Abstract
The members of Orbivirus genus within the Reoviridae family are arthropod-borne viruses which are responsible for high morbidity and mortality in ruminants. Bluetongue virus (BTV) which causes disease in livestock (sheep, goat, cattle) has been in the forefront of molecular studies for the last three decades and now represents the best understood orbivirus at a molecular and structural level. The complex nature of the virion structure has been well characterised at high resolution along with the definition of the virus encoded enzymes required for RNA replication; the ordered assembly of the capsid shell as well as the protein and genome sequestration required for it; and the role of host proteins in virus entry and virus release. More recent developments of Reverse Genetics and Cell-Free Assembly systems have allowed integration of the accumulated structural and molecular knowledge to be tested at meticulous level, yielding higher insight into basic molecular virology, from which the rational design of safe efficacious vaccines has been possible. This article is centred on the molecular dissection of BTV with a view to under- standing the role of each protein in the virus replication cycle. These areas are important in themselves for BTV replication but they also indicate the pathways that related viruses, which includes viruses that are pathogenic to man and animals, might also use providing an informed starting point for intervention or prevention.
Keywords: Virus structure, Replication, Molecular biology, Host factors
1. Introduction
Bluetongue virus (BTV) is a complex, non-enveloped, double stranded RNA (dsRNA) virus and is the archetypal member of the genus Orbivirus of the family Reoviridae. The family Reoviridae comprises of segmented dsRNA viruses, possessing icosahedral non-enveloped capsids with a double-layered architecture. This large group of dsRNA viruses represent the causative agents of several economically and medically significant diseases and as such present a great challenge in virological understanding.
BTV, the prototype of the Orbivirus genus is a pathogen of livestock and is common throughout the world including Europe, causing serious periodic outbreaks. Consequently it has been studied extensively as a model system for related viruses and substantial progress has been achieved in sequence, biochemical and structural studies of BTV. Indeed, BTV was the first virus of this genus to be characterised at a genetic level and in its structure and replication.
Through the testing study of this virus many innovations have been achieved which have now become the standard tools of molecular virology. BTV provided the first whole genome sequence of a dsRNA virus and the first virus-like particle (VLP), a game changing discovery, now in widespread use for many viruses both for vaccine development and as a tool for basic research. Indeed VLP based assay system has facilitated to dissect the structure of BTV and the virus assembly pathway. Simultaneously, the structure of an integral virus core protein was solved, which became a paradigm in the field. The structure was the first for a large RNA virus and was later merged with cryo-electron microscopy (cryo-EM) data to give a unique high-resolution 3D image of the viral core. This structure constituted the first complex viral capsid and biological assembly to be solved at atomic resolution. Latterly the virus replication cycle, notably virus entry, replication and egress have been examined. Recent significant developments include the first helper virus free, in vitro T7 reverse genetics system for dsRNA viruses, which will allow major studies of how the virus causes disease (and generation of new vaccines), and the development of an in vitro cell-free assembly system which gives rise to infectious virus particles in the absence of cellular factors. Further the first 3D image of the whole virus has been obtained, illustrating how BTV may enter into the host cells.
These breakthroughs have provided the initial model system from which the understanding of the Orbivirus genus as a whole and to some extend the other members of the family has been possible. This review is focused on the molecular biology of the current understanding of BTV replication, the role of each viral protein in virus replication, the basic virological study of which has allowed rational designing of highly attenuated efficacious vaccines against BTV infection. This article summarises the current understanding of BTV replication at molecular and structural levels.
2. The virion structure
The structure and function paradigm of biological assemblies dictates that in order to comprehensively understand the biology of the virus, it is informative to know the structure and organisation of the virus. To this effect, significant progress has been made to identify the organisation of BTV at an atomic level. Initial transmission electron microscopy (TEM) of purified virus particles and density migration of chemically treated virions indicated an icosahedral double shelled structure of the virus particle, existing as both whole particles and cores from which the outer layer proteins were removed (Huismans et al., 1987b; Martin and Zweerink, 1972; Van Dijk and Huismans, 1980). Indeed, from the expression of individual subsets of structural proteins, using a recombinant baculovirus system, it was possible to reconstitute particles dis- playing the biophysical characteristics of particles obtained from virus preparations (French and Roy, 1990; French et al., 1990; Loudon and Roy, 1991). From this and protein profiling of purified particles and cores, the order and organisation of the virion has been established.(Matsuo and Roy, 2013).
