Abstract
Background
Sharing needles and ancillary injecting equipment is a primary risk exposure for hepatitis C virus (HCV) infection among people who inject drugs (PWID); however, infectivity of these exposures is not well quantified. We aimed to estimate per-event HCV infectivity associated with receptive needle sharing (RNS) among susceptible PWID.
Methods
Participants in a prospective cohort study of young adult PWID who were anti-HCV and HCV RNA negative at baseline and attended at least 2 follow-up study visits between 2003 and 2014 were eligible. Data were selected from the first HCV-negative through the first HCV-positive visit (or last HCV-negative among those uninfected). Anti-HCV and HCV-RNA tests were used to determine infection status. A probabilistic exposure model linking observed HCV infection outcomes to self-reported exposure events was applied to estimate infectivity.
Results
Among 344 participants, a maximum likelihood estimate considering RNS yielded a pooled population per RNS event HCV probability of 0.25% (95% confidence interval [CI], 0.10%–0.43%), and 1.12% (95% CI, 0.48%–2.35%) among those who acquired any HCV infection (primary or reinfection).
Conclusions
HCV is highly infectious in association with RNS, a primary injection-related risk exposure. Our infectivity estimate among participants who acquired any HCV infection is 1.7 times higher than that estimated for HIV infection in PWID and 2.24 times higher than that estimated among health care workers exposed through needle sticks. The strengths of this study include the assessment of receptive needle sharing events, the prospective design, and relatively short recall and testing periods. These results can inform transmission models and research to prevent HCV infection.
Keywords: HCV transmission, per-contact infectivity, injection drug use, per-contact probability
Hepatitis C virus (HCV) is the most prevalent bloodborne virus in the United States, with consequences ranging from acute illness to chronic liver disease and mortality [1]. Injection drug use is the most common route of HCV transmission, and infection rates have been increasing, especially among younger people who inject drugs (PWID), a group that may be less aware of the highly infectious nature of the virus and the potential for transmission/acquisition from shared injecting paraphernalia [2]. HCV is both very infectious and stable at room temperature and can remain infectious on surfaces and in liquid, syringes, and ancillary injecting equipment for several days depending on temperature and syringe volume [3–5]. Modeling studies of HCV transmission in PWID have used estimated injection-related transmission probabilities ranging from 1% to 3% [6–9], but these were not derived from observed data in PWID.
Sharing injecting paraphernalia, including both needles and syringes (referred to here as needles) and ancillary drug preparation equipment (cookers or cottons, referred to here as ancillary equipment), is common among PWID, and a significant proportion of, if not all, HCV infections are attributed to these exposures [10]. Receptive needle sharing (RNS) is associated with almost 2-fold higher relative hazard of incident HCV, with a pooled risk ratio (PRR) of 1.97 (95% confidence interval [CI], 1.57–2.49) from 22 studies [10]. An analysis among 92 prisoners followed prospectively in Australia estimated per-event HCV infection probability via injection equipment sharing as 0.57% (95% CI, 0.32%–1.05%) [11]. Equipment sharing in that study was defined as sharing any injecting paraphernalia, inclusive of both needles and ancillary equipment in the past year, without specifying receptive vs distributive behavior. The HCV status of sharing partners was not known, but HCV prevalence in the prison was stated as being between 40% and 80%. In the iatrogenic setting, the probability of HCV transmission associated with a needle stick exposure from a known HCV-positive patient has also been estimated at 0.5% [12].
Sharing ancillary equipment is also a recognized risk exposure for HCV transmission, but infectivity is assumed to be lower relative to needles [13, 14]. In a simulation model using injecting behavior and HCV prevalence data (estimated at 67% to 73% in Glasgow, Scotland [15]) estimated that HCV transmission probability associated with sharing ancillary equipment was ~8-fold lower than through sharing needles: 0.19%–0.30% vs 2.5%, respectively. This difference is plausibly related to reduced infectiousness of the former sharing mode. Indeed, a recent study using an in vitro experimental model found that HCV was recovered at much lower levels and with low (short duration) infectivity from ancillary equipment compared with needles, and no virus was recovered from “cookers” used in drug preparation [16].
Despite this collective research, there remain gaps in information regarding transmission probability of HCV in association with the separate and specific exposures of RNS and of sharing ancillary equipment among PWID. Some studies do not distinguish between different exposure routes, and the behaviors are also likely to be highly collinear, to be practiced jointly by some individuals, and to be potentially misclassified [17]. Here we estimate the per-event infectivity and transmission risk of HCV in association with RNS and other exposures using data collected in an ongoing prospective observational study of young adult PWID at risk of HCV infection, thus overcoming the limitations of retrospective studies, and those that do not distinguish between the different paths of injecting (receptive vs distributive). This information will help to inform prevention programs and studies aimed at reducing HCV transmission.
