Abstract
Achieving a cure for HIV remains a priority in HIV research. Two cases of ‘sterilizing cure’ have been observed—in Timothy Ray Brown and the “London” patient; both patients received allogeneic hematopoietic stem cell transplantation (HSCT) from donors homozygous for the CCR5-delta 32 deletion, which impairs function of an HIV coreceptor on host cells. Other strategies that have been evaluated for achieving sterilizing cure or functional cure—ie, sustained virologic remission in the absence of antiretroviral therapy (ART)—include: HSCT with wild-type CC chemokine receptor (CCR5); early ART to limit size of the HIV latent reservoir; shock and kill strategies using latency reversing agents and/or anti-HIV broadly neutralizing antibodies; and gene therapy, including attempts to modify CCR5 genes, HIV proviruses in autologous host cells, or enhanced T cells. This article summarizes a presentation by Jonathan Li, MD, MMSc, at the International Antiviral Society-USA (IAS-USA) continuing education program held in Atlanta, Georgia, in March 2019.
Keywords: HIV cure, CCR5-delta 32 deletion, London patient, post-treatment controllers, early ART, shock and kill, gene therapy
HIV cure remains a priority of research in HIV infection management and treatment. Pursuing this goal requires identifying and developing strategies to overcome mechanisms of HIV persistence to induce and maintain HIV remission.
Update on the Epidemic
The advent of effective combination antiretroviral therapy (ART) has resulted in marked declines in HIV mortality and incidence. However, there remains much work to be done in battling the epidemic. Joint United Nations Programme on HIV and AIDS (UNAIDS)/World Health Organization (WHO) data indicate that there are more than 37 million people globally living with HIV infection. Of these, 23 million are on ART.1 There are 1.8 million new infections and nearly 800,000 AIDS-related deaths each year. The WHO introduced the 90-90-90 initiative, aimed at achieving awareness of infection status in 90% of infected individuals, having 90% of those individuals maintained on treatment, and achieving virologic suppression in 90% of those on treatment. However, it is currently estimated that only 75% of infected individuals know of their infection status, 59% are on treatment, and 47% have viral suppression.2 There are numerous issues that contribute to suboptimal ART treatment and retention in care. Many infected individuals are asymptomatic, leading to delayed diagnosis, denial, or complacency. Other factors that contribute to gaps in the cascade of care include challenges in accessing affordable and consistent care, ART intolerability, pill fatigue, drug-drug interactions, stigma, life chaos, substance abuse, and challenges in connecting with hard-to-reach populations. Further, there are long-term complications of HIV despite ART, including evidence for accelerated aging and increased risks of cognitive dysfunction, cardiovascular disease, renal disease, and other complications.3–6 All of these factors provide a strong rationale for pursuing cure for HIV infection.
Mechanisms of HIV Persistence
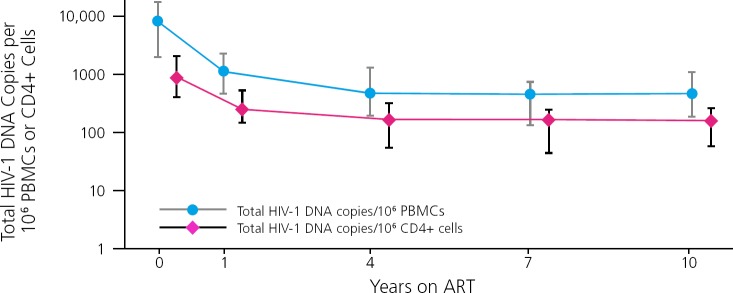
Data from long-term follow-up of a cohort of patients on ART indicate that the HIV reservoir, indicated by levels of HIV DNA in CD4+ cells and peripheral blood mononuclear cells, initially declines during ART but plateaus at around 4 years and remains stable thereafter (Figure 1).7 Key factors in HIV persistence include viral integration into the host cell genome and that the integrated provirus can become latent or silent, such that little viral RNA or proteins are being expressed that would permit the immune system to recognize and target the infected cells.
Figure 1.
