Abstract
Background:
Increased continuous glucose monitor (CGM) use presents both the benefit and burden of increased data for clinicians to rapidly analyze. The ambulatory glucose profile (AGP) is an evolving a universal software report for CGM data analysis.
Objectives/Hypotheses:
We utilized the Juvenile Diabetes Research Foundation-CGM dataset to evaluate the AGP across a broad spectrum of patients to show how AGP can be used clinically to assist with CGM-related decision making. We hypothesized that AGP metrics would be different across age and HbA1c strata.
Subjects:
AGPs were generated from the JDRF-CGM trial dataset for all periods during which there were ≥10 days of CGM coverage in the 2 weeks adjacent to an HbA1c measurement yielding 1101 AGPs for 393 unique subjects.
Methods:
AGPs were stratified by age group (8–14, 15–24, and ≥25 years) and HbA1c (within or above target for age) and compared for between group differences in AGP metrics via two-factor ANOVA. Glycemic differences between time periods were analyzed via segmented regression analysis.
Results:
Glucose exposure (average and estimated A1c) and variability (standard deviation and interquartile range) were different between the low and high HbA1c levels. Within a given HbA1c level all age groups were significantly different from each other with older patients having lower averages with less variability than younger patients.
Conclusions:
AGP analysis of the JDRF-CGM data highlights significant differences in glycemic profiles between pediatric and adult age groups and between well and less well-controlled patient populations.
Keywords: ambulatory glucose profile, continuous glucose monitor, diabetes
1 |. INTRODUCTION
Advances in continuous glucose monitoring (CGM) devices over the past several years have improved their accuracy, reduced the number of required calibrations, and incorporated remote monitoring features.1 Studies have shown that substantial benefits of CGM include improved glycemic control, reduced hemoglobin A1c (HbA1c), reduced hypoglycemia, and reduced burden of care for diabetes.2–10 CGM studies of early generation devices have shown usage rates among the pediatric population of 3%−4% whereas more recent data using newer devices have shown rates of 17%−25% among some populations (K. Miller and R. Beck, personal communication, May 2016).11,12 CGM use may continue to become more common as real-time treatment decisions are increasingly made from non-adjunctive CGM data without fingerstick BG values.13 While real-time CGM use is increasing, the rate of data downloading remains low as ≤15% of patients download their devices weekly and <30% of patients download monthly.12 Among patients <18 years old it has been reported that 36% of patients never or rarely download their devices.14 Increased CGM use has presented both a benefit and challenge for physicians in clinical practice. In clinic CGM, downloads provide an abundant source of data with values plotted every 1 to 15 minutes throughout the day and night. This large dataset enables clinicians to see a more continuous glycemic picture than that provided by self-monitoring of blood glucose (SMBG) with previous “black box” portions of the day now filled in by copious data. At the same time, already busy clinicians must rapidly review, analyze, and synthesize this data for use in treatment advice and dosing adjustments. This challenge is further compounded by the lack of standard metrics and data reporting among the different manufacturers of CGM devices.
This issue was addressed by a panel of diabetes specialists in 2012 with the development of recommendations for the ambulatory glucose profile (AGP), a universal software report for CGM data for use in both research and clinical settings.15 The AGP derives from work by the International Diabetes Center.16,17 The AGP combines all CGM data from several days (generally 14 days) into a single composite 24-hour period and then applies mathematical algorithms to help display glycemic patterns.18 Graphically, the AGP clearly displays the median curve (50th percentile) representing glucose stability, the 25th and 75th percentile curves which display glucose variability via the interquartile range (IQR), and the 10th and 90th percentile curves which display glucose excursions via the interdecile range.19 Numerically the AGP also presents average glucose, estimated HbA1c, glucose standard deviation and IQR, as well as percent time in various ranges and the option for “Close-Up” statistics on area under the curve (AUC) analysis, variability, and hyperglycemia/hypoglycemia episodes.15
To evaluate the longitudinal benefit of CGM systems in lowering HbA1c and reducing hypoglycemia the Juvenile Diabetes Research Foundation (JDRF) sponsored a randomized multicenter clinical trial of CGM efficacy and safety in adults and children with type 1 diabetes between January 2007 and January 2009.5,20–23 The data from the JDRF-CGM trial is publically available for further analysis via the Jaeb Center for Health Research (http://diabetes.jaeb.org/).24 We utilized the JDRF-CGM data to evaluate the AGP across a broad spectrum of pediatric and young adult patients and to further analyze patients across the study-specified age (8–14, 15–24, and ≥25 years) and whether HbA1c met ADA and ISPAD targets for age (<18 years old <7.5% or ≥7.5%, ≥18 years old <7.0% or ≥7.0%).25,26 We hypothesized that AGP metrics would be different across the 6 stratified groups as unique glycemic challenges are present in pre-pubertal, pubertal, and post-pubertal populations. Our aim is to show how the AGP can be used in a clinical setting to assist with clinical decision making with CGM and how CGM data differs in school age and adolescent/young adults compared with adults with type 1 diabetes.
