Abstract
Background
The objective of this study was to investigate whether 100% antiretroviral therapy (ART) adherence in men with HIV (MWH) is associated with normalization of concentrations of biomarkers of inflammation and immune activation compared with HIV-uninfected men.
Methods
We analyzed person-visits with available biomarker data from the Multicenter AIDS Cohort Study (MACS) among MWH receiving ART with HIV RNA <50 copies/mL and among HIV-uninfected men. Self-reported adherence was classified as 100% if no missed ART doses in the past 4 days were reported. We evaluated associations between ART adherence and concentrations of 24 serum biomarkers compared with HIV-uninfected visits using a generalized gamma model, adjusting for potential confounders.
Results
Person-visits (2565 from MWH reporting 100% ART adherence and 1588 from HIV-uninfected men) from a total of 1469 men were included in the analysis. Serum concentrations of interleukin-6 (IL-6), soluble interleukin-6 receptor (sIL-6R), IL-1β, interferon-γ (IFN-γ), chemokine C-C motif ligand 2 (CCL2), and CCL14 from person-visits among MWH who reported 100% adherence were similar to HIV-uninfected person-visits. Comparatively higher concentrations of 11 biomarkers and lower concentrations of 7 biomarkers were observed in person-visits from MWH who reported 100% ART adherence, compared with HIV-uninfected person-visits.
Conclusions
Although MWH with virologic suppression who reported 100% ART adherence exhibited overall higher concentrations of biomarkers of inflammation and immune activation compared with HIV-uninfected men, some biomarker concentrations were similar in both groups. These findings suggest that optimal ART adherence could have clinical implications beyond achieving and sustaining viral suppression.
Keywords: adherence, antiretroviral therapy, HIV, inflammation
Antiretroviral therapy (ART) use has allowed for longer and healthier lifespans in persons with HIV (PWH). However, ART optimization requires substantial and durable adherence to achieve and maintain virologic suppression. Although modern ART regimens have become more forgiving of suboptimal adherence with respect to virologic suppression (which is usually viewed as an advantage) [1], the gap between virally suppressive and optimal adherence may have biological implications that remain largely unknown.
Suboptimal adherence may drive the residual inflammation, immune activation, and coagulopathy that have been associated with the development of serious non-AIDS events in PWH despite sustained virologic suppression [2, 3]. This adherence–inflammation association has been demonstrated in several clinical and research cohorts using different adherence measures [4–7]. Data from the Multicenter AIDS Cohort Study (MACS) revealed that men with HIV (MWH) who reported <100% ART adherence (using 4-day and 6-month recall periods) had 11% to 21% higher plasma concentrations of interleukin (IL)-10, tumor necrosis factor–alpha (TNF-α), IL-6, IL-2, interferon-gamma (IFN-γ), and C-reactive protein (CRP) compared with MWH who reported 100% adherence [4]. These findings led us to hypothesize that PWH who are optimally ART-adherent could have an inflammatory profile that more closely resembles persons without HIV.
To address this question, we investigated whether 100% self-reported ART adherence is associated with normalization of biomarkers of inflammation and immune activation among virologically suppressed MWH when compared with HIV-uninfected men.
METHODS
Study Design and Participants
We evaluated selected person-visits between October 1998 and September 2009 from men enrolled in the MACS. The MACS is an ongoing multicenter study of HIV-1 infection among men who have sex with men, conducted at 4 sites within the United States: Baltimore, Maryland/Washington, DC; Chicago, Illinois; Los Angeles, California; and Pittsburgh, Pennsylvania [8]. MACS participants are evaluated at 6-month intervals, with comprehensive study visits (person-visits) that include standardized interviews, physical examinations, and blood sampling for laboratory analyses and storage for future research. MACS study protocols at all clinical sites were approved by local institutional review boards, and all participants signed an informed consent before enrollment or any study procedures.
Serum biomarker data were available from selected MACS participants and person-visits from a previously described study, where samples were selected at 1-year intervals for men with known HIV seroconversion dates and from visits immediately before and after ART initiation (and every 2 years) for all ART recipients [9]. Four visits from 250 participants who were not HIV-infected were assayed as controls (obtained between 1984 and 2009) [9]. For all person-visits selected for the current study in men with HIV infection on ART, simultaneous HIV viral load (VL) and biomarker data were available. To focus our analyses on MWH receiving virally suppressive ART, we restricted the current study to person-visits where ART use was reported and plasma HIV RNA levels were <50 copies/mL (Roche Amplicor assay). ART was defined as a drug regimen that contained at least 3 antiretrovirals, including 2 nucleoside reverse-transcriptase inhibitors (NRTIs) combined with either an unboosted protease inhibitor (PI), a boosted PI (b/PI), or a non-nucleoside reverse-transcriptase inhibitor (NNRTI). NRTI-only regimens were also included. Given the calendar period evaluated (1984–2009), this analysis did not include person-visits from men receiving integrase strand transfer inhibitor (INSTI)–based ART. For comparison, we included person-visits (with biomarker data) contributed by HIV-uninfected men in that same period. These HIV-uninfected person-visits were drawn from the 250 original HIV-uninfected controls as well as from person-visits from MACS participants before documented HIV seroconversion.
