Abstract
Therapeutic vaccines and broadly neutralizing antibodies (bNAbs) represent potential approaches to antiretroviral-free treatment of HIV. Although therapeutic vaccines have been able to produce transient reductions in viral load during analytic treatment interruptions (ATIs), thus far none has been able to induce long-term remission. Pairing with latency reversal agents and immune modulators may improve vaccine efficacy. The bNAbs are investigated as a promising approach to achieving durable virologic control in the absence of antiretroviral therapy. Combinations of antibodies are necessary for increasing overall breadth and potency of coverage and preventing emergence of resistance. The next generation of antibodies includes engineered bispecific and trispecific antibodies that target 2 or 3 independent viral sites. This article is based on a presentation by Magdalena E. Sobieszczyk, MD, MPH, at the International Antiviral Society-USA (IAS-USA) continuing education program held in New York in March 2019.
Keywords: HIV, therapeutic vaccines, broadly neutralizing antibodies, viral rebound, antibody targets, bNAbs, bispecific, trispecific
Therapeutic Vaccines and Antibodies
The rationale for investigating antiretro-viral-free approaches to HIV treatment is severalfold:
It is impossible to eradicate HIV from latent viral reservoirs with antiretroviral therapy (ART) alone
It is important to have treatment options including agents with potential for less frequent dosing
There are gaps in ART delivery
ART is associated with long-term adverse effects
Adherence and retention in care remain a challenge
The rationale for therapeutic HIV vaccines and therapeutic use of anti-HIV broadly neutralizing antibodies (bNAbs) includes evidence from individuals whose immune system naturally controls HIV without ART (ie, long-term nonprogressors, elite controllers) that effective host-mediated anti-HIV immunity is possible. This raises the issue of whether it is possible to augment host immune response to kill infected CD4+ T cells and neutralize circulating virus in the absence of ART.
Therapeutic HIV Vaccines
At a minimum, the goals of a therapeutic vaccine would be to simplify ART regimens and allow for periodic analytic treatment interruption (ATI). Optimal objectives would include the ability to eliminate the need for ART either by eradicating the virus or by inducing host immune responses capable of controlling virus replication.
However, in the many placebo-controlled studies thus far that have included interruption of ART to measure therapeutic vaccine efficacy, no therapeutic vaccines have been successful in achieving durable suppression of HIV viremia.1–6 For example, a recently reported study showed that a DNA/rVSV therapeutic vaccine was unsuccessful in achieving sustained sup-Pression of virus after ART interruption in individuals who initiated ART early in infection (Figure 1).2 Similarly, a trial of the MVA-B vaccine showed no substantial effect on viral load rebound after ATI or on the viral reservoir with or without use of a latency reversal agent.3 Recent studies suggest that eliciting a broad immune response may be associated with greater impact on viral rebound following ATI. For example, a trial examining a DC-HIV vaccine (dendritic cells loaded with heat-inactivated autologous HIV) showed that the vaccine induced broad immune responses and a substantial reduction in viral load during ATI. However that the effect was transient4; in a separate study using a dendritic cell platform, broader immune responses correlated with better plasma viral load after ATI.7 And 2 trials investigating the ALVAC-HIV vaccine and Lipo-6T showed that vaccine-induced CD4+ and CD8+ T cell responses were associated with virologic control and delayed time to resumption of ART following ATI, compared with placebo.5,6
Figure 1.