2 |. METHODS
2.1 |. JDRF-CGM trial data
The JDRF-CGM trial dataset was accessed from the Jaeb Center database. The trial Control and Treatment groups were combined for this analysis. From this dataset, periods for which there was an available HbA1c and at least 10 days of CGM coverage in the adjacent 2 weeks were selected for AGP analysis. Overall, this yielded data for 393 subjects contributing 1101 distinct AGPs. The AGPs were then stratified by 2 factors with 3 and 2 levels, respectively: age group (8–14, 15–24, and ≥25 years) and HbA1c (within target for age and above target for age). It is worth noting that the original JDRF-CGM trial reported HbA1c stratified by <7.0% and ≥7.0% whereas this analysis utilized the recommended A1c cutoff of <7.5% for patients <18 years old and <7.0% for patients ≥18 years old as is recommended by the International Society for Pediatric and Adolescent Diabetes (ISPAD)25 and the American Diabetes Association (ADA).26
2.2 |. Statistical analysis
Statistics are presented as mean ± standard deviation or as a percentage. For significance testing a P-value of <.05 was considered statistically significant. For each age-A1c group the CGM values were compiled for each hour of the day and the median (50th percentile), interquartile (25th and 75th percentile) and interdecile (10th and 90th percentile) lines were plotted to generate the standard AGP graphical report. Estimated HbA1c (eA1c) was calculated using the method published by Nathan et al.27
For each individual AGP the standard reporting metrics were determined, these were: average glucose, estimated HbA1c (eA1c), glucose standard deviation, glucose IQR, percent time in range (<50, <60, <70, 70–140, 70–180, >180, >250, and >400 mg/dL), average number of CGM readings per day, hourly AUC (daytime [7:00 am–11:00 pm], night-time [11:00 pm-7:00 am], and 24 hour), coefficient of variation, and hypoglycemia and hyperglycemia metrics (hours per day, episodes per day, and mean duration) for each hypoglycemic (<70 mg/dL) and hyperglycemic (>180 mg/dL) range. The AGP metrics were then averaged for each group and two-factor ANOVA was used to look for between group differences. If differences were found, then two-sided Student’s t tests were used to identify significantly different groups.
For the median curve for each stratified group, segmented regression analysis28 was conducted to analyze for significant glycemic differences during different portions of the day. Hours of the day were broken into 6-time periods (12:00–5:59 am, 6:00–9:59 am, 10:00–12:59 am, 1:00–4:59 pm, 5:00–8:59 pm, 9:00–11:59 pm), based on visual inspection of the trends in the mean hourly percentile curves. Segmented regression was used to obtain estimates of the overall intercept and the slope for each group during each of the time periods. ANOVA was used to first test whether the intercepts and slopes were significantly different. If the P-value for the slope is significant, then the groups are significantly different in the rate of change in glucose over time during that time period.
3 |. RESULTS
3.1 |. AGP demographic characteristics
In total 1101 unique AGPs were generated representing 393 patients or 2.8 profiles per patient. These AGPs contained data for a CGM period of 12.3 ± 1.3 days. Demographic characteristics for each patient at the time of randomization are presented in Table 1. The data in this analysis comes from both experimental arm patients (69.2%) and control arm patients (30.8%), which approximately matches the JDRF-CGM randomization scheme of 2:1 between the experimental and control arms.
TABLE 1.
