Central Illustration
Key Words: ACE2, clinical trials, COVID-19, renin angiotensin system, SARS-CoV-2
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; AT1R, angiotensin II type 1 receptor; CI, confidence interval; CoV, coronavirus; COVID-19, coronavirus disease-2019; FDA, Food and Drug Administration; IFN, interferon; IL, interleukin; IQR, interquartile range; MERS, Middle East respiratory syndrome; RAS, renin-angiotensin system; RNA, ribonucleic acid; sACE2, soluble angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome-coronavirus-2; TMPRSS2, transmembrane protease serine 2
Highlights
-
•
SARS-CoV-2, the virus that causes COVID-19, is a novel CoV that infects humans by binding to ACE2, which degrades angiotensin II, and hence plays a critical role in modulating the renin angiotensin system (RAS).
-
•
The emerging epidemiology of COVID-19 suggests that patients with cardiovascular risk factors, including older age, cardiovascular disease, or cancer may be more susceptible to infection and suffer from worse clinical outcomes.
-
•
Because of the limited understanding with respect to the interaction of RAS inhibitors and SARS-CoV-2 infectivity, we endorse current society recommendations to continue RAS antagonists for clinical indications for which these agents are known to be beneficial.
-
•
Treatments for COVID-19 that are undergoing clinical trials range from therapies that block the entry of SARS-CoV-2 into host cells, to repurposed antiviral therapies such as protease inhibitors and nucleoside analogs that block viral replication by inhibiting viral RNA-dependent RNA polymerase.
Summary
The coronavirus disease-2019 (COVID-19) pandemic has resulted in a proliferation of clinical trials designed to slow the spread of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). Many therapeutic agents that are being used to treat patients with COVID-19 are repurposed treatments for influenza, Ebola, or for malaria that were developed decades ago and are unlikely to be familiar to the cardiovascular and cardio-oncology communities. Here, the authors provide a foundation for cardiovascular and cardio-oncology physicians on the front line providing care to patients with COVID-19, so that they may better understand the emerging cardiovascular epidemiology and the biological rationale for the clinical trials that are ongoing for the treatment of patients with COVID-19.
The coronavirus disease-2019 (COVID-19) pandemic has resulted in a proliferation of clinical trials that are designed to slow the spread of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), the virus that causes COVID-19. These therapies range from vaccines, to repurposed treatments for influenza, to drugs that were not effective in patients with Ebola, to treatments for malaria that were developed decades ago. Recognizing that patients with underlying cardiovascular risk factors, cardiovascular disease, or cancer have an increased risk for adverse outcomes with COVID-19, and recognizing that these vulnerable populations may be enrolled in COVID-19 clinical trials, here we present a critical review of the rationale for the different therapeutics that are currently being employed. As background, we first review the epidemiology of COVID-19, followed by the biology of CoV. We then briefly define the complex interplay between the CoV and the renin-angiotensin system (RAS), which is directly relevant to the care of the majority of patients with cardiovascular disease or cancer who are receiving drugs that modulate this system. Finally, we review the mechanisms of action of the multiple therapies that are currently being studied in clinical trials. Given the breadth of information that is emerging, we will not discuss the role of vaccines.
Epidemiology of COVID-19
The current impact of the novel CoV, SARS-CoV-2 is unquantifiable. The number of confirmed cases and deaths from the global COVID-19 pandemic increase daily (1,2). Although there is a great deal that still remains to be understood, initial reports from 552 hospitals in China describing 1,099 of the 7,736 patients infected with COVID-19 provide some insight into the disease (3). In this multicenter retrospective analysis, the majority were Wuhan residents or had contact with Wuhan residents, although 25.9% were neither. The median age of patients was 47 years (interquartile range [IQR]: 35 to 58 years), and 41.9% were female. Patients with more severe disease, compared with those with nonsevere disease, tended to be older and tended to suffer from at least 1 comorbidity. In this retrospective analysis, patients commonly received intravenous antibiotics (58.0%). Oseltamivir was administered in 35.8%, systemic steroids in 18.6%, and oxygen in 41.3% of patients. The median duration of hospitalization was 12.0 days (IQR: 10.0 to 14.0 days); however, the majority of the patients (93.6%) remained hospitalized at the time of data analysis and as such, the clinical course still largely remains to be defined.
COVID-19 and cardiovascular complications
Epidemiologic data thus far suggest that patients with cardiovascular risk factors, including older age, cardiovascular disease, or cancer are more susceptible to infection and suffer from worse clinical outcomes (4). COVID-19 can also directly result in a number of cardiovascular complications, including fulminant myocarditis, myocardial injury, heart failure, and arrhythmia (3,5,6). There have been a number of published case reports of clinically suspected myocarditis as suggested by: markedly elevated troponin levels, ST-segment elevation on electrocardiogram without obstructive coronary disease, severely decreased left ventricular systolic function, and shock (7), with cardiac magnetic resonance imaging evidence of diffuse myocardial edema and gadolinium enhancement (8). However, in another isolated autopsy report from a patient who suffered from SARS-CoV-2–related pneumonia and cardiac arrest, no obvious histological changes in the myocardium were observed with the exception of few interstitial mononuclear inflammatory infiltrates (9).
Elevated troponin levels have also been observed in those with worse clinical outcomes. In a retrospective, single-center analysis of 416 hospitalized patients with confirmed COVID-19, 19.7% displayed evidence of cardiac injury, as defined by elevated high-sensitivity troponin I levels greater than the 99th percentile upper limit. Those with confirmed cardiac injury tended to be older (median age of 74 vs. 60 years) and suffer from hypertension (59.8% vs. 23.4%), diabetes (24.4% vs. 12.0%), coronary heart disease (29.3% vs. 6.0%), heart failure (14.6% vs. 1.5%), or cancer (8.5% vs. 0.6%) (10).
COVID-19 in patients with cardiovascular risk factors or disease
Patients with cardiovascular risk factors or disease are at increased risk of suffering from worse clinical outcomes with COVID-19. In an analysis of 2 cohorts from Jinyintan Hospital and Wuhan Hospital of 191 patients, patients with hypertension, diabetes, or coronary heart disease were at increased risk of in-hospital mortality (11). The prevalence of hypertension among nonsurvivors was 48% as compared to 30% in survivors; 31% versus 19% for diabetes, and 13% versus 8% for cardiovascular disease. These comorbidities were also more likely to be present in patients who required intensive care unit admission (4). Other studies, including a recently published meta-analysis of 46,248 infected patients, have corroborated the observation that patients with cardiovascular risk factors or cardiovascular disease have worse clinical outcomes (12) and also suggest that hypertension (17 ± 7%; 95% confidence interval [CI]: 14% to 22%), diabetes (8 ± 6%; 95% CI: 6% to 11%), and cardiovascular disease (5 ± 4%; 95% CI: 4% to 7%) were prevalent comorbidities among infected patients. Recent studies have also demonstrated that age and hypertension were predictors of an increased likelihood of cardiovascular complications, and cardiovascular complications were associated with a 4.26-fold increased risk of death (95% CI: 1.9 to −9.49) (10).
COVID-19 in patients with heart transplantation
There have been case series published on COVID-19 infection in heart transplant recipients. Two confirmed cases suggest similar presentations to nontransplant recipients and both patients demonstrated clinical improvement. A questionnaire of 87 heart transplant recipients in China, of which importantly 96.6% undertook quarantine procedures, did not suggest a markedly elevated rate of SARS-CoV-2 infection in this population (13,14).
COVID-19 in patients with cancer
In a retrospective medical review of 1,524 patients with cancer who were admitted to the Department of Radiation and Medical Oncology in Zhongnan Hospital of Wuhan University from December 30, 2019, to February 17, 2020, the infection rate of SARS-CoV-2 in patients with cancer was 0.79% (95% CI: 0.3% to 1.2%) (15). In contrast, the estimated cumulative incidence of all COVID-19 cases in Wuhan was 0.37%. As a result, the odds of infection in patients with cancer were estimated to be 2.31 (95% CI: 1.89 to 3.02) greater. Patients with cancer who were infected had a median age of 66 years and were more likely to have non–small cell lung cancer (58.3%). Five of these patients were being treated with chemotherapy, immunotherapy, or radiation therapy. Three deaths were recorded.
In a multicenter, prospective cohort study of 2,007 cases from 575 hospitals, 1% of the 1,590 COVID-19 cases had a history of cancer (15). This in contrast to an incidence of cancer in the Chinese population of 0.29% per 100,000 people. Again, among those infected, lung cancer was most common, and patients tended to be older. Patients with cancer also suffered from an increased risk of adverse events that tended to occur earlier, including admission to the intensive care unit, need for invasive ventilation, or death, which occurred in 7 of 18 patients (39%), compared with 124 of 1,572 patients without cancer (8%). Patients with cancer who were recently treated with chemotherapy or surgery were also more likely to suffer from clinically severe adverse events. However, there is a critical need for additional studies to validate these early observations.