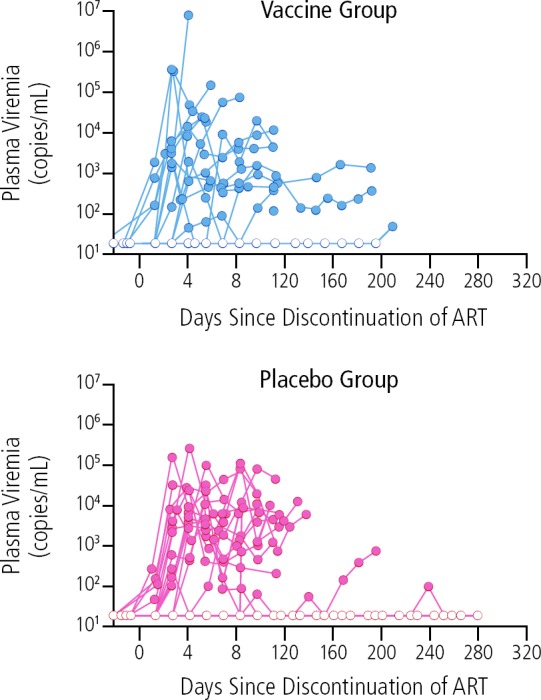
Absence of effect of DNA/rVSV therapeutic vaccination compared with placebo on control of HIV rebound following interruption of antiretroviral therapy (ART). Adapted from Sneller et al.2
Despite such disappointments, the field is looking now at combining therapeutic vaccines with other agents, such as toll-like receptor 7 (TLR7) agonists and latency reversal agents. For example, a provocative study in SIV-infected rhesus monkeys showed that use of the therapeutic Ad26/MVA vaccine alone induced broad cellular immune responses, but resulted in no clinically significant decrease in viral load setpoint after ATI.8 However, with the addition of a TLR7 agonist to the vaccine, there was a 1.7510-log copies/mL reduction in viral load, a 2.5-fold delay in viral rebound, and 33% of animals maintained undetectable viral load after ATI.
Thus, although there have been no randomized controlled trials of therapeutic vaccination that have induced any remission after ATI, it is now presumed that vaccines are needed that induce broad host immune responses to recognize diverse escape viral variants after viral rebound. Furthermore, therapeutic vaccines are being paired with potent latency reversal agents (eg, vorinostat) or immune modulators (TLR7 agonist) with the aim of potentially inducing evidence of remission.
Broadly Neutralizing Antibodies
The long-established function of passively administered bNAbs has been to inhibit viral entry into host cells by blocking key binding sites on the viral envelope (neutralizing activity). More recent studies of these antibodies demonstrate their capacity to engage the host immune system through Fc effector functions to mediate killing of infected cells via antibody-dependent cell-mediated cytotoxicity (ADCC), enhancing immune responses against HIV and potentially clear latently infected cells, thereby potentially presenting a strategy for targeting the reservoir.
A minority of HIV-infected individuals (5%–10%) develop the ability to neutralize various heterologous viruses from different subtypes within 2 to 3 years after infection. Very broad and potent neutralizing antibodies have been isolated from these individuals. These antibodies bind to relatively conserved regions of the HIV envelope. Passive transfer of bNAbs is being investigated for treatment, eradication/cure, prevention, and to guide prevenz vaccine design.
Much work has focused on isolating bNAbs and identifying what targets on the HIV envelope they attach to in order to block viral entry into CD4 cells. A number of targets on the virus have been identified as bNAb attachment points that are capable of neutralizing a wide range of HIV isolates, including the CD4 binding site, membrane proximal external region (MPER), the gp41 and gp120 interface, the V3 loop, and the V1/V2 loop (Figure 2).9 The figure shows some of the bNAbs in clinical development that target these sites.
Figure 2.
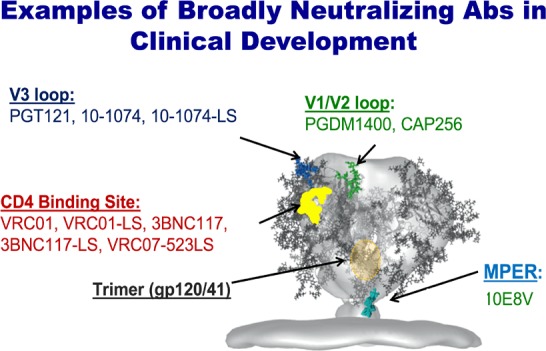
Examples of broadly neutralizing antibodies (bNAbs) in development and their viral target sites. Adapted from Stewart-Jones et al.31
Several seminal studies have shown antiviral activity with bNAb candidates. For example, passive infusion of the anti-CD4 binding site 3BNC117 antibody showed significant reduction of virema10 and, in another study, suppressed viral rebound among 13 ART-treated participants with 3BNC117-sensitive viruses.10 Patients underwent ATI and received 2 or 4 infusions of the antibody. The infusions were associated with a viral rebound delay of an average of 8.4 weeks in all participants versus 2.6 weeks in matched historical controls. The rebound virus was found to have resistance to 3BNC117 with low diversity in most patients.