METHODS
Population and Setting
This analysis was conducted using data from the UFO Study, a prospective observational cohort study of young adult PWID. Details of the UFO Study procedures have been described elsewhere [18]. In brief, participants were recruited using street-based outreach ongoing since 2000 with 3 major waves of cohort enrollment. Those enrolled into follow-up were under 30 years of age, reported injecting drugs in the previous month, understood and spoke English, did not report “knowing” they were HCV-infected, did not plan to leave the area within 3 months (the latter 2 criteria were added in 2003), and tested negative for HCV ribonucleic acid (RNA). Quarterly risk assessments included in-depth interviews to assess risk exposures and testing for antibodies to HCV (anti-HCV) and HCV RNA. All participants were provided risk reduction counseling concomitant with HCV (and HIV) testing and compensation for baseline (up to $15) and follow-up visits ($20–$40). The study provided some on-site medical care, immunizations, and referrals to primary care, mental health, and drug treatment.
Data Collection
At baseline and quarterly study visits, blood samples were obtained to test for anti-HCV using an enzyme immunoassay (EIA-3; Ortho Clinical Diagnostics, Raritan, NJ, USA), and after 2012, Orasure (OraSure Technologies, Bethlehem, PA, USA) and HCV RNA, a nucleic acid amplification test (Gen-Probe Inc., San Diego, CA, USA, and marketed by Novartis Vaccines and Diagnostics, Emeryville, CA, USA). Self-reported sociodemographic information, injection-related risk exposures (duration, frequency, and types of drugs used, exposure to previously used needles and other ancillary equipment), sexual behavior, use of HIV and HCV prevention services (eg, syringe service programs and/or pharmacies), and drug treatment were collected using an interviewer-administered questionnaire at baseline and follow-up visits. Serological and behavioral data were collected between 2003–2008 and 2010–2014.
Primary Outcome
The primary outcome assessed was incident HCV infection, defined as a new HCV RNA–positive and/or anti-HCV-positive test result following a previously documented negative HCV test. Participants who became HCV positive were censored after first positive test for each exposure window (described below in “Exposure Variables: Definitions and Exposure Window”), whereas those who remained HCV negative contributed data up until their last study visit.
Exposure Variables: Definitions and Exposure Window
Our analysis focuses on potential exposure to HCV via RNS, defined as use of a needle after it was used by another person. Participants were asked to recall their 3 most frequent injecting partners in the past month as well as the number of times the participant engaged in RNS with each partner in the past month, and answers were recorded for each injecting partner. The sum of the 3 answers was calculated to represent each participant’s total number of RNS exposures over the past month.
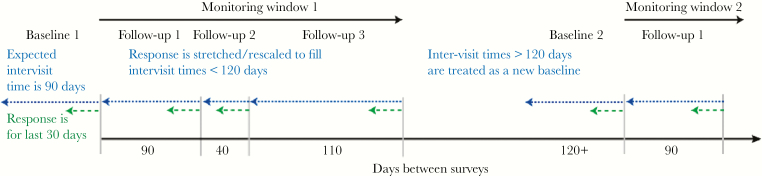
The total number of RNS exposures was adjusted relative to the actual intervisit times (exposure windows), which were expected to be 90 days. The reported 30-day RNS exposure values were therefore multiplied by a stretch factor to account for exposure windows longer or shorter than the expected 90 days (Figure 1). For participants with an intervisit gap longer than 120 days, data were treated as a new baseline exposure window. Reasons for this strategy included (1) funding gaps resulting in the gaps in observation and (2) the possibility of unrecorded infection and spontaneous clearance events during gaps in observation [19, 20]. For each exposure window, the recalled RNS exposure data that were collected at the same visit as the first HCV RNA–positive test were used in the model.
Figure 1.
Adjustment of inter-visit times using a “stretch” factor. The expected inter-visit time is 90 days. In the example below, there were actually 40 days between the second and third surveys. Thus, the reported number of 30-day exposures (green dashed lines) at the third survey is lengthened by a multiplicative factor of 40/30 to represent the number of exposures since a participant’s last visit rather than the number of exposures for the past 30 days. Similarly, the fourth survey’s reported number of exposures is increased by 110/30 to represent the number of exposures since the participant’s last visit (110 days prior to the fourth survey). For participants with an inter-visit gap greater than 120 days, data were treated as a new baseline.