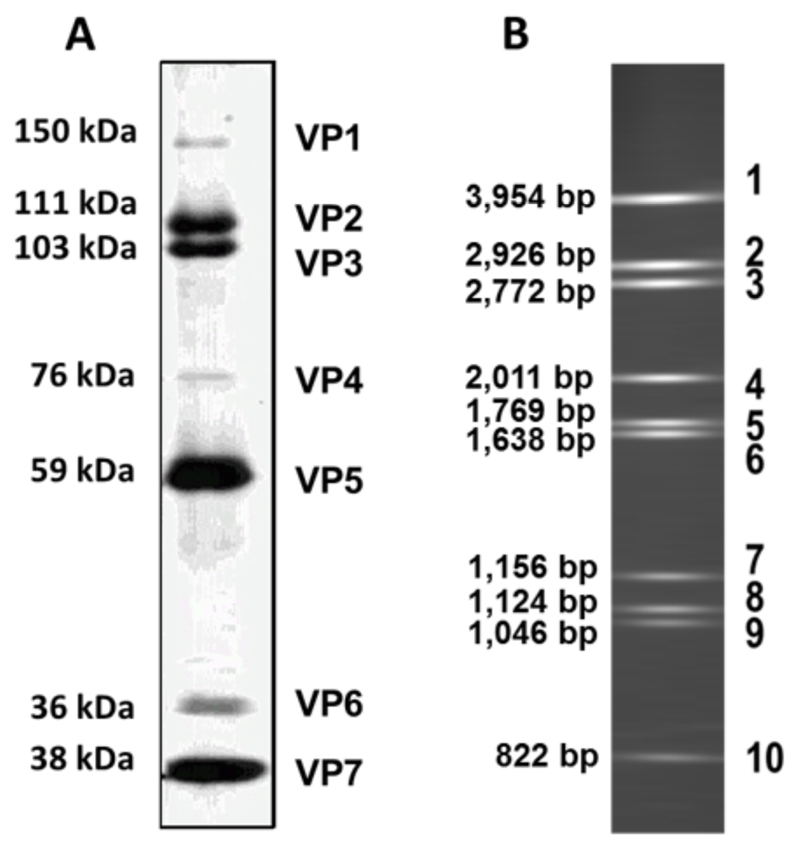
The particle consists of seven structural proteins three of which (VP1, VP4 and VP6) are minor components having low relative concentrations in the particle in relation to the major components of VP2, VP3, VP5 and VP7 (Fig. 1A). These minor components are not necessary to reconstitute virus-like and core-like particles (CLP) by recombinant baculovirus expression, indicating that they are not components of the protein capsid and serve as encapsidated cargo.
Fig. 1. The virion constituents.
(A) SDS-PAGE of purified virions. The minor core proteins VP1, VP4 and VP6 are present at a relatively lower concentration to that of the remaining structural proteins. (Sizes are calculated based on molecular weights for BTV serotype 10). (B) The ten segments of the dsRNA profile of purified core particles. (Sizes for BTV serotype 10).
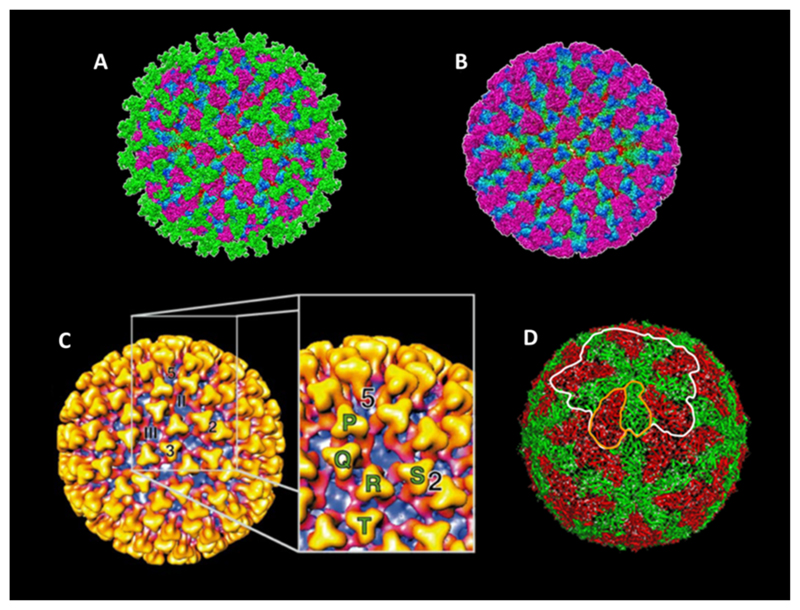
The core particle itself comprises of VP3 and VP7 which encapsidate the ten genomic dsRNA segments (Fig. 1B) along with minor protein components. The core is then decorated with a further protein capsid layer to form mature virus. This constitutes VP2 and VP5, which act as outer capsid proteins and are surface exposed to a varying extent (Fig. 2A and B). Higher resolution cryo-EM (Hewat et al., 1994; Nason et al., 2004; Prasad et al., 1992) and X-ray crystal structure (Grimes et al., 1998) of the core particle revealed the finer organisation of the core at this level. These show that the core is comprised of a T2 sub-core composed of 60 dimers of VP3 (Fig. 2D). Upon this a T = 13 arrangement of 260 VP7 trimers is supported through hydrophobic interactions, with an asymmetric unit consisting of five quasi equivalent trimer positions denoted P, Q, R, S and T (Fig. 2C). The core exhibits multiple solvent channels one at each fivefold axis and 120 shared amongst the six-fold axis (Grimes et al., 1997, 1998; Prasad et al., 1992).
Fig. 2. Structure of the particle and inner capsid layers.
(A) The 7 A ° Cryo-EM structure of the complete particle. Triskelion shaped trimers of VP2 (green) are exposed on the outer surface. VP5 trimers (purple) sit under VP2 trimers between the triskelion arms. Regions of the underlying VP7 (blue to green gradient) and VP3 (red) can be seen. (Adopted from Zhang et al., 2010.) (B) The structure of the mature particle lacking the VP2 trimer layer (colouring as in A). (Adopted from Zhang et al., 2010 (C)). The 23 A ° Cryo-EM structure of the core particle. VP7 trimers (gold to red gradient) are surface exposed with regions of the VP3 sub-core layer (purple) visible below. 2, 3 and 5 denote the icosahedral 2-fold, 3-fold and 5-fold symmetry axis respectively. II and III denote the position of class II and class III channels. The cut away annotates the P, Q, R, S and T crystallographic positions. (Adapted from Grimes et al., 1997 (D)). The 3.5 A ° X-ray crystal structure of the sub-core particle. Two isoforms of VP3 are present, A (green) and B (red). These form a dimer (outlined in orange) which associate to form a decamer (outlined in white), twelve of which compose the sub-core layer. (Adapted from Grimes et al., 1998).