The CoV Family
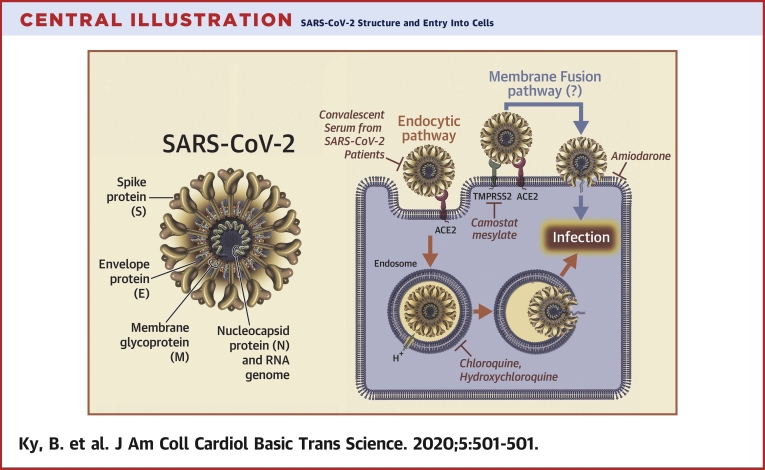
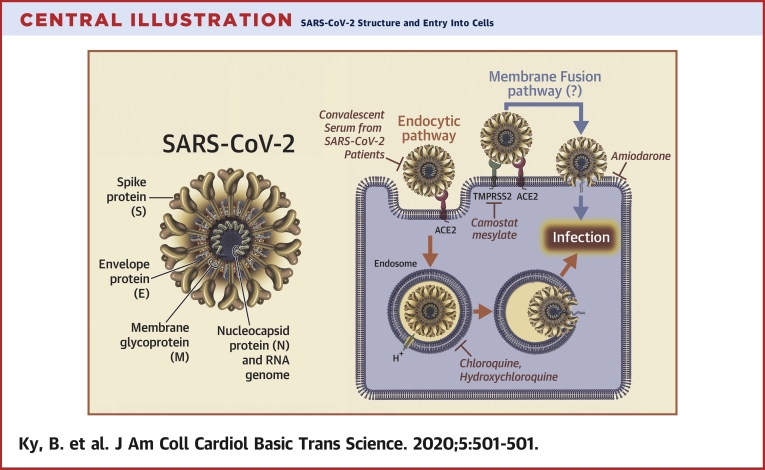
CoVs represent a large family of hundreds of enveloped, single-stranded, positive-sense ribonucleic acid (RNA) viruses that establish an infection primarily by targeting the mucosal surfaces of respiratory and intestinal tracts of a wide range of mammals and birds. There are 4 main subgroupings of CoVs: alpha, beta, gamma, and delta (16). The 7 CoVs that are capable of infecting humans include 229E (alpha CoV), NL63 (alpha CoV), OC43 (beta CoV), HKU1 (beta CoV), Middle East respiratory syndrome (MERS)-CoV (beta CoV), SARS-CoV (beta CoV), and SARS-CoV-2 (beta CoV). The prototype human CoV isolates 229E and OC43 have been causally linked to the common cold. SARS-CoV is the cause of the SARS, whereas MERS-CoV was established as the cause of MERS. Identification and sequencing of the virus responsible for COVID-19 established that it was a novel CoV that shared 88% sequence identity with 2 bat-derived SARS-like CoVs (16). Subsequently, the 2019 novel CoV was shown to share a 79.5% sequence homology with SARS-CoV and was subsequently renamed SARS-CoV-2 (16). The genome of the CoVs encodes 4 major structural proteins: the spike (S) protein, nucleocapsid protein, membrane protein, and the envelope protein (Central Illustration). The S protein is responsible for facilitating entry of the CoV into the target cell (16,17) and is composed of a short intracellular tail, a transmembrane anchor, and a large ectodomain that consists of a receptor binding S1 subunit and a membrane-fusing S2 subunit (16).
Central Illustration.
SARS-CoV-2 Structure and Entry Into Cells
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus genome encodes 4 major structural proteins: the spike (S) protein; the nucleocapsid (N) protein; the membrane (M) protein; and the envelope (E) protein. The S protein is responsible for facilitating entry of the CoV into the target cell. The routes employed by SARS-CoV include endocytosis and membrane fusion. The route employed by SARS-CoV-2 is via endocytosis; whether SARS-CoV-2 enters cells by membrane fusion is not known. Binding of the S protein of SARS-CoV to angiotensin-converting enzyme 2 (ACE2) leads to the uptake of the virions into endosomes, where the viral S protein is activated by the pH-dependent cysteine protease cathepsin L. Activation of the S protein by cathepsin L can be blocked by bafilomycin A1 and ammonium chloride, which indirectly inhibit the activity of cathepsin L by interfering with endosomal acidification. Chloroquine and hydroxychloroquine are weak bases that diffuse into acidic cytoplasmic vesicles such as endosomes, lysosomes, or Golgi vesicles and thereby increases their pH. MDL28170 inhibits calpain and cathepsin L. SARS-CoV can also directly fuse with host cell membranes, after processing of the virus spike protein by transmembrane protease serine 2 (TMPRSS2), a type II cell membrane serine protease. Camostat mesylate is an orally active serine protease inhibitor. Modified from Simmons et al. (25). RNA = ribonucleic acid.
CoV Virology
Given that far more is known with respect to the virology of SARS-CoV than of SARS-CoV-2, and given that these 2 CoVs appear to have some overlapping biology and clinical presentations, we will discuss these 2 viruses together, with an emphasis on the most recent studies that have revealed unique biological aspects of SARS-CoV-2. We will review viral attachment, entry, and replication of SARS-CoV and SARS-CoV-2 in host cells. This discussion will be integrated with a review of the ongoing clinical trials that target these different aspects of the biology of SARS-CoV-2 (see Tables 1, 2, 3, 4, and 5).
Table 1.
Select Treatment Trials Targeting RAS
| Drug Name | Mechanism of Action | NCT Number | Title | Study Population | Targeted Enrollment | Study Design | Primary Outcome Measure |
|---|---|---|---|---|---|---|---|
| Losartan | Anti-RAS | NCT04312009 | Losartan for Patients With COVID-19 Requiring Hospitalization | Age ≥18 yrs with presumptive positive laboratory test for SARS-CoV-2; admission to the hospital with a sequential organ failure assessment score ≥1 and increased oxygen requirement; randomization within 24 h of presentation | 200 | Randomized, double-blind, placebo controlled | Difference in oxygenation status at 7 days |
| Losartan | Anti-RAS | NCT04311177 | Losartan for Patients With COVID-19 Not Requiring Hospitalization | Age ≥18 yrs with presumptive positive laboratory test for SARS-CoV-2 or URI or fever | 516 | Randomized, double-blind, placebo controlled | Hospital admission up to 15 days |
For an up-to-date listing of trials, search for “COVID-19” at the ClinicalTrials.gov website.
COVID-19 = coronavirus disease-2019; NCT = national clinical trial; OFA = organ failure assessment; RAS = renin-angiotensin system; SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2; URI = upper respiratory infection.
Table 2.