Antiretroviral Adherence Evaluation
ART adherence at each person-visit in MWH was classified using self-reported data collected at that study visit, as previously reported in the MACS [1, 4]. Men were asked about the number of pills taken over the prior 4 days for each medication in their ART regimen. To assess 4-day adherence, a percentage of expected adherence was calculated using the following formula: (∑# of times drug taken over 4 days)/(∑# of times per day drug prescribed*4) [1, 4]. If different values of adherence were reported across ART drugs, the lowest adherence percentage calculated was used. Men were also asked whether this 4-day ART intake was typical of their use since their last study visit [1, 4]. Men were classified as 100% adherent if they reported no missed doses in the past 4 days and if they reported that this adherence pattern was typical since their last study visit. Any different response was assigned <100% adherence [4]. We used the term “4-day adherence” for this metric, though it encompasses information pertaining to the 6-month period since the last visit.
Biomarkers of Inflammation and Immune Activation
Serum biomarkers of inflammation and immune activation were quantified using the MesoScale Discovery (MSD, Gaithersburg, MD, USA) and Luminex (Luminex, Austin, TX, USA) platforms or immunonephelometric assays (high-sensitivity C-reactive protein) by a clinical reference laboratory (Quest Diagnostics, Madison, NJ, USA), as previously described in the MACS [4, 9, 10]. CD4+/CD8+ T-cell immunophenotyping was performed using flow cytometry as previously reported in the MACS [11].
Statistical Analysis and Covariate Definitions
Our primary comparison of biomarker concentrations was between 2 groups of person-visits: (1) visits contributed by MWH taking ART at which HIV VL was <50 copies/mL and 100% ART adherence was reported and (2) visits contributed by HIV-uninfected men. We also compared biomarker concentrations in a third group of person-visits contributed by MWH at which there was an undetectable HIV VL but <100% ART adherence was reported. Individual participants could contribute multiple person-visits if eligible, and men could contribute to any of the 3 comparison groups if they became HIV-infected while under observation and/or varied in reported adherence from visit to visit and met eligibility criteria.
Because the distributions of biomarker concentrations were heterogeneous, we modeled them parametrically using the flexible generalized gamma model, an approach we have used previously with these data [4, 9]. In multivariable models, we allowed covariates to affect the location (β) parameter while holding the scale (σ) and shape (λ) parameters constant. Herein, we present results from these models by translating covariate effects on the β parameter as a constant percent shift in biomarker distribution across percentiles. Concentrations below the lower limit of detection (LLD) for a given assay were handled by modeling the inverse of concentrations and thus employing standard methods for right-censored survival data.
We included covariates as possible confounders in our models based on associations with exposure and outcome; those described below were included in final models. All covariate data were obtained from the MACS database. Age (continuous), race (white/nonwhite), and tobacco smoking at the time of visit (yes/no) were ascertained at study visits via self-report. Infection with hepatitis C virus (HCV) was defined by the presence of detectable plasma HCV RNA, and infection with hepatitis B virus (HBV) was defined by the presence of HBV surface antigen. Depressive symptoms were defined as a Center for Epidemiologic Studies Depression score ≥16. Diabetes mellitus was defined as an HbA1c level ≥6.5%, a fasting glucose level ≥126 mg/dL, or the use of antidiabetic medications. Anemia was defined as a hemoglobin concentration below the 5th percentile of the general population. Hypertension was defined as either systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or the use of antihypertensive medications. Absolute CD4+ T lymphocyte counts (cells/μL) were determined by flow cytometry.
We used models that accommodated repeated measurements per individual. Because we tested relationships between adherence and 24 different biomarker concentrations, we adjusted for multiple tests by controlling the false discovery rate at 5% using the Benjamini-Hochberg procedure [4, 12]. Spearman rank analysis was utilized to determine the correlation coefficient (ρ) between the biomarkers in the study population. Analyses were conducted using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA), and Stata 13 (StataCorp LP, College Station, TX, USA).