Cryo-EM utilising differential density mapping using cores and CLPs has revealed a flower-like structure that sits beneath each fivefold axis. This is believed to represent the replication complex composed of VP1, VP4 and VP6 of which the precise structural nature remains to be revealed (Nason et al., 2004). Biochemical data however, supports a model in which dimers of VP4 associate with a molecule of VP1 and an undefined stoichiometry of VP6 (Ramadevi et al., 1998b). The structure of the dsRNA genome segments is also apparent through similar methods, with stacked lines of density lying parallel to the capsid structure. Tentatively this suggests a model in which each genome segment is associated with a single replication complex present at each five-fold vertex. The genomic dsRNA coiled around these complexes and concentrically towards the centre of the virion. It is likely however, that icosahedral aver- aging in image reconstruction masks the finer detail of genome packaging and interaction with the inner VP3 subcore layer (Gouet et al., 1999; Nason et al., 2004). A similar model has been proposed for rotavirus, another member of the family, based on cryo-EM data in which interaction of genomic segments with the inner subcore layer and replication complex causes concentric ordering at the icosahedral fivefold axis (Pesavento et al., 2001; Prasad et al., 1996).
Due to the intrinsic instability of the outer capsid protein layer, which is easily dissociated by proteolysis and mildly acidic conditions (Verwoerd et al., 1972), a high resolution view of its structural organisation has proved challenging. Initially it was revealed by a 40 angstrom (Å) and subsequently 22 Å cryo-EM density map that two density forms are present with this layer, sail-like structures forming triskelions assigned to be VP2 and globular-like densities localised beneath predicted to be VP5 (Hewat et al., 1992a; Nason et al., 2004).
Recent progress in cryo-EM density reconstitution techniques have allowed for a refinement of the resolution to 7 Å, enabling finer detail of the outer capsid to be revealed (Zhang et al., 2010). The 60 trimers of VP2 of the outer most proteins exposed to the solvent environment under which 120 trimers of VP5 are arranged (Fig. 2A). Unlike Reovirus and Rotavirus, these outer capsid proteins do not assimilate the under lyingT-13 arrangement of VP7 sub shell, displaying an arrangement that is unique to BTV (Settembre et al., 2011; Zhang et al., 2010). The high resolution density map revealed features of VP2, which exhibits an L shaped profile with the base forming a hub domain of the triskelion trimers. This region exhibit sa galectin-like fold similar to that of the VP8* cleavage product of RotavirusVP4 (Dormitzer et al., 2002). The upward arm of the L represents a tip domain which projects from the surface of the virion and is highly solvent accessible exhibiting a β-sheet rich fold (Zhang et al., 2010). The VP2 triskelions make contact with the underlying VP7 trimers through projections from the tip and hub domains.
The arrangement of VP5 is also unique, with trimers surrounding the VP2 trimers and positioned underlying the gaps in the triskelion arms of VP2 (Fig. 2A). The trimers possess a highly compact globular fold predominated by α-helices with a central coiled-coil motif facilitating trimerisation, analogous to membrane viral fusion proteins such as the stalk of Human immune deficiency virus (HIV) gp41 (Chan et al., 1997; Zhang et al., 2010). Interaction between VP5 trimers and VP7 sub shell are predicted to be weak owing to small density ‘fingers’ of contact, additionally Interactions between VP2 and VP5 are of a similarly density weak nature (Zhang et al., 2010). The paucity of inter-trimer contacts of the outer capsid corresponds well with its observed fragility.
In summary the virion is a non-enveloped icosahedral particle consisting of a genome of ten dsRNAs that are closely associated with replication complexes of three minor proteins. These are encapsidated by a double-shelled particle of protein layers of varying structural stability. The inner core comprises VP3 and VP7 that are strongly associated and the outer capsid layer, which displays weak interactions, is composed of VP2 and VP5.
3. Overview of the replication cycle
BTV and related orbiviruses constitute the only non-membrane mammalian viruses to be vectored by an insect species, the biting midge genus Culicoides (Mellor et al., 2000). Virus is inoculated into the host bloodstream by Culicoides saliva during a blood meal. Upon which it disseminates leading to primary infection in endothelial cells and mononuclear phagocytes, namely dendritic cells present in the lymph node draining the site of inoculation (Barratt-Boyes and MacLachlan, 1994).
