Abstract
A high proportion of individuals with HIV infection currently are diagnosed at an advanced stage of disease (late presenters), increasing their risk for immune reconstitution inflammatory syndrome (IRIS). IRIS typically occurs within 6 months of initiation of antiretroviral therapy (ART) in patients with low CD4+ cell counts and can occur before any marked elevation in CD4+ count is achieved on ART. In addition to low CD4+ count at ART initiation, 2 other major clinical predictors of IRIS are preexisting opportunistic infection (including subclinical infection) and shorter treatment period for opportunistic infection prior to starting ART. Mycobacterial infection-associated IRIS, including tuberculosis (TB)-associated IRIS, and cryptococcal infection-associated IRIS are the most common forms of the syndrome. Corticosteroid prophylaxis and early treatment can be effective in reducing incidence of TB-IRIS and severity of symptoms in select patients. Sterilization of the cerebrospinal fluid should be achieved prior to starting ART in patients with TB meningitis and cryptococcal meningitis. This article summarizes a presentation by Irini Sereti, MD, MHS, at the International Antiviral Society-USA (IAS-USA) continuing education program held in Washington, DC, in April 2019.
Keywords: HIV, antiretroviral therapy, IRIS, immune reconstitution, CD4+, tuberculosis, cryptococcal meningitis, corticosteroids
The risk of immune reconstitution inflammatory syndrome (IRIS) is highest in individuals with HIV infection who initiate antiretroviral therapy (ART) with advanced HIV disease (ie, in those with lower CD4+ cell counts who have history of or an active opportunistic illness). It is unfortunate that a high proportion of HIV-infected individuals are still diagnosed and begin ART during advanced disease. Despite some improvement in earlier diagnosis and initiation of treatment from preceding years, a recent study of trends across 55 countries estimated that in 2015, 37% of patients initiating ART had advanced HIV infection indicated by CD4+ cell counts of less than 200/µL. US data from 2007 and 2008 indicate that 35% of new diagnoses occurred at a CD4+ count of less than 200/µL, including 42% of new diagnoses in black individuals and 46% in Hispanic individuals.
Morbidity and mortality are markedly increased among patients initiating ART at low CD4+ cell counts, particularly during the first 6 months to 1 year after starting ART. Contributors to excess mortality include ongoing opportunistic infections during recovery of CD4+ cell count, infectious and thromboembolic complications, toxicity associated with polypharmacy, and IRIS.
IRIS Risk and Presentation
IRIS can be defined as a worsening of manifestations or abrupt or atypical presentation of infections or tumors related to infections after HIV patients start ART. The syndrome can be ‘paradoxical’, in that a pre-existing condition worsens as ART improves CD4+ cell count and immune function, or can occur as the unmasking of an occult infection. The reported incidence of IRIS has ranged from approximately 3% to almost 50%, depending on patient population, study cohort, or rate of recognition of this complication. IRIS typically occurs within 6 months of ART initiation in the setting of successful virologic suppression and when treatment of an opportunistic infection has resulted in a successful microbiologic outcome (in paradoxical IRIS). Thus, for example, a patient with tuberculosis (TB) can be TB culture-negative when IRIS occurs; it is not the infection, but the immune response to the residual antigen that is ‘out of control’.
The 3 major clinical predictors of IRIS are: severe CD4+ cell depletion at initiation of ART; pre-existing opportunistic infection (even if subclinical); and shorter treatment period for an opportunistic infection before starting ART, which can be associated with presence of higher antigen loads.
Presentations of IRIS are shown in Figure 1. The upper left shows a patient who had TB and developed persistent and relapsing IRIS in the form of necrotic lymphadenopathy; the patient had already undergone several drainages when the photograph was taken. The upper right shows a woman who came to the US from Mexico and presented with disseminated histoplasmosis and mycobacterium avium complex (MAC, and exhibited enlarged and inflamed lymph nodes. The bottom left shows a patient who also had an unmasking MAC lymphadenitis that caused necrotic lymphadenopathy that was compressing the trachea and jugular vein; the condition required corticosteroid treatment and lymph node drainage. The bottom right shows a patient who had miliary TB, as well as TB arthritis and osteomyelitis, and who developed severe persistent IRIS that caused blockage of the thoracic duct.1
Figure 1.