Sensitivity Analyses
To examine whether person-visits from a subset of men reporting optimal treatment success (ie, self-reported perfect adherence and early and sustained viral suppression) exhibit biomarker levels more similar to HIV-uninfected person-visits than to the entire group of 100% adherent person-visits, we performed a supplementary analysis that restricted the MWH study population to an “elite” group of highly adherent participants and person-visits. This group met the following criteria: (a) initiated ART at a high CD4+ T-lymphocyte count (>500 cells/mm3), (b) reported 100% ART adherence at every study visit since ART initiation, (c) never had a post-ART visit with an HIV VL >1000 copies/mL, and (d) never had a gap longer than 2 years between study visits. This elite adherent group was compared with all HIV-uninfected person-visits from the main analysis.
RESULTS
Study Population
The demographic characteristics of the participants and person-visits included in this analysis are presented in Table 1. Overall, 1469 men contributed 4530 person-visits from 1984 to 2009, with a median age that ranged between 42 and 49 years across the visits. Of these visits, 1588 visits involved 652 men who were HIV-uninfected at that visit, 2565 were from 905 HIV-suppressed MWH receiving ART and reporting 100% 4-day adherence, and 377 were from 255 HIV-suppressed MWH receiving ART and reporting <100% 4-day adherence.
Table 1.
Characteristics of Study Participants and Person-Visits, Stratified by HIV Status and Self-Reported 4-Day ART Adherence
| Participants, No. (%) or Median (IQR) | |||
|---|---|---|---|
| HIV-Uninfected at an Included Visita | 100% ART Adherence at an Included Visita | <100% ART Adherence at an Included Visita | |
| Unique MACS participants (n = 1469)a | 652 | 905 | 255 |
| Hispanic | 47 (7) | 139 (15) | 39 (15) |
| Black, non-Hispanic | 138 (21) | 220 (24) | 65 (25) |
| White, non-Hispanic | 463 (71) | 532 (59) | 147 (58) |
| Other race | 4 (1) | 14 (2) | 4 (2) |
| No. of visits per participanta | 2 (1–4) | 2 (1–4) | 1 (1–2) |
| Person-Visits, No. (%) or Median (IQR) | |||
| HIV Uninfected at Visita | 100% ART Adherence at Visita | <100% ART Adherence at Visita | |
| Person-visits (n = 4530)a | 1588 | 2565 | 377 |
| Calendar year at visit | 2002 (1988–2006) | 2006 (2004–2008) | 2006 (2003–2008) |
| Age at visit, y | 42 (34–49) | 49 (43–54) | 47 (43–54) |
| Cumulative years of ART at time of visit | - | 5.2 (2.9–7.9) | 5.6 (3.1–8.1) |
| Type of ART at visit | |||
| Boosted PI-based | - | 858 (33) | 146 (39) |
| Unboosted PI-based | - | 385 (15) | 62 (16) |
| NNRTI-based, no PI | - | 1242 (48) | 149 (40) |
| NRTI/other-based, no PI | - | 80 (3) | 20 (5) |
| CD4+ T-cell count at visit | - | 582 (422–773) | 582 (440–769) |
| >500 cells/μL | - | 1578 (63) | 226 (62) |
| 351–500 cells/μL | - | 530 (21) | 98 (27) |
| 201–350 cells/μL | - | 299 (12) | 30 (8) |
| ≤200 cells/μL | - | 100 (4) | 12 (3) |
| Other factors at time of visitb | |||
| Obesity | 221 (14) | 296 (12) | 37 (10) |
| Hepatitis B infection | 42 (3) | 115 (4) | 18 (5) |
| Hepatitis C infection | 253 (16) | 199 (8) | 25 (7) |
| Tobacco smoking | 639 (40) | 718 (28) | 100 (27) |
| Diabetes mellitus | 106 (7) | 268 (10) | 43 (11) |
| Anemia | 158 (10) | 338 (13) | 43 (11) |
| Hypertension | 345 (22) | 580 (23) | 73 (19) |
| Statin use | 91 (11) | 754 (32) | 110 (31) |
| Depressive symptoms | 379 (24) | 577 (22) | 102 (27) |
Abbreviations: ART, antiretroviral therapy; IQR, interquartile range; MACS, Multicenter AIDS Cohort Study; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
aSome participants contributed to visits in more than 1 category.
bObesity was defined as body mass index ≥30 kg/m2. Diabetes mellitus was defined as hemoglobin A1C ≥6.5%, fasting glucose ≥126 mg/dL, or use of antidiabetic medications. Anemia was defined as hemoglobin <5th percentile of the general population. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg or use of antihypertensive medications. Depressive symptoms were defined as Center for Epidemiologic Studies–Depression score ≥16.