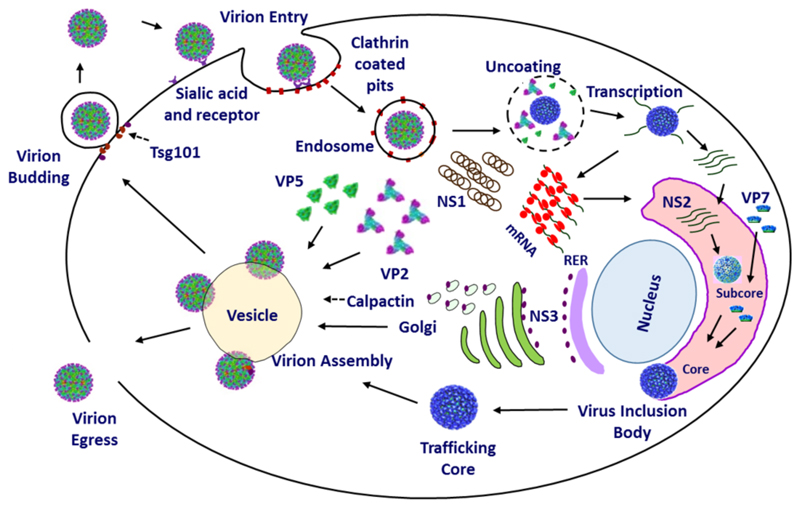
The virus life cycle is classical in the sense that it follows well characterised, discrete steps of replication comprehensively described in other viruses i.e. entry, replication, assembly and egress. Fig. 3 depicts an overview of this process. Infection occurs by cell adhesion via binding to surface glycoproteins owing to the reported hemagglutination activity (Eaton and Crameri, 1989), bound virus is internalised by the host cell endocytic machinery, through which the virus translocates into the cytoplasm shedding its outer capsid and subsequently becoming transcriptionally active (Huismans et al., 1987b).
Fig. 3. An overview of the replication cycle.
Virus entry occurs via Sialic acid attachment by VP2 followed by clathrin mediated endocytosis. Particles are then trafficked to the endosome upon which acidic pH mediates VP5 membrane permeabilisation leading to virion un-coating and egress of the core particle to the host cell cytosol. Transcription and translation of viral proteins occurs leading to cellular morphogenesis by non-structural protein. NS1 forms tubules and NS2 assembles the viral inclusion body (VIB) where components for core assembly are concentrated. Assembled core particles leave the VIB and are trafficked on exocytotic vesicles by NS3 interaction with calpactin. During this process the outer capsid proteins VP5 and VP2 are acquired for a complete particle. Particles egress the cell via budding mediated by Tsg101 interaction with NS3 or via host cell lysis.
Ten viral single-stranded RNAs (ssRNA) are produced by transcription of genomic segments (Fukusho et al., 1989). These are then translated by host cell ribosomes yielding the seven viral structural proteins VP1 through to VP7 and three or four non- structural proteins, NS1, NS2, NS3 and NS4 (Mertens et al., 1984; Ratinier et al., 2011). NS1 preferentially promotes translation of BTV ssRNAs enhancing viral titre (Boyce et al., 2012). NS2 forms viral inclusion bodies (VIBs) which recruit viral ssRNAs and viral protein components required for genomic packaging, replication and core assembly (Kar et al., 2007). Upon assembly cores are released from VIBs and enter into the exocytosis pathway, facilitated by NS3, whereby the outer capsid proteins VP5 and VP2 are acquired and the mature virion particles egress from the host cell to propagate infection (Beaton et al., 2002; Wirblich et al., 2006).
This over view describes the key features of BTV life cycle however, each process requires a multitude of complex biological and biochemical interactions within viral components and with host cell components. The characterisation of some of these processes have provided an overall understanding of BTV assembly, replication and trafficking processes at a molecular level, allowing not only effective vaccine design but also offering insight into the general biology of dsRNA viruses as a whole. To this extent the following discusses the molecular details of BTV replication cycle in finer detail.
4. Virus entry and the outer capsid proteins
4.1. VP2
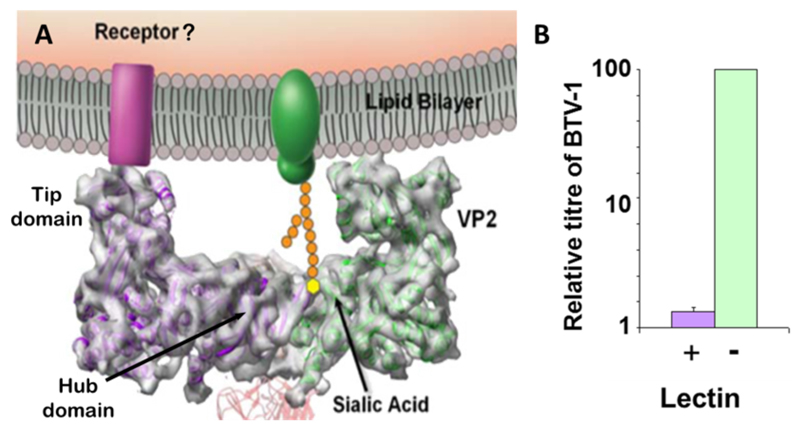
VP2 is the most solvent-exposed protein of the outer capsid (Fig. 2A, green trimers) and is therefore the protein that primarily interacts with the environmental medium of the virus (Hassan et al., 2001; Hassan and Roy, 1999; Zhang et al., 2010). Pertaining to this, a high antigenic selection pressure is imposed upon VP2 by the host humoral immune response. Extensive sequencing data and immunological profiling has demonstrated that VP2 is highly variable and falls into 25 (possibly 26) distinct serotypes with specific serotypes exhibiting geographical partitioning (Maan et al., 2007, 2011). Sequence alignments of VP2 demonstrate regions of higher sequence variation suggesting specific regions of VP2 protein are subject to higher selection pressure (Ghiasi et al., 1987). With the availability of a 7 Å structure it is possible to speculate that these regions could comprise the highly solvent-exposed tip domain (see Fig. 4A), however without a higher resolution structure to map sequence to structure this remains unsubstantiated.
Fig. 4. BTV cell attachment.