Persistence of the HIV reservoir, indicated by levels of HIV DNA in CD4+ cells and peripheral blood mononuclear cells (PBMCs), during antiretroviral therapy (ART). Adapted from Besson, et al.7
Other factors may contribute to HIV persistence. For example, some researchers believe that there continues to be active viral replication in patients on ART, particularly within certain tissues or compartments where ART levels may be suboptimal.8,9 However, this is a relatively controversial hypothesis, with the predominance of evidence demonstrating the lack of active viral replication in patients on fully suppressive ART.10–12 Another cause of HIV persistence lies in the infection of long-lived cells as memory CD4+ cells and hematopoietic progenitor cells.13 Further, infected cells, particularly CD4+ cells, can undergo homeostatic or clonal proliferation, expanding the HIV reservoir.14–18 Finally, B cell follicles within lymph nodes act as an “immune sanctuary” that prevents access of HIV-specific CD8+ cells, potentially allowing persistence of virus within lymph nodes.19,20
Cure—Success Stories
One example of cure is Timothy Ray Brown (initially known as the “Berlin patient”). To understand this case it needs to be understood that HIV requires a CD4 receptor and a coreceptor to gain entry to the host cell, especially the CC chemokine receptor 5 (CCR5). Individuals have been identified who are naturally resistant to acquiring HIV infection with the most frequently transmitted CCR5-tropic virus due to the presence of a deletion in the CCR5 gene, called the CCR5-delta 32 deletion, that impairs expression of the CCR5 receptor.21
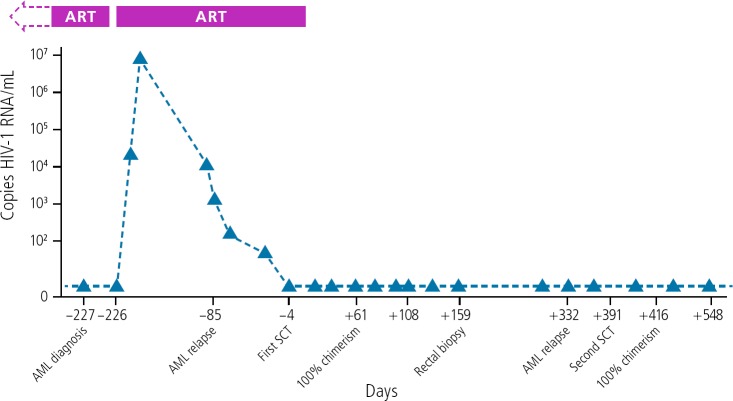
Timothy Ray Brown was living with HIV infection when he was diagnosed with acute myeloid leukemia (AML). To treat the leukemia, he was to undergo allogeneic hematopoietic stem cell transplantation (HSCT), requiring conditioning chemotherapy and whole-body irradiation. In the search for a donor for the transplant, his physicians identified one who was both an HLA match for Mr Brown and who was homozygous for the CCR5-delta 32 deletion. As shown in Figure 2, Mr Brown's viral load increased during ART interruption for chemotherapy, and decreased when ART was resumed prior to transplantation.22 ART was stopped when he received a first transplantation, with no viral rebound. He subsequently experienced leukemic relapse and underwent a second transplantation, and has now been HIV-free for more than 12 years off ART.
Figure 2.
HIV remission in Timothy Brown by allogenic hematopoietic skin cell transplant from a donor with the chemokine receptor 5—delta 32 deletion. Abbreviations: AML, acute myeloid leukemia; ART, antiretroviral therapy; SCT, gene for a human hormone secretion. Adapted from Hutter et al.22
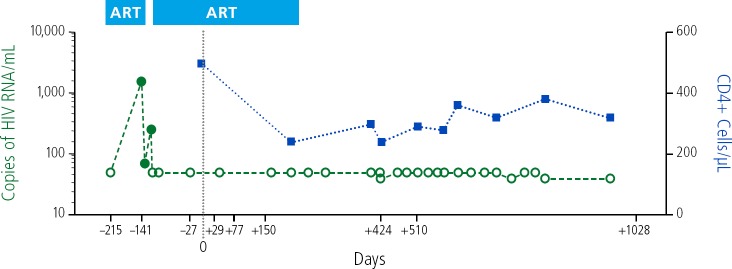
More recently, details have been presented about the “London patient.” This patient had Hodgkin lymphoma and also received allogeneic HSCT with cells from a donor homozygous for the CCR5 delta 32 deletion. The patient had viral rebound during ART interruption and achieved resuppression with resumption of ART. After approximately 18 months, ART was stopped, and the patient has been free of viral rebound for more than 2 years off ART (Figure 3).23
Figure 3.