We additionally examined data on both ancillary equipment sharing and backloading with the intent of including it in a model that would account for all these exposures, in addition to RNS. We discovered through an extensive simulation study that a generalization to include multiple independent exposure types (ie, needle sharing, equipment sharing, and syringe backloading) simultaneously in the maximum likelihood estimation (MLE) model used later produced strongly biased exposure probability. The model could estimate the per-contact probability of infection associated with one exposure type accurately, but probabilities of any additional exposure types were considerably inflated. Similar estimation issues occurred when we implemented the prevalence of HCV as an additional parameter in a separate 2-parameter model. These estimation problems are likely related to identifiability issues arising from both complex parametrizations and lack of supporting data. Thus, the univariate single-exposure model is used throughout this work. Therefore, as noted above, we focused this paper on RNS exposures, as they remain a crucial and primary route of HCV transmission.
Population Included in Analysis
Participants who were anti-HCV and HCV RNA negative at baseline and had HCV results for at least 2 study visits were included in the analysis and censored for each monitoring window when HCV infection was detected. Infection events included all participants with primary infection (no serological evidence of any previous infection) and those with confirmed reinfection after documented spontaneous clearance of HCV infection [18, 21]. Participants with evidence of chronic HCV throughout their time in the cohort follow-up were excluded. We estimated per-contact infectivity in 4 subgroups: (1) participants with incident primary HCV infection; (2) participants with phylogenetically confirmed reinfection events following spontaneous clearance; (3) participants with any HCV infection, including primary infection and reinfections; and (4) all participants in the analysis data set, including those in subgroups 1, 2, and 3 above, in addition to those who remained HCV negative throughout the entire study period.
Statistical Model and Analysis of Maximum Likelihood Estimate for Per-Contact Infectivity Rates
The estimated per-RNS event infectivity rate, βN, of HCV acquisition for receptive needle sharing was estimated via maximum likelihood. The following likelihood function, L, was used for a sample size of N participants and Si interviews per participant i:
This likelihood models the HCV transmission process as a series of Bernoulli trials for each participant visit interval, with outcome transmission probability, fij, for each representing the conditional probability of transmission occurrence as a function of βN and the number of reported RNS events, nij:
The outcome indicator yij represents the HCV infection status for each visit interval, where yij = 0 for HCV-negative participants and yij = 1 for HCV-positive participants. This transmission model implicitly assumes that all sharing events carry the same risk of infection.
The confidence interval of the per-contact infectivity of HCV associated with RNS was estimated using equal-tailed nonparametric bootstrap intervals at the 95% confidence level. To construct a bootstrap estimate of the maximum likelihood estimate sampling distribution, 1000 bootstrap resamples were obtained by sampling with replacement from PWID within the exposure data.
RESULTS
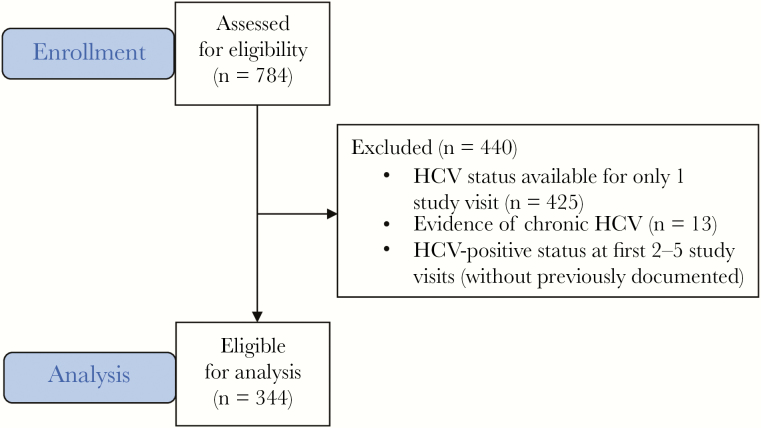
The UFO Study followed 784 participants between 2003 and 2014, of whom 344 (43.9%) were eligible for inclusion in this analysis (Figure 2). A total of 440 (56.1%) participants did not meet the inclusion criteria due to having no or limited follow-up data (425, 54.2%), HCV-positive status at all study visits with no previously documented HCV-negative test (13, 1.7%), or HCV-positive status for their first 2–5 study visits before clearing HCV infection, without a documented HCV-negative status before infection (2, 0.3%).
Figure 2.
Flowchart of study participants included in the analysis.
Table 1 shows baseline demographic characteristics and risk exposures of the participants included (n = 344) and excluded from the analysis (n = 440). The sample was predominantly male (61.6%), the median age at first injection (interquartile range [IQR]) was 18 years (16–21) years, and participants reported injecting mostly heroin (71.5%). Among those who reported injecting heroin at some point over the previous 3 months, the median (IQR) number of heroin injections in the last month was 15 (4–30). In the previous 3 months, about one-third (32.8%) of participants reported borrowing a used needle from another, and similarly 32.4% reported engaging in receptive ancillary equipment sharing. Among those included were 35 participants who became HCV positive without reporting any needle or ancillary equipment exposures in the preseroconversion window period.