Select Treatment and Prophylaxis Trials Targeting Viral Cell Entry
| Drug Name | Mechanism of Action | NCT Number | Title | Study Population | Targeted Enrollment | Study Design | Primary Outcome Measure |
|---|---|---|---|---|---|---|---|
| Treatment Trials | |||||||
| Camostat | Viral entry | NCT04321096 | The Impact of Camostat Mesylate on COVID-19 Infection (CamoCo-19) | Age 18–110 yrs, COVID-19–confirmed hospitalized patients (<48 h) or if hospital-acquired COVID-19 is suspected, <48 h since onset of symptoms | 180 | Randomized, double-blind placebo controlled, phase IIa trial | Time to clinical improvement at 30 days |
| Hydroxychloroquine | Viral entry | NCT04315896 | Hydroxychloroquine Treatment for Severe COVID-19 Pulmonary Infection (HYDRA Trial) | Age 18–80 yrs, COVID-19 confirmed by RT-PCR in any respiratory sample; severe disease defined by pulse O2< 91%, 3% decline from baseline pulse O2, or need for increased supplemental O2, mechanical ventilation, or sepsis | 500 | Randomized, double-blind, placebo controlled | All-cause hospital mortality at 120 days |
| Hydroxychloroquine | Viral entry | NCT04316377 | Norwegian Coronavirus Disease 2019 Study (NO COVID-19) | Age >18 yrs, hospitalized, moderately severe disease (NEWS score ≤6); SARS-CoV-2–positive test | 202 | Randomized, open, single arm | Rate of decline in SARS-CoV viral load at 96 h |
| Prophylaxis Trials | |||||||
| Chloroquine phosphate | Viral entry | NCT04303507 | Chloroquine/Hydroxychloroquine Prevention of Coronavirus Disease (COVID-19) in the Healthcare Setting (COPCOV) | Age ≥16 yrs; health care worker or front-line participant with patient contact working in a health care facility; inpatient or relative of a patient and likely exposed to COVID-19; agree to not self-medicate with potential antivirals | 40,000 | Randomized, double-blind, placebo controlled | Number of symptomatic COVID-19 infections Severity of symptoms |
| Hydroxychloroquine | Viral entry | NCT04308668 | Post-exposure Prophylaxis/Pre-emptive Therapy for SARS-Coronavirus-2 (COVID-19 PEP) | Age >18 yrs; exposure to a COVID-19 case within 4 days as either a health care worker or household contact; symptomatic COVID-19 case with confirmed diagnosis within 4 days of symptom onset; or symptomatic health care worker with known COVID-19 contact and within 4 days of symptom onset | 3,000 | Randomized, double-blind, placebo controlled | Incidence of COVID-19 disease at 14 days Ordinal Scale of COVID-19 disease severity at 14 days |
| Hydroxychloroquine | Viral entry | NCT04318444 | Hydroxychloroquine Post Exposure Prophylaxis for Coronavirus Disease (COVID-19) | Age >18 yrs; household contact of index case: currently residing in the same household as an individual evaluated at NYP via outpatient, ED, or inpatient services who (1) tests positive for COVID-19, or (2) is defined as suspected case, or PUI, by the treating physician | 1,600 | Randomized, double-blind, placebo controlled | Symptomatic, lab-confirmed COVID-19 |
| Hydroxychloroquine | Viral entry | NCT04318015 | Hydroxychloroquine Chemoprophylaxis in Healthcare Personnel in Contact With COVID-19 Patients (PHYDRA Trial) | Age >18 yrs; health care personnel exposed to patients with COVID-19 respiratory disease (physicians, nurses, chemists, pharmacists, janitors, stretcher-bearer, administrative, and respiratory therapists) | 400 | Randomized, double-blind, placebo controlled | Symptomatic COVID-19 infection rate at 60 days |
For an up-to-date listing of trials, search for “COVID-19” at the ClinicalTrials.gov website.
ED = emergency department; NEWS = National Early Warning Score; NYP = New York Presbyterian; PUI = person under investigation; RT-PCR = reverse transcriptase-polymerase chain reaction; other abbreviations as in Table 1.
Table 3.
Select Treatment Trials Targeting Viral Replication
| Drug Name | Mechanism of Action | NCT Number | Title | Study Population | Targeted Enrollment | Study Design | Primary Outcome Measure |
|---|---|---|---|---|---|---|---|
| Umifenovir | Antiretroviral | NCT04260594 | Clinical Study of Arbidol Hydrochloride Tablets in the Treatment of Pneumonia Caused by Novel Coronavirus | Age ≥18 yrs; subjects with pneumonia diagnosed as 2019-nCoV infection; detection of 2019-nCoV nucleic acid–positive by RT-PCR in respiratory tract or blood samples; virus gene sequence of respiratory tract or blood samples is highly homologous to the known 2019-nCoV | 380 | Randomized, single-arm, open-label umifenovir | Negative viral conversion rate at 7 days |
| ASC09 + ritonavir; lopinavir + ritonavir | Antiretroviral | NCT04261907 | Evaluating and Comparing the Safety and Efficiency of ASC09/Ritonavir and Lopinavir/Ritonavir for Novel Coronavirus Infection | Age between 18 to 75 yrs; lab (RT-PCR) and clinically confirmed case of 2019-nCoV pneumonia; hospitalized with a new onset respiratory illness (≤7 days since illness onset) | 160 | Randomized, open-label ASC09/ritonavir or lopinavir/ritonavir | The incidence of adverse outcomes, defined by at least 1 of the following: pulse O2 ≤93% without O2 supplementation, PaO2-to-FiO2 ratio ≤300 or RR ≥30 breaths/min assessed at 14 days |
| Darunavir + cobicistat | Antiretroviral | NCT04252274 | Efficacy and Safety of Darunavir and Cobicistat for Treatment of COVID-19 (DC-COVID-19) | Pneumonia caused by 2019-nCoV | 30 | Randomized, open-label, single-arm | The viral clearance rate of throat swabs, sputum, or lower respiratory tract secretions at day 7 |
| Lopinavir + ritonavir; umifenovir | Antiretroviral | NCT04252885 | Efficacy of Lopinavir Plus Ritonavir and Arbidol Against Novel Coronavirus Infection (ELACOI) | Age 18–80 yrs; confirmation of SARS-CoV-2 infection by RT-PCR with normal kidney and liver function | 125 | Randomized, open-label (1:1:1) to lopinavir + ritonavir; or umifenovir; or standard care | The rate of viral inhibition, as determined by RT-PCR at days 2, 4, 7, 10, 14, and 21 |
| Lopinavir + ritonavir | Antiretroviral | NCT04330690 | Treatments for COVID-19: Canadian Arm of the SOLIDARITY Trial (CATCO) | Age >6 months with confirmed SARS-CoV-2 by RT-PCR, admitted to hospital | 440 | Randomized, open-label (1:1) of lopinavir + ritonavir or standard care | Efficacy of intervention at 29 days as determined by 10-point ordinal scale of clinical status |
| Remdesivir | Antiretroviral | NCT04280705 | Adaptive COVID-19 Treatment Trial (ACTT) | Age 18–99 yrs, PCR-confirmed novel coronavirus infection by lab assay; illness as defined by abnormal radiographic imaging, clinical assessment, and pulse O2 ≤94%, requiring O2, or requiring mechanical ventilation | 572 | Adaptive, randomized, double-blind placebo controlled | Time to recovery at day 29 |
| Remdesivir | Antiretroviral | NCT04292899 | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Severe Coronavirus Disease (COVID-19) | Age ≥18 yrs; confirmation of SARS-CoV-2 infection by RT-PCR ≤4 days before randomization; current hospitalization with pulse O2 ≤94% | 6,000 | Randomized, open-label study of remdesivir 5 days; or remdesivir 10 days | Odds of clinical improvement on a 7-point ordinal scale by day 11 |
| Remdesivir | Antiretroviral | NCT04292730 | Study to Evaluate the Safety and Antiviral Activity of Remdesivir (GS-5734) in Participants With Moderate Coronavirus Disease (COVID-19) Compared to Standard of Care Treatment | Age ≥18 yrs; confirmation of SARS-CoV-2 infection by RT-PCR ≤4 days before randomization; current hospitalization with fever, pulse O2 >94%, radiographic evidence of pulmonary infiltrates | 1,600 | Randomized, open-label study of remdesivir 5 days; or remdesivir 10 days; or standard of care | Odds of clinical improvement on a 7-point ordinal scale by day 11 |
For an up-to-date listing of trials, search for “COVID-19” at the ClinicalTrials.gov website.
Table 4.
