Abstract
Background
Procalcitonin testing has been adopted by antimicrobial stewardship programs as a means of reducing inappropriate antibiotic use, including within intensive care units (ICUs). However, concerns regarding procalcitonin’s sensitivity exist. The purpose of this study is to calculate the sensitivity of procalcitonin for bacteremia among hospitalized patients.
Methods
This was a retrospective cohort study of adult patients admitted to an academic medical center between July 1, 2018, and June 30, 2019, with ≥1 positive blood culture within 24 hours of admission and procalcitonin testing within 48 hours. Low procalcitonin was defined as <0.5 µg/L.
Results
A total of 332 patients were included. The sensitivity of procalcitonin for bacteremia was 62% at the sepsis threshold of 0.5 µg/L, 76% at a threshold of 0.25 µg/L, and 92% at a threshold of 0.1 µg/L. Of the 125 patients with low procalcitonin, 14% were initially admitted to the ICU and 9% required the use of vasopressors. In that same group, the top 3 organisms isolated were Staphylococcus aureus (39%), Escherichia coli (17%), and Klebsiella spp. (7%). Compared with those patients with elevated procalcitonin, patients with low procalcitonin were significantly more likely to have >24-hour delayed receipt of antibiotic therapy (3% vs 8%; P = .04), including among patients admitted to the ICU (1% vs 18%; P = .02).
Conclusions
The sensitivity of procalcitonin for bacteremia is unacceptably low for a rule-out test. Antimicrobial stewardship programs should use caution before promoting the withholding of antibiotic therapy for patients with low initial procalcitonin values.
Keywords: bacteremia, procalcitonin, antimicrobial stewardship, diagnostic stewardship, rapid diagnostics
Procalcitonin is often utilized by antimicrobial stewardship programs as a means of identifying patients at low risk for bacterial infection. However, in this retrospective cohort study, procalcitonin was poorly sensitive for bacteremia irrespective of infection source or causative bacterium.
The overuse of antibiotic agents and corresponding rise in bacterial resistance are a well-known global health care threat. Antimicrobial stewardship programs are tasked with promoting the safe and rational use of antibiotics in order to best preserve their activity. Thus, these programs have a vested interest in diagnostic markers capable of providing information about the likelihood of bacterial infection. Procalcitonin is an inflammatory biomarker with increased specificity for bacterial infection compared with traditional inflammatory biomarkers such as erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP). In clinical trials, use of procalcitonin algorithms, many of which include recommendations to withhold antibiotics for patients with procalcitonin levels below a specified threshold, have demonstrated reductions in antibiotic utilization without a negative impact on clinical outcomes for patients with suspected lower respiratory tract infection [1] and sepsis [2], though these results have not been consistently observed across all studies [3, 4].
Although procalcitonin has been increasingly adopted by stewardship programs to reduce the initiation and continuation of unnecessary antibiotic therapy (with a PubMed search for “procalcitonin” and “stewardship” revealing more articles published within the past 3 years than all other years combined), concerns exists that procalcitonin may not have adequate sensitivity for bacterial inflammation across all infection sources, particularly when the inflammatory response is anticipated to be predominantly localized to the infection site (eg, cellulitis). Delays in active antibiotic therapy among critically ill patients with bacterial infection are known to be associated with deleterious patient outcomes [5], and false-negative results may reduce provider belief in procalcitonin testing and decrease overall algorithm adherence, limiting the test’s utility.
At a minimum, to ensure the safe use of procalcitonin-guided therapy among truly infected patients, procalcitonin testing should have adequate sensitivity to detect bacteremia. Several authors have claimed in previous studies that a low serum procalcitonin may be used to “accurately [rule] out the diagnosis of bacteremia” [6] given the test’s high negative predictive value [6–8]. Recently, a meta-analysis of >500 patients with bacteremia across 13 clinical trials using procalcitonin-guided antibiotic management demonstrated a modest reduction in antibiotic therapy duration without increasing mortality or length of stay [9]. However, not all of the included studies used procalcitonin testing to determine the need for antibiotic initiation, algorithm adherence was noted to be lower in high-risk populations, less than 1% of bacteremias were caused by methicillin-resistant Staphylococcus aureus (MRSA), and the rate of false-negative results was not reported. Thus, the objectives of the study were to assess the real-world sensitivity of procalcitonin for bacteremia among hospitalized patients and to evaluate the role of procalcitonin as an indicator for withholding antibiotic therapy.