Table 1.
Comparison of Baseline Interview Characteristics and Injection Risk Exposures of Study Participants Included in Analysis (n = 344) and Excluded From Analysis (n = 440)
| Participants Included (n = 344) | Participants Excluded (n = 440) | P Valuea | |
|---|---|---|---|
| Characteristic/Exposure | No. (%) | No. (%) | |
| Sex | .019 | ||
| Male | 212 (61.6) | 312 (70.9) | |
| Female | 102 (29.7) | 103 (23.4) | |
| Transgender or unreported | 30 (8.7) | 25 (5.7) | |
| Race/ethnicity | .158 | ||
| White/Caucasian/European American | 215 (62.5) | 310 (70.5) | |
| Black/African American | 12 (3.5) | 15 (3.4) | |
| Latino/a or Hispanic or Latin American | 16 (4.7) | 10 (2.3) | |
| Asian/Asian American/Filipino/a or Pacific Islander | 5 (1.5) | 3 (0.7) | |
| Native American | 7 (2.0) | 7 (1.6) | |
| Other | 89 (25.9) | 95 (21.6) | |
| Injected the following substance in the last 3 mob | |||
| Heroin | 246 (71.5) | 360 (81.8) | <.001 |
| Meth/amphetamine (speed) | 223 (64.8) | 256 (58.2) | .067 |
| Cocaine | 87 (25.3) | 123 (28.0) | .351 |
| Crack | 76 (22.1) | 109 (24.8) | .357 |
| Speedballs | 104 (30.2) | 168 (38.2) | .018 |
| Goofballs | 83 (24.1) | 109 (24.8) | .811 |
| Borrowed a used needle from another person (RNS) in last 3 mo | 113 (32.8) | 184 (41.8) | .010 |
| Engaged in receptive ancillary drug preparation equipment sharing in last 3 mo | 111 (32.3) | 185 (42.0) | .003 |
| Engaged in receptive backloading in last 3 mo | 208 (60.5) | 269 (61.1) | .709 |
| Needle used to backload was new (unused) | 68 (32.7) | 110 (40.9) | .263 |
| Median (IQR) | Median (IQR) | ||
| Age at enrollment, y | 24 (21–26) | 24 (21–26) | .219 |
| Age at first injection, y | 18 (16–21) | 17 (15–20) | .006 |
| Highest school grade completed | 12 (11–13) | 12 (10–13) | .489 |
| Days injected any substance including medication in past mo | 20 (6–30) | 20 (7–30) | .407 |
| No. of days injected substance in last moc | |||
| Heroin | 15 (4–30) | 18 (5–30) | .121 |
| Meth/amphetamine (speed) | 8 (3–15) | 5 (2–15) | .023 |
| Cocaine | 2 (1–4) | 2 (1–5) | .498 |
| Crack | 3.5 (1–7) | 2 (1–6) | .533 |
| Speedballs | 2 (1–7) | 3 (1–9.5) | .485 |
| Goofballs | 2 (1–5) | 2 (1–8) | .270 |
| Injections per day on injecting days in last mod | 3 (2–4) | 2. 5 (2–4) | .663 |
| No. of people with whom the subject injected in past moe | 3 (1–5) | 3 (2–6) | .240 |
| No. of people whose used needle the subject borrowed in the last 3 mof | 1 (1–2) | 1 (1–2) | .147 |
| No. of times engaged in RNS with the 3 people with whom subject injected (RNS) the most in the past mof,g | 2 (0–8) | 3 (1–7.5) | .346 |
Abbreviations: IQR, interquartile range; RNS, receptive needle sharing.
aChi-square test for categorical variables and Wilcoxon rank sum test (for comparison of medians) for continuous variables.
bTotal column percentage exceeds 100% due to participants reporting using more than 1 substance.
cAmong those who reported injecting the corresponding substance in the last 3 months.
dAmong those who reported injecting anything including medication in the last month (n = 560).
eAmong those who reported injecting with others at any point in the last 3 months (n = 528).
fAmong those who reported borrowing a previously used needle in the last 3 months (n = 204).
gNumber of times engaged in RNS in the last month: nij = nij1 + nij2 + nij3 for each participant, i, and each survey, j, where 1, 2, and 3 represent each of the 3 people with whom the participant reported engaging in RNS most frequently.