As noted, individual men could contribute multiple person-visits to multiple categories. The median contribution (interquartile range [IQR]) was 3 (2–4) person-visits per participant across all categories. MWH on ART had been receiving ART for a median (IQR) of 5 (3–8) years at each person-visit. The median (IQR) CD4+ T-lymphocyte count at person-visits where 100% ART adherence was reported was similar to person-visits where <100% ART adherence was reported (582 [422–773] cells/mm3 vs 582 [440–769] cells/mm3) (Table 1) and ranged between 312 cells/mm3 and 322 cells/mm3 before ART initiation in both groups (data not shown).
Biomarker Concentrations at Person-Visits From MWH Reporting 100% ART Adherence Relative to HIV-Uninfected Person-Visits
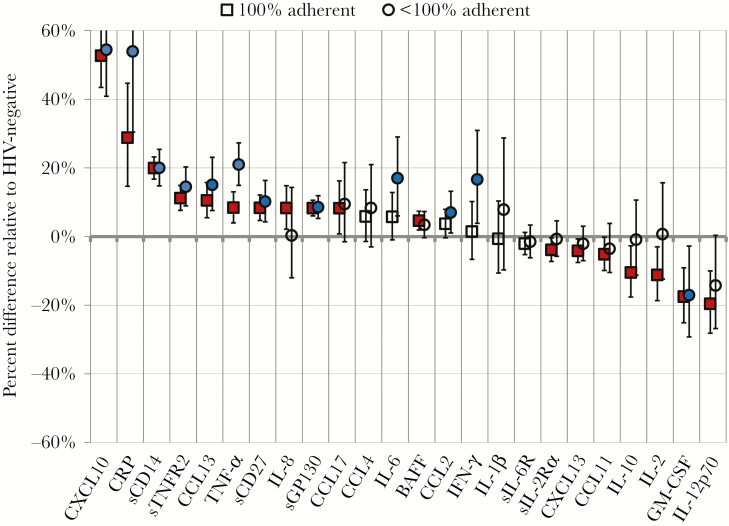
Biomarker concentrations by quartile are shown in Supplementary Table 1. In adjusted models, no significant differences in serum biomarker concentrations among person-visits of HIV-suppressed men where 100% ART adherence was reported compared with person-visits of HIV-uninfected men were observed for the following biomarkers: IL-6 (6%), CCL4 (6%), CCL2 (4%), IFN-γ (1%), IL1-β (–1%), and sIL-6R (–2%; all P > .05) (Supplementary Table 2). Significantly higher concentrations of 11 biomarkers were observed among person-visits of HIV-suppressed men where 100% ART adherence was reported compared with person-visits of HIV-uninfected men (Figure 1; Supplementary Table 2): CXCL10 (53%), CRP (28%), sCD14 (20%), sTNFR2 (11%), CCL13 (11%), TNF-α (8%), sCD27 (8%), sGP130 (8%), IL-8 (8%), CCL17 (8%), and BAFF (5%; all P < .05). After adjusting for multiple comparisons, significantly higher biomarker concentrations in 9 biomarkers were still observed among person-visits of HIV-suppressed men where 100% ART adherence was reported: CXCL10, CRP, sCD14, sTNFR2, CCL13, TNF-α, sCD27, sGP130 (all P < .001), and BAFF (P = .001). HIV-suppressed person-visits where 100% ART adherence was reported revealed lower concentrations of 7 biomarkers relative to person-visits from men without HIV: sIL-2Rα (–4%), CXCL13 (–4%), CCL11 (–5%), IL-10 (–11%), IL-2 (–11%), GM-CSF (–18%), and IL-12p70 (–20%; all P < .05). After adjusting for multiple comparisons, only GM-CSF (–18%) and IL-12p70 (–20%) remained significantly lower (both P < .001) (Supplementary Table 2). Significant correlation among many of the assayed biomarkers was observed (Supplementary Table 3), with high correlation (ρ > 0.40) among some biomarkers with similar function (eg, IL-6 vs CRP, IL-6 vs TNF-α, and sCD27 vs sTNFR2).
Figure 1.
Percent shifts in distribution of biomarker concentrations in person-visits from MWH with 100% and <100% 4-day self-reported ART adherence, compared with HIV-uninfected person-visits. Biomarker data were analyzed at person-visits where HIV-infected men reported taking ART and had plasma HIV RNA levels <50 copies/mL. Person-visits were considered adherent (squares) if self-reported adherence for the previous 4 days was 100%, and nonadherent (circles) if reported adherence for the previous 4 days was <100%. Generalized gamma models were adjusted for age, race, hepatitis B and C virus infection, smoking, depressive symptoms, diabetes mellitus, anemia, hypertension, and CD4+ T-lymphocyte cell count. Error bars represent 95% confidence intervals. Red-filled squares and blue-filled circles indicate hazard ratios that are statistically significant at P < .05. Abbreviations: ART, antiretroviral therapy; BAFF, B-cell activating factor; CCL, chemokine C-C motif ligand; CRP, C-reactive protein; CXCL, chemokine C-X-C motif ligand; GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN-γ, interferon γ; IL, interleukin; MWH, men with HIV; sCD14, soluble CD14; sCD27, soluble CD27; sgp130, soluble glycoprotein 130; sIL-2Rα, soluble IL-2 receptor-α; sIL-6R, soluble IL-6 receptor; sTNF-R2, soluble tumor necrosis factor receptor 2; TNF-α, tumor necrosis factor α.