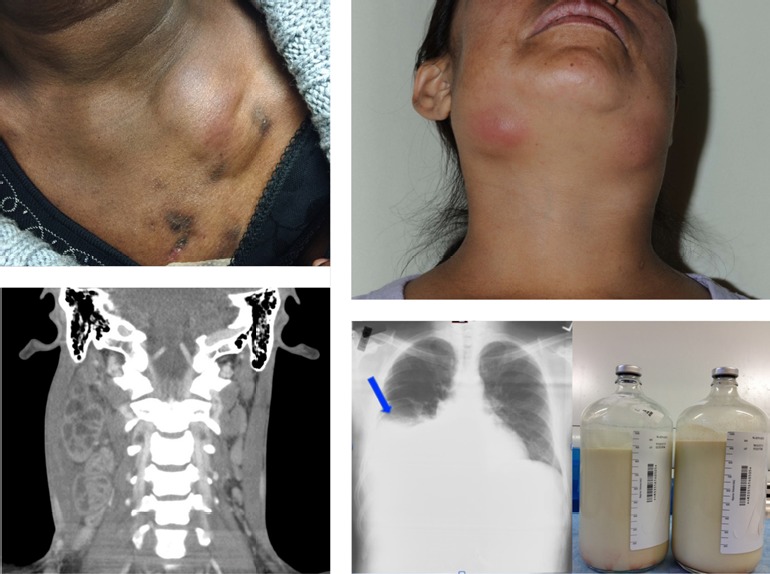
Upper left: a patient with tuberculosis (TB) who developed persistent and relapsing immune reconstitution inflammatory syndrome (IRIS) in the form of necrotic lymphadenopathy. The patient had undergone several drainages when the photograph was taken. Upper right: a patient who came to the US from Mexico and presented with disseminated histoplasmosis and mycobacterium avium complex (MAC) and exhibited enlarged and inflamed lymph nodes after ART initiation. Bottom left: a patient who had unmasking MAC lymphadenitis that caused necrotic lymphadenopathy that was compressing the trachea and jugular vein. The condition required corticosteroid treatment and lymph node drainage. Bottom right: a patient with miliary TB, TB arthritis, and osteomyelitis who developed severe persistent IRIS that caused blockage of the thoracic duct and chylothorax. Adapted from Hsu et al,1 and from Barber et al17
IRIS Pathogenesis
IRIS is characterized by production of inflammatory cytokines by CD4+ cells. Figure 2 shows computed tomography (CT) scans of a patient originally from Cameroon who presented with mostly extrapulmonary TB.2 The scan before the initiation of ART shows lymphadenopathy, but the patient's condition had markedly improved while on treatment for TB. After starting ART, the patient developed worsening lymphadenopathy, high fever, and elevated C-reactive protein (CRP). The bottom of the figure shows proportions of interferon-gamma (the cytokine that is measure in gamma release assays) expressing CD4+ cells in response to TB antigens. The blue spots indicate the CD4 + cells that produced inter-feron-gamma and the inflammatory cytokine tumor necrosis factor (TNF). As can be seen, there was minimal production of the inflammatory cytokines prior to ART, whereas extremely high concentrations occurred after the start of ART in the context of a rapid expansion and improved functionality of CD4+ cells. IRIS is also characterized by increased monocyte production of the inflammatory cytokines TNF, interferon-gamma, interleukin (IL)-18, and IL-6, the latter of which results in augmented CRP production in the liver. Measurement of CRP can be used to test for suspected IRIS in the clinical setting.
Figure 2.
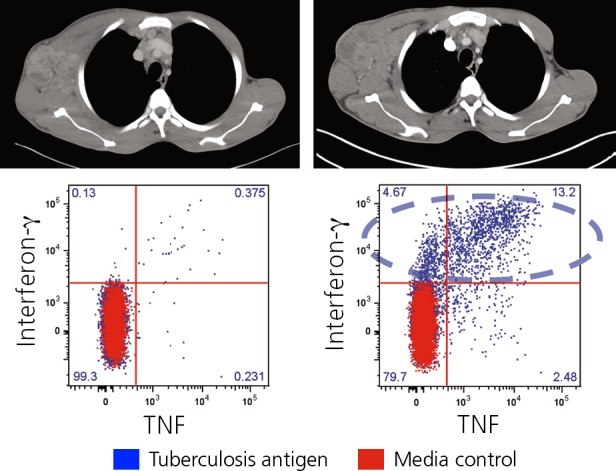
Rapid expansion of activated CD4+ T cells during tuberculosis-associated immune reconstitution inflammatory syndrome. Top shows computed tomography scans prior to (left) and after initiation (right) of antiretroviral therapy with axillary lymphadenopathy. Bottom shows concentrations of CD4+ cells producing inflammatory cytokines. Adapted from Boulougoura and Sereti.2
The author and colleagues have also found in a small pilot study that 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) can be used to predict and diagnose IRIS in patients with opportunistic infections. Glucose up take is high in inflammatory cells. Figure 3 shows results of 18F-FDG-PET before and after the start of ART in a patient who had MAC at baseline and subsequently developed IRIS. It can be seen that FDG uptake is increased post-ART. Overall, patients who developed IRIS were more likely to have more areas of increased FDG uptake prior to ART, with an additionally marked increase in uptake and distribution being observed after ART.3 These increases in concentration and distribution of inflammatory cells were correlated with levels of inflammatory cytokines in patients with IRIS.