(A) The model of cell surface interaction of VP2. The side on profile of a VP2 triskelion trimer is shown. Two VP2 molecules can be seen (green and purple) attached to an underlying VP7 trimer (red). Note the L shaped profile of the trimer. The hub domain binds a sialidated cell surface protein (green) in a receptor pocket. The tip domain may interact with an additional co-receptor (purple). (Adapted from Zhang et al., 2010). (B) Sialic acid interaction is necessary but not sufficient for cell entry. Pre-treatment of HeLa cells with wheat germ agglutinin (lectin) drastically decreases but does not inhibit the replication of BTV.
As discussed the outer surface location of VP2 exposes it to the environment, which imposes antigenic selection pressure, this surface positioning however allows it to act as an environmental sensor which can mediate the initial contact between virus and host cell, thus facilitating entry. Indeed neutralising antibodies can be generated solely using purified VP2 from virions or recombinant protein (Huismans et al., 1987a; Inumaru and Roy, 1987). Viral entry is predominantly modelled to occur first through cell attachment, followed by induction of virus particle uptake by the cell.
Initial cell attachment is believed to occur through non-specific interactions between virions and cell surface molecules, due to the intrinsic physical properties of the virus such as charge and hydrophobicity. The generalised receptor heparan sulphate has been proposed to mediate initial cell contact for mammalian non-enveloped viruses of the Picornaviridae (Spillmann, 2001). However, this is based on the electrostatic potential of the capsid surface which can be mapped owing to high resolution crystal structures for these viruses (Fry et al., 1999). In the absence of high resolution structure of mature BTV particle, the role of heparan sulphate, or other non-specific cell surface factors, in viral ‘surfing’ remains speculative.
Aside the exact mechanism of initial cell contact, once the BTV particle has been localised at the host cell surface, specific receptor interactions occur between the viral receptor binding protein and the cell surface receptor. BTV has long been demonstrated to exhibit hemagglutination activity and this activity maps to purified VP2 which has been shown to interact with glycophorin A in vitro (Hassan and Roy, 1999).
The 7 Å structure of VP2 reveals structural features that facilitate this activity. The hub domain, as discussed in virion structure, exhibits a galectin-like fold shared with that of the sialic acid binding domain VP8*, the cleavage product of Rotavirus VP4. This protein has been demonstrated both structurally and functionally to bind sialic acid (Isa et al., 1997; Kraschnefski et al., 2009). The hub domain of VP2 is thus likely to bind sialic acid of an as yet unidentified cell surface glycoprotein (Fig. 4A), with each VP2 triskelion possessing three binding sites, one from each VP2 hub domain monomer. Biochemical analysis reveals that VP2 binds type 2 oligosaccharide structures (Galβ1-4GlcNAcβ1-3) in glycan arrays (Bhattacharya and Roy, 2010).
This data has been recapitulated in vivo through cell culture, which demonstrates a tenfold loss in virus titre when cells are pre-treated with sialic acid binding wheat germ agglutinin (Fig. 4B). This suggests sialic acid interaction is necessary but not sufficient for cell entry, indicating a role of an unidentified co-receptor in the entry process (Zhang et al., 2010). This co-receptor could potentially be bound by the projection of the tip domain (Fig. 4A).
Alternatively the tip domain could act to sterically inhibit anti- body access to the receptor binding region located in the triskelion hub. The strategy of burial of receptor binding sites has been adopted by other non-enveloped viruses, for example Poliovirus in which receptor binding occurs within a deep ‘canyon’ like feature near the fivefold axis of the capsid surface (Harber et al., 1995; Rossmann, 1989). Notwithstanding the specific mechanism of cell surface attachment a wealth of evidence supports VP2 may facilitate a similar process.
Subsequent to cell attachment, the virion must enter the cell through host cell pathways (Marsh and Helenius, 2006). The mechanism of BTV internalisation has been interrogated in cell culture. Initially, specific RNA interference knockdown of the μ2 subunit of the pathway essential clathrin-AP2 adaptor complex in HeLa cells, indicated that BTVs cell entry is mediated by the clathrin coated pit pathway (Forzan et al., 2007). In addition the clathrin specific inhibitor Chlorpromazine, significantly reduced BTV replication (Forzan et al., 2007). Taken together this data strongly suggests that BTV utilises the clathrin mediated endocytotic pathway for cell entry.
Moreover, inhibition of vacuolar H+ ATPase by Bafilomycin A1 significantly reduces virus titre, indicating that not only is endocytosis required for productive infection but entry into an acidifying environment is essential (Forzan et al., 2007). This draws parallels with the mechanism of entry of non-enveloped viruses such as Influenza virus, in which clathrin mediated endocytosis delivers the virus into an acidic microenvironment. This enables a pH mediated conformational change of Haemagglutinin (HA) fusion protein causing the membrane fusion necessary for delivery of the RNA genome into the host cell and productive replication (Guinea and Carrasco, 1995).
The mechanism by which clathrin mediated endocytosis is induced remains unclear, as binding to a receptor seems insufficient to induce pit formation and clathrin recruitment. Speculatively, the solution may lie in the multiplicity of binding sites of the virion surface, it has been observed that Influenza virus particles do not bind to pre-formed pits but induce pit formation around the curvature of the virion (Rust et al., 2004). This could partly be a result of the clustering of receptor molecules due to the high density of binding sites of the virion surface, which in turn could trigger signalling events to induce pit formation.