Select Treatment and Prophylaxis Trials Targeting the Immune System
| Drug Name | Mechanism of Action | NCT Number | Title | Study Population | Targeted Enrollment | Study Design | Primary Outcome Measure |
|---|---|---|---|---|---|---|---|
| Treatment Trials | |||||||
| IFN-α1β | Immunomodulatory | NCT04293887 | Efficacy and Safety of IFN-α1β in the Treatment of Novel Coronavirus Patients | Age ≥18 yrs with clinically diagnosed coronavirus pneumonia within 7 days, including RT-PCR evidence of coronavirus and symptoms | 328 | Randomized, open-label, single-arm | Incidence of side effects within 14 days including dyspnea, pulse O2 ≤94%, and RR ≥24 breaths/min |
| Methylprednisolone | Immunomodulatory | NCT04273321 | Efficacy and Safety of Corticosteroids in COVID-19 | Age >18 yrs, diagnosis of novel coronavirus pneumonia (COVID-19) | 400 | Randomized, open-label, single-arm | Incidence of treatment failure in 14 days |
| Methylprednisolone | Immunomodulatory | NCT04244591 | Glucocorticoid Therapy for COVID-19 Critically Ill Patients With Severe Acute Respiratory Failure | Age >18 yrs, RT-PCR–confirmed infection, symptoms for >7 days, PaO2/FiO2 <200, positive pressure ventilation or HFNC higher than 45 l/min for <48 h, requiring ICU admission | 80 | Randomized, open-label of glucocorticoid therapy or standard of care | Murray lung injury score at 7 days |
| Sarilumab | Immunomodulatory | NCT04315298 | Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID-19 | Age ≥18 yrs; confirmation of SARS-CoV-2 infection by RT-PCR; current hospitalization with evidence of pneumonia and severe disease, critical disease, or multiorgan system dysfunction | 400 | Adaptive, randomized, double-blind, placebo-controlled with high and low doses | Percent change in C-reactive protein levels at 4 days Percentage of patients reporting clinical severity rated on a 7-point ordinal scale |
| Siltuximab | Immunomodulatory | NCT04329650 | Efficacy and Safety of Siltuximab vs. Corticosteroids in Hospitalized Patients With COVID-19 Pneumonia | Age ≥18 yrs; confirmation of SARS-CoV-2 infection by RT-PCR; current hospitalization with evidence of pneumonia; maximum O2 support of 35% | 100 | Randomized, open-label of siltuximab or methylprednisolone | Proportion of patients requiring ICU admission at 29 days |
| Tocilizumab | Immunomodulatory | NCT04317092 | Tocilizumab in COVID-19 Pneumonia (TOCIVID-19) | No age or sex limit; SARS-CoV-2 infection by RT-PCR, current hospitalization secondary to pneumonia; pulse O2 ≤93%, requiring O2, or requiring mechanical ventilation (invasive or noninvasive) | 400 | Open-label, single-arm | Mortality at 1 month |
| Tocilizumab | Immunomodulatory | NCT04320615 | A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA) | Age ≥18 yrs; hospitalized with COVID-19 pneumonia per WHO criteria; pulse O2 ≤93% or PaO2/FiO2 <300 | 330 | Randomized, double-blind placebo controlled | Clinical status using a 7-category ordinal scale at 28 days |
| Anakinra, siltuximab, or tocilizumab | Immunomodulatory | NCT04330638 | Treatment of COVID-19 Patients With Anti-interleukin Drugs (COV-AID) | Age ≥18 yrs; hospitalized with confirmed COVID-19 diagnosis by RT-PCR or other laboratory test; hypoxia defined by PaO2/FiO2; CXR or CT scan with bilateral infiltrates | 342 | Randomized, open-label (1:1:1:1) to anakinra, or siltuximab, or anakinra + siltuximab, or tocilizumab, or anakinra + tocilizumab | Time to clinical improvement at 15 days |
| Prophylaxis Trial | |||||||
| Recombinant human IFN-α1β and thymosin α1 | Immunomodulatory | NCT04320238 | Experimental Trial of rhIFNα Nasal Drops to Prevent 2019-nCOV in Medical Staff | Age 18 to 65 yrs, formally serving as medical staff in Taihe Hospital | 2,944 | 2-arm, open-label to IFN-α1β in a low-risk group and IFN-α1β and thymosin α1 in a high-risk group | New COVID-19 diagnosis at 28 days |
For an up-to-date listing of trials, search for “COVID-19” at the ClinicalTrials.gov website.
Table 5.
Select Treatment Trials With Multiple Targets
| Drug Name | Mechanism of Action | NCT Number | Title | Study Population | Targeted Enrollment | Study Design | Primary Outcome Measure |
|---|---|---|---|---|---|---|---|
| Lopinavir + ritonavir; ribavirin; IFN-β1b | Antiretroviral and immunomodulatory | NCT04276688 | Lopinavir/Ritonavir, Ribavirin and IFN-beta Combination for nCoV Treatment | Age ≥18 yrs hospitalized for virologically confirmed 2019-nCoV infection with NEWS ≥1 on recruitment; febrile with symptoms and duration of symptoms ≤10 days | 127 | Randomized, open-label (1:1:1) to lopinavir + ritonavir; or ribavirin; or IFN-β1b | Time to negative nasopharyngeal viral RT-PCR assessed up to 1 month |
| Lopinavir + ritonavir; hydroxychloroquine | Antiretroviral and viral entry | NCT04307693 | Comparison of Lopinavir/Ritonavir or Hydroxychloroquine in Patients With Mild Coronavirus Disease (COVID-19) | Age 16–99 yrs with confirmed mild COVID-19 (NEWS 0–4) | 150 | Randomized, open-label (1:1:1) lopinavir + ritonavir; or hydroxychloroquine; or standard care | Viral load at hospital days 3, 5, 7, 10, 14, 18 |
| Remdesivir + hydroxychloroquine; remdesivir; hydroxychloroquine | Antiretroviral and viral entry | NCT04321616 | Efficacy of Different Anti-viral Drugs in COVID-19 Infected Patients | Age ≥18 yrs with confirmed SARS-CoV-2 by RT-PCR, admitted to hospital or ICU | 700 | Randomized, Open-Label (1:1:1) remdesivir; or hydroxychloroquine; or remdesivir + hydroxychloroquine adaptive controlled design; comparison with standard of care | In-hospital mortality at 3 weeks |
| Combinations of oseltamivir, chloroquine, darunavir, ritonavir, lopinavir, oseltamivir, favipiravir | Antiretroviral and viral entry | NCT04303299 | Various Combination of Protease Inhibitors, Oseltamivir, Favipiravir, and Hydroxychloroquine for Treatment of COVID19: A Randomized Control Trial (THDMS-COVID-19) | Age 16–100 yrs with COVID-19 diagnosis | 320 | Randomized, open-label, oseltamivir + chloroquine; or darunavir + ritonavir + oseltamivir; or lopinavir + ritonavir + oseltamivir; or favipiravir + lopinavir + ritonavir; or darunavir + ritonavir + oseltamivir + chloroquine; or darunavir + ritonavir + favipiravir + chloroquine | Time to negative detection of SARS-CoV-2 in nasopharyngeal swab at 24 weeks |
| Favipiravir; chloroquine phosphate | Antiretroviral and viral entry | NCT04319900 | Clinical Trial of Favipiravir Tablets Combined With Chloroquine Phosphate in the Treatment of Novel Coronavirus Pneumonia | Age 18–75 yrs; diagnosed with nCoV pneumonia with a course of illness no more than 14 days; if the course is >14 days, no progression by chest radiograph within 7 days; respiratory symptoms; positive COVID-19 RT-PCR within 3 days | 150 | Randomized, double-blind 3-arm study of favipiravir + chloroquine; or favipiravir; or placebo | Time to improvement of respiratory symptoms Number of days of viral shedding Frequency of improvement of respiratory symptoms |
| Remdesivir; lopinavir + ritonavir; IFN-β1a; hydroxychloroquine | Antiretroviral, viral entry, and immunomodulatory | NCT04315948 | Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy) | Age ≥18 yrs, laboratory-confirmed SARS-CoV-2 infection as determined by RT-PCR or other assay <72 h prior to randomization, hospitalized patients with illness of any duration, and pulmonary exam abnormalities and pulse O2 ≤ 94%, requiring O2, or requiring mechanical ventilation, or acute respiratory failure requiring mechanical ventilation and/or supplemental O2 | 3,100 | Adaptive, randomized, open-label 1:1:1:1:1 to remdesivir; or lopinavir/ritonavir; or lopinavir/ritonavir + INF-β1α; or hydroxychloroquine; or standard of care | Percentage of subjects reporting disease severity on a 7-point ordinal clinical scale reflective of hospitalization and oxygenation status; or death at 15 days |
| Favipiravir + tocilizumab | Antiretroviral and immunomodulatory | NCT04310228 | Favipiravir Combined With Tocilizumab in the Treatment of Corona Virus Disease 2019 | Age 18–65 yrs, COVID-19 diagnosis, increased IL-6 | 150 | Randomized, open-label (1:1:1) of favipiravir + tocilizumab; or favipiravir; or tocilizumab | Clinical cure rate at 3 months |
Angiotensin-converting enzyme 2 (ACE2) is the entry receptor for SARS-CoV and SARS-CoV-2. Viruses enter cells by binding to host cell-encoded proteins that facilitate the entry of the virus into the cell, as well as allow the virus to survive and replicate within the cell. Some viruses, including certain strains of CoVs are capable of down-modulating the entry receptor once they gain access to the cell. Receptor down-modulation is a strategy broadly used by many viruses to escape the immune system, as well as establish the best environment for viral replication and spread (18). Receptor down-modulation may also disrupt many of the natural physiologic functions of the host cell, resulting in cell death leading to organ level dysfunction.
The entry receptor utilized by both SARS-CoV and SARS-CoV-2 is ACE2 (Central Illustration), which is a type I transmembrane carboxypeptidase with 40% homology to ACE. ACE plays a critical role in activation of the RAS, by processing angiotensin I (angiotensin 1-10) to angiotensin II (angiotensin 1-8), the major effector peptide of RAS, which mediates its effects through selective interactions with G-protein–coupled angiotensin II type 1 (AT1) and type 2 (AT2) receptors (19). ACE, however, has not been implicated in the entry of human CoVs into cells.