Figure 3.
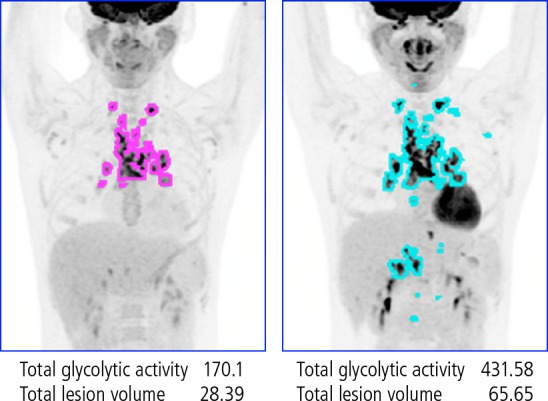
18F-flurodeoxyglucose positron emission tomography before (left) and after (right) the start of ART in a patient who had Mycobacterium avium complex at baseline and subsequently developed immune reconstitution inflammatory syndrome (IRIS). Flurodeoxyglucose uptake is markedly increased during IRIS. Adapted from Hammoud et al.3
Underlying Diseases in IRIS
Among the underlying opportunistic conditions associated with IRIS, the most common are mycobacterial infections, including TB and MAC and other non-TB infections, and cryptococcal infections. Others include cytomegalovirus retinitis, progressive multifocal leukoencephalopathy (PML), herpesvirus infection, Kaposi sarcoma, non-Hodgkin lymphoma, candidiasis, viral hepatitis, human papillomavirus infection, and Pneumocystis infection.
TB-Associated IRIS. TB is one of the most common underlying diseases worldwide in IRIS cases (although MAC is more common in the US). The reported incidence of TB-associated IRIS ranges from 7% to 50%. In most reports, it is associated with the initiation of ART, with onset most commonly being observed 2 to 6 weeks after the start of treatment. The incidence is higher at very low CD4+ counts (ie, <50/µL). The spectrum of the syndrome includes exacerbation of existing disease, development of new manifestations or new sites of disease, and dissemination or death. ART cannot be delayed in patients with CD4+ counts below 50/µL, since such delay is associated with increased AlDS-defining illnesses and mortality. An exception is in patients with TB meningitis; the recommendation in this setting is to sterilize the CSF before starting ART. Multidrug-resistant TB should be included in the differential diagnosis.4–7
The International Network for the Study of HIV-associated IRIS (INSHI) definition of TB-associated IRIS includes the major clinical criteria of: new or enlarging lymph nodes, cold abscesses, or other focal tissue involvement; new or worsening radiologic features of TB; new or worsening central nervous system (CNS) TB; and new or worsening serositis (pleural effusion, ascites, or pericardial effusion). Minor clinical criteria (>2 required) consist of: new or worsening constitutional symptoms; new or worsening respiratory symptoms; and new or worsening abdominal pain accompanied by peritonitis, hepatomegaly, splenomegaly, or abdominal adenopathy.8
Cryptococcal Infection-Associated IRIS. The INSHI definition of cryptococcal IRIS includes the antecedent criteria of cryptococcal infection that has improved with antifungal treatment and the clinical criteria of clinical deterioration within 12 months of starting ART, with the development of meningitis, intracranial lesions, skin lesions, pulmonary nodules, or lymphadenopathy. Nonadherence to ART and other diagnoses, including other infections or malignancies, must be excluded.9
Box 1.