In another study, the anti-CD4 binding site antibody VRCO1 was used in HIV-infected patients who were not screened for VRC01 sensitivity.11 Patients underwent ATI and 3 to 8 infusions. The median time to plasma viral rebound was 4 to 5.6 weeks, depending on the number of infusions only slightly delaying rebound compared with historical controls. Viral rebound was poly-clonal, despite high antibody levels in most patients and attributed to preexisting resistance. A recently reported study of this antibody in Thai adults who initiated ART during acute HIV infection did not significantly delay viral rebound after ART discontinuation.12
In general, it has been shown that the antibodies have on average been able to reduce HIV viral load by approximately 1.5 log10 copies/mL after a single infusion of one antibody in patients with viremia.9,10,13,14 However, with ATI, viral rebound ultimately occurs and, at least in the monotherapy setting, viruses resistant to the bNAbs emerge after several weeks, strengthening rationale for combination bNAbs. Findings reported at the 2019 Conference on Retroviruses and Opportunistic Infections (CROI) with use of the PGT121 antibody that targets the V3 loop bear noting, however. In viremic patients not on ART, response in terms of virologic control depended on baseline viral load. Those with a high baseline viral load before infusion of the bNAb had an approximately 1.7-log10 copy/mL drop, with rebound resistant virus in most responders. Patients with low baseline viral load exhibited somewhat better viral suppression, with 2 experiencing suppression for more than 6 months after a single infusion of the antibody.15
Steps toward optimizing bNAbs include identification or engineering of more broad and potent antibodies, modifying Fc portion to, for example, extend the half-life of the antibodies, using combinations of antibodies, and developing single antibodies with multiple targets through the engineering of bispecific and trispecific antibodies. Greater potency and breadth of bNAbs are needed to increase the proportion of a viral strain neutralized by the antibody and to neutralize a greater proportion of all circulating strains of the various viral subtypes. Figure 3 indicates the potency and breadth of effect of bNAbs that have been studied to date.16
Figure 3.
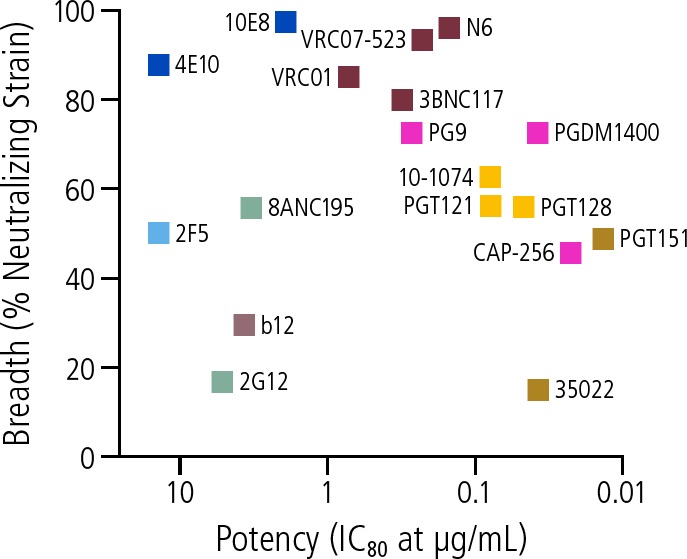
Examples of potency and breadth of effect of broadly neutralizing antibodies (bNAbs) in development. IC80 = 80% inhibitory concentration. Adapted from Gama and Koup.16
Clinical studies are exploring bNAbs with extended half-lives. It has been shown that introduction of an LS mutation—a 2 amino acid motif in the Fc region of the antibody—increases the affinity of that antibody for the neonatal Fc receptor. This allows for recirculation or recycling of the antibody in the serum and extends its plasma half-life. Such a modification can result in the ability to dose the antibody less frequently. It has also been shown to increase the levels of the antibody at the mucosal surface in animal studies, which could play a role in prevention of acquisition of HIV. Studies of the LS mutation antibody versions are ongoing both for prevention and treatment.17,19
Other modifications to the Fc region may increase the effector functions of the antibody, potentially increasing ADCC or phagocytosis and potentially targeting the HIV reservoir. A report at the 2019 CROI described an engineered variant of the antibody PGT121 that enhanced killing of HIV-infected CD4+ T-cells by natural killer cells.20
Combining antibodies may have particular promise for improving therapeutic potential. In studies to date, rebound viruses after bNAb treatment did not demonstrate increased resistance to other antibodies that target different envelope epitopes. Combinations of 2 or more bNAbs have been shown to result in more robust and sustained antiviral effects by increasing overall breadth and potency of coverage and preventing the emergence of resistance.