Consistent with a previous report directly comparing adherent with nonadherent person-visits [4], person-visits where <100% adherence was reported compared with visits from HIV-uninfected person-visits revealed relatively higher concentrations of CRP, TNF-α, IL-6, IFN-γ, IL-10, and IL-2 compared with person-visits where 100% adherence was reported (Figure 1; Supplementary Table 2) [4]. The CD4+/CD8+ T-cell ratio, which has been associated with clinical outcomes and immune recovery in persons with HIV [13, 14], showed no differences in person-visits where 100% ART adherence was reported when compared with person-visits where <100% adherence was reported, although both groups had a lower ratio relative to men without HIV (data not shown). Similarly, no consistent significant differences were observed when comparing NNRTI-based with boosted PI-based ART in the study population (data not shown).
Sensitivity Analyses of Biomarker Levels in Participants With Elite ART Adherence Relative to HIV-Uninfected Person-Visits
A subset of 219 person-visits, contributed by 86 MWH, met the definition of “elite” adherence specified in the “Methods.” The characteristics of this study population are displayed in Supplementary Table 4. In this group of participants and person-visits, representing optimal ART adherence and clinical responses to ART, we observed findings that were broadly similar to the results from our primary analysis. Of the 11 biomarkers with concentrations that were elevated in the analysis of all 100% adherent person-visits relative to HIV-uninfected person-visits, 9 were also significantly higher among elite adherent person-visits: CXCL10 (49%), CRP (29%), sCD14 (26%), IL-8 (23%), sTNFR2 (14%), sCD27 (14%), sGP130 (11%), CCL13 (10%), and BAFF (6%; all P < .05) (Supplementary Figure 1, Supplementary Table 5). Of these, 5 biomarkers (CXCL10, sCD14, IL-8, sCD27, and sGP130) remained significant after adjusting for multiple comparisons (P < .001). Serum concentrations of IL-12p70 (–10%), IL-2 (–12%), IL-10 (–15%), CXCL13 (–3%), and GM-CSF (–15%) remained lower at elite adherent person-visits relative to HIV-uninfected person-visits, as they had been in the primary analysis, but these differences were not statistically significant (Supplementary Table 5). Finally, in this subpopulation of person-visits, no significant differences in IL-6, IFN- γ, sIL-6R, CCL4, CCL2, and IL-1β were observed, similar to the findings in the main analysis.
Discussion
Our findings demonstrate that MWH who are considered successfully treated (ie, are virologically suppressed on ART and report 100% adherence) still exhibit a wide array of differences in concentrations of biomarkers of inflammation and immune activation relative to HIV-uninfected men. These findings, including higher concentrations of several biomarkers of inflammation such as TNF-α, sTNFR2, CRP, sCD14, and IL-8, are consistent with our previous observations in all virologically suppressed post-ART visits [9]. Conversely, the person-visits at which 100% adherence was reported demonstrated lower concentrations of the anti-inflammatory cytokine IL-10 and the T-cell-stimulatory cytokine IL-2 and similar concentrations of IFN-γ, the pro-inflammatory cytokine IL-6, and the monocyte chemoattractant CCL2, compared with HIV-uninfected person-visits. Concentrations remained significantly elevated for 5 biomarkers (sCD14, IL8, CXCL10, sCD27, sGP130) even when analyses were adjusted for multiple tests and restricted to adherent person-visits from a much smaller subset that represented a “best-case” scenario in HIV treatment: early ART initiation, persistent virologic suppression, and no reports of nonadherence. These findings confirm that some forms of immune dysregulation persist among persons with treated HIV infection, even with apparent virologic suppression and a high degree of adherence to ART. However, our findings specific to IL-6—which did not differ between HIV-uninfected person-visits and those where 100% adherence was reported but which were significantly higher in person-visits with <100% adherence—suggest that some measures of dysregulation may be “normalized” only in those individuals who are highly adherent to ART. To our knowledge, this is the first report in which optimal ART adherence has been associated with normalization of some measures of residual inflammation and immune activation among MWH who are virologically suppressed on ART.