Illustrative Case 1 — Not Your Usual Hiccups
A 38-year-old patient with a recent diagnosis of HIV infection presented with persistent hiccups and anemia. The patient was found to have thrush, weight loss, chills, fatigue, and cough. The CD4+ cell count was 10/µL and HIV RNA level was 350,000 copies/mL. Acid-fast bacillus (AFB) assay of bronchoalveolar fluid was positive, with Mycobacterium avium complex (MAC), although blood culture was negative. The patient improved on azithromycin and ethambutol. The patient was started on elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate. Two weeks later, the patient had worsening hiccups; a CD4+ cell count of 63/ µL, an HIV-RNA level of 237 copies/mL, and high-sensitivity c-reactive protein (CRP) up to 51 mg/L from baseline of 14 mg/L. Four weeks later, the patient had high fevers, hiccups, CRP of 120 mg/L, CD4+ count of 163/µL, and indeterminate TB test results. A repeat chest computed tomography (CT) was performed. The figure shows CT scans before and after antiretroviral therapy (ART), with evident cavitation, and the course of the patient's CRP levels. As noted, corticosteroid treatment should not be started without additional workup. The best course of management at this point would be to repeat bronchoscopy or sputum testing to exclude other diagnoses or progressing or resistant MAC and possibly to add moxifloxacin to the antibacterial regimen.
Box 1 Figure.
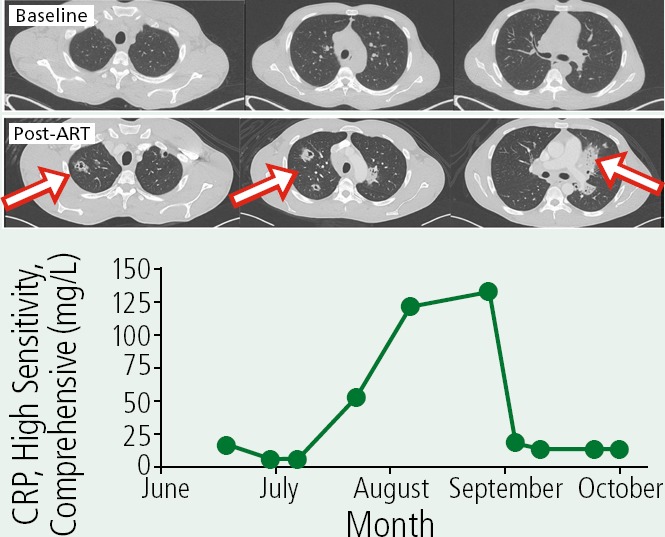
Computed tomography scans before and 1 month after initiating antiretroviral therapy (ART) demonstrating the development of infiltrates and cavilty lesions (arrows) and course of changes in high-sensitivity C-reactive protein (CRP).
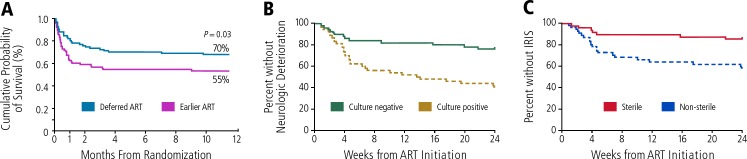
As in the case of TB meningitis, ART should not be started until cerebrospinal fluid (CSF) is sterile and infection controlled in patients with cryptococcal meningitis. As shown in Figure 4A, the COAT (Cryptococcal Optimal ART Timing) trial in patients with cryptococcal meningitis showed 6-month overall survival of 55% and 70% (P=.03) in the earlier ART and delayed ART groups, respectively. The survival curves for the treatment groups diverged sharply within the first month of the trial10; such findings support the potential lethality of cryptococcal IRIS and the need to effectively treat the infection before initiating ART. As shown in Figure 4B, another study found that patients with cryptococcal meningitis who were CSF culture-negative at the initiation of ART had a greater than 60% reduction in risk of clinical deterioration than those who were culture-positive and that those with sterile CSF had a greater than 60% reduction in risk of IRIS than those without sterile CSF.11
Figure 4.
A: Overall survival In the COAT (Cryptococcal Optimal ART Timing) trial comparing early and delayed antiretroviral therapy (ART) In patients with cryptococcal meningitis. Adapted from Boulware, N Eng J Med, 2014.10 B and C: Trial in patients with cryptococcal meningitis showing reduced risk of neurologic deterioration in patients with culture-negative results and reduced risk of immune reconstitution inflammatory syndrome (IRIS) in patients with sterile cerebrospinal fluid at the start of ART. Adapted from Chang et al.11
Box 2.