Compared with the 344 participants included, the 440 participants who did not meet inclusion criteria were more likely to be male (70.9% vs 61.6%; P = .019), to report younger age at first injection (median [IQR], 17 [15–20] vs 18 [16–21]; P = .006), and to report fewer days injecting meth/amphetamine in the past month (median [IQR], 5 [2–15] vs 8 [3–15]; P = .023); a higher proportion reported injecting heroin (81.8% vs 71.5%; P < .001) and speedballs (38.2 vs 30.2%; P = .018) in the previous 3 months. A higher proportion of those excluded compared with included engaged in RNS (41.8% vs 32.8%; P = .010) and receptive ancillary equipment sharing in the last 3 months (42% vs 32.3%; P = .003). There were no statistically significant differences between the groups by race/ethnicity (P = .158), receptive backloading (0.709), age at enrollment (P = .219), education (0.489), number of injecting days in the past month (P = .407) or injections per day in the past month (P = .663), number of people with whom they injected in the past month (P = .240), or number of times in the last month engaged in RNS with the 3 people with whom they injected most frequently (P = .346).
Among the 344 participants included, there were 118 (34.3%) with incident HCV infection, including primary infection (n = 101) and reinfections (n = 17) over the observation period. Among those with primary infection (n = 101), 11 (10.9%) cleared their infection at some point during their participation in the study (Supplementary Figure 1). Among participants with primary infection, the per-event infectivity of HCV associated with RNS (expressed as βN ×100) was 0.89% (95% CI, 0.20%–2.27%), and among those who cleared and experienced reinfection, the per-event infectivity of HCV associated with RNS was 1.62% (95% CI, 0.44%–8.14%) (Table 2). The per-contact infectivity of HCV by RNS exposure among participants who acquired a primary infection or reinfection was 1.12% (95% CI, 0.48%–2.36%). Over the entire at-risk study population, including those who acquired and did not acquire infection, the per-contact infectivity of HCV associated with RNS was 0.25% (95% CI, 0.10%–0.43%). In Supplementary Figure 2, we show the probability of a participant remaining seronegative in a monitoring window for RNS exposures given that the participant was observed to become seropositive, with right censoring for participants remaining seronegative (shown in Supplementary Figure 2). Results show very high risk of becoming HCV infected with few sharing events.
Table 2.
Per-Contact Infectivity (%) and 95% CI of HCV Acquisition Associated With Receptive Needle Sharing
| Group | No. | Infectivity, % | 95% CI, % |
|---|---|---|---|
| All participants pooled, including those who remained HCV negative | 344 | 0.25 | 0.10–0.43 |
| Participants with only primary HCV infection | 101 | 0.89 | 0.20–2.27 |
| Participants with reinfection (following clearance) | 17 | 1.62 | 0.44–8.14 |
| Participants with any HCV infection (primary or reinfection) | 118 | 1.12 | 0.48–2.36 |
Infectivity is from maximum likelihood estimate; confidence interval is estimated using the nonparametric bootstrap.
Abbreviations: CI, confidence interval; HCV, hepatitis C virus.
DISCUSSION
This study provides, to our knowledge, the first per-RNS event estimates of HCV infectivity specific to RNS exposures from prospective data in a population of young adult PWID. Our overall estimate of per-RNS event infectivity, 0.25% (95% CI, 0.10%–0.43%), represents the probability of acquisition of infection following needle sharing with any injecting partner irrespective of infection status. The overall prevalence of HCV viremia has ranged from 30% to 33% in this population since the study began in 2000 [22, 23], so up to one-third of sharing events could be with an infected person. However, sharing events may not be random; serosorting by HCV status can occur [24, 25], resulting in fewer HCV exposures. Thus, this overall approximation likely underestimates the probability of infection from RNS with an infected partner as the events counted in the denominator include RNS with uninfected participants. This underestimation can be viewed as attenuation from the true value caused by random measurement error in the event counts, a phenomenon familiar from similar models from the HIV transmission context [26].
The per-contact infectivity of RNS with infected partners is likely better represented in the subset of those with who acquired primary HCV and/or were reinfected after an RNS event as 1.12% (95% CI, 0.48%–2.36%) as it reflects having had contact with an HCV-positive person. Stratifying these 2 groups yields similar infectivities of 0.89% (95% CI, 0.20%–2.27%) for participants with primary infection only and 1.62% (95% CI, 0.44%–8.14%) for participants with at least 1 reinfection. Still, an estimate that includes RNS with persons who are not infected is most representative of the study population, as not all PWID are necessarily infected with HCV, not all previously used needles are contaminated with HCV when being reused by another person, and serosorting may occur [24, 25]. In addition, given the high incidence of HCV in young adult PWID, it is not possible to know the HCV status of one’s partners without very frequent testing. Furthermore, our overall estimate (0.25%; 95% CI, 0.10%–0.43%) is lower than the estimate of per-contact infectivity associated with any sharing of injection paraphernalia reported in a correctional population in Australia (0.57%; 95% CI, 0.32%–1.05%) [11], although these confidence intervals overlap. The higher infectivity estimate in that study is plausible, given that it was associated with a broad exposure characterization (any paraphernalia sharing—receptive or distributive), high background prevalence of viremia, and lower availability of sterile needles in the prison setting.