HIV remission in the London patient following CC chemokine receptor 5-delta 32/delta 32 deletion hematopoietic stem-cell transplantation. ART indicates antiretroviral therapy Adapted from Gupta et al.23
Differences between Mr Brown and the London patient include the fact that Mr Brown was initially heterozygous for the CCR5 delta 32 deletion, whereas the London patient was homozygous for wild-type CCR5 before the transplant. For AML, Mr Brown underwent 2 HSCTs, total body irradiation, and full intensity conditioning chemotherapy. For lymphoma, the London patient underwent a single HSCT, received no irradiation, and underwent reduced-intensity conditioning. The mechanisms for T-cell depletion in the 2 patients also differed. Perhaps the take-home lesson from the comparison is that the highly toxic conditioning regimen and whole-body irradiation Mr Brown received may not be necessary for HIV eradication, that the same result can be achieved with less intense conditioning and without radiation. Together, the cases point to the importance of the donor being homozygous for the CCR5-delta 32 deletion, but additional studies will be needed to assess the impact of other factors, such as graft-versus-host reactions.
Timothy Brown and the London patient represent achievement of a sterilizing HIV cure, in which there is no functional HIV remaining in the body. However, HSCTs are associated with substantial morbidity and mortality. A more realistic goal may be a functional cure, consisting of sustained virologic remission and HIV suppression in the absence of ART. Such sustained remission has been observed in HIV controllers or long-term non-progressors and in HIV post-treatment controllers (PTCs). A great deal of data from AIDS Clinical Trials Group (ACTG) studies indicate that viral rebound generally occurs between 2 and 4 weeks after interrupting suppressive ART.24 However, con-siderable variation has also been ob-served in time to rebound and there are individuals who are able to maintain viral suppression for a prolonged period after ART discontinuation. Several years ago, the French VISCONTI study identified 14 individuals who started ART early in infection and who maintained virologic suppression after interrupting ART.25 The identification of these PTCs has led to increased interest in finding strategies for HIV remission
Strategies for Inducing HIV Remission
Among the approaches being investigated for inducing HIV remission and transforming patients into PTCs are: bone marrow transplantation (BMT) with CCR5 wild-type donors; early HIV treatment; shock and kill strategies, and gene therapy, among others.
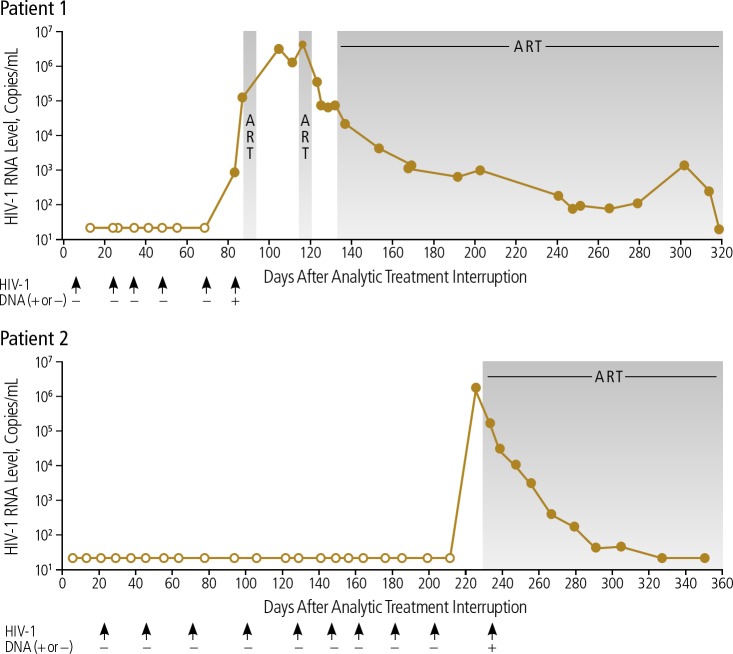
Individuals who naturally have the CCR5-delta 32 deletion are rare; a gene frequency of approximately 10% is found in those of European descent.26 Thus, people with HIV are far more likely to receive a HSCT from a donor with wild-type CCR5 cells. The cases of 2 “Boston BMT patients” showed that allogeneic HSCT from donors with wild-type CCR5 resulted in delayed HIV rebound. After transplantation, no evidence of HIV DNA was detected.27 After analytic treatment interruption, 1 patient maintained virologic suppression for 3 months and the other for 8 months (Figure 4).28 Although no sterilizing cure was achieved, the findings indicate that depletion of the HIV reservoir can result in suppression longer than the typical 2 to 4 weeks after stopping ART. Both patients were found to have acute retroviral rebound syndrome, likely as their post-transplant immune system was functionally naive to HIV.
Figure 4.