METHODS
This study was an institutional review board–approved, retrospective chart review of the electronic medical record at St. Joseph’s Hospital and Medical Center, a 600-bed tertiary care facility and level 1 trauma center in Phoenix, Arizona.
Inclusion and Exclusion Criteria
Adult patients admitted between July 1, 2018, and June 30, 2019, were eligible for study inclusion. Patients were required to have ≥1 positive blood culture within 24 hours of hospital admission and serum procalcitonin testing within 48 hours of the initial blood draw. Patients with a single blood culture positive for common skin contaminants (eg, Corynebacterium spp., Bacillus spp., and coagulase-negative staphylococci with the exception of Staphylococcus lugdenensis) were excluded.
Data Collection and Laboratory Diagnostics
For each patient, data were collected regarding demographics, medical comorbidities, initial infection severity (based on initial intensive care unit [ICU] disposition and/or receipt of vasopressor therapy within 24 hours of presentation), culture and sensitivity results of bacterial cultures, and select outcome measures. These included systemic inflammatory response syndrome (SIRS) criteria (highest value within 24 hours of the initial blood culture), as well as ESR and CRP values, when available. Procalcitonin measurements were performed in-house using the Architect Brahms PCT assay (Abbott, Abbott Park, IL, USA) as part of routine care. At the study facility, initial procalcitonin testing was recommended within institutional guidelines for suspected lower respiratory infections only; however, the ordering of procalcitonin was unrestricted and could be utilized with any patient by provider discretion.
Study Objectives and Definitions
The objectives of the study were to assess the sensitivity of procalcitonin for bacteremia and to compare the characteristics of patients testing negative for bacterial infection per procalcitonin (Group A, procalcitonin <0.5 µg/L) with those of the patients testing positive (Group B, procalcitonin ≥0.5 µg/L), particularly with respect to infection source and isolated organism. For the primary analysis, a low procalcitonin level was defined as procalcitonin value <0.5 µg/L to correspond with the recommendation of “antibiotics discouraged” in trials assessing the utility of procalcitonin for guiding antibiotic therapy for sepsis [10]. Additionally, patients in Group A were further divided into 3 subgroups to compare their clinical features: (1) procalcitonin <0.1 µg/L, corresponding to a recommendation of “antibiotics strongly discouraged”; (2) procalcitonin 0.1 to <0.25 µg/L, corresponding to a recommendation of “antibiotics discouraged” in trials assessing the utility of procalcitonin for guiding antibiotic therapy for suspected lower respiratory tract infection [11]; and (3) procalcitonin 0.25 to <0.5 µg/L. Patients were grouped according to their initial procalcitonin value; however, any follow-up procalcitonin levels obtained within 48 hours were also recorded by the study investigators for patients with an initially low level, selected as a pragmatic cutoff point to encompass the likely peak value and consistent with previous education at the study facility that a repeat procalcitonin level may be considered after 24–48 hours for patients with suspected lower respiratory tract infection and an initially low procalcitonin value.
Statistical Analysis
Categorical data are expressed as absolute and relative frequencies, with continuous data expressed as either means and standard deviations or medians and interquartile ranges (IQRs). The X2 or Fisher exact test was used to compare categorical variables (2 × 2 contingency table), and the Student t test or Mann-Whitney test was used to compare continuous data, as appropriate. P < .05 was considered statistically significant for all tests. All analyses were performed using IBM SPSS Statistics 25 (IBM, Armonk, NY, USA).