A recently reported study examined the effects of 3 infusions of the combination of 2 antibodies 3BNC117 and 10–1074 during ATI in patients who were virologically suppressed on ART for more than 24 months who had been prescreened for sensitivity to the antibodies. The median duration of viral suppression was 21 weeks for antibody-sensitive virus, a longer duration of suppression than has been seen with single antibodies.21 A noteworthy finding is that 2 patients maintained virologic suppression long after antibody levels had waned. This observation may suggest that the effector function of the antibodies is acting to augment host immune response, supporting the investigation of whether bNAbs can be a component of cure strategies. Additionally, this combination was also evaluated in viremic, treatment naive individuals, and resulted in an average HIV viral load decrease of 2.05 logio copies/mL in individuals with virus sensitive to both antibodies.22
In short, antibody combinations are likely to be necessary to increase overall breadth and potency of effect and to prevent the emergence of resistance. The number of bNAbs required for effective treatment may differ based on the indication; for example, in active viremia the combination of 3 or 4 bNAbs may be required to cover the swarm of viruses present. This also raises the issue of the need to screen for bNAb sensitivity prior to therapy to potentially reduce the number of bNAbs required for treatment and to amplify efficacy.
Finally, the next generation of bNAbs will likely include engineered bispecific and trispecific antibodies, single molecules that target multiple different epitopes on the virus or a cellular receptor like CD4. Such an approach could potentially lower the likelihood of escape mutations and provide enhanced neutralization potency and breadth in a single molecule. An example of a bispecific antibody in development is 10E8.4/iMab, shown in Figure 4.23,24 One arm of the antibody binds an epitope in the membrane proximal external region (MPER) of gp41; the other arm binds the HIV CD4 receptor molecule on T-helper cells. The antibody entered first in human clinical testing in spring of 2019.25
Figure 4.
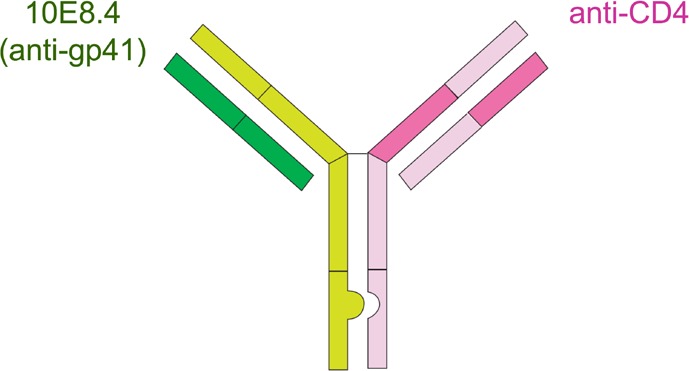
Example of an engineered bispecific antibody–10E8.4/iMab represented on the left. See text for details. Adapted from Huang et al.23
- One arm of IgG binds an epitope in the membrane proximal external region (MPER) of gp41
- The other arm binds either the HIV-1 CD4 receptor on T-helper cell
Phase 1, first in human, clinical study for treatment and prevention is starting 2019
Trispecific antibodies are engineered to bind to 3 sites on the HIV envelope. An example of one that recently entered clinical testing in individuals with HIV infection combines specificities of 3 antibodies binding to the CD4 binding site, V1/V2 loop, and gp41 MPER.26 Animal data presented at the 2019 CROI showed that the antibody exerted potent Fc effector functions, promising for mediating ADCC and phagocytosis, and potent suppression of viral replication in viremic simian/HIV-infected animals.27,28
Because current bNAbs are engineered as human proteins, antidrug antibody (ADA) responses can be infrequently observed, potentially interfering with activity and resulting in emergence of resistance to this immune therapy. In clinical studies to date, ADA responses have not been detected.29,30
Recent advances in bNAb development are exciting and might offer alternative approaches to HIV therapy or possibly cure. The role of bNAbs in this area will be established by further preclinical and clinical studies. As these products move forward in development, however, and before they are widely adopted for the treatment and possible eradication, it is important to consider their cost, frequency and practicality of dosing intravenously or subcutaneously, their acceptability to diverse populations.