Our study has some limitations, including the assumption that the per-contact probability is constant. Contact patterns, for example, members or nonmembers of a social network, may impact infectivity [27]. Our use of exposure data from reported injecting partners and not random injecting partners could result in under- or overestimation of infectivity rates, based on differential behaviors or exposure with established vs nonestablished partnerships, such as cleaning syringes (the effectiveness of which is inconclusive [28, 29]). Within injecting partnerships, we have seen evidence of assortative risk and increased HCV incidence by perceived HCV status and age of injecting partners [24, 25, 30]. However, we have also shown that participants informed of new HCV infections do not change their injecting behaviors [31, 32]. Limiting exposure recall to 3 injecting partners could also result in underestimation. Infectivity could also be impacted by factors such as viremia level in the infected transmitting injecting partner, or being in the acute seronegative viremic infection period [33, 34]. We note that there has not been evidence of increasing or decreasing HCV incidence over time in the UFO Study cohort, suggesting some risk constancy. Another important consideration of this study is that the cohort enrolls only PWID under age 30, who, compared with older PWID, are more likely to be in an early phase of their injecting career and to have higher incidence of HCV relative to older PWID [2, 35]. Similarly, the risk in PWID populations in other locations may vary due to background HCV prevalence, thus impugning generalizability of findings to other locations.
As mentioned in the “Results,” 35 (10%) participants became infected with HCV but reported no RNS exposures (nor ancillary equipment sharing) before infection. Of these 35 participants, 31 had a primary infection and 4 were reinfected at least once during study observation. We intended to run a sensitivity analysis imputing exposure data from previous interviews, but 34 of 35 participants reported 0 exposures to RNS or ancillary equipment sharing at all of their previous study interviews. Other possible but unlikely routes of HCV acquisition include ancillary equipment sharing and backloading [16], as well as sexual transmission [36], although underreporting of RNS exposures is highly likely.
Higher-risk PWID were less likely to be included in this analysis, due in large part to loss to follow-up, which may contribute to underestimates of infectivity. Nondifferential underreporting of high-risk exposure such as RNS associated with social desirability of responses could also bias the infectivity estimate downward. Accordingly, our estimates likely represent a lower bound estimate of infectivity. This study is strengthened, however, by the relatively short 3-month recall and HCV testing periods. Thus, infection events are likely discrete events due to the frequent sampling periods and testing to ascertain infection status, clearance, and reinfection. Nevertheless, we acknowledge that more frequent testing or interviewing could result in more precise measures and possibly less underestimation [19, 37].
This analysis fills an important information gap on infectivity associated with a recognized and well-defined exposure associated with HCV transmission. HCV is extremely viable; it can survive in a syringe for up to 63 days [4] and up to 5 days on inanimate surfaces [5]. The probability of HCV transmission in association with injection drug use is generally assumed to be higher than that associated with HIV, not only in association with the higher background prevalence but also with the per-act probability. Hudgens et al. [38] estimated that the overall probability/transmission risk of acquiring HIV subtype 2 associated with needle sharing (with any partner irrespective of HIV status) among Thai injectors at 0.63% (or 63/10 000 exposures; 95% CI, 42–92/10 000 exposures) higher than our pooled population estimate (0.25%), but half that of our infectivity estimate among those who acquired any HCV (1.12%) and similar to the estimate found for HCV acquisition in association with any equipment sharing among prisoners in Australia [11]. HCV in association with injecting is higher than observed in health care workers exposed through needle sticks (0.50%; 95% CI, 0.39%–0.65%) [12]. It should be noted that higher estimates of infection probability have been estimated in association with hollow bore needles, deep injury, higher viremia, and HIV infection in the index case (>6 log copies/mL vs ≤4 log copies/mL) [39]. Our higher estimate of infection probability of 1.12% probability of any infection (primary or reinfection) likely reflects a more accurate acquisition risk given the significantly higher prevalence of HCV in the injecting population and the long survivability of the virus, which is higher than the per-event probability of HCV in the pooled susceptible population.