Illustrative Case 2: Is It the Drugs or the Virus?
A 30-year-old patient presented with a Kaposi sarcoma skin lesion. The CD4+ cell count was 7/µL and the patient was hepatitis A virus (HAV) antibody-negative, hepatitis C virus (HCV) antibody-negative, HBsAg-positive, HBsAb-negative, HBcAbpositive, and HBeAg-positive. The patient was started on antiretroviral therapy (ART) with efavirenz/emtricitabine/tenofovir disoproxil fumarate, a regimen also active against HBV, prophylaxis with trimethoprim/sulfamethoxazole and azithromycin, and received HAV vaccine. The figure shows the patient's decreasing HBV viral load, increasing CD4+ cell count, decreasing HIV viral load level, and a marked flare in alanine amino-transferase (ALT), aspartate transaminase (AST), and alkaline phosphatase levels (ALK Phos) that subsequently resolved during the months after starting ART.
As the liver enzyme levels increased, efavirenz, trimethoprim/sulfamethoxazole, and azithromycin were stopped in case they contributed or caused liver toxicity and raltegravir was added to ongoing emtricitabine and tenofovir disoproxil fumarate, however, the drug changes did not appear to change the course of flare. Based on this, the hepatitis flare was likely associated with hepatitis B virus (HBV) IRIS, which is more common at high HBV viral load levels and can be associated with HbeAg clearance. This patient was indeed subsequently found to have converted to HBeAg-negative status.
Box 2 Figure.
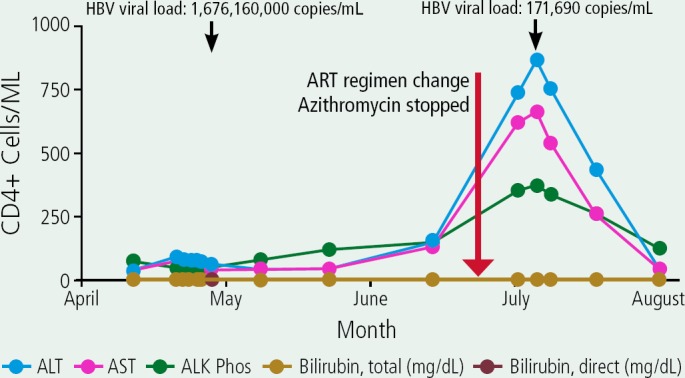
Case 2: Decreasing hepatitis B virus (HBV) viral load, increasing CD4+ cell count, decreasing HIV viral load, and marked flare in alanine transaminase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALK Phos) that subsequently resolved during the months after starting antiretroviral therapy (ART). The patient had been started ART with efavirenz/emtricitabine/tenofovir disoproxil fumarate and antibacterial treatment with trimethoprim/sulfamethoxazole and azithromycin. For ART, efavirenz was stopped and raltegravir added to emtricitabine and tenofovir disoproxil fumarate.
Box 3.
Illustrative Case 3—Doing Well but Having Trouble Reading:
A 33-year-old patient with tuberculosis (TB) with lung infiltrates and mediastinal lymphadenopathy, CD4+ cell count of 10/µL, HIV RNA level of 1.1 million copies/mL, HBsAg+ status, and hepatitis B virus (HBV) viral load of 165,000 copies/mL was started on antiretroviral therapy (ART) with efavirenz, emtricitabine, and tenofovir disoproxil fumarate, RIPE (rifampicin, isoniazid, pyrazinamide, and ethambutol), and trimethoprim/sulfamethoxazole. The patient developed a drug reaction with eosinophilia and systemic symptoms (DRESS) from rifampicin and TB treatment was switched to isoniazid, ethambutol, and moxifloxacin. Clinical improvement was observed, the CD4+ cell count increased to 84/µL, and the HIV RNA level decreased to less than 40 copies/mL. However, the patient complained of blurred vision and was found to have continuous vertical nystagmus that did not improve with focusing. The patient underwent head magnetic resonance imaging and was found to have a tuberculoma located in the middle of the medulla that was causing the nystagmus. The patient was treated with low-dose prednisone, with resolution of the tuberculoma and nystagmus.