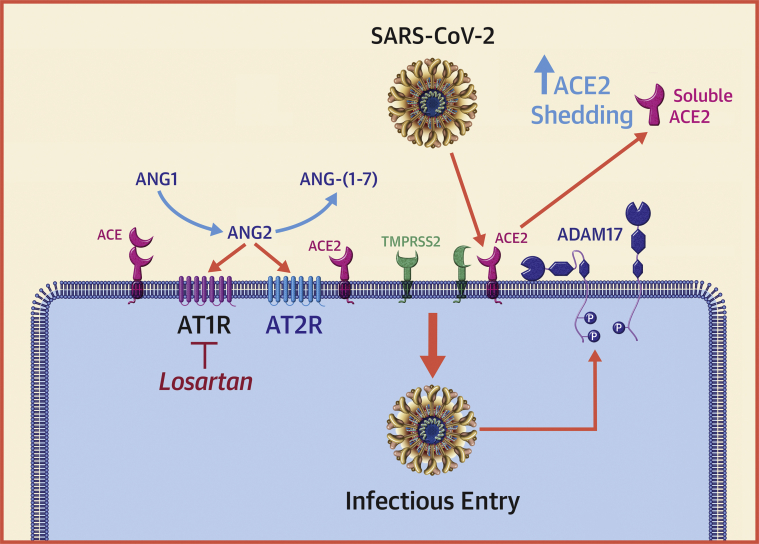
ACE2 is highly expressed in the mouth, tongue, and types I and II alveolar epithelial cells in the lungs. ACE2 is also abundantly expressed by cardiovascular endothelium, cardiac myocytes, cardiac fibroblasts, as well as epithelial cells of the kidney and testis. The major substrate of ACE2 is angiotensin II, which is cleaved to angiotensin 1-7 (Figure 1) and functions through association with the G-protein–coupled receptor Mas receptor. The ACE2–angiotensin (1, 2, 3, 4, 5, 6, 7)–Mas receptor axis is regarded as the counter-regulatory arm of the RAS by opposing the effects of the ACE–angiotensin II axis–AT1. Although the precise role of ACE2 is still being evaluated, studies been shown that ACE2 exerts protective effects in the pulmonary and the cardiovascular systems, where it serves to oppose the deleterious effects of RAS activation (20, 21, 22).
Figure 1.
Interaction of CoVs With the RAS
Angiotensin-converting enzyme (ACE) converts angiotensin I (ANG1) (angiotensin 1-10) to angiotensin II (ANG2) (angiotensin 1-8), which is the major effector peptide of the renin-angiotensin system (RAS). ANG2 mediates its effects through selective interactions with G-protein–coupled angiotensin II type 1 receptor (AT1R) and G-protein–coupled angiotensin II type 1 type 2 receptor (AT2R). ANG2 is degraded to ANG-(1-7) by angiotensin-converting enzyme 2 (ACE2), ANG-(1-7) binds to the Mas receptor (not shown). The ACE2–ANG-(1-7)–Mas receptor axis opposes the effects of ACE–ANG2–AT1 axis. The binding of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein to ACE2 induces ACE2 shedding by activating a disintegrin and metalloproteinase-17 (ADAM-17). A decrease in ACE2 levels would be expected to result in a decrease in the levels ANG-(1-7) levels (cytoprotective) and a corresponding increase in tissue levels of ANG2 (proinflammatory and profibrotic). Transmembrane serine protease 2 (TMPRSS2), a type II cell membrane serine protease that activates the spike protein of SARS-CoV-2 and allows it to bind to ACE2. Modified from Simmons et al. (25). CoVs = coronaviruses; P = phosphorylation.
Infection with SARS-CoV and SARS-CoV-2 is triggered by binding of the S protein on the surface of the CoV to ACE2 that is expressed on the cell surface. The receptor binding domain of the S protein of SARS-CoV-2 is located on the S1 subunit, which undergoes a conformational change when it binds to ACE2, which facilitates viral attachment to the surface of target cells (17). Binding of SARS-CoV-2 to ACE2 can result in uptake of virions into endosomes (Central Illustration). Viral entry into the cell requires priming of the S protein by the serine protease transmembrane protease serine 2 (TMPRSS2), which cleaves the viral S protein at the S1/S2 and the S2′ site and allows fusion of viral and cellular membranes (23). The S proteins of SARS-CoV-2 can also use pH-sensitive endosomal proteases (cathepsin B and L) for priming and entry into cells. Interestingly, the binding affinity of the SARS-CoV-2 S ectodomain to ACE2 is 10- to 20-fold higher than the binding of the SARS-CoV ectodomain to ACE2 (17). The increase in stickiness of the SARS-CoV-2 capsid S protein makes disease transmission more likely and might explain the increased person-to-person transmission with SARS-CoV-2 compared with that of SARS-CoV. Insofar as the viral S proteins are the part of the virus that interacts with the immune system, they may serve as a promising target for vaccines. Relevant to this discussion, convalescent sera from patients with SARS have been shown to block the entry of SARS-CoV-2 entry into cultured cells, albeit with less efficiency that SARS-CoV (23). However, monoclonal antibodies raised against the receptor binding domain of the S1 protein of SARS-CoV do not bind to the receptor binding domain of the S1 protein of SARS-CoV-2, suggesting that SARS-directed antibodies are not cross reactive and that SARS-CoV-2 proteins are necessary to develop effective antibodies. Although ACE inhibitors do not inhibit ACE2, Hoffman et al. (23) demonstrated that anti-ACE2 antibody prevented entry of viral vectors into cell lines expressing the SARS-CoV-2 S protein.
Interaction of CoV with the RAS
An additional layer of complexity to understanding the pathophysiology of the SARS-CoV-2 in humans stems from the complexity of the interactions of CoVs with the RAS (Figure 1), as well as the widespread use of drugs that interfere with the RAS, including ACE inhibitors, angiotensin-receptor antagonists, or angiotensin receptor-neprilysin inhibitors. Each of these drugs has different effects on the expression of the various components of the RAS in different tissue beds. Here we will briefly discuss these important interactions, as well as their implications for the treatment of patients with COVID-19.
Previous studies have shown that SARS-CoV S proteins induce the expression of a cell surface metalloenzyme termed a disintegrin and metalloproteinase-17, which was originally described as the enzyme that cleaves membrane-bound tumor necrosis factor-α from the cell surface and allows it circulate in the soluble form of tumor necrosis factor-α (24). As shown in Figure 1, activation of a disintegrin and metalloproteinase-17 results in the proteolytic cleavage of ACE2 (referred to as shedding) from the cell surface, with the release of the catalytically active soluble angiotensin-converting enzyme 2 (sACE2) ectodomains into the circulation (22,25). A decrease in ACE2 levels on the cell surface would be expected to result in a decrease in the levels of angiotensin 1-7 (cytoprotective) and a corresponding increase in tissue levels of angiotensin II (proinflammatory and profibrotic). The importance of SARS-CoV2–induced down-regulation of cell surface ACE2 was demonstrated in experimental studies, wherein administration of recombinant human ACE2 protein, genetic deletion of the AT1 receptor, or administration of an AT1 receptor antagonist were shown to be protective in acute lung injury models (21,22). These and other observations have suggested that the use of AT1 receptor antagonists may be beneficial in patients with COVID-19 (26), and consistent with this, losartan is currently being tested in randomized, double-blind placebo controlled studies as a potential therapy in hospitalized infected patients (Table 1). Relevant to this discussion, the ACE inhibitors in clinical use do not directly affect ACE2 activity (27). The biological significance of circulating sACE2 is not known. Of note, sACE2 retains its ability to bind the S protein of SARS-CoV and was shown to prevent entry of SARS-CoV into cells in vitro (28). Thus, sACE2 may act as a decoy receptor that prevents SARS-CoV-2 from binding to ACE2 on the cell surface. APN01 is a human recombinant sACE2 that has been shown to block the early stages of SARS-CoV-2 infections in cell culture and human tissue organoid cultures (29). APN01 has already undergone safety and tolerability testing in a phase II trial of healthy volunteers (NCT00886353), but at the time of this writing is not being tested clinically in patients with COVID-19.
The recognition that many patients with COVID-19 have underlying medical conditions that are treated with ACE inhibitors and AT1 receptor antagonists (30), coupled with the knowledge that higher urinary ACE2 levels have been observed in patients treated with AT1 receptor antagonists (26), has given rise to the concern that pharmacologic up-regulation of ACE2 by RAS inhibitors may influence the infectivity of SARS-CoV-2 in a patient population that is already at high risk for severe COVID-19 infection (31). However, as noted in a recent review (32) on this topic, the experimental and clinical data often yield conflicting results with respect to the role of ACE inhibitors and AT1 receptor antagonists on ACE2 levels in different pathophysiological contexts. These conflicting results suggest that the effects on RAS inhibitors on ACE2 are complex and nuanced and should not be assumed to be the same for all RAS inhibitors, nor should it be assumed that changes in ACE2 levels in the heart or other tissues necessarily reflect changes in ACE2 levels in the lung, which is the portal of entry for SARS-CoV-2. Given that we have limited understanding with respect to the interaction of RAS inhibitors, ACE2 levels, and SARS-CoV-2 infectivity in humans, we do not believe that it is possible to make definitive statements that go beyond the joint statement issued on March 17, 2020, by the Heart Failure Society of America, American College of Cardiology, and American Heart Association, that recommended “continuing renin-angiotensin-aldosterone system antagonists for those patients who are currently prescribed such agents for indications for which these agents are known to be beneficial” (33).
