related stressors. When individuals have partners who endorse diabetes as an individual issue, the experience of stressful events may be more closely tied to their own perceived competence in self-care. The experience of diabetes distress itself may be more individualistic and less influenced by partners.
Although self-efficacy interacted with partner-shared illness appraisal to predict general psychological distress and daily diabetes stressors, this interaction was unrelated to self-care behavior and HbA1c. Because many self-care behaviors that affect HbA1c are performed alone, it is possible that confidence in one’s own abilities to successfully perform self-care behaviors is more critical than partner-shared appraisals. Thus, partner-shared appraisals may not be sufficient to affect the relation between self-efficacy and either self-care or HbA1c.
There were a number of study strengths. A particular strength of this work is an advancement of our understanding of how the relation of self-efficacy to health may be influenced by the interpersonal context—in this case, an individual difference factor that likely reflects partner involvement in diabetes. Research has focused on individual self-efficacy and the role of interpersonal coping in the context of a chronic illness, but our study advances the literature by recognizing that these factors may operate differently in conjunction with one another. An additional study strength is that we used a sample of adults with T1D that spanned all of adulthood. Much previous research on T1D has focused on childhood and adolescence. Studies of adults often focus on type 2 diabetes, but those findings cannot necessarily be extrapolated to type 1. It is critical to understand how T1D affects individuals across the lifespan, and this study is one of the few studies that advances our understanding of how adults with T1D manage their illness.
However, this study was not without limitations. One limitation is that the sample was primarily White and well-educated. Therefore, we are uncertain how self-efficacy and partner-shared appraisals would interact with one another in a more diverse sample. It is important for future studies to understand how this work extrapolates to a more diverse sample because results indicated that those with low self-efficacy were more likely to be Hispanic or Latino compared to those persons with higher levels of self-efficacy. An additional limitation of this study was the cross-sectional nature of the data. We do not know how self-efficacy and partner-shared appraisals serve to influence psychological distress and diabetes stressors over time.
In conclusion, we found that persons with T1D who had low levels of self-efficacy reported more general psychological distress, more diabetes distress, more frequent daily diabetes stressors, and poorer self-care and HbA1c. However, the effects on psychological distress and the experience of daily diabetes stressors were mitigated when persons with T1D had partners who perceived diabetes to be a shared issue. Future research should investigate the mechanisms by which partner-shared illness appraisals compensate for low individual efficacy.
Acknowledgements
Portions of these data were presented at the 2018 Meeting of the Society of Behavioral Medicine.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health (DP3 DK103999).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Bandura A (2000) Exercise of human agency through collective efficacy. Current Directions in Psychological Science 9(3): 75–78. [Google Scholar]
- Bandura A and Adams NE (1977) Analysis of self-efficacy of behavior change. Cognitive Therapy and Research 1(4): 287–310. [Google Scholar]
- Berg CA and Upchurch R (2007) A developmental-contextual model of couples coping with chronic illness across the adult life span. Psychological Bulletin 133(6): 920–954. [DOI] [PubMed] [Google Scholar]
- Berg CA, Butner JE, Butler JM, et al. (2013) Parental persuasive strategies in the face of daily problems in adolescent type 1 diabetes management. Health Psychology 32(7): 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly EA and Wray LA (2010) The role of collective efficacy in exercise adherence: A qualitative study of spousal support and type 2 diabetes management. Health Education Research 25(2): 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T and Mermelstein R (1983) A global measure of perceived stress. Journal of Health and Social Behavior 24: 385–396. [PubMed] [Google Scholar]
- Diener ED, Emmons RA, Larsen RJ, et al. (1985) The satisfaction with life scale. Journal of Personality Assessment 49(1): 71–75. [DOI] [PubMed] [Google Scholar]
- Helgeson VS, Jakubiak B, Van Vleet M, et al. (2018) Communal coping and adjustment to chronic illness: Theory update and evidence. Personality and Social Psychology Review 22(2): 170–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MD, Anderson JR, Walker A, et al. (2013) Common dyadic coping is indirectly related to dietary and exercise adherence via patient and partner diabetes efficacy. Journal of Family Psychology 27(5): 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DK, Glasgow RE, Toobert DJ, et al. (2010) Self-efficacy, problem solving, and social-environmental support are associated with diabetes self-management behaviors. Diabetes Care 33(4): 751–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin AB, LaGreca AM, Geffken GR, et al. (2009) Validity and reliability of an adolescent and parent rating scale of type 1 diabetes adherence behaviors: The Self-Care Inventory (SCI). Journal of Pediatric Psychology 34(9): 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons RF, Mickelson KD, Sullivan MJL, et al. (1998) Coping as a communal process. Journal of Social and Personal Relationships 15: 579–605. [Google Scholar]
- Nouwen A, Urquhart Law G, Hussain S, et al. (2009) Comparison of the role of self-efficacy and illness representations in relation to dietary self-care and diabetes distress in adolescents with type 1 diabetes. Psychology and Health 24(9): 1071–1084. [DOI] [PubMed] [Google Scholar]
- Polonsky WH, Fisher L, Earles J, et al. (2005) Assessing psychosocial distress in diabetes: Development of the Diabetes Distress Scale. Diabetes Care 28(3): 626–631. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977) The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1: 385–401. [Google Scholar]
- Robbins ML, Mehl MR, Smith HL, et al. (2013) Linguistic indicators of patient, couple, and family adjustment following breast cancer. Psycho-Oncology 22: 1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbaugh MJ, Mehl MR, Shoham V, et al. (2008) Prognostic significance of spouse we talk in couples coping with heart failure. Journal of Consulting and Clinical Psychology 76(5): 781–789. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh MJ, Shoham V, Skoyen JA, et al. (2012) We-talk, communal coping, and cessation success in a couple-focused intervention for health-compromised smokers. Family Process 51(1): 107–121. [DOI] [PubMed] [Google Scholar]
- Sarkar U, Fisher L and Schillinger D (2006) Is self-efficacy associated with diabetes self-management across race/ethnicity and health literacy? Diabetes Care 29(4): 823–829. [DOI] [PubMed] [Google Scholar]
- Talbot F, Nouwen A, Gingras J, et al. (1997) The assessment of diabetes-related cognitive and social factors: The Multidimensional Diabetes Questionnaire. Journal of Behavioral Medicine 20(3): 291–312. [DOI] [PubMed] [Google Scholar]
- Tracy EL, Berg CA, Baucom KJW, et al. (2018) Daily Sleep Quality and Duration and Daily Stressors in Couples Coping with Type 1 Diabetes. Health Psychology. Epub ahead of print 29 October. DOI: 10.1037/hea0000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Ven NC, Weinger K, Yi J, et al. (2003) The confidence in diabetes self-care scale: Psychometric properties of a new measure of diabetes-specific self-efficacy in Dutch and US patients with type 1 diabetes. Diabetes Care 26(3): 713–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge JS and Ranby KW (2018) Influence of relationship partners on self-efficacy for self-management behaviors among adults with type 2 diabetes. Diabetes Spectrum. Epub ahead of print May DOI: 10.2337/ds17-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajdel M, Helgeson V, Seltman H, et al. (2018) Links of communal coping to relationships and health: A latent variable approach. Paper presented at the 38th annual meeting of the society of behavioral medicine, New Orleans, LA, April. [Google Scholar]