RESULTS
Among 1289 total adult inpatients with positive blood cultures during the study time frame, 332 (26%) met inclusion criteria. The sensitivity of procalcitonin for bacteremia was 62% at the sepsis threshold of 0.5 µg/L, 76% at a threshold of 0.25 µg/L, and 92% at a threshold of 0.1 µg/L. Three-quarters of procalcitonin testing was performed simultaneously or within 1 hour of the blood culture draw, and 97% of all initial procalcitonin levels were collected within 24 hours. Procalcitonin values ranged from 0.02 µg/L to 166.23 µg/L. Receipt of antibiotic therapy before the drawing of the procalcitonin level was associated with a higher median procalcitonin (3.02 µg/L vs 1.06 µg/L; P = .02). A total of 125 (38%) included patients had a low (<0.5 µg/L) initial procalcitonin value (Group A). The median (IQR) procalcitonin values were 0.18 (0.1–0.315) µg/L for Group A and 4.02 (1.49–13.63) µg/L for Group B (P < .001). Only 8 (6%) patients with a low initial procalcitonin value had a repeat level ordered within 48 hours; 4 of these repeated levels were decreased or unchanged from the initial procalcitonin value, and only 2 increased above the 0.5-µg/L threshold. There were no significant differences between the 2 groups with respect to patient demographics or comorbidities (Table 1), with the exception that patients in Group B were more likely to have diabetes (43% vs 31%; P = .032) or require chronic renal replacement therapy at baseline (10% vs 3%; P = .028).
Table 1.
Comparison of Patient Characteristics and Clinical Course Between Low-Procalcitonin (<0.5 µg/L, Group A) and High-Procalcitonin (≥0.5 µg/L, Group B) Patient Groups
| Group A (n = 125) | Group B (n = 207) | P Value | |
|---|---|---|---|
| Age, mean (SD), y | 56.5 (17.6) | 56.6 (16.7) | .945 |
| Male, No. (%) | 71 (56.8) | 116 (56.0) | .892 |
| White, No. (%) | 103 (82.4) | 165 (79.7) | .547 |
| Hispanic/Latino, No. (%) | 45 (36.0) | 73 (35.3) | .871 |
| Comorbidities, No. (%) | |||
| Diabetes mellitus | 39 (31.2) | 89 (43.0) | .032 |
| Malignancy | 11 (8.8) | 25 (12.1) | .352 |
| Chronic lung disease | 10 (8.0) | 13 (6.3) | .550 |
| Chronic immunosuppressiona | 9 (7.2) | 18 (8.7) | .629 |
| Cirrhosis | 9 (7.2) | 20 (9.7) | .441 |
| Heart failure | 9 (7.2) | 18 (8.7) | .629 |
| Chronic renal replacement therapy | 4 (3.2) | 20 (9.7) | .028 |
| Chronic oral corticosteroid useb | 3 (2.4) | 8 (3.9) | .545 |
| ICU disposition, No. (%) | 18 (14.4) | 78 (37.7) | <.001 |
| Bacteremia source, No. (%) | |||
| Skin/bone | 52 (41.6) | 54 (26.1) | .003 |
| Urinary | 27 (21.6) | 61 (29.5) | .116 |
| Endovascular/line-related | 14 (11.2) | 22 (10.6) | .871 |
| Respiratory | 9 (7.2) | 26 (12.6) | .123 |
| Intra-abdominal | 9 (7.2) | 19 (9.2) | .530 |
| Central nervous system | 2 (1.6) | 2 (1.0) | .634 |
| Other/unknown | 12 (9.6) | 23 (11.1) | .664 |
| No. of positive blood cultures, No. (%) | |||
| 1 of 2 | 48 (38.4) | 69 (33.3) | .349 |
| 2 of 2 | 74 (59.2) | 128 (61.8) | .634 |
| Repeat blood cultures drawn, No. (%) | 122 (97.6) | 189 (91.3) | .022 |
| Repeat blood cultures positive, No. (%) | 18 (14.8) | 36 (19.0) | .329 |
| ESR >20 mm/h, No. (%)c,d | 39 (86.7) | 37 (82.2) | .561 |
| CRP >10, No. (%)c,d | 47 (95.9) | 52 (100) | .233 |
| Tmax ≥38°C, No. (%)c | 77 (61.6) | 108 (52.2) | .094 |
| WBC ≥12, No. (%)c | 57 (45.6) | 141 (68.1) | <.001 |
| Respiratory rate ≥20, No. (%)c | 98 (78.4) | 188 (90.8) | .002 |
| Mechanically ventilated, No. (%) | 5 (4.0) | 32 (15.5) | .001 |
| Heart rate ≥90, No. (%)c | 110 (88.0) | 188 (90.8) | .411 |
| Need for vasopressor support, No. (%) | 11 (8.8) | 50 (24.2) | <.001 |
| Antibiotic delay >24 h, No. (%) | 10 (8.9) | 6 (3.3) | .038 |
| Antibiotic duration, median (IQR), d | 14 (10–42) | 14 (11–28) | .111 |
| Length of stay, median (IQR), d | 6 (4–9) | 6 (4–10) | .301 |
| In-hospital mortality, No. (%) | 1 (0.8) | 16 (7.7) | .004 |
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ICU, intensive care unit; IQR, interquartile range; PCT, procalcitonin; WBC, white blood cell count.