Patient demographic characteristics at randomization
| Characteristic | Mean ± SD |
|---|---|
| Age at randomization (y) | 26.4 ± 16.1 |
| Female (%) | 56.7 |
| HbA1c (%) | 7.4 ± 0.9 |
| CGM use (days) | 12.3 ± 1.2 |
| Weight (kg) | 65.7 ± 18.9 |
| BMI (kg/m2) | 23.7 ± 4.4 |
| Diabetes duration (y) | 14.2 ± 12.1 |
| Race (%) | |
| White | 94.4 |
| Black/AA | 1.5 |
| Asian | 0.5 |
| American Indian | 0.3 |
| Pacific Islander | 0.3 |
| Multi-racial | 2.0 |
| Not reported | 1.0 |
| Hispanic (%) | 3.8 |
| Pump users (%) | 81.2 |
| Treatment group (%) | |
| Control | 30.8 |
| RT-CGM | 69.2 |
RT-CGM, real time-continuous glucose monitor.
3.2 |. Stratified AGP analysis
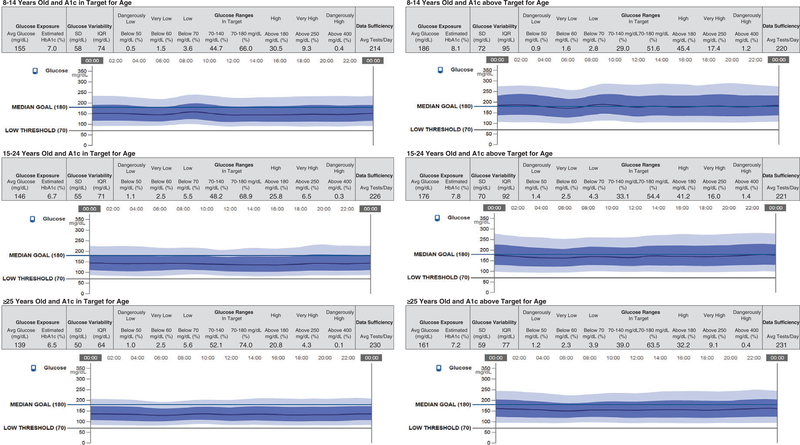
Strata-based composite AGPs were constructed for each age-A1c group and are displayed in Figure 1. As the AGP report is intended for descriptive visual inspection, additional statistical analysis was conducted using two-factor ANOVA to quantify observed trends (Table 2). This analysis investigated between group differences as well as A1c and age group interactions. Only the glucose exposure, glucose variability, and select glucose ranges were analyzed in this fashion, these metrics included: average glucose, eA1c, measured A1c, standard deviation in glucose, IQR, % time below 70 mg/dL, % time in a narrow target range 70–140 mg/dL, % time in wider target range 70–180 mg/dL, % time above 180 mg/dL, and % time above 250 mg/dL. The interaction between age group and A1c group was statistically significant for each of these 10 metrics.
FIGURE 1.
Composite ambulatory glucose profiles (AGPs) for all A1c and age groups
TABLE 2.
Composite AGP statistics by strata different letters denote significantly different groups based on pair-wise t-tests
| HbA1c in target for age |
HbA1c above target for age |
||||||
|---|---|---|---|---|---|---|---|
| 8–14 years-old n=211 | 15–24 years-old n=116 | ≥25 years-old n=260 | 8–14 years-old n=116 | 15–24 years-old n=158 | ≥25 years-old n=240 | F-value | |
| Glucose average (mg/dL) | 155c | 146b | 139a | 186f | 176e | 161d | <0.0001 |
| Estimated HbA1c (%) | 7.0c | 6.7b | 6.5a | 8.1f | 7.8e | 7.2d | <0.0001 |
| Measured HbA1c (%) | 6.7b | 6.7b | 6.5a | 8.1e | 7.9d | 7.5c | <0.0001 |
| Glucose SD (mg/dL) | 58 | 55b | 50a | 72d | 70d | 59c | <0.0001 |
| IQR (mg/dL) | 74b,c | 71b | 64a | 95d | 92d | 77c | <0.0001 |
| Glucose ranges | |||||||
| Below 50 mg/dL | 0.5 | 1.1 | 1.0 | 0.9 | 1.4 | 1.2 | |
| Below 60 mg/dL | 1.5 | 2.5 | 2.5 | 1.6 | 2.5 | 2.3 | |
| Below 70 mg/dL | 3.6a,b | 5.5c | 5.6c | 2.8a | 4.3b | 3.9b | <0.0001 |
| 70–140 mg/dL | 44.7d | 48.2e | 52.1f | 29.0a | 33.1b | 39.0c | <0.0001 |
| 70–180 mg/dL | 66.0b,c | 68.9c | 74.0d | 51.6a | 54.4a | 63.5b | <0.0001 |
| Above 180 mg/dL | 30.5c | 25.8b | 20.8a | 45.4e | 41.2d | 32.2c | <0.0001 |
| Above 250 mg/dL | 9.3c | 6.5b | 4.3a | 17.4d | 16.0d | 9.1c | <0.0001 |