Confocal microscopy has revealed that once endocytosed BTV migrates to the early endosome where by VP5 and VP7 become separately localised, indicating uncoating of the virion and dissemination of the core into the cytoplasm (Forzan et al., 2007). This process is not well understood, however, it must be mediated by components of the viral outer capsid.
4.2. VP5
Experimental evidence shows VP5 acts as a fusion protein. The recombinant protein is toxic when expressed in Spodoptera frugiperda (Sf9) cells and can mediate cell toxicity by membrane permeablisation in culture (Hassan et al., 2001). Additionally, this protein functions as a membrane fusion protein when linked to a transmembrane domain, causing syncytial formation in Sf9 cells upon acidic pH shock (Forzan et al., 2004).
Analysis of the sequence of VP5 shows the archetypal features characterised for membrane fusion proteins. The N-terminus exhibits a 30 residue amphiphilic helix which is preceded by a long heptad repeat stretch predicted to form trimeric coiled- coils, indicative of a fusion protein like function. Interestingly, sequence homology indicates that the N-terminal helix is more closely related to antimicrobial peptides than viral fusion proteins, possibly suggesting a novel mode of action (Hassan et al., 2001). This N-terminal coiled domain is followed by a highly α-helical C-terminal globular domain which may facilitate attachment of the protein to the VP7 core layer (Zhang et al., 2010). Owing to the high α-helical content of this protein it is tempting to suggest that it falls into the class I category of fusion proteins such as Orthomyxovirus HA and Paramyxovirus F proteins (Colman and Lawrence, 2003) and as such may demonstrate similarity in its mechanism of action.
As a general model fusion proteins are expressed as a precursor that requires proteolytic processing to form a molecule that is kinetically trapped in a metastable high energy state. The process of cell entry through receptor binding, acidification, further proteolysis or a combinatorial effect relieves the kinetic barrier allowing conformational change of the protein to its lowest energy state, thus enabling membrane fusion as a consequence.
This activity has been well characterised structurally and biologically for fusion proteins of enveloped viruses (Kielian and Rey, 2006; Weissenhorn et al., 2007; White et al., 2008), however this model also seems true for non-enveloped fusion proteins. The fusion processes have been characterised for Reovirus μ1 and Rotavirus VP4, both of which require proteolytic processing. In the case of Reovirus, μ1 is cleaved to form an N-terminal myristoylated fusion peptide μ1N and C-terminal μ1C. In the case of VP4, priming for fusion occurs by proteolytic cleavage to VP8*, the receptor binding domain and VP5 which acts as the fusion protein, much in analogy to enveloped virus fusion proteins (Dormitzer et al., 2002; Ivanovic et al., 2008; Liemann et al., 2002; Yoder et al., 2009).
To date no evidence suggests that BTV VP5 is proteolytically processed and consequently must fold into a metastable conformation upon translation. It must, therefore, be tightly regulated in its conformational change to a lower energy state and fusion process.
Recombinant VP2 has been shown to inhibit fusiogenic activity and cell toxicity of VP5 in culture, it is likely that interactions with VP2 in the outer capsid act to control the fusion activity of VP5 (Forzan et al., 2004; Hassan et al., 2001). In this scenario, in variation to membrane fusion proteins in which receptor binding and fusion domains are covalently linked, the receptor binding and fusion functions of BTV are encoded in separate polypeptides that are associated in a non-covalently linked lattice.
Thus VP2 has an additional role as sensor, acting as a regulatory molecule that modulates the activity of VP5 in the correct environment. The structure of the virion as discussed above supports a model in which VP2 triskelions sterically inhibit VP5, lying on top of VP5 trimers partially occluding them (Zhang et al., 2010). It is necessary therefore, for VP2 to undergo a conformational change in order to reveal VP5 and abrogate inhibition. It is plausible that the combination of receptor binding and the acidic environment of the early endosome may mediate this process. Notably, it has been demonstrated that partial proteolysis of VP2 occurs in saliva of Culicoides and mammalian derived blood (Darpel et al., 2011; Marchi et al., 1995). This susceptibility to proteolysis may be an additional mechanism by which VP5 activation is induced.
Endosomal cathepsins cause the proteolytic activation of membrane fusion proteins such as Ebola virus GP2 and Paramyxoviruses Hendra and Nipha (Pager and Dutch, 2005; Pager et al., 2006; Schornberg et al., 2006). Cathepsins may act upon VP2 in the endo- some thereby partially degrading the structure to activate VP5. Regardless of the precise mechanism, VP5 fusogenic activity must be activated when membrane proximal. VP2 must play a role in anchoring the virion to the endosomal membrane through receptor binding whilst other environmental factors modify its structure to activate VP5.
The manner in which VP5 enables membrane penetration of the core without the presence of a viral membrane with which to fuse has proven enigmatic. Recombinant VP5 is clearly able to form pores in membranes as demonstrated in cell culture toxicity assays (Hassan et al., 2001). It may be that initial pore formation occurs via individual trimers through insertion of amphiphilic helices. Pore expansion is then driven by the curvature of the virion via the protein coat model in which curvature of the protein network interacting with the membrane drives viral budding (Kozlov and Chernomordik, 2002), inducing curvature stress, leading to membrane scission. Once released into the cytoplasm the membrane coat containing VP5 and VP2 are shed from the core, which is then primed for transcription.
In summary, VP2 acts as a receptor binding protein attaching the virion to cell surface by interaction with glycoproteins. This induces clathrin mediated endocytosis of the virus particle. The virus is then delivered to the early endosome where acidity, proteolysis and receptor interaction induce VP2 conformational change inducing VP5 membrane permeablisation activity. This process facilitates core egress from the endosome.