aAIDS, chemotherapy in the past 30 days, ≥20 mg of prednisone or equivalent daily for a minimum of 2 weeks, or receipt of other highly immunosuppressive agent within 3 months.
b≥5 mg prednisone or equivalent steroid dose daily for >1 month.
cBased on highest recorded value within 24 hours of initial blood culture.
dAmong patients with reported values.
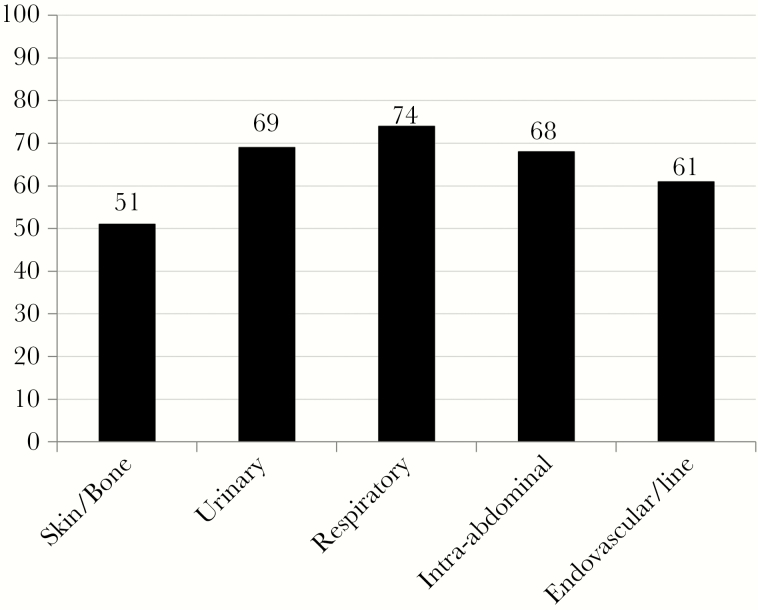
The 3 most common infection sources were skin and skin structure infection (32%), which included patients with osteomyelitis or prosthetic joint infection, urinary tract infection (27%), and endovascular or line-related infection (11%). Patients in Group A were more likely to have a skin or bone infection than Group B patients (P = .003). For the 5 most common infection types, procalcitonin sensitivity for bacteremia was lowest with a skin or bone infection (51% sensitivity at the 0.5-µg/L threshold) and highest for respiratory tract infection (74% at the 0.5-µg/L threshold and 80% at the 0.25-µg/L threshold) (Figure 1).
Figure 1.
Sensitivity (%) of Procalcitonin by Infection Source (0.5 μg/L Threshold).
The majority of patients had 2 of 2 initial blood culture sets positive, whereas approximately one-third of patients had 1 of 2 sets positive. Patients in Group A were more likely to have repeat blood cultures (98% vs 91%; P = .022), of which 15% were positive. S. aureus was the most commonly isolated organism in Group A, at 39%, of which 41% was MRSA (Table 2). This was significantly higher than the rate of S. aureus observed in Group B (39% vs 21%; P < .001). There was no significant difference between the 2 groups with respect to the proportion of S. aureus that was MRSA. After S. aureus, Escherichia coli and Klebsiella spp. were the next most common organisms isolated among the patients in Group A. Less than 5% of bacteremias were polymicrobial. Overall, procalcitonin was more sensitive for gram-negative bacteremia than gram-positive bacteremia (70% vs 56%; P = .008).
Table 2.