It is well accepted that HCV prevention should include strategies to reduce the number and frequency of high-risk exposures to HCV through RNS and that widespread availability of unused injecting paraphernalia via syringe service programs can reduce HCV incidence [40]. Treatment for opioid dependence can reduce incidence through reductions in injecting frequency [40, 41]. Provision of sterile water to promote safe drug apportionment has also been proposed [16]. Mathematical modeling has shown that HCV treatment has high potential to reduce HCV prevalence among PWID [42]. Infectivity estimates can further inform these and other models of prevention impact. These estimates should help to inform policies to increase the availability of clean and sterile injecting equipment to reduce RNS and HCV infection rates.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Dr. Helen Wearing for her review and comments on the manuscript.
Financial support. This study was supported by grant R01DA016017 (Page) from the National Institute on Drug Abuse and grant K24 AA022586 from the National Institutes of Alcohol Abuse and Alcoholism (Hahn), National Institutes of Health. Y.L. received additional support from Dialysis Clinic Inc. (DCI)—a national nonprofit corporation.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ly KN, Hughes EM, Jiles RB, Holmberg SD. Rising mortality associated with hepatitis C virus in the United States, 2003-2013. Clin Infect Dis 2016; 62:1287–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zibbell JE, Asher AK, Patel RC, et al. Increases in acute hepatitis c virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health 2018; 108(2):175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ciesek S, Friesland M, Steinmann J, et al. How stable is the hepatitis C virus (HCV)? Environmental stability of HCV and its susceptibility to chemical biocides. J Infect Dis 2010; 201:1859–66. [DOI] [PubMed] [Google Scholar]
- 4. Paintsil E, He H, Peters C, Lindenbach BD, Heimer R. Survival of hepatitis C virus in syringes: implication for transmission among injection drug users. J Infect Dis 2010; 202:984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doerrbecker J, Friesland M, Ciesek S, et al. Inactivation and survival of hepatitis C virus on inanimate surfaces. J Infect Dis 2011; 204:1830–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mather D, Crofts N. A computer model of the spread of hepatitis C virus among injecting drug users. Eur J Epidemiol 1999; 15:5–10. [DOI] [PubMed] [Google Scholar]
- 7. Hutchinson SJ, Bird SM, Taylor A, Goldberg DJ. Modelling the spread of hepatitis C virus infection among injecting drug users in Glasgow: implications for prevention. Int J Drug Policy 2006; 17:211–21. [Google Scholar]
- 8. Hahn JA, Wylie D, Dill J, et al. Potential impact of vaccination on the hepatitis C virus epidemic in injection drug users. Epidemics 2009; 1:47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corson S, Greenhalgh D, Hutchinson SJ. A time since onset of injection model for hepatitis C spread amongst injecting drug users. J Math Biol 2013; 66:935–78. [DOI] [PubMed] [Google Scholar]
- 10. Pouget ER, Hagan H, Des Jarlais DC. Meta-analysis of hepatitis C seroconversion in relation to shared syringes and drug preparation equipment. Addiction 2012; 107:1057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boelen L, Teutsch S, Wilson DP, et al. Per-event probability of hepatitis C infection during sharing of injecting equipment. PLoS One 2014; 9:e100749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jagger J, Puro V, De Carli G. Occupational transmission of hepatitis C virus. JAMA 2002; 288:1469; author reply 1469–71. [DOI] [PubMed] [Google Scholar]
- 13. Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. J Infect Dis 2002; 186:1558–64. [DOI] [PubMed] [Google Scholar]
- 14. Thorpe L, Ouellet L, Hershow R, Bailey S, Williams I, Monterosso E. The multiperson use of non-syringe injection equipment and risk of hepatitis C infection in a cohort of young adult injection drug users, Chicago 1997–1999 [poster]. Ann Epidemiol 2000; 10:472–3. [DOI] [PubMed] [Google Scholar]
- 15. Corson S, Greenhalgh D, Taylor A, Palmateer N, Goldberg D, Hutchinson S. Modelling the prevalence of HCV amongst people who inject drugs: an investigation into the risks associated with injecting paraphernalia sharing. Drug Alcohol Depend 2013; 133(1):172–9. [DOI] [PubMed] [Google Scholar]
- 16. Heimer R, Binka M, Koester S, et al. Recovery of infectious hepatitis C virus from injection paraphernalia: implications for prevention programs serving people who inject drugs. J Infect Dis 2018; 217:466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koester S, Heimer R, Barón AE, et al. Re: “Risk of hepatitis C virus among young adult injection drug users who share injection equipment.” Am J Epidemiol 2003; 157:376; author reply 376–8. [DOI] [PubMed] [Google Scholar]