Summary
The bNAbs are a potentially promising approach toward durable control of HIV rebound in the absence of ART. They have been shown to be generally safe and well tolerated in clinical studies to date, and are also being actively pursued for prevention as passive prevention and as a platform for design of vaccines. To date, no randomized controlled trials of therapeutic vaccination have shown the ability to induce long-term remission after ATIs. Combination strategies of bNAbs, therapeutic vaccines, and immunomodulators (eg, TLR7 agonist) may be needed to diminish the HIV reservoir.
Footnotes
Presented by Dr Sobieszczyk in March 2019. First draft prepared from transcripts by Matthew Stenger. Reviewed and edited by Dr Sobieszczyk in $$October$$ 2019.
Financial affiliations in the past 12 months: Dr Sobieszczyk has no relevant financial affiliations to disclose
References
- 1. Tung FY, Tung JK, Pallikkuth S, Pahwa S, Fischi MA. A therapeutic HIV-1 vaccine enhances anti-HIV-1 immune responses in patients under highly active antiretroviral therapy. Vaccine. 2016;34(19): 2225–2232. [DOI] [PubMed] [Google Scholar]
- 2. Sneller MC, Justement JS, Gittens KR, et al. A randomized controlled safety/efficacy trial of therapeutic vaccination in HIV-infected individuals who initiated antiretroviral therapy early in infection. Sci Transl Med. 2017;9(419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mothe B, Climent N, Plana M, et al. Safety and immunogenicity of a modified vaccinia Ankara-based HIV-1 vaccine (MVAB) in HIV-1-infected patients alone or in combination with a drug to reactivate latent HIV-1. J Antimicrob Chemother. 2015; 70(6):1833–1842. [DOI] [PubMed] [Google Scholar]
- 4. Garcia F, Climent N, Assoumou L, et al. A therapeutic dendritic cell-based vaccine for HIV-1 infection. Infect Dis. 2011; 203(4):473–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levy Y, Gahery-Segard H, Durier C, et al. Immunological and virological efficacy of a therapeutic immunization combined with interleukin-2 in chronically HIV-1 infected patients. AIDS. 2005;19(3): 279–286. [PubMed] [Google Scholar]
- 6. Levy Y, Durier C, Lascaux AS, et al. Sustained control of viremia following therapeutic immunization in chronically HIV-1-infected individuals. AIDS. 2006;20(3): 405–413. [DOI] [PubMed] [Google Scholar]
- 7. Levy Y, Thiebaut R, Montes M, et al. Dendritic cell-based therapeutic vaccine elicits polyfunctional HIV-specific T-cell immunity associated with control of viral load. Eur J Immunol. 2014;44(9): 2802–2810. [DOI] [PubMed] [Google Scholar]
- 8. Borducchi EN, Cabral C, Stephenson KE, et al. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature. 2016;540(7632): 284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scheid JF, Horwitz JA, Bar-On Y, et al. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535(7613): 556–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Caskey M, Klein F, Lorenzi JC, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557): 487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bar KJ, Sneller MC, Harrison LJ, et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med. 2016;375(21):2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crowell TA, Colby DJ, Pinyakorn S, et al. Safety and efficacy of VRC01 broadly neutralising antibodies in adults with acutely treated HIV (RV397): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet HIV. 2019;6(5): e297–e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lynch RM, Boritz E, Coates EE, et al. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med. 2015;7(319):319ra206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caskey M, Schoofs T, Gruell H, et al. Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat Med. 2017; 23(2):185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stephenson KE, Julg B, Ansel J, et al. Therapeutic activity of PGT121 monoclonal antibody in HIV-infected adults [Abstract 145LB]. Top Antivir Med. 2019;27(1s):54s. [Google Scholar]
- 16. Gama L, Koup RA. New-generation high-potency and designer antibodies: role in HIV-1 treatment. Annu Rev Med. 2018; 69:409–419. [DOI] [PubMed] [Google Scholar]
- 17. Clinical Trials. First-in-human study of 10-1074-LS alone and in combination with 3BNC117-LS. https://clinicaltrials.gov/ct2/show/NCT03554408. Accessed on January 22, 2020.