Comparison of Isolated Organisms Between Low-Procalcitonin (<0.5 µg/L, Group A) and High-Procalcitonin (≥0.5 µg/L, Group B) Patient Groups
| Organism, No. (%) | Group A (n = 125)a | Group B (n = 207)a | PCT Sensitivity, % |
|---|---|---|---|
| Gram(+) aerobe, any | 81 (64.8) | 105 (50.7) | 56.5 |
| Gram(-) aerobe, any | 43 (34.4) | 103 (49.8) | 70.1 |
| Staphylococcus aureus | 49 (39.2) | 44 (21.3) | 47.3 |
| MRSA | 20 (16.0) | 17 (8.2) | 45.9 |
| MSSA | 29 (23.2) | 27 (13.0) | 48.2 |
| Escherichia coli | 22 (17.6) | 62 (30.0) | 73.8 |
| Klebsiella species | 9 (7.2) | 14 (6.8) | 60.9 |
| Viridans streptococci/NVS | 9 (7.2) | 16 (7.7) | 64.0 |
| Streptococcus agalactiae | 8 (6.4) | 2 (1.0) | - |
| Enterococcus species | 4 (3.2) | 8 (3.9) | - |
| Streptococcus pyogenes | 4 (3.2) | 13 (6.3) | - |
| Staphylococcus, not aureus | 4 (3.2) | 5 (2.4) | - |
| Streptococcus pneumoniae | 3 (2.4) | 14 (6.8) | - |
| Haemophilus influenzae | 3 (2.4) | 1 (0.5) | - |
| Pseudomonas aeruginosa | 2 (1.6) | 10 (4.8) | - |
| Acinetobacter species | 2 (1.6) | 2 (1.0) | - |
| Anaerobe | 6 (4.8) | 13 (6.3) | - |
| Other gram(-) aerobe | 5 (4.0) | 14 (6.8) | - |
| Other gram(+) aerobe | 0 | 3 (1.4) | - |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; NVS, nutritionally variant streptococci.
aPercentages may total >100% due to polymicrobial bacteremias.
Fourteen percent of patients in Group A were admitted to the ICU, and 9% required the use of vasopressor therapy (Table 1). Tachycardia and tachypnea were the most commonly observed SIRS criteria in this group (88% and 78% of patients, respectively), whereas leukocytosis was observed the least often at 46%. Sixty-two percent of Group A patients were febrile within 24 hours compared with 52% of patients in Group B. Elevations in ESR and CRP were common across both groups (>80%). Overall, Group B patients were more likely to require ICU admission and vasopressor or ventilator support, and these patients also had a higher observed mortality rate.
All patients received antibiotic therapy, and there were no differences in total antibiotic duration (median, 14 days) or length of stay (median, 6 days) between the groups. Compared with Group B, patients in Group A were more likely to have a >24-hour delay from the time of initial blood draw to the initial receipt of antibiotic therapy (3% vs 8%; P = .04), including among patients initially admitted to the ICU (1% vs 18%; P = .02). With the exception of age, there were no observed differences in clinical features among the 3 low-procalcitonin subgroups (Table 3).
Table 3.
Comparison of Select Clinical Parameters Among Low-Procalcitonin Thresholds
| PCT <0.1 µg/L (n = 28) | PCT 0.1–<0.25 µg/L (n = 54) | PCT 0.25–<0.5 µg/L (n = 43) | P Value | |
|---|---|---|---|---|
| Age, mean (SD), y | 66.5 (15.4) | 53.8 (17.2) | 53.3 (17.2) | .002 |
| ICU disposition, No. (%) | 5 (17.9) | 8 (14.5) | 5 (11.6) | .761 |
| ESR >20 mm/h, No. (%)a,b | 9 (81.8) | 18 (85.7) | 12 (92.3) | .741 |
| CRP >10, No. (%)a,b | 12 (92.3) | 21 (100) | 14 (93.3) | .453 |
| Tmax ≥38°C, No. (%)a | 15 (53.6) | 35 (64.8) | 27 (62.8) | .599 |
| WBC ≥12, No. (%)a | 10 (35.7) | 24 (44.4) | 23 (53.5) | .331 |
| Respiratory rate ≥20, No. (%)a | 20 (71.4) | 44 (81.5) | 34 (79.1) | .572 |
| Mechanically ventilated, No. (%) | 1 (3.6) | 4 (7.4) | 0 | .179 |
| Heart rate ≥90, No. (%)a | 26 (92.9) | 44 (81.5) | 40 (93.0) | .148 |
| Need for vasopressor support, No. (%) | 3 (10.7) | 4 (7.4) | 4 (9.3) | .873 |
| Antibiotic duration, median (IQR), d | 17.5 (11–40.3) | 14 (12–28) | 16 (10–42) | .929 |
| Length of stay, median (IQR), d | 6 (4–7.8) | 6 (4–8.3) | 6 (3–9) | .785 |
Abbreviations: CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ICU, intensive care unit; IQR, interquartile range; PCT, procalcitonin; WBC, white blood cell count.
aBased on highest recorded value within 24 hours of initial blood culture.
bAmong patients with reported values.