- 18. Page K, Hahn JA, Evans J, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis 2009; 200:1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vickerman P, Grebely J, Dore GJ, et al. International Collaboration of Incident HIV and Hepatitis C in Injecting Cohorts (InC3); InC Collaborative Group The more you look, the more you find: effects of hepatitis C virus testing interval on reinfection incidence and clearance and implications for future vaccine study design. J Infect Dis 2012; 205:1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grebely J, Page K, Sacks-Davis R, et al. InC3 Study Group The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 2014; 59:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Page K, Osburn W, Evans J, et al. Frequent longitudinal sampling of hepatitis C virus infection in injection drug users reveals intermittently detectable viremia and reinfection. Clin Infect Dis 2013; 56:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus infection and needle exchange use among young injection drug users in San Francisco. Hepatology 2001; 34:180–7. [DOI] [PubMed] [Google Scholar]
- 23. Page K, Morris MD, Hahn JA, et al. Injection drug use and hepatitis C virus infection in young adult injectors: using evidence to inform comprehensive prevention. Clin Infect Dis 2013; 57(Suppl 2:S32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hahn JA, Evans JL, Davidson PJ, et al. Hepatitis C virus risk behaviors within the partnerships of young injecting drug users. Addiction 2010; 105:1254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Page K, Evans JL, Hahn JA, et al. HCV incidence is associated with injecting partner age and HCV serostatus mixing in young adults who inject drugs in San Francisco. PLoS One 2019; 14:e0226166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jewell NP, Shiboski SC. Statistical analysis of HIV infectivity based on partner studies. Biometrics 1990; 46:1133–50. [PubMed] [Google Scholar]
- 27. Yin Q, Shi T, Dong C, Yan Z. The impact of contact patterns on epidemic dynamics. PLoS One 2017; 12:e0173411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kapadia F, Vlahov D, Des Jarlais DC, et al. Second Collaborative Injection Drug User Study (CIDUS-II) Group Does bleach disinfection of syringes protect against hepatitis C infection among young adult injection drug users? Epidemiology 2002; 13:738–41. [DOI] [PubMed] [Google Scholar]
- 29. Hagan H, Thiede H. Does bleach disinfection of syringes help prevent hepatitis C virus transmission? Epidemiology 2003; 14:628–9; author reply 629. [DOI] [PubMed] [Google Scholar]
- 30. Page K, Evans JL, Hahn JA, Vickerman P, Shiboski S, Morris MD. HCV incidence is associated with injecting partner age and HCV serostatus mixing in young adults who inject drugs in San Francisco. PLoS One 2019; 14:e0226166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsui JI, Vittinghoff E, Hahn JA, et al. Risk behaviors after hepatitis C virus seroconversion in young injection drug users in San Francisco. Drug Alcohol Depend 2009; 105:160–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spelman T, Morris MD, Zang G, et al. International Collaborative of Incident HIV and Hepatitis C in Injecting Cohorts (InC3 Study) A longitudinal study of hepatitis C virus testing and infection status notification on behaviour change in people who inject drugs. J Epidemiol Community Health 2015; 69:745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hahn JA, Tully DC, Evans JL, et al. Role of HCV viremia in corroborated HCV transmission events within young adult injecting partnerships. Open Forum Infect Dis 2019; 6(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Major M, Gutfraind A, Shekhtman L, et al. Modeling of patient virus titers suggests that availability of a vaccine could reduce hepatitis C virus transmission among injecting drug users. Sci Transl Med 2018; 10:eaao4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hagan H, Pouget ER, Des Jarlais DC, Lelutiu-Weinberger C. Meta-regression of hepatitis C virus infection in relation to time since onset of illicit drug injection: the influence of time and place. Am J Epidemiol 2008; 168:1099–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Terrault NA, Dodge JL, Murphy EL, et al. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatology 2013; 57:881–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edlin BR, Shu MA, Winkelstein E, et al. More rare birds, and the occasional swan. Gastroenterology 2009; 136:2412–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hudgens MG, Longini IM Jr, Vanichseni S, et al. Subtype-specific transmission probabilities for human immunodeficiency virus type 1 among injecting drug users in Bangkok, Thailand. Am J Epidemiol 2002; 155:159–68. [DOI] [PubMed] [Google Scholar]
- 39. Yazdanpanah Y, De Carli G, Migueres B, et al. Risk factors for hepatitis C virus transmission to health care workers after occupational exposure: a European case-control study. Clin Infect Dis 2005; 41:1423–30. [DOI] [PubMed] [Google Scholar]
- 40. Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev 2017; 9:CD012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsui JI, Evans JL, Lum PJ, et al. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med 2014; 174:1974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin NK, Vickerman P, Foster GR, et al. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. J Hepatol 2011; 54:1137–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.