The residual immune dysregulation among optimally adherent MWH compared with men without HIV does not imply that the importance of ART adherence is, in any way, diminished. Although 100% adherence among MWH with suppressed viremia was still associated with higher concentrations of several inflammatory biomarkers when compared with person-visits from HIV-uninfected men, these biomarker concentrations were lower when compared with persons-visit reporting <100% adherence, consistent with previous reports [4–7]. Most importantly, our findings that concentrations of certain biomarkers in person-visits from MWH who reported 100% ART adherence were comparable to those observed in HIV-uninfected men is noteworthy. In particular, the findings specific to IL-6—which has been consistently associated with high morbidity and mortality among treated persons with HIV [2, 15]—provide clinical context for these results, suggesting that high ART adherence may contribute to the reduction in residual inflammation observed in MWH on suppressive ART by improving at least 1 pathway in this population and that optimal ART adherence (beyond suppression) could have an impact on mortality.
Although provocative, the mechanisms behind our findings are yet to be fully determined. It has been previously proposed that HIV replication below the limit of detection when adherence is <100% is a potential mechanism driving these observations [4, 5], which could explain the reduction of IL-6 or IFN-γ to levels similar to HIV-uninfected person-visits when adherence was reported to be 100%. However, studies evaluating whether residual viral replication is a driver of inflammation have yielded mixed results. For example, some studies have demonstrated a relationship between variations in ART adherence and residual systemic viral replication, using ultrasensitive single-copy assays [16–18], that has been associated with enhanced inflammation [19–22] and cardiovascular morbidity [23, 24]. However, a recent study in a cohort of 110 long-standing, virologically suppressed individuals did not find an association between residual replication (measured using single-copy assays) and biomarkers of inflammation [25], or between cumulative ART exposure (assessed through antiretroviral hair concentrations) and these outcomes [26]. These discrepant results demonstrate the need for further research in the field and the need to comprehensively assess ART adherence and exposure when designing and interpreting results of studies on HIV persistence and chronic inflammation.
The normalization in the concentrations of some biomarkers of inflammation observed in this study among participants who reported early and durable 100% ART adherence raises the question of whether improving ART adherence in PWH who are virally suppressed could lead to decreases in inflammation. Although this has not been yet demonstrated, 2 ART intensification trials—where additional antiretrovirals were added to already suppressive ART—offer indirect evidence suggesting that this may be a possibility. In these studies, reductions in CD4+ T-cell activation and residual viremia were observed among participants randomized to placebo intensification [6, 27, 28], an observation that could be explained by increases in ART adherence related to participation in a clinical trial (ie, Hawthorne effect) [29]. Of note, our study identified a subset of biomarkers (ie, IL-8, BAFF) for which serum concentrations were higher in person-visits from MWH who reported 100% ART adherence vs person-visits from HIV-uninfected men and those who reported <100% adherence. Although a direct mechanism for these findings remains unclear, these results are consistent with recent data demonstrating higher plasma concentrations of several biomarkers of endothelial and immune activation in PWH with long-standing suppression who had high levels of tenofovir exposure quantified using tenofovir diphosphate measured in dried blood spots [30]. Further research to evaluate whether adherence variations can significantly impact residual inflammation, immune activation, and clinical outcomes is required.
Our study has several strengths. First, this analysis included a large, well-characterized study population that contributed prospectively collected samples, allowing for an assessment of a comprehensive panel of biomarkers of inflammation and immune activation. Second, the MACS is particularly well-suited for this study because person-visits from HIV-uninfected men were drawn from the same source population (and, in some cases, from the same individuals) as person-visits from MWH. Third, our findings are clinically relevant, as they suggest that management of complications associated with chronic inflammation (and perhaps even treatments directed at inflammation) will be necessary for optimal care of PWH, even when they are virologically suppressed and fully adherent to ART. The main limitations of our report include the inherent subjective nature of self-report as a measure of ART adherence. In addition, there is a possibility of uncontrolled confounding, including the possibility that highly adherent MWH could be healthier in unmeasured ways (eg, exercise or diet) and that the men included in this analysis may have variable competing non-HIV risks that promote inflammation. In addition, this analysis focused on non-INSTI-based ART regimens, which may have a more modest effect on inflammation compared with INSTIs [31]. Furthermore, and limiting the generalizability of our findings, the number of study visits that met the definition of “elite” adherence was small, and the study was restricted to men, with only a small proportion of MWH who were younger than 30 years of age. Finally, our assessments of differences in biomarker concentrations are statistical and not clinical, as little is known about the causal links between actual biomarker concentrations and clinical outcomes.