DISCUSSION
Tests with a high negative predictive value are often regarded as excellent rule-out tests, with a negative test result having a high likelihood of being a true negative. For procalcitonin, its high negative predictive value would indicate that the likelihood of bacterial infection with a below-threshold result is low, and therefore antibiotic therapy may rationally be withheld. However, before adopting a diagnostic test into facility treatment guidelines on the basis of a high negative predictive value, antimicrobial stewardship programs should also ensure that the test has demonstrated adequate sensitivity in the patient population of interest. When the pretest probability of bacterial infection is low in a population, the number of false negatives may be obscured by the large number of true negatives, resulting in an artificially high negative predictive value despite low sensitivity. In this real-world sample of inpatients at a large academic medical center, procalcitonin testing within 48 hours of admission demonstrated poor sensitivity for bacteremia, indicating that procalcitonin should not be routinely used as a rule-out test for systemic infection. Over one-third of all inpatient bacteremias in which a procalcitonin was obtained were not detected using the most common threshold of 0.5 µg/L, and even if the strictest cutoff value documented in the literature of 0.1 µg/L was applied, nearly 10% of bacteremias would still be missed. Further, a low procalcitonin value was associated with delayed initiation of appropriate antibiotic therapy in this population, an association that persisted even when restricting the analysis to the most critically ill patient subgroups. Although this study was not designed to look at any potentially deleterious outcomes of this delay in antibiotic therapy, increases in mortality have previously been reported among ICU patients who did not receive early antibiotic therapy based on a procalcitonin protocol for chronic obstructive pulmonary disease [12].
Supporting that the patients in the low-procalcitonin group had true bacteremia, the most commonly isolated bacteria were S. aureus and Enterobacteriaceae, organisms that are virtually always treated as true pathogens when found in the blood [13]. Additionally, patients in the low-procalcitonin group were treated for equivalent antibiotic durations as those in the high-procalcitonin group, with similar lengths of inpatient stay. As anticipated based on prior studies [14], procalcitonin sensitivity was higher for gram-negative organisms compared with gram-positive organisms. However, among the organisms present in sufficient numbers to perform analysis, in no case did sensitivity exceed 80%. Similarly, although procalcitonin sensitivity was particularly low for bacteremia secondary to skin and skin structure infection, sensitivity did not exceed 80% for any infection source. Although arguably the strongest data for procalcitonin exist in the setting of lower respiratory tract infection, the most recent American Thoracic Society and Infectious Diseases Society of America guidelines for the diagnosis and treatment of community-acquired pneumonia strongly recommend against withholding antibiotic therapy on the basis of initial serum procalcitonin due to concerns about the test’s sensitivity [15].
Higher procalcitonin concentrations have been correlated with greater disease severity and organ dysfunction [16], which may invite the hypothesis that even if some patients with bacteremia are missed using procalcitonin algorithm-guided antibiotic therapy, these patients are less likely to be severely septic and therefore less likely to suffer negative consequences from any potential delays in infection diagnosis or antibiotic initiation. Indeed, in this study, the patients with elevated procalcitonin values appeared overall to have more severe illness, with a greater proportion requiring intensive care interventions. However, a sizeable proportion of patients in the low-procalcitonin group also appeared critically ill, representing ~20% of all included patients requiring ICU admission and vasopressor support. Further, when comparing patient characteristics across all 3 low-procalcitonin thresholds, there was no apparent numerical trend suggesting that the patients with very low procalcitonin values (<0.1 µg/L) had a decreased likelihood of being critically ill compared with the patients with more borderline values. Therefore, the possibility of severe infection cannot be excluded even at the 0.1-µg/L threshold.