Hepatitis Flares. IRIS in the form of hepatitis flares is most commonly observed in patients with hepatitis B (HBV) or hepatitis C virus (HCV) infection. The incidence of such IRIS is as high as 20% in people with chronic hepatitis and fatalities have been reported. Risk of IRIS is increased with higher HBV viral burden (adjusted odds ratio per logio increase, 1.36; P=.003) or HCV viral load (adjusted odds ratio per log10 increase, 1.30; P=.04). It has also been observed in patients who are HBV core antigen (HBcAg) antibody-positive (HBcAb+) alone. The syndrome has been associated with increased levels of 1L-10, 1L-18, and CXCL10. Despite the clinical deterioration or liver function test abnormalities, it may lead to clearance of HBV envelope antigen (HBeAg) or seroconversion. Differential diagnosis should include drug toxicities.12–14
Considerations in the Management of IRIS
Patients at higher risk for IRIS are those with very low CD4+ counts, disseminated disease (associated with higher antigen loads), lack of immune response in cryptococcal meningitis, or severe anemia. IRIS is a diagnosis of exclusion. It is important not to ‘blindly’ start corticosteroid treatment, since patients are still immunocompromised after starting ART. The onset of IRIS does not require achieving of high CD4+ cell counts; in fact, the syndrome can be observed in the absence of any marked increase after starting ART. All ART regimens have been associated with IRIS; early indications of a lower risk with HIV integrase strand transfer inhibitors have not been borne out in clinical trial populations.
The Occam's razor “law of parsimony” does not apply in HIV-late presenters within HIV infection; as such, IRIS—patients may have 2 or more infectious states that may account for findings and all need to be addressed. 1t is also important to note that 1R1S can occur in individuals who do not have HIV infection for reasons other than the initiation of ART. The syndrome has been observed in patients with disseminated mycobacterial infection who have received a transplant for primary immune deficiency,15 in patients with PML after natalizumab treatment, in TB monoinfected patients who can experience paradoxical worsening after initiation of TB medications, and in other settings in which immune suppression is followed by immune recovery.
Clinical management of IRIS includes observation or symptomatic relief in mild cases. 1n more severe cases, drug treatment includes nonsteroidal antiinflammatory drugs and corticosteroids. Lymph node drainage and use of a lumbar drain (in cases of cryptococcal meningitis) may be necessary. As noted, it is crucial that cryptococcal meningitis be diagnosed early and that it be treated until CSF is sterile. To prevent unmasking IRIS, cryptococcal antigen (CRAg) and acid-fast bacillus (AFB) screening should be performed in patients in high-prevalence areas. ART should be continued during management.
An example of the benefit of early prednisone treatment in 1R1S is provided by a trial in which patients who rapidly developed mild to moderate symptomatic TB 1R1S after starting ART were randomly assigned to prednisone (n = 54) or placebo (n = 48). At 4 weeks after starting ART, symptom relief was observed in 80% vs 56% of patients (P=.03), with 7% vs 13% patients being converted to open-label treatment within 2 weeks.16
Another, more recent, study showed that prophylactic prednisone was associated with significant reduction in risk for paradoxical TB-1R1S (Figure 5). 1n the trial, patients with TB and with CD4+ counts less than 100/µL were randomly assigned to receive prophylactic prednisone at 40 mg for 2 weeks after starting ART, and then 20 mg for 2 weeks (n=120) or placebo (n=120). The cumulative incidence of TB-associated IRIS at 12 weeks was 32.5% vs 46.7% (relative risk, 0.70; P=.02; overall hazard ratio, 0.61; P=.03).17 It should be noted that the trial was performed in outpatients with overall milder cases of TB.
Figure 5.