Delayed viral rebound in the Boston bone marrow transplant (BMT) patients, who received donor cells with wild-type CC chemokine receptor-5 (CCR5). ART indicates antiretroviral therapy (indicated by shaded areas). Adapted from Henrich et al.28
Early HIV treatment has been shown to reduce the size of the HIV reservoir to a level below that seen in patients starting ART during chronic infection.29,30 It has not been clear whether the reduction with early treatment might preferentially lead to post-treatment control. In the VISCONTI study, all participants had received early ART, and there was no comparison group of participants who had started ART during chronic infection. In the CHAMP (Control of HIV After Antiretroviral Medication Pause) study, 14 North American clinical trials were analyzed and 67 participants were identified who were PTCs.31 PTCs accounted for 13% of participants who had early ART versus 4% of participants who began ART during chronic infection (P<.01), suggesting that early ART may lower the barrier to achieving HIV remission. Of note, studies of ART initiation during the very earliest stages of HIV infection (ie, Fiebig I) did not identify any PTCs, which raises the possibility that a slight delay in ART initiation may lead to a more robust immune response.32
The shock and kill strategy is an approach to reawaken latently infected cells through use of a latency reversing agent that can stimulate HIV RNA and protein production, while enhancing the immune system's ability to kill these cells, for example, through the use of a therapeutic vaccine or an HIV broadly neutralizing antibody (bNAB). The approach would be used while patients are maintained on suppressive ART, so that no new infection of cells will occur despite the stimulation of virus production. A recent study in SHIV-infected monkeys examined this approach using a toll-like receptor 7 (TLR7) agonist (GS-9620) as a latency reversing agent combined with an anti-HIV bNAb.33 After treatment interruption, rebound was observed in fewer monkeys receiving both the latency reversing agent and the antibody than in those receiving either alone or neither. There is also some evidence that the use of bNAbs alone may be able to induce long-term HIV remission.34
One gene therapy approach that has been investigated is use of zinc-finger nucleases targeting the CCR5 gene. In one study, CD4+ cells were taken from individuals with HIV infection, modified in the laboratory with the zinc-finger nucleases with the aim of modifying the CCR5 gene to contain the deletions, and reinfused into the patient.35 All participants had viral rebound during treatment interruption, although one participant exhibited suppression before the interruption ended and ART was resumed. This participant was initially heterozygous for the CCR5-delta 32 deletion. It is believed that this particular strategy is not efficient in modifying the cells to contain the CCR5 deletions, but that achieving the modification may be more likely in individuals already heterozygous for the deletion. Some data from ongoing studies support the idea that alteration of viral kinetics with this approach is more likely in heterozygous patients. However, much work remains to be done in this field.
Other approaches that are being evaluated include enhancing T-cell activity with the use of chimeric antigen receptors (CARs), an approach that is currently being utilized in the oncology field and is being evaluated for HIV.36 There are also attempts to silence the HIV provirus, including through the use of CRISPR proviral gene editing37 or inhibition of the HIV Tat function.38
Footnotes
Presented by Dr Li in March 2019. First draft prepared from transcripts by Matthew Stenger. Reviewed and edited by Dr Li in November 2019.
Financial affiliations in the past 12 months: Dr Li has served as a consultant to Jan Biotech. (Updated 11/22/19
References
- 1. World Health Organization (WHO). HIV/AIDS: data and statistics. https://www.who.int/hiv/data/en/. Accessed on November 26, 2019.
- 2. World Health Organization (WHO). HIV testing and care continuum (2018). https://www.who.int/hiv/data/2018hiv-continuum-care.png. Accessed on November 26, 2019.
- 3. Hasse B, Ledergerber B, Furrer H, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 4. Freiberg MS, Chang CC, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Althoff KN, Rebeiro P, Brooks JT, et al. Disparities in the quality of HIV care when using US Department of Health and Human Services indicators. Clin Infect Dis. 2014;58(8):1185–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanna DB, Ramaswamy C, Kaplan RC, et al. Trends in cardiovascular disease mortality among persons with HIV in New York City, 2001–2012. Clin Infect Dis. 2016;63 (8):1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Besson GJ, Lalama CM, Bosch RJ, et al. HIV-1 DNA decay dynamics in blood during more than a decade of suppressive antiretroviral therapy. Clin Infect Dis. 2014;59(9): 1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A. 2014;111(6):2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. R Lorenzo-Redondo, Fryer HR, Bedford T, et al. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530(7588):51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kearney MF, Spindler J, Shao W, et al. Lack of detectable HIV-1 molecular evolution during suppressive antiretroviral therapy. PLoS Pathog. 2014;10(3):e1004010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Zyl GU, Katusiime MG, Wiegand A, et al. No evidence of HIV replication in children on antiretroviral therapy. J Clin Invest. 