Whilst discussion has been made on the entry of mammalian cells, BTV is vectored by Culicoides and has been shown to replicate in insect cells in culture and in whole insect models. Experimental evidence indicates that the core is the more infectious species to Culicoides derived KC cells (Mertens et al., 1996). This suggests that that the core itself is able to interact with the insect cell surface and facilitate its’ own entry in to the insect cell cytosol. An RGD motif in VP7 that maps to a solvent exposed loop in its upper domain has been implicated in the core interaction with insect cells. Mutations of this tri-peptide significantly affect the interaction of CLPs with KC cell surface (Tan et al., 2001).
RGD motifs have been shown in other viruses such as Foot and Mouth disease virus to mediate cell attachment and internalisation due to interaction with integrins (Fox et al., 1989; Mason et al., 1994). The same could apply for BTV cores, however this remains to be tested by mutational analysis of replicating virus genome. Once internalised, the process by which the core translocates into the insect cell cytosol remains unknown.
5. Viral genome transcription and replication
As discussed the core consists of the dsRNA genome and replication complexes encapsidated by VP3 dimer and VP7 trimer layers. This assembly exhibits multiple solvent accessible channels that vary in depth and occur as three different types based on symmetry locations (Prasad et al., 1992). Type I channels are found at the icosahedral fivefold axis under which replication complexes are located, type II channels localise around the fivefold axis and type III at the three fold axis (Fig. 2C). Cryo-EM and X-ray crystal structures reveal that some of these channels penetrate through to the interior of the core (Grimes et al., 1997, 1998; Nason et al., 2004; Prasad et al., 1992).
X-ray structural analysis using crystal soaks with various substrates has revealed that penetrating channels act as entry and exit sites for the substrates and products required and formed during genome transcription and replication (Diprose et al., 2001). Furthermore, crystal soaking revealed that the fivefold channel is enlarged in the presence of Mg2+. The removal of the outer capsid layer during entry reveals translocation channels when the core is in the cell cytosol thus activating transcription when the necessary substrates are available. Transcriptase activity can be reconstituted in vitro by removal of the outer capsid layer through proteolysis and exposure to Mg2+, additionally Mn2+ has been shown to increase transcriptase activity (Van Dijk and Huismans, 1980).
The stimulatory activity of divalent cations could simply be by acting as cofactors for the transcription and capping reactions. However, this could in addition be mediated through allosteric regulation of the replication complex, by inducing conformational changes in the core itself, influencing the enzymatic activity of the transcription enzymes.
BTV cores produce ten positive sense ssRNA transcripts corresponding to each of the ten dsRNA segments (Fukusho et al., 1989). These vary in sizes from 3944 base pairs (bp) for segment 1 down to 833 bp for segment 10 (values for BTV serotype10). Each possess a 5′ cap 1 structure and lack polyadenylation at their 3′ terminus (Ramadevi et al., 1998a). Transcripts are released from the core particle into the host cell cytoplasm where they act as templates both for translation and for negative strand viral RNA synthesis to generate genomic dsRNAs (Mertens et al., 1984; Van Dijk and Huismans, 1980, 1988).
The current view supposes that transcripts are extruded from Class I pore at the icosahedral fivefold axis. Indeed as mentioned above, these pores undergo conformational enlargement in the presence of Mg2+ and crystal soaks with a ssDNA oligomer corresponding to the 5′ sequence of segment 7 show density localisation of the oligomer at these channels (Diprose et al., 2001). Cryo EM of actively transcribing rotavirus particles displays a similar model in which extra density is seen at the fivefold vertex indicative of release mRNA at type I channels (Lawton et al., 1997). Additionally antibodies that bind VP6, the surface protein of the double layer particles (DLPs), and occlude these channels inhibit transcriptional competency of closely related rotavirus (Lawton et al., 1999).
Recent experimental data using Rotavirus has demonstrated that for DLPs segments are not promiscuous, in that each segment is associated with a specific channel and thus replication complex (Periz et al., 2013). Due to the highly similar architecture of BTV cores and Rotavirus DLPs the same singular association of segments with replication complex and fivefold vertex is likely to be true. Whilst this demonstrates singularity of association of segments with transcription complexes, it will be of great insight to deter- mine whether the overall organisation and association of segments with pores within the core is identical in each virion particle, providing clues to as to the nature of the assembly process. Additionally as BTV displays only 10 genomic segments there remain 2 vertices for which this model is ambiguous. It is possible that they remain unoccupied due to steric constraints of genome packaging, in which case it is likely that they are devoid of replication complexes to prevent competition between complexes. Alternatively, they may be filled with an additional segment however this is less likely given the observed experimental data.
5.1. The replication complex
The replication complex consists of VP1, VP4 and VP6 (reviewed Roy, 2008). A wealth of biochemical data from expression and purified recombinant proteins has elucidated the function of each component.
5.2. The RNA polymerase VP1
VP1, the largest (150 kDa) protein of BTV, has been shown to act as an RNA dependent RNA polymerase (RdRp) when expressed as a recombinant protein (Boyce et al., 2004; Urakawa et al., 1989). Recombinant VP1 can copy each of the positive sense ssRNA segments fully in a single in vitro reaction to produce a dsRNA duplex that is RNase I resistant, RNase III sensitive (Boyce et al., 2004). This replicase activity is independent of other core proteins unlike that of Rotavirus VP1 which requires the presence of VP2 (Patton et al., 1997). Additionally dsRNA synthesis has been demonstrated to occur de novo without self-priming, through production of single genomic unit length ssRNA upon denaturation of dsRNA product (Boyce et al., 2004). This suggests BTV VP1 possesses a high intrinsic polymerase priming and processivity allowing priming independent dsRNA synthesis unlike the reliance on dinucleotide priming observed for Reovirus λ3 and Rotavirus VP1 (Chen and Patton, 2000; Tao et al., 2002).