Entry of SARS-CoV and SARS-CoV-2 into cells
The entry of enveloped viruses into host cells occurs through 2 primary mechanisms: the first is direct fusion of the viral membrane with the plasma membrane of the host cells, which allows the virus to directly deliver its genomic material into the cytosol; and the second is that the virus hijacks the cell's endocytic machinery, by binding to a cell surface receptor, which then triggers endocytosis of the virus-receptor complex (Central Illustration). In the endocytic pathway, the endocytosed virions are subjected to an activation step within the endosome, which is typically mediated by the acidic environment of the endosome, resulting in fusion of the viral and endosomal membranes, which allows for the release of the viral genome into the cytosol. Several viruses, including human immunodeficiency virus and SARS-CoV use direct membrane fusions at the cell surface or endocytosis to enter cells. As noted, recent studies suggest that SARS-CoV-2 binds to ACE2, which leads to endocytosis of the receptor-virus complex (23). What is not known at this time is whether SARS-CoV-2 is also capable of directly fusing with the lipid membrane of cells. However, based on the similarities of how SARS-CoV and SARS-CoV-2 behave, it is likely that their modes of entry into cells will be similar. Understanding these differences in cell entry has implications for developing novel therapeutics.
Therapeutics targeting endocytosis
The entry of SARS-CoV into cells was shown to occur by direct fusion of the viral membranes with the plasma membrane of the host cell (Central Illustration), through a process that requires processing of the viral S protein by TMPRSS2 at or near the cell surface. Processing of the S protein exposes the fusion peptide of the S protein that inserts into the cell membrane, which brings the envelope of the viral membrane into closer approximation with the membrane of the host cell, thereby facilitating fusion (34). At the time of this writing, the uptake of SARS-CoV-2 into cells has been shown to occur through endocytosis of the SARS-CoV-2–ACE2 complex, which also requires priming of the S protein by TMPRSS2. It is not known whether SARS-CoV-2 also enters though direct fusion.
Based on the evidence linking TMPRSS2-mediated SARS-CoV-2 activation to SARS-CoV-2 infectivity (23,35), the small molecule serine protease inhibitor camostat mesylate may also be an attractive target for clinical trials with SARS-CoV-2 (Table 2). Camostat mesylate has already been shown to inhibit replication of influenza and parainfluenza viruses and to prevent the development of pneumonia and viral myocarditis in infected mice (36). Given that the SARS-CoV-2 S protein is activated by the pH-dependent cysteine protease cathepsin L, this processing step may be sensitive to inhibition with drugs that indirectly inhibit cathepsin L activity by interfering with endosomal acidification (e.g., bafilomycin A1) or by compounds that directly block the proteolytic activity of cathepsin L.
It has also been suggested that the antimalarial drugs chloroquine and hydroxychloroquine might exert a potent antiviral effect by virtue of their ability to increase endosomal pH. Inside cells, chloroquine and hydroxychloroquine are rapidly protonated and concentrated in endosomes. The positive charge of the chloroquine increases the pH of the endosome, which prevents cathepsin-induced priming of the viral S protein. Both chloroquine and hydroxychloroquine decrease SARS-CoV-2 replication in cultured cells; however, hydroxychloroquine is more potent than chloroquine (37). In a small single-arm study of patients with confirmed COVID-19, treatment with hydroxychloroquine was associated with a significant difference in clearing of viral nasopharyngeal carriage of SARS-CoV-2 within 3 to 6 days when compared with that of untreated control subjects that were studied at 3 to 6 days. Azithromycin when added to hydroxychloroquine was significantly more efficient for virus elimination (38). However, both therapies can result in QT prolongation, and as such, caution needs to be exercised when using these therapies together. Chloroquine and hydroxychloroquine can also manifest in cardiotoxicity, including cardiomyopathy, both systolic and diastolic, atrioventricular block, and bundle branch block (39). Hydroxychloroquine will be used as one of the treatment arms in the World Health Organization (WHO) multinational SOLIDARITY (Efficacy of Different Antiviral Drugs in SARS-CoV-2) trial (40) and is also currently being investigated in a number of other studies (Tables 2 and 5). Interestingly, amiodarone, which is a cationic amphiphile, was shown to inhibit Ebola virus infection in vitro in target cells, using concentrations of amiodarone that overlapped those detected in the sera of patients treated for arrhythmias. Both amiodarone and its main metabolite, monodesethyl amiodarone, were shown to interfere with the fusion of the viral envelope with the endosomal membrane, thus blocking viral replication (41). Amiodarone has also been shown to inhibit SARS-CoV infection and spreading in vitro by altering the late compartments of the endocytic pathway by acting after the transit of the virus through endosomes (42).
Replication of SARS-CoV in host cells
Because of the exceptionally large size of the CoV RNA genome (∼30 kb) and the complexity of CoV-host cell interactions, coupled with the novelty of the SARS-CoV-2 genome, very little is known regarding SARS-CoV-2 replication in cells, let alone how the virus interacts with the host. Given that antiviral strategies are being considered for treatment of patients with COVID-19, here we will review what is generally understood about SARS-CoV replication in mammalian cells, recognizing that this information may change as we learn more about SARS-CoV-2 (see Figure 2).
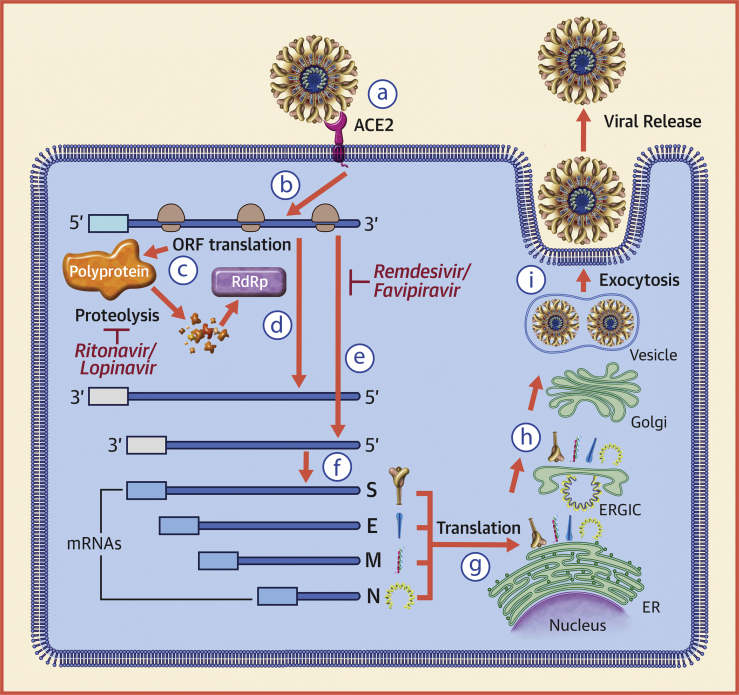
Figure 2.
The Replication Strategy of SARS-CoV
(a) The severe acute respiratory syndrome coronavirus (SARS-CoV) spike (S) glycoprotein attaches to the angiotensin-converting enzyme 2 (ACE2) receptor on the cell surface. On entering the cytoplasm, the viral core particle, which contains the positive (5′ to 3′) strand genomic ribonucleic acid (RNA), is released into the cytoplasm of the cell (b). The positive-strand viral RNA is translated on host ribosomes to generate a large polyprotein (c) that undergoes proteolytic processing to generate multiple viral proteins, including an RNA-dependent RNA polymerase (RdRp). The RNA-dependent RNA polymerase generates a full-length, antisense negative-strand (3′ to 5′) viral RNA strand (d) that serves as template for replicating positive-strand viral genomic RNA, as well as shorter negative-strand RNAs (e) that serve as templates for synthesizing messenger ribonucleic acids (mRNAs) that code for structural proteins of the virus (f), including the S, membrane (M), envelope (E), and nucleocapsid (N) proteins. Translation of viral mRNAs occurs using the host endoplasmic reticulum (ER) (g). Once the viral structural proteins, S, E, and M, are translated and inserted into the ER, they move along the secretory pathway to the endoplasmic reticulum-Golgi intermediate compartment (ERGIC) (h). The viral proteins become encapsulated and bud into membranes containing viral structural proteins, where mature virions are assembled. (i) Following assembly, virions are transported to the cell surface in vesicles and released by exocytosis. Modified from Turner et al. (43). ORF = open reading frame.