2017;127(10):3827–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenbloom DIS, Hill AL, Laskey SB, Siliciano RF. Re-evaluating evolution in the HIV reservoir. Nature. 2017;551(7681):E6–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carter CC, Onafuwa-Nuga A, McNamara LA, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16(4):446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bui JK, Sobolewski MD, Keele BF, et al. Proviruses with identical sequences comprise a large fraction of the replication-competent HIV reservoir. PLoS Pathog. 2017;13(3):e1006283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wiegand A, Spindler J, Hong FF, et al. Single-cell analysis of HIV-1 transcriptional activity reveals expression of proviruses in expanded clones during ART. Proc Natl Acad Sci U S A. 2017;114(18):E3659–E3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hiener B, Horsburgh BA, Eden JS, et al. Identification of genetically intact HIV-1 proviruses in specific CD4(+) T cells from effectively treated participants. Cell Rep. 2017;21(3):813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang Z, Gurule EE, Brennan TP, et al. Expanded cellular clones carrying replication-competent HIV-1 persist, wax, and wane. Proc Natl Acad Sci U S A. 2018;115(11): E2575–E2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reeves DB, Duke ER, Wagner TA, Palmer SE, Spivak AM, Schiffer JT. A majority of HIV persistence during antiretroviral therapy is due to infected cell proliferation. Nat Commun. 2018;9(1):4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fukazawa Y, Lum R, Okoye AA, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21(2):132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Banga R, Procopio FA, Noto A, et al. PD-1 (+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med. 2016;22(7):754–761. [DOI] [PubMed] [Google Scholar]
- 21. Samson M, Libert F, Doranz BJ, Rucker J. Resistance to HIV-1 infection in caucasian individuals in bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382(6593):722–725. [DOI] [PubMed] [Google Scholar]
- 22. Hutter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N EnglJMed. 2009;360(7):692–698. [DOI] [PubMed] [Google Scholar]
- 23. Gupta RK, Abdul-Jawad S, McCoy LE, et al. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature. 2019;568(7751): 244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS. 2016;30(3):343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated anti-retroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martinson JJ, Chapman NH, Rees DC, Liu YT, Clegg JB. Global distribution of the CCR5 gene 32-basepair deletion. Nat Genet. 1997;16(1):100–103. [DOI] [PubMed] [Google Scholar]
- 27. Henrich TJ, Hu Z, Li JZ, et al. Long-term reduction in peripheral blood HIV type 1 reservoirs following reduced-intensity conditioning allogeneic stem cell transplantation. J Infect Dis. 2013;207(11): 1694–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Henrich TJ, Hanhauser E, Marty FM, et al. Antiretroviral-free HIV-1 remission and viral rebound after allogeneic stem cell transplantation: report of 2 cases. Ann Intern Med. 2014;161(5):319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Buzon MJ, Martin-Gayo E, Pereyra F, et al. Long-term antiretroviral treatment initiated at primary HIV-1 infection affects the size, composition, and decay kinetics of the reservoir of HIV-1-infected CD4 T cells. J Virol. 2014;88(17):10056–10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ananworanich J, Chomont N, Eller LA, et al. HIV DNA set point is rapidly established in acute HIV infection and dramatically reduced by early ART. EBioMedicine. 2016;11:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Namazi G, Fajnzylber JM, Aga E, et al. The control of HIV after antiretroviral medication pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis. 2018;218(12):1954–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Colby D, Chomont N, Kroon E, et al. HIV RNA rebound postinterruption in persons suppressed in Fiebig I acute HIV. Poster presented at: 24th Conference on Retroviruses and Opportunistic Infections (CROI); February 13–16, 2017; Seattle, Washington.
- 33. Borducchi E, Abbink P, Nkolola J, Lewis MG, Geleziunas R, Barouch D. PGT121 combined with GS-9620 delays viral rebound in SHIV-infected rhesus monkeys. [CROI Abstract 73LB] In Special Issue: Abstracts From the 2018 Conference on Retroviruses and Opportunistic Infections. Top Antivir Med. 2018;26(suppl 1):29s. [Google Scholar]
- 34. Mendoza P, Gruell H, Nogueira L, et al. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. 2018;561(7724):479–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tebas P, Stein D, Tang WW, et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N Engl J Med. 2014;370(10):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim GB, Hege K, Riley JL. CAR talk: how cancer-specific CAR T cells can instruct how to build CAR T cells to cure HIV. Front Immunol. 2019;10:2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dash PK, Kaminski R, Bella R, et al. Sequential LASER ART and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat Commun. 2019;10(1):2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kessing CF, Nixon CC, Li C, et al. In vivo suppression of HIV rebound by didehydrocortistatin A, a “block-and-lock” strategy for HIV-1 treatment. Cell Rep. 2017; 21 (3):600–611. [DOI] [PMC free article] [PubMed] [Google Scholar]