Although previous data are often difficult to interpret given differing patient populations and common use of nonstandard procalcitonin cutoff values, the results of this study are consistent with the existing literature. In a meta-analysis of 58 studies including 3420 bacteremic patients (including only 118 patients from the United States), procalcitonin was only 76% sensitive for bacteremia using a 0.5-µg/L cutoff [7]. Not included in the previous meta-analysis, a large study from Austria including 666 bacteremic patients meeting SIRS criteria had a very similar procalcitonin sensitivity for bacteremia as that observed in the present study, at 64%, though their study had a larger proportion of patients with bacteremia from coagulase-negative staphylococci, at 12%, and did not report on infection source. Also, some bacteremias (7%) were missed even at the 0.1-µg/L threshold [17]. At a 0.5-µg/L cutoff, procalcitonin was also only 60% sensitive for bacteremia in a study including 302 patients in China with monobacterial nosocomial pneumonia and positive blood cultures [18].
The limitations of the present study should be acknowledged. First, it is known that procalcitonin may take several hours to reach the thresholds utilized to rule out infection; thus, some procalcitonin algorithms suggest that a confirmatory repeat procalcitonin level within 24 hours may be considered if the first level is low. Very few patients in this study with initially low procalcitonin values had repeat testing ordered, which is appropriate if it was determined by the managing team that antibiotics should be initiated regardless based on the patient’s overall clinical picture or based on the early return of positive blood cultures. It is possible that the sensitivity of procalcitonin for bacteremia would be higher if serial testing was used. However, it is questionable whether the gains in sensitivity would be substantial and outweigh the increased cost, given that repeat testing resulted in an above-threshold procalcitonin value for only 2 of the 8 patients who had repeat testing performed in this study. Additionally, this study included only patients with bacteremia, suggesting that the infection had time to emerge from a localized infection to one causing bacteremia and systemic inflammation. Conversely, if procalcitonin testing is delayed from initial presentation, the patient’s peak procalcitonin level may be missed, resulting in a falsely low value. In this study, patients were eligible for inclusion with procalcitonin testing up to 48 hours after the collection of their initial blood cultures, when procalcitonin levels would likely be declining for patients with resolving infection. However, almost all patients in this study had procalcitonin testing conducted within 24 hours after their positive blood cultures were drawn, with the majority of levels collected together with the initial blood cultures. Allowing a wider window of eligibility also allows for results more reflective of real-world clinician use of procalcitonin testing.
Second, although procalcitonin performed poorly at identifying bacteremia in this single-center analysis, the authors do not intend to imply that procalcitonin testing should never be used. This study did not address response to therapy in patients with elevated procalcitonin values or the ability to discontinue antibiotics as procalcitonin levels decline. In that regard, procalcitonin may be another tool, like total white blood cell count (WBC). Analogous to procalcitonin in this study, the WBC was only elevated in 50%–60% of patients. However, like a normal initial WBC count in a patient who is clinically infected, monitoring for a trend down in either WBC or procalcitonin when the value is initially normal is not appropriate for monitoring clinical response.
Effective antimicrobial stewardship always involves some measure of acceptable risk and consideration of the tradeoffs among potential benefits and risks to the individual, the hospital, and society. However, antimicrobial stewardship programs should recognize that procalcitonin testing is not a panacea. Provider acceptance of procalcitonin testing, particularly trust in low values, has been shown to often be low in real-world studies, leading to increased testing costs not offset by reductions in antibiotic expenditures or length of stay [19]. Conversely, poor algorithm adherence may have masked potential patient safety issues in previous trials. False negative results, such as those observed in the present study, are also likely to decrease prescriber confidence in procalcitonin testing. This may have the further consequence of decreased prescriber adherence to facility algorithms recommending discontinuation of antibiotic therapy in the setting of a substantial drop in procalcitonin from baseline.
CONCLUSIONS
A threshold of 0.5 µg/L was poorly sensitive for bacteremia, regardless of organism or infection site. No established cutoff value had adequate sensitivity to capture all critically ill bacteremic inpatients. Low procalcitonin values were associated with delayed receipt of antibiotic therapy, including among high-risk patient subgroups. Antimicrobial stewardship programs should not use procalcitonin as a means of withholding potentially inappropriate antibiotic therapy in patients being admitted to the hospital.