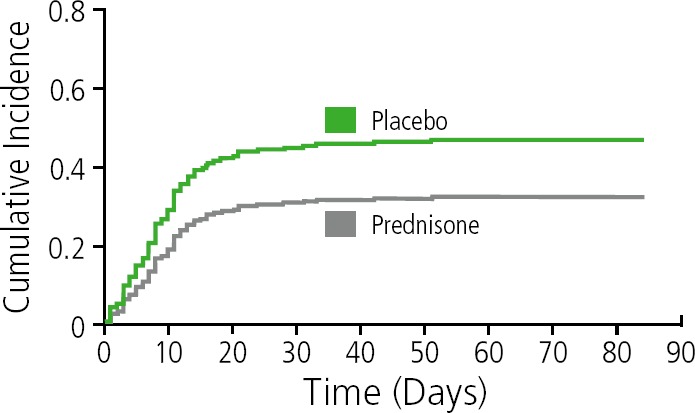
Risk of tuberculosis-associated immune reconstitution inflammatory syndrome in patients receiving prophylactic prednisone or placebo. Adapted from Meintjes et al1
Conclusions
Opportunistic infections remain a reality in regions with high prevalence of HIV. IRIS is an inflammatory reaction that can be managed with maintenance of ART, but may require immune suppression with corticosteroid treatment in patients who are already very ill. Management of immunosuppressed H1V patients should include screening for opportunistic infections. In cases of cryptococcal meningitis, it is crucial to sterilize CSF before starting ART. It is important to note that unmasking cryptococcal meningitis-associated IRIS may increase in frequency with test and treat roll-out in high-prevalence areas. Prednisone can be effective in prevention and early treatment of TB-associated IRIS in select patients (ie, in non-hospitalized, non-critically ill patients).
Footnotes
Presented by Dr Sereti in April 2019. First draft prepared from transcripts by Matthew Stenger. Reviewed and edited by Dr Sereti in January 2020.
Financial affiliations in the past 12 months: Dr Sereti has no relevant financial affiliations to disclose. (Updated 01/20/20)
References
- 1. Hsu DC, Faldetta KF, Pei L, et al. A paradoxical treatment for a paradoxical condition: infliximab use in three cases of mycobacterial IRIS. Clin Infect Dis. 2016; 62(2):258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boulougoura A, Sereti L. HIV infection and immune activation: the role of coinfections. Curr Opin HIV AIDS. 2016;11(2): 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hammoud DA, Boulougoura A, Papadakis GZ, et al. Increased metabolic activity on 18F-fluorodeoxyglucose positron emission tomography-computed tomography in human immunodeficiency virus-associated immune reconstitution inflammatory syndrome. Clin Infect Dis. 2019;68(2): 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walker NF, Stek C, Wasserman S, Wilkinson RJ, Meintjes G. The tuberculosis-associated immune reconstitution inflammatory syndrome: recent advances in clinical and pathogenesis research. Curr Opin HIV AIDS. 2018;13(6):512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naidoo K, Yende-Zuma N, Padayatchi N, et al. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Ann Intern Med. 2012;157(5):313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laureillard D, Marcy O, Madec Y, et al. Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome after early initiation of antiretroviral therapy in a randomized clinical trial. AIDS. 2013;27(16):2577–2586. [DOI] [PubMed] [Google Scholar]
- 7. Luetkemeyer AF, Kendall MA, Nyirenda M, et al. Tuberculosis immune reconstitution inflammatory syndrome in A5221 STR1DE: timing, severity, and implications for H1V-TB programs. JAIDS. 2014; 65(4):423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in H1V-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10(11):791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370(26):2487–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chang CC, Dorasamy AA, Gosnell BL, et al. Clinical and mycological predictors of cryptococcosis-associated immune reconstitution inflammatory syndrome. AIDS. 2013;27(13):2089–2099. [DOI] [PubMed] [Google Scholar]
- 12. Lewin EB. A paradigm for the control of influenza. J Infect Dis. 2010;202(11):1619–1622. [DOI] [PubMed] [Google Scholar]
- 13. Andrade BB, Hullsiek KH, Boulware DR, et al. Biomarkers of inflammation and coagulation are associated with mortality and hepatitis flares in persons coinfected with HIV and hepatitis viruses. Infect Dis. 2013;207(9):1379–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crane M, Oliver B, Matthews G, et al. 1mmunopathogenesis of hepatic flare in H1V/hepatitis B virus (HBV)-coinfected individuals after the initiation of HBV-active antiretroviral therapy. J Infect Dis. 2009; 199(7):974–981. [DOI] [PubMed] [Google Scholar]
- 15. Manion M, Dimitrova D, Pei L, et al. IRIS as a post-transplantation complication in primary immunodeficiency with disseminated M. avium. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed]
- 16. Meintjes G, Wilkinson R, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24: 2381–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barber DL, Andrade BB, Sereti L, Sher A. Immune reconstitution inflammatory syndrome: the trouble with immunity when you had none. Nat Rev Microbiol. 2012;10(2):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meintjes G, Stek C, Blumenthal L, et al. Prednisone for the prevention of paradoxical tuberculosis-associated IRIS. N Engl J Med. 2018;379(20):1915–1925. [DOI] [PubMed] [Google Scholar]