| Above 400 mg/dL | 0.4 | 0.3 | 0.1 | 1.2 | 1.4 | 0.4 | |
| Avg. tests per day | 214 | 226 | 230 | 220 | 221 | 231 | |
| Wake 7 am to 11 pm AUC (mg/dL*h) | 148 | 140 | 133 | 181 | 170 | 156 | |
| Sleep 11 pm to 7 am AUC (mg/dL*h) | 149 | 143 | 134 | 181 | 170 | 157 | |
| 24-h AUC (mg/dL*h) | 148 | 141 | 134 | 181 | 170 | 156 | |
| Coefficient of variation (%) | 37.2 | 37.6 | 36.2 | 38.6 | 40.1 | 37.0 | |
| Hypoglycemia <70 mg/dL | |||||||
| Avg. hours per day (h) | 0.7 | 0.8 | 0.8 | 0.8 | 0.8 | 0.7 | |
| Mean episodes per day | 1.2 | 1.7 | 1.6 | 0.8 | 1.4 | 1.2 | |
| Mean duration (h) | 1.4 | 1.0 | 1.1 | 1.6 | 1.3 | 1.1 | |
| Hyperglycemia >180 mg/dL | |||||||
| Avg. hours per day (h) | 3.0 | 2.9 | 2.6 | 3.4 | 3.8 | 3.3 | |
| Mean episodes per day | 2.5 | 2.2 | 2.0 | 3.6 | 2.8 | 2.5 | |
| Mean duration (h) | 1.4 | 1.6 | 1.6 | 1.3 | 1.7 | 1.7 | |
AGP, ambulatory glucose profile; AUC, area under the curve; IQR, interquartile range.
The average glucose and eA1c were different between the in target and above target A1c levels, as would be expected as well as between each age group. Within the low and high A1c levels, the ≥25 age group was significantly better controlled than the 15–24 group which was better controlled than the 8–14 group (139 vs 146 vs 155 mg/dL and 161 vs 176 vs 186 mg/dL; 6.5% vs 6.7% vs 7.0% and 7.2% vs 7.8% vs 8.1%).
Glucose variability, as measured by standard deviation and IQR was also different between the low and high A1c levels. The ≥25 group had significantly less variability (SD 50 and 59 mg/dL; IQR 64 and 77 mg/dL) than either the 15–24 group (SD 55 and 70 mg/ dL; IQR 71 and 92 mg/dL) or the 8–14 group (SD 58 and 72 mg/dL; IQR 74 and 95 mg/dL) within each A1c level. The 15–24 and 8–14 groups were not significantly different from each other within each A1c level.
Percent time in the narrow target range of 70–140 mg/dL was also different between the 2 A1c levels and for each age group. For A1c values within target, all age groups were significantly different with the youngest group having the lowest percent time in range and the oldest group having the highest percent time in range (44.7%, 48.2%, and 52.1%). For A1c values above target, a similar trend was seen with the youngest age group having the lowest percent time in target range and the oldest group having the highest percent time in target range (29.0%, 33.1%, and 39.0%). For the wider target range 70–180 mg/dL the ≥25-year-old group spent significantly more time in range than either of the 2 younger age groups which were similar to each other for both the in target (74.0%, 68.9%, and 66.0%) and above target (63.5%, 54.4%, and 51.6%) A1c groups. This difference of ~7.5% time in range represents in improvement of almost 2 hours per day in target range. The percent time <70 mg/dL was significantly lower for the 8–14-year-old group than the 2 older groups, which were not different from each other at both A1c levels.