Acknowledgments
The authors would like to acknowledge Kathleen Fairman, MA, MPhil, PhD, for her assistance with the statistical analysis.
Financial support. This study was supported by internal funding from Midwestern University.
Potential conflicts of interest. The authors declare that there is no conflict of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Schuetz P, Wirz Y, Sager R, et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev 2017; 10:CD007498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iankova I, Thompson-Leduc P, Kirson NY, et al. Efficacy and safety of procalcitonin guidance in patients with suspected or confirmed sepsis: a systematic review and meta-analysis. Crit Care Med 2018; 46:691–8. [DOI] [PubMed] [Google Scholar]
- 3. Huang DT, Yealy DM, Filbin MR, et al. ; ProACT Investigators Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med 2018; 379:236–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van der Does Y, Limper M, Jie KE, et al. Procalcitonin-guided antibiotic therapy in patients with fever in a general emergency department population: a multicentre non-inferiority randomized clinical trial (HiTEMP study). Clin Microbiol Infect 2018; 24:1282–9. [DOI] [PubMed] [Google Scholar]
- 5. Ibrahim EH, Sherman G, Ward S, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000; 118:146–55. [DOI] [PubMed] [Google Scholar]
- 6. Chirouze C, Schuhmacher H, Rabaud C, et al. Low serum procalcitonin level accurately predicts the absence of bacteremia in adult patients with acute fever. Clin Infect Dis 2002; 35:156–61. [DOI] [PubMed] [Google Scholar]
- 7. Hoeboer SH, van der Geest PJ, Nieboer D, Groeneveld AB. The diagnostic accuracy of procalcitonin for bacteraemia: a systematic review and meta-analysis. Clin Microbiol Infect 2015; 21:474–81. [DOI] [PubMed] [Google Scholar]
- 8. Riedel S, Melendez JH, An AT, et al. Procalcitonin as a marker for the detection of bacteremia and sepsis in the emergency department. Am J Clin Pathol 2011; 135:182–9. [DOI] [PubMed] [Google Scholar]
- 9. Meier MA, Branche A, Neeser OL, et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: a patient-level meta-analysis of randomized trials. Clin Infect Dis 2019; 69:388–96. [DOI] [PubMed] [Google Scholar]
- 10. Bouadma L, Luyt CE, Tubach F, et al. ; PRORATA Trial Group Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet 2010; 375:463–74. [DOI] [PubMed] [Google Scholar]
- 11. Schuetz P, Christ-Crain M, Thomann R, et al. ; ProHOSP Study Group Effect of procalcitonin-based guidelines vs standard guidelines on antibiotic use in lower respiratory tract infections: the ProHOSP randomized controlled trial. JAMA 2009; 302:1059–66. [DOI] [PubMed] [Google Scholar]
- 12. Daubin C, Valette X, Thiollière F, et al. ; BPCTrea Study Group Procalcitonin algorithm to guide initial antibiotic therapy in acute exacerbations of COPD admitted to the ICU: a randomized multicenter study. Intensive Care Med 2018; 44:428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinstein MP, Towns ML, Quartey SM, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis 1997; 24:584–602. [DOI] [PubMed] [Google Scholar]
- 14. Thomas-Rüddel DO, Poidinger B, Kott M, et al. ; MEDUSA Study Group Influence of pathogen and focus of infection on procalcitonin values in sepsis patients with bacteremia or candidemia. Crit Care 2018; 22:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019; 200:e45–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta 2002; 323:17–29. [DOI] [PubMed] [Google Scholar]
- 17. Hoenigl M, Raggam RB, Wagner J, et al. Procalcitonin fails to predict bacteremia in SIRS patients: a cohort study. Int J Clin Pract 2014; 68:1278–81. [DOI] [PubMed] [Google Scholar]
- 18. Yan ST, Sun LC, Lian R, et al. Diagnostic and predictive values of procalcitonin in bloodstream infections for nosocomial pneumonia. J Crit Care 2018; 44:424–9. [DOI] [PubMed] [Google Scholar]
- 19. Cole JL. Experience during the first year of procalcitonin implementation: a precautionary tale for smaller facilities. Infect Control Hosp Epidemiol 2018; 39:1142–3. [DOI] [PubMed] [Google Scholar]