In conclusion, our findings reinforce the view of HIV infection as a disturbance mediated through inflammatory and immune activation pathways that is only partially reversed via virally suppressive ART, even among MWH who are highly adherent. However, these findings give rise to further speculation as to whether this immune dysregulation is partially responsive to full and sustained ART adherence. Given the persisting disparities in morbidity and mortality that remain in PWH, even among “well-treated” persons, this study suggests a potential role for optimization of ART adherence to further attenuate these discordances and thereby exert a beneficial clinical impact on MWH receiving ART.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Multicenter AIDS Cohort Study (Principal Investigators). Johns Hopkins University Bloomberg School of Public Health (Joseph Margolick, Todd Brown), U01-AI35042; Northwestern University (Steven Wolinsky), U01-AI35039; University of California, Los Angeles (Roger Detels, Otoniel Martinez-Maza), U01-AI35040; University of Pittsburgh (Charles Rinaldo, Jeremy Martinson), U01-AI35041; the Center for Analysis and Management of MACS, Johns Hopkins University Bloomberg School of Public Health (Lisa Jacobson, Gypsyamber D’Souza), UM1-AI35043. The MACS website is located at http://aidscohortstudy.org/.
Financial support. Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS). The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. The research was also supported by the HIV Prevention Trials Network (HPTN), sponsored by the NIAID, NIDA, NIMH, and the Office of AIDS Research, of the NIH, Department of Health and Human Services (DHHS; UM1-AI068613). J.C.M. is supported in part by NIH/NIAID K23 AI104315 and R21 AI124859. T.T.B. is supported in part by NIH/NIAID K24 AI120834.
Disclaimer. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the NIH, Johns Hopkins ICTR, or NCATS.
Potential conflicts of interest. T.T.B. has served as a consultant for Gilead Sciences, Merck, ViiV Healthcare, BMS, EMD-Serono, and Theratechnologies. F.J.P. has served as a speaker and/or consultant for Gilead Sciences, Janssen, ViiV, Merck and Co, and Theratechnologies. B.J.C.M. has a research grant from Gilead Sciences. All other authors: no conflicts reported. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. J.R.C.M. led the conception and study design, generated the concept proposal, led the research team and interpretation of results, wrote the first manuscript draft, and performed all the edits for all the subsequent drafts. T.T.B. co-led the study conception, design, interpretation, and analysis, made substantial edits, and contributed to critical revision of the manuscript. F.J.P. contributed to cohort management, assisted with interpretation of the results, made substantial edits, and contributed to critical revision of the manuscript. B.J.C.M. contributed to the cohort, assisted with interpretation of the results, and performed manuscript editing and revision. E.C.B. participated in study design, performed oversight of the biomarker testing and analysis, assisted with interpretation of the results, performed edits, and contributed to critical revision of the manuscript. L.P.J. assisted with study design and data management, made substantial edits, and contributed to critical revision of the manuscript. N.I.W. co-led the study conception, design, and data acquisition, performed the statistical analyses, generated the tables and figures, assisted with data analysis and interpretation of the results, made substantial edits, and contributed to critical revision of the manuscript.
Prior presentation. These data have been previously presented (in part) at the 20th International Workshop of Co-morbidities and Adverse Drug Reactions in HIV; October 13–14, 2018; New York, NY. Abstract ADRLH-81.
References
- 1. Viswanathan S, Detels R, Mehta SH, et al. Level of adherence and HIV RNA suppression in the current era of highly active antiretroviral therapy (HAART). AIDS Behav 2015; 19:601–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Borges ÁH, O’Connor JL, Phillips AN, et al. ; INSIGHT SMART Study and ESPRIT Groups Interleukin 6 is a stronger predictor of clinical events than high-sensitivity C-reactive protein or D-dimer during HIV infection. J Infect Dis 2016; 214:408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunt PW, Lee SA, Siedner MJ. Immunologic biomarkers, morbidity, and mortality in treated HIV infection. J Infect Dis 2016; 214(Suppl 2):S44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castillo-Mancilla JR, Brown TT, Erlandson KM, et al. Suboptimal adherence to combination antiretroviral therapy is associated with higher levels of inflammation despite HIV suppression. Clin Infect Dis 2016; 63:1661–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castillo-Mancilla JR, Phillips AN, Neaton JD, et al. ; INSIGHT SMART Study Group Association of suboptimal antiretroviral therapy adherence with inflammation in virologically suppressed individuals enrolled in the SMART study. Open Forum Infect Dis 2018; 5(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castillo-Mancilla JR, Morrow M, Boum Y, et al. Brief report: higher ART adherence is associated with lower systemic inflammation in treatment-naive ugandans who achieve virologic suppression. J Acquir Immune Defic Syndr 2018; 77:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Castillo‐Mancilla JR, Phillips AN, Neaton JD, et al. Incomplete ART adherence is associated with higher inflammation in individuals who achieved virologic suppression in the START study. J Int AIDS Soc 2019; 22:e25297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126:310–8. [DOI] [PubMed] [Google Scholar]