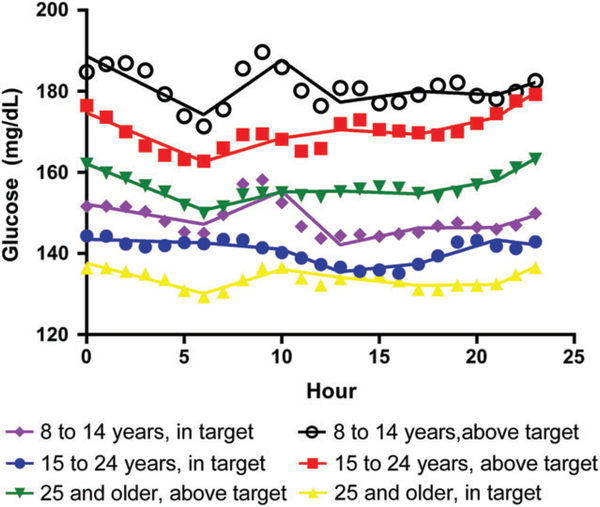
3.3 |. Segmented regression analysis
For more detailed analysis of trends in the median glucose curves, segmented regression produced estimates of the daily intercept and slope for each strata time period (Figure 2 and Table 3). Significant differences between groups for average rate of change of BG were seen during the 12:00 to 5:59 am, 6:00 to 9:59 am, and 10:00 am to 12:59 pm time periods as well as for the daily starting BG value (intercept). From 12:00 to 5:59 am, for all groups in the A1c above target level median glucose fell significantly while for the A1c in target level median glucose fell significantly for the >25-year old group but was essentially stable for the 8–14 and 15–24 year old groups. From 6:00 to 9:59 AM, for all groups in the A1c above target level median glucose rose significantly while for the A1c in target level median glucose rose significantly for the 8–14 and >25-year-old groups but was essentially stable for the 15–24-year-old group.
FIGURE 2.
Segmented regression analysis by A1c and age group. Hourly averages with best-fit regression line through each time segment
TABLE 3.
Median parameter estimates from segmented regression models showing period starting glucose or intercept (Bo, mg/dL) and slope (B1, mg/dL/h)
| HbA1c in target for age |
HbA1c above target for age |
||||||
|---|---|---|---|---|---|---|---|
| Hours of day | 8–14 years old | 15–24 years old | ≥25 years old | 8–14 years old | 15–24 years old | ≥25 years old | P-value |
| Intercept | 152.2 ± 1.8 | 143.5 ± 0.9 | 137.6 ± 0.9 | 188.6 ± 2.2 | 174.7 ± 1.5 | 162.1 ± 0.7 | <.0001 |
| 12:00–5:59 am | −0.8 ± 0.5 | −0.1 ± 0.2 | −1.2 ± 0.2a | −2.4 ± 0.6a | −2.0 ± 0.4a | −1.9 ± 0.2a | .0003 |
| 6:00–9:59 am | 2.0 ± 0.7a | −0.4 ± 0.3 | 1.5 ± 0.3a | 3.3 ± 0.8a | 1.4 ± 0.6a | 1.1 ± 0.3a | .0002 |
| 10:00 am–12:59 pm | −4.4 ± 0.9a | −1.8 ± 0.4a | −0.7 ± 0.4 | −3.4 ± 1.1a | 0.7 ±0.8 | 0.1 ± 0.4 | <.0001 |
| 1:00–4:59 pm | 1.0 ± 0.7 | 0.5 ± 0.3 | −0.5 ± 0.3 | 0.7 ± 0.9 | −0.3 ± 0.6 | −0.2 ± 0.3 | .3210 |
| 5:00–8:59 pm | 0.0 ± 0.7 | 1.5 ± 0.3a | 0.1 ± 0.3 | −0.2 0.9 | 1.1 0.6 | 0.8 0.3a | .2502 |
| 9:00–11:59 pm | 1.5± 1.6 | −0.6± 0.8 | 2.2± 0.8a | 1.5 2.1 | 3.1 1.4a | 2.7 0.6a | .4485 |
Abbreviation: HbA1c, glycated hemoglobin.
Denotes that slope is significantly different than zero (P < .05).