Once the genomic RNA of SARS-CoV is released into the cytoplasm of the host cell, the positive-strand viral RNA is translated on host ribosomes into a large polypeptide termed the replicase, which undergoes proteolytic cleavage to yield proteins that are required from genome replication, including a viral RNA-dependent RNA polymerase. The viral RNA-dependent RNA polymerase generates a full-length, antisense negative-strand viral RNA template, which is used for replicating positive strand viral genomic RNA, as well as shorter subgenomic negative strand RNAs that serve as templates for synthesizing messenger RNAs that code for structural proteins of the virus, including the S, membrane, envelope, and nucleocapsid proteins. Translation of viral messenger RNAs occurs using the host endoplasmic reticulum. Once the viral structural proteins, S, envelope, and membrane, are translated in the endoplasmic reticulum, they move along the secretory pathway to the endoplasmic reticulum-Golgi intermediate compartment. There, the viral proteins become encapsulated and bud into membranes containing viral structural proteins. Following assembly and maturation, virions are transported to the cell surface in vesicles and released by exocytosis (43,44).
Therapeutics for viral replication
There are a number of antiviral drugs that are being repurposed for the treatment of SARS-CoV-2. A partial list of these antiviral drugs are discussed next.
Nucleoside analogs
Remdesivir (GS-5734, Gilead Sciences, Inc., Foster City, California) is a nucleoside analog that exhibits broad antiviral activity. Remdesivir is a prodrug that is metabolized to its active form GS-441524, which interferes with the action of viral RNA-dependent RNA polymerase, resulting in a decrease in viral RNA production. It is not known, however, whether remdesivir terminates RNA chains or causes mutations in them. Remdesivir was effective against multiple types of CoVs in cell culture and a mouse model of SARS (45); however, it did not show an effect in patients with Ebola. Remdesivir is currently being tested in several clinical trials for hospitalized patients with COVID-19 and pneumonia (Tables 3 and 5). Remdesivir is also 1 of the 4 treatment arms in the multinational SOLIDARITY trial, which is the World Health Organization’s sponsored multinational randomized, open clinical trial to evaluate the safety and comparative efficacy of hydroxychloroquine, remdesivir, the combination of lopinavir and ritonavir, and the combination lopinavir and ritonavir plus interferon-beta (40). SOLIDARITY will use an adaptive design, which will allow for discontinuation of drugs that lack effectiveness, as well as adding new drugs that appear promising. This type of trial design offers flexibility and efficiency, particularly in the identification of early signals related to either efficacy or toxicity, while maintaining study validity (46).
Favipiravir (Avigan, Fujifilm Toyama Chemical, Tokyo, Japan) is another nucleoside analog antiviral drug that inhibits viral RNA-dependent RNA polymerase. Like remdesivir, it is a prodrug that is metabolized to its active form, favipiravir-ribofuranosyl-5'-triphosphate. Although favipiravir has undergone phase III clinical trials for the treatment of influenza, it is not yet approved by the U.S. Food and Drug Administration (FDA). Japan has granted approval for favipiravir for treating viral strains unresponsive to current antivirals. In preliminary studies, favipiravir was shown to have more potent antiviral activity than lopinavir/ritonavir (47).
Ribavirin (Copegus, Genentech Inc., San Francisco, California) is a prodrug that acts as nucleoside inhibitor. The metabolites of ribavirin resemble adenosine or guanosine nucleosides that then become incorporated into viral RNA and inhibit RNA-dependent replication in RNA viruses. Ribavirin is currently FDA-approved for the treatment of chronic hepatitis C virus infection in combination with peginterferon alfa-2a (Pegasys, Genentech).
Protease inhibitors
Lopinavir is a protease inhibitor class that is used in fixed-dose combination with another protease inhibitor, ritonavir (lopinavir/ritonavir [Kaletra, AbbVie Inc., North Chicago, Illinois]) for the treatment of human immunodeficiency virus. Results from a randomized, open-label study of 199 hospitalized adult patients with confirmed SARS-CoV-2 infection assigned 1:1 to lopinavir 400 mg–ritonavir 100 mg twice daily for 14 days with standard of care or standard of care alone were recently published (48). All patients had an oxygen saturation of 94% on room air or a ratio of partial pressure of oxygen to the fraction of inspired oxygen <300 mg Hg. The primary endpoint was time to clinical improvement, where clinical improvement was defined based on an ordinal scale or survival from the hospital. The study was designed for 80% power with a 2-sided significance level of α of 0.05 to detect an 8-day difference in median time to clinical improvement. Here, the median time to clinical improvement was 16.0 days (IQR: 13.0 to 17.0 days) in the lopinavir/ritonavir group compared with 16.0 days (IQR: 15.0 to 18.0 days) with standard care. The mortality at 28 days in the treatment group was similar to that observed in the standard care group (19.2% vs. 25%; difference: −5.8%; 95% CI: −17.3% to 5.7%), as was the detectable viral load. However, there were some suggestions of potential benefit with lopinavir/ritonavir with a shorter intensive care unit stay (median: 6 days vs. 11 days) and a shorter time to hospital discharge (median: 12 days vs. 14 days). As noted, the fixed-dose combination of lopinavir/ritonavir is 1 treatment arm in the SOLIDARITY trial (Table 3) (40).
Immunomodulatory therapies
Interferons (IFNs) are cytokines that activate the innate immune system in response to viral infection. Type I interferons (IFN-α/β) are synthesized by most cell types in the body response to a viral infection, whereas type II interferon (IFN-γ) is produced by immune cells following antigen stimulation. Both type I and type II IFNs provoke the synthesis of proteins that have antiviral and immunomodulatory effects. Recombinant IFN-β has been shown to inhibit SARS-CoV replication in vitro more effectively than either IFN-α or IFN-γ (49,50). Interestingly, IFN-γ down-regulates the expression of ACE2 on the cell surface and protects type I pneumocytes from SARS-CoV infection (51). The combination of lopinavir/ritonavir and IFN-β1b is being evaluated in the treatment of laboratory-confirmed MERS requiring hospitalization (52) and will also be evaluated in the SOLIDARITY trial (40).
A number of additional immunomodulatory agents are also currently being evaluated, including the interleukin (IL)-6 inhibitor, tocilizumab, and glucocorticosteroids (Tables 4 and 5), given the cytokine storm syndrome that has been observed in subgroups with severe COVID-19 (53) with increased levels of IL-2, IL-6, IL-7, and additional inflammatory cytokines (54). One meta-analysis suggested that the mean IL-6 levels were 2.9-fold (95% CI: 1.17 to 7.19-fold) greater in patients with complicated compared with noncomplicated COVID-19 (54). Tocilizumab (Actemra, Genentech) is FDA-approved for the treatment of severe cytokine release syndrome in patients treated with chimeric antigen receptor T-cell therapy and is also approved for the treatment of rheumatoid arthritis (55, 56, 57, 58). Tocilizumab is a monoclonal antibody that binds the IL-6 receptor, both the membrane-bound and soluble forms, thus inhibiting both classic and trans-IL-6 downstream signaling. Similarly, the IL-6 humanized murine chimeric monoclonal antibody siltuximab, although not FDA-approved for the treatment of cytokine release syndrome, has also been used in the treatment of cytokine release syndrome and is also being studied as a potential therapy in severe COVID-19 infections. Siltuximab (Sylvant, Janssen Biotech Inc., Horsham, Pennsylvania) binds directly to IL-6 and prevents the activation of immune effector cells. Sarilumab (Kevzara, Sanofi US, Bridgewater, New Jersey) is a human monoclonal antibody against the IL-6 receptor that was developed for the treatment of rheumatoid arthritis that is also being evaluated for severe COVID-19.
There are no systematically obtained clinical data yet that support a benefit to the use of steroids, and some reports have suggested a possible detriment with delayed viral clearance and increased risk of infection with MERS and SARS, although the role of steroids in COVID-19 is an area of active investigation (Table 4) (59).
COVID-19 and Cardiovascular Disease
The COVID-19 pandemic has presented innumerable challenges to health care organizations and health care providers. Given that the vast majority of patients with cardiovascular disease are at high risk for SARS-CoV-2 infection, the cardiovascular and cardio-oncology communities will play a major role in caring for patients with COVID-19 now and for the foreseeable future. As a community, we have a long tradition of enrolling patients into clinical trials that evaluate therapeutic agents whose mechanisms of action are familiar, which facilitates reaching clinical equipoise when enrolling patients in clinical trials. In the coming months, our communities will be asked to contribute patients to clinical trials where the mechanisms of action of the therapeutic agents are less familiar and the knowledge base required for providing care for COVID-19 is accelerating at a dizzying pace. Here we have tried to provide a foundation for physicians who are on the front line of providing care to patients with COVID-19, so that they can better understand the emerging cardiovascular epidemiology of COVID-19, as well as the biological rationale for the plethora of clinical trials that are either being designed or are currently recruiting patients.