- 18. Clinical Trials. VRC 605: safety and pharmacokinetics of a human monoclonal antibody, VRC-HIVMAB075-00-AB (VRC07-523LS), administered intravenously or subcutaneously to healthy adults. https://clinicaltrials.gov/ct2/show/NCT03015181. Accessed on January 22, 2020.
- 19. Clinical Trials. Evaluating the safety and serum concentrations of a human monoclonal antibody, VRC-HIVMAB075-00-AB (VRC07-523LS), administered in multiple doses and routes to healthy, HIV-uninfected adults. https://clinicaltrials.gov/ct2/show/NCT03387150. Accessed on January 22, 2020.
- 20. Thomsen ND, Balakrishnan M, Pace CS, et al. GS-9722: first-in-class effector-enhanced broadly neutralizing antibody for HIV cure [CROI Abstract 356]. In special Issue: Abstracts From the 2019 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med. 2019; 27(1s):129s. [Google Scholar]
- 21. Mendoza P, Gruell H, Nogueira L, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. 2018;561(7724):479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bar-On Y, Gruell H, Schoofs T, et al. Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat Med. 2018;24(11): 1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang Y, Yu J, Lanzi A, et al. Engineered bispecific antibodies with exquisite HIV-1-neutralizing activity. Cell. 2016;165(7): 1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Padte NN, Yu J, Huang Y, Ho DD. Engineering multi-specific antibodies against HIV-1. Retrovirology. 2018;15(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clinical Trials. 10E8.4/iMab bispecific antibody in HIV-uninfected and HIV-infected adults. https://clinicaltrials.gov/ct2/show/NCT03875209. Accessed on January 22, 2020.
- 26. Clinical Trials. Pharmacokinetics of SAR441236. https://clinicaltrials.gov/ct2/show/NCT03705169. Accessed on January 22, 2020.
- 27. Xu L, Pegu A, Rao E, et al. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science. 2017;358(6359):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pegu A, Xu L, Demouth M, et al. Potent antiviral activity of trispecific broadly neutralizing HIV antibodies [CROI Abstract 28LB]. In Special Issue: Abstracts From the 2019 Conference on Retrovi-ruses and Opportunistic Infections. Top Antivir Med. 2019;27(1s):12s. [Google Scholar]
- 29. Mayer KH, Seaton KE, Huang Y, et al. Safety, pharmacokinetics, and immunological activities of multiple intravenous or subcutaneous doses of an anti-HIV monoclonal antibody, VRC01, administered to HIV-uninfected adults: Results of a phase 1 randomized trial. PLoS Med. 2017;14(11): e1002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cohen YZ, Butler AL, Millard K, et al. Safety, pharmacokinetics, and immunogenicity of the combination of the broadly neutralizing anti-HIV-1 antibodies 3BNC117 and 10–1074 in healthy adults: A randomized, phase 1 study. PLoS One. 2019;14(8): e0219142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stewart-Jones GB, Soto C, Lemmin T et al. Trimeric HIV-1-env structures define glycan shields from clades A.B. and G. Cell. [DOI] [PMC free article] [PubMed]