4 |. DISCUSSION
Increased use of real-time continuous glucose monitoring among pediatric patients holds significant potential to improve glycemic control in this population as well as reduce the burden of hypoglycemia and overall diabetes care. CGM use is becoming more commonplace and pediatric glycemic control guidelines are beginning to shift towards recommendations for use in children, particularly those with hypoglycemia unawareness.25,29 However, appropriate incorporation of CGM data into clinical practice remains a challenge for both patients and providers. Here we utilize the JDRF-CGM trial dataset to show both the utility of the AGP standardized reporting template and to highlight population differences in glycemic profiles by age and A1c strata.
Side-by-side comparison of Figure 1 against Table 2 highlights the clinical utility of the AGP reporting method. As exemplified by the 8–14-year old groups, rapid visual inspection of the modal glucose curves shows that for the A1c in target group about two thirds of values fall in the target 70–180 range while for the A1c above target group approximately half of the values fall in the target range. Visual inspection of these groups shows that there is an early morning BG rise followed by late morning glucose decline. These findings are supported by the percentage of time in range, AUC, and segmented slope analysis from Table 2. AGP can thus be seen as a tool to allow rapid visual display of trends that would otherwise require complex tabular analysis to convey.
Between group comparisons of key AGP metrics in this analysis revealed both expected and surprising trends. Average glucose was unsurprisingly different between the low and high A1c levels for each age group. Within each A1c level, however, the oldest age group had significantly better control than the youngest age group supporting the challenge of tight glycemic control in children and the reality of higher glycemic targets in this age group. The middle age group, containing older teens and young adults, was similar to both the oldest and youngest group for the low A1c group and was different from both groups for the higher A1c group. These trends support that teens and young adults can achieve glycemic control that is similar to that seen in adults, but for teens and young adults with poor control, their glycemic challenges are different from those seen for adults and those of younger children.
A more practical description of these results is that for the lower A1c level all age groups spent approximately 17–18 hours per day in the target range (70–180 mg/dL). For the higher A1c level, the oldest group spent approximately 15 hours per day in target range and the other 2 age-groups spent about 12–13 hours per day in target range. It can thus be seen that an improvement of 2–3 hours per day in target range is correlated with a shift from above target to in target A1c values. Measures of glycemic variability, glucose standard deviation and IQR, followed the same trends seen for average glucose. This supports the growing observations that glycemic variability is strongly correlated with tight glucose control. Interestingly, the well-controlled youngest group had significantly less variability than the 2 poorly controlled older groups. This suggests that it is possible to limit glycemic variability even in younger children.
Appropriate glycemic measurement statistics for clinical and research use remains a debate in the field with average glucose, eA1c, and percent time in target range all proposed.15,30,31 The JDRF AP consortium recently published a consensus statement recommending the use of HbA1c, mean CGM glucose, % CGM time 70–140 mg/dL and % time 70–180 mg/dL as main metrics among several others.31 In this analysis we see that % CGM time 70–140 mg/dL showed very different glycemic trends then average glucose or eA1c. It is worth emphasizing that based on glucose meter data a time in target range of 50% or greater is generally required to achieve target HbA1c.32 This again supports that tight control in younger children which is similar to that seen for adults is achievable but for less well controlled children and young adults, glycemic control is significantly different from that seen for adults.
Hypoglycemia (<70 mg/dL) trends within this analysis revealed several very surprising findings. Within each A1c level, the youngest age group actually had lower rates of hypoglycemia than the older groups. This could be due to the higher glycemic averages in these groups. The hypoglycemia rates for the lower A1c level were also higher than for the higher A1c level. These findings contrast with findings from other recent reports which have stated that higher glycemic targets and A1c targets do not result in lower rates of hypoglycemia, although these reports used severe hypoglycemia in contrast to CGM data.33–35
Segmented regression analysis of the AGP median curves showed several notable trends in this population dataset. It is important to note that these slopes represent rates of change for a population over a time period and not for individuals as would be seen on a CGM receiver. Median glucose for all ages in the A1c above target group fell significantly from 12:00 to 5:59 AM. This trend supports focus on the overnight period as higher risk for glucose decline and resultant hypoglycemia in all age groups particularly among more poorly controlled patients. Significant large rises in average glucose were seen from 6:00 to 9:59 am in the youngest age followed by significant large declines in average glucose from 10:00 am to 12:59 pm in both A1c groups. This trend of significant rise and then fall in BG during the early morning into late afternoon period for the youngest group highlights the significant insulin resistance seen in growing children, the challenge of glycemic control early in the day in this population, and the likely need for different insulin dosing in this population compared to older age groups. This findings are consistent with findings from the STAR 3 trial which showed that improvement in the breakfast meal period with sensor augmented pump therapy accounted for 59% of A1c improvement and that improvement in CGM values overnight was significantly associated with improvement in breakfast CGM values.36
AGP analysis of JDRF-CGM trial data illustrates the rapid clinical assessment of large complex data provided by this analytic summary. Analysis of data using these methods highlights significant differences in glycemic profiles between various pediatric and adult age groups and between well controlled non-well controlled patient populations. As use of CGM devices continues to rapidly expand, efficient incorporation of their data into clinic practice will become central to providing optimal patient care to children with type 1 diabetes.