Acknowledgment
The authors would like to acknowledge Elizabeth Thompson for her assistance in reviewing the ClinicalTrials.gov website.
Footnotes
This work was supported by grants from the National Institutes of Health (R21HL141802, R34HL146927, and R01HL118018 to Dr. Ky; and R01HL107594 and U10 HL110309 to Dr. Mann) and the American Heart Association (TPA34910059 to Dr. Ky). This paper will co-publish in JACC: Basic to Translational Science and JACC: CardioOncology.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
Bonnie Ky, Email: bonnie.ky@pennmedicine.upenn.edu.
Douglas L. Mann, Email: dmann@wustl.edu.
References
- 1.STAT The Covid-19 Tracker. https://www.statnews.com/2020/03/26/covid-19-tracker/ Available at: Accessed April 14, 2020.
- 2.John Hopkins University of Medicine Coronavirus Resource Center COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. https://coronavirus.jhu.edu/map.html Available at: Accessed April 14, 2020.
- 3.Guan W.J., Ni Z.Y., Hu Y., for the China Medical Treatment Expert Group for COVID-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1861–1862. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li B., Yang J., Zhao F. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020 Mar 11 doi: 10.1007/s00392-020-01626-9. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganatra S., Hammond S.P., Nohria A. The novel coronavirus disease (COVID-19) threat for patients with cardiovascular disease and cancer. J Am Coll Cardiol CardioOnc. 2020 Mar 20 doi: 10.1016/j.jaccao.2020.03.001. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P. The heart in COVID19: primary target or secondary bystander? J Am Coll Cardiol Basic Trans Science. 2020;5:537–542. doi: 10.1016/j.jacbts.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. 2020 Mar 16 doi: 10.1093/eurheartj/ehaa190. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inciardi R.M., Lupi L., Zaccone G. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1096. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi S., Qin M., Shen B. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 Mar 25 doi: 10.1001/jamacardio.2020.0950. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J., Zheng Y., Gou X. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C.Y., Chen S.F., Hollander S.A. Donor heart selection during the COVID-19 pandemic: a case study. J Heart Lung Transplant. 2020;39:498–499. doi: 10.1016/j.healun.2020.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolley A.E., Mehra M.R. Dilemma of organ donation in transplantation and the COVID-19 pandemic. J Heart Lung Transplant. 2020;39:410–411. doi: 10.1016/j.healun.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landi A., Iannucci V., Nuffel A.V., Meuwissen P., Verhasselt B. One protein to rule them all: modulation of cell surface receptors and molecules by HIV Nef. Curr HIV Res. 2011;9:496–504. doi: 10.2174/157016211798842116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hartupee J., Mann D.L. Neurohormonal activation in heart failure with reduced ejection fraction. Nat Rev Cardiol. 2017;14:30–38. doi: 10.1038/nrcardio.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harding S.E., Vescovo G., Jones S.M., Gurden J., Poole-Wilson P.A. Contractile responses of isolated adult rat and rabbit cardiac myocytes to isoproterenol and calcium. J Mol Cell Cardiol. 1988;20:635–647. doi: 10.1016/s0022-2828(88)80121-4. [DOI] [PubMed] [Google Scholar]
- 21.Imai Y., Kuba K., Rao S. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann M., Kleine-Weber H., Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol. 2010;45:146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons G., Zmora P., Gierer S., Heurich A., Pohlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antiviral Res. 2013;100:605–614. doi: 10.1016/j.antiviral.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020 Mar 4 doi: 10.1002/ddr.21656. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rice G.I., Thomas D.A., Grant P.J., Turner A.J., Hooper N.M. Evaluation of angiotensin-converting enzyme (ACE), its homologue ACE2 and neprilysin in angiotensin peptide metabolism. Biochem J. 2004;383:45–51. doi: 10.1042/BJ20040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia H.P., Look D.C., Tan P. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009;297:L84–L96. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monteil V., Kwon H., Prado P. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical grade human ACE2. Cell. 2020 Apr 2 doi: 10.1016/j.cell.2020.04.004. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 Feb 24 doi: 10.1016/S2213-2600(20)30079-5. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382:1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bozkurt B., Kovacs R., Harrington B. HFSA/ACC/AHA Statement Addresses concerns Re: Using RAAS Antagonists in COVID-19. Latest in Cardiology [ACC news story] March 17, 2020 doi: 10.1016/j.cardfail.2020.04.013. https://www.acc.org/latest-in-cardiology/articles/2020/03/17/08/59/hfsa-acc-aha-statement-addresses-concerns-re-using-raas-antagonists-in-covid-19 Available at: Accessed April 14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen F.S. How viruses invade cells. Biophys J. 2016;110:1028–1032. doi: 10.1016/j.bpj.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawase M., Shirato K., van der Hoek L., Taguchi F., Matsuyama S. Simultaneous treatment of human bronchial epithelial cells with serine and cysteine protease inhibitors prevents severe acute respiratory syndrome coronavirus entry. J Virol. 2012;86:6537–6545. doi: 10.1128/JVI.00094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhirnov O.P., Klenk H.D., Wright P.F. Aprotinin and similar protease inhibitors as drugs against influenza. Antiviral Res. 2011;92:27–36. doi: 10.1016/j.antiviral.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Yao X., Ye F., Zhang M. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 Mar 9 doi: 10.1093/cid/ciaa237. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gautret P., Lagier J.C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 Mar 20 doi: 10.1016/j.ijantimicag.2020.105949. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Page R.L., 2nd, O'Bryant C.L., Cheng D. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e32–e69. doi: 10.1161/CIR.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 40.Kuperschmidt K., Cohen J. WHO launches a global megatrial of the four most promising coronavirus treatments. March 22, 2020. https://www.sciencemag.org/news/2020/03/who-launches-global-megatrial-four-most-promising-coronavirus-treatments Available at: Accessed April 14, 2020.
- 41.Salata C., Baritussio A., Munegato D. Amiodarone and metabolite MDEA inhibit Ebola virus infection by interfering with the viral entry process. Pathog Dis. 2015;73:ftv032. doi: 10.1093/femspd/ftv032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stadler K., Ha H.R., Ciminale V. Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level. Am J Respir Cell Mol Biol. 2008;39:142–149. doi: 10.1165/rcmb.2007-0217OC. [DOI] [PubMed] [Google Scholar]
- 43.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fehr A.R., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheahan T.P., Sims A.C., Graham R.L. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017;9:eaaal3653. doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chow S.C. Adaptive clinical trial design. Annu Rev Med. 2014;65:405–415. doi: 10.1146/annurev-med-092012-112310. [DOI] [PubMed] [Google Scholar]
- 47.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 48.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020 Mar 18 doi: 10.1056/NEJMoa2001282. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spiegel M., Pichlmair A., Muhlberger E., Haller O., Weber F. The antiviral effect of interferon-beta against SARS-coronavirus is not mediated by MxA protein. J Clin Virol. 2004;30:211–213. doi: 10.1016/j.jcv.2003.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haagmans B.L., Kuiken T., Martina B.E. Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med. 2004;10:290–293. doi: 10.1038/nm1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arabi Y.M., Alothman A., Balkhy H.H., for the MIRACLE Trial Group Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:81. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehta P., McAuley D.F., Brown M., for the UK HLH Across Specialty Collaboration COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coomes E.A., Haghbayan H. Interleukin-6 in COVID-9: a systematic review and meta-analysis. https://www.medrxiv.org/content/10.1101/2020.03.30.20048058v1 Available at: Accessed April 3, 2020. [DOI] [PMC free article] [PubMed]
- 55.Neelapu S.S., Tummala S., Kebriaei P. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ghosh A.K., Chen D.H., Guha A., Mackenzie S., Walker J.M., Roddie C. CAR T cell therapy–related cardiovascular outcomes and management: systemic disease or direct cardiotoxicity? J Am Coll Cardiol CardioOnc. 2020;2:97–109. doi: 10.1016/j.jaccao.2020.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riegler L.L., Jones G.P., Lee D.W. Current approaches in the grading and management of cytokine release syndrome after chimeric antigen receptor T-cell therapy. Ther Clin Risk Manag. 2019;15:323–335. doi: 10.2147/TCRM.S150524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mahmoudjafari Z., Hawks K.G., Hsieh A.A., Plesca D., Gatwood K.S., Culos K.A. American Society for Blood and Marrow Transplantation Pharmacy Special Interest Group survey on chimeric antigen receptor T cell therapy administrative, logistic, and toxicity management practices in the United States. Biol Blood Marrow Transplant. 2019;25:26–33. doi: 10.1016/j.bbmt.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 59.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]