- 9. Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McKay HS, Margolick JB, Martínez-Maza O, et al. Multiplex assay reliability and long-term intra-individual variation of serologic inflammatory biomarkers. Cytokine 2017; 90:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aziz N, Jamieson BD, Quint JJ, et al. Longitudinal intra- and inter-individual variation in T-cell subsets of HIV-infected and uninfected men participating in the LA Multi-Center AIDS Cohort Study. Medicine (Baltimore) 2019; 98:e17525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 1995; 57:289–300. [Google Scholar]
- 13. Lu W, Mehraj V, Vyboh K, et al. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV-positive patients. J Int AIDS Soc 2015; 18:20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog 2014; 10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Borges ÁH, O’Connor JL, Phillips AN, et al. ; INSIGHT SMART and ESPRIT Study Groups and the SILCAAT Scientific Committee Factors associated with plasma IL-6 levels during HIV infection. J Infect Dis 2015; 212:585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li JZ, Gallien S, Ribaudo H, et al. Incomplete adherence to antiretroviral therapy is associated with higher levels of residual HIV-1 viremia. AIDS 2014; 28:181–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Podsadecki TJ, Vrijens BC, Tousset EP, et al. Decreased adherence to antiretroviral therapy observed prior to transient human immunodeficiency virus type 1 viremia. J Infect Dis 2007; 196:1773–8. [DOI] [PubMed] [Google Scholar]
- 18. Pasternak AO, de Bruin M, Jurriaans S, et al. Modest nonadherence to antiretroviral therapy promotes residual HIV-1 replication in the absence of virological rebound in plasma. J Infect Dis 2012; 206:1443–52. [DOI] [PubMed] [Google Scholar]
- 19. Mavigner M, Delobel P, Cazabat M, et al. HIV-1 residual viremia correlates with persistent T-cell activation in poor immunological responders to combination antiretroviral therapy. PLoS One 2009; 4:e7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ostrowski SR, Katzenstein TL, Pedersen BK, et al. Residual viraemia in HIV-1-infected patients with plasma viral load <or = 20 copies/ml is associated with increased blood levels of soluble immune activation markers. Scand J Immunol 2008; 68:652–60. [DOI] [PubMed] [Google Scholar]
- 21. Falasca F, Di Carlo D, De Vito C, et al. Evaluation of HIV-DNA and inflammatory markers in HIV-infected individuals with different viral load patterns. BMC Infect Dis 2017; 17:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruggiero A, Cozzi-Lepri A, Beloukas A, et al. ; ERAS Study Group Factors associated with persistence of plasma HIV-1 RNA during long-term continuously suppressive firstline antiretroviral therapy. Open Forum Infect Dis 2018; 5(X):XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boyd A, Meynard JL, Morand-Joubert L, et al. ; Collaboration in HIV, Inflammation and Cardiovascular Disease Study Association of residual plasma viremia and intima-media thickness in antiretroviral-treated patients with controlled human immunodeficiency virus infection. PLoS One 2014; 9:e113876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pereyra F, Lo J, Triant VA, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012; 26:2409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gandhi RT, McMahon DK, Bosch RJ, et al. ; ACTG A5321 Team Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 2017; 13:e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gandhi M, Gandhi RT, Stefanescu A, et al. ; A5321 Team Cumulative antiretroviral exposure measured in hair is not associated with measures of HIV persistence or inflammation among individuals on suppressive ART. J Infect Dis 2018; 218:234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hunt PW, Shulman NS, Hayes TL, et al. The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood 2013; 121:4635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hatano H, Hayes TL, Dahl V, et al. A randomized, controlled trial of raltegravir intensification in antiretroviral-treated, HIV-infected patients with a suboptimal CD4+ T cell response. J Infect Dis 2011; 203:960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deschamps AE, Van Wijngaerden E, Denhaerynck K, et al. Use of electronic monitoring induces a 40-day intervention effect in HIV patients. J Acquir Immune Defic Syndr 2006; 43:247–8. [DOI] [PubMed] [Google Scholar]
- 30. Castillo-Mancilla J, MaWhinney S, Coyle RP, et al. Cumulative ART exposure is associated with endothelial and immune activation in HIV. Poster presented at: CROI 2019; March 4–8, 2019; Seattle, WA. Poster #0657. [Google Scholar]
- 31. Hileman CO, Kinley B, Scharen-Guivel V, et al. Differential reduction in monocyte activation and vascular inflammation with integrase inhibitor-based initial antiretroviral therapy among HIV-infected individuals. J Infect Dis 2015; 212:345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.