ACKNOWLEDGMENTS
We would like to give special thanks to Nathan Crouther, with Abbott Diabetes Care, for the preparation of the dataset and the AGP analysis tools. There was no direct financial support for this project. Dr Forlenza and Dr Maahs are paid consultants for Abbott Diabetes Care and Dr Dunn is an employee of Abbott Diabetes Care. Abbott Diabetes Care utilizes AGP in its clinical reports, but has no financial interest in this project. Drs Forlenza & Maahs also conduct research sponsored by the NIH, the JDRF, the NSF, Medtronic Diabetes, Dexcom, Roche, Johnson and Johnson, Insulet, Tandem, Bigfoot, and Novo-Nordisk. Dr Maahs is on the advisory board for Insulet. Dr Pyle has no conflicts to report.
Funding information
NIH; JDRF; NSF; Medtronic Diabetes;Dexcom; Roche; Johnson and Johnson; Insulet;Tandem; Bigfoot; Novo-Nordisk
REFERENCES
- 1.Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016;18 suppl. 2:S23–s213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ryan EA, Germsheid J. Use of continuous glucose monitoring system in the management of severe hypoglycemia. Diabetes Technol Ther. 2009;11:635–639. [DOI] [PubMed] [Google Scholar]
- 3.Bailey TS, Zisser HC, Garg SK. Reduction in hemoglobin A1C with real-time continuous glucose monitoring: results from a 12-week observational study. Diabetes Technol Ther. 2007;9:203–210. [DOI] [PubMed] [Google Scholar]
- 4.Garg S, Zisser H, Schwartz S, et al. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29:44–50. [DOI] [PubMed] [Google Scholar]
- 5.Beck RW, Hirsch IB, Laffel L, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32:1378–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battelino T, Liabat S, Veeze HJ, Castaneda J, Arrieta A, Cohen O. Routine use of continuous glucose monitoring in 10 501 people with diabetes mellitus. Diabet Med. 2015;32:1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011;34:795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deiss D, Bolinder J, Riveline JP, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29:2730–2732. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch IB, Abelseth J, Bode BW, et al. Sensor-augmented insulin pump therapy: results of the first randomized treat-to-target study. Diabetes Technol Ther. 2008;10:377–383. [DOI] [PubMed] [Google Scholar]
- 10.Forlenza GP, Buckingham B, Maahs DM. Progress in diabetes technology: developments in insulin pumps, continuous glucose monitors, and progress towards the artificial pancreas. J Pediatr. 2016;169:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange Clinic Registry. Diabetes care. 2015;38:971–978. [DOI] [PubMed] [Google Scholar]
- 12.Wong JC, Foster NC, Maahs DM, et al. Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care. 2014;37:2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox LA, Balkman E, Englert K, Hossain J, Mauras N. Safety of using real-time sensor glucose values for treatment decisions in adolescents with poorly controlled type 1 diabetes mellitus: a pilot study. Pediatr Diabetes. 2016. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck RW. Downloading diabetes device data: empowering patients to download at home to achieve better outcomes. Diabetes technology & therapeutics. 2015;17:536–537. [DOI] [PubMed] [Google Scholar]
- 15.Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the Ambulatory Glucose Profile (AGP). Diabetes Technol Ther. 2013;15:198–211. [DOI] [PubMed] [Google Scholar]
- 16.Mazze RS, Lucido D, Langer O, Hartmann K, Rodbard D. Ambulatory glucose profile: representation of verified self-monitored blood glucose data. Diabetes Care. 1987;10:111–117. [DOI] [PubMed] [Google Scholar]
- 17.Mazze RS, Strock E, Wesley D, et al. Characterizing glucose exposure for individuals with normal glucose tolerance using continuous glucose monitoring and ambulatory glucose profile analysis. Diabetes Technol Ther. 2008;10:149–159. [DOI] [PubMed] [Google Scholar]
- 18.Mazze RS. Making sense of glucose monitoring technologies: from SMBG to CGM. Diabetes Technol Ther. 2005;7:784–787. [DOI] [PubMed] [Google Scholar]
- 19.Matthaei S. Assessing the value of the ambulatory glucose profile in clincial practice. Br J Diabetes Vasc Dis. 2014;14:148–152. [Google Scholar]
- 20.Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. [DOI] [PubMed] [Google Scholar]
- 21.JDRF CGM Study Group. JDRF randomized clinical trial to assess the efficacy of real-time continuous glucose monitoring in the management of type 1 diabetes: research design and methods. Diabetes Technol Ther. 2008;10:310–321. [DOI] [PubMed] [Google Scholar]
- 22.Beck RW, Buckingham B, Miller K, et al. Factors predictive of use and of benefit from continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2009;32:1947–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.JDRF-CGM Study Group. Effectiveness of continuous glucose monitoring in a clinical care environment: evidence from the Juvenile Diabetes Research Foundation continuous glucose monitoring (JDRF-CGM) trial. Diabetes Care. 2010;33:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.JDRF Continuous Glucose Monitoring Clinical Trial. 2016. http://diabetes.jaeb.org/. Accessed December 8 2015. [Google Scholar]
- 25.Rewers MJ, Pillay K, de Beaufort C, et al. Assessment and monitoring of glycemic control in children and adolescents with diabetes. Pediatr Diabetes. 2014;15 suppl 20:102–114. [DOI] [PubMed] [Google Scholar]
- 26.American Diabetes Association (ADA). Standards of medical care in diabetes – 2016. Diabetes Care. 2016;39:S1–S112.26696671 [Google Scholar]
- 27.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vieth E. Fitting piecewise linear regression functions to biological responses. J Appl Physiol (Bethesda, Md: 1985). 1989;67:390–396. [DOI] [PubMed] [Google Scholar]
- 29.Ly TT, Maahs DM, Rewers A, et al. Assessment and management of hypoglycemia in children and adolescents with diabetes. Pediatr Diabetes. 2014;15 suppl. 20:180–192. [DOI] [PubMed] [Google Scholar]
- 30.Kohnert KD, Heinke P, Vogt L, Salzsieder E. Utility of different glycemic control metrics for optimizing management of diabetes. World J Diabetes. 2015;6:17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maahs DM, Buckingham BA, Castle JR, et al. Outcome measures for artificial pancreas clinical trials: a consensus statement. Diabetes care. 2016;39:1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brewer KW, Chase HP, Owen S, Garg SK. Slicing the pie. Correlating HbA—values with average blood glucose values in a pie chart form. Diabetes Care. 1998;21:209–212. [DOI] [PubMed] [Google Scholar]
- 33.Maahs DM, Hermann JM, DuBose SN, et al. Contrasting the clinical care and outcomes of 2,622 children with type 1 diabetes less than 6 years of age in the United States T1D Exchange and German/Austrian DPV registries. Diabetologia. 2014;57:1578–1585. [DOI] [PubMed] [Google Scholar]
- 34.O’Connell SM, Cooper MN, Bulsara MK, Davis EA, Jones TW. Reducing rates of severe hypoglycemia in a population-based cohort of children and adolescents with type 1 diabetes over the decade 2000–2009. Diabetes Care. 2011;34:2379–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karges B, Rosenbauer J, Kapellen T, et al. Hemoglobin A1c Levels and risk of severe hypoglycemia in children and young adults with type 1 diabetes from Germany and Austria: a trend analysis in a cohort of 37,539 patients between 1995 and 2012. PLoS Med. 2014;11:e1001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maahs DM, Chase HP, Westfall E, et al. The effects of lowering night-time and breakfast glucose levels with sensor-augmented pump therapy on hemoglobin A1c levels in type 1 diabetes. Diabetes Technol Ther. 2014;16:284–291. [DOI] [PubMed] [Google Scholar]