Further studies in vitro revealed that dinucleotide priming can enhance replication rate suggesting that this step is rate limiting for de novo synthesis of RNA (Matsuo and Roy, 2011). More interestingly a degree of specificity of replicase activity has been observed. VP1 can synthesise dsRNA from BTV and Rotavirus ssRNA transcripts but not from non-virally derived sequences unless fused with BTV sequences at 5′ and 3′ termini (Matsuo and Roy, 2011). This implies common secondary structural elements are required for RdRp replication, present in members of the Reoviridae family. This structural dependency may prove a mechanism by which interferon response is controlled by BTV minimising VP1 synthesised nonspecific dsRNA.
Whilst a high resolution structure is lacking, VP1 has been modelled based on the available Reovirus λ 3 structure which has revealed a typical RdRp organisation with a central polymerase domain, an N-terminal domain and a C-terminal domain, this model is supported by mutagenesis combined with dissection of biological activity of each domain (Wehrfritz et al., 2007). This data suggest a common organisation to RdRps of the Reoviradae, however finer structural differences could facilitate the observed biochemical discrepancies in processivity and priming.
5.3. The capping enzyme VP4
As identified in vitro VP1 produces dsRNA from ssRNA templates, however these are not capped in discrepancy to core transcripts, as such capping activity lies in a separate protein. Recombinant VP4 (76 kDa) alone synthesises cap 1 structures on ssRNA species in vitro, that are identical to those found on authentic BTV mRNA (Martinez-Costas et al., 1998; Ramadevi and Roy, 1998; Ramadevi et al., 1998a). Thus complete capping is achieved by a single viral protein. This is notably different to other viral capping enzymes, e.g. those of Vaccinia virus, where capping is dependent on a complex of three proteins that carry out separate catalytic steps along the capping pathway (Venkatesan et al., 1980).
The formation of cap structure requires three key enzymatic activities. Firstly an RNA triphosphatase (RTase) function is required to prepare the RNA substrate for capping via removal of the □ phosphate of the 5′ terminal ribonucleobase of the RNA template. Secondly a guanylyltransferase (GTase) activity caps the formed 5′ diphosphate with guanylymonophosphate (GMP) via a 5′–5′ phosphodiester linkage. Third and finally a guanine- N7-Methyltransferase (N7MTase) function methylates the N7 position of the capping guanosine using an S-adenosylmethionine cosubstrate. An additional nucleoside-2′ -O-methyltransferase (2′OMTase) is also required for BTV and Reovirus transcripts, to methylate the 2′ -hydroxyl group of the ribose of the first nucleotide to form cap 1 structure.
The X-ray crystal structure of VP4 reveals the molecular detail that allow this protein to perform the entire capping process, the structure possesses an elongated hourglass morphology with defined domains along the length of the protein (Sutton et al., 2007). Two of these domains map to folds with significant structural homology to known methyl transferases and have been assigned OMTase and N7MTase based on their structural similarity to Vaccinia virus VP39 (OMTase) and Ecm1 (mRNA cap N7MTase) respectively (Fabrega et al., 2004; Hodel et al., 1996). A C-terminal domain of unique architecture is putatively assigned the GTase domain.
An additional domain with kinase-like structural homology is present at the N-terminus which lacks catalytic residues and kinase P-loop. In conjunction to this it demonstrates higher sequence variation across serotypes, and as such it is speculated to play a scaffolding role in the protein structure, possibly mediating an interaction with VP1 to feed the nascent RNA chain into the capping catalytic assembly within VP4. Whilst informative this structural analysis does not account for an RTase function and assignments of function require functional validation both in vivo and in vitro.
The helicase VP6: The dsRNA genome of BTV imposes constraints on template accessibility for transcription. It is necessary, therefore, for the virus to encode a helicase to allow for unwinding of the dsRNA duplex ahead of the polymerase and to dissociate the transcripts from template RNA.
The remaining minor protein of the replication complex is that of VP6, which is significantly smaller than VP1 and VP4 with Mr of 36 kDa. Sequence alignments suggest that VP6 possess some of the hall marks of DEAD box class helicases with a Walker A and DEAD box like motifs, however this is highly speculative as motifs are non-canonical (Kar and Roy, 2003). In vitro characterisation of recombinant protein demonstrates VP6 has high affinity for both ssRNA and dsRNA and possesses a nucleotide triphophatase activity (Stäuber et al., 1997). Further, in an in vitro helicase assay VP6 is able to unwind dsRNA duplexes uni-directionally, with a preference for duplexes with 5′ and or 3′ ssRNA overhangs (Stäuber et al., 1997). Size exclusion chromatography, in addition to sedimentation analysis using a glycerol gradient, demonstrated that VP6 can form hexamers with ring-like structures visible under TEM (Kar and Roy, 2003).
Similar hexameric morphology has been characterised for viral DNA helicases such as SV40 large T antigen and T7 gp4 (Singleton et al., 2000; Wessel et al., 1992) as well as RNA helicases such as P4 of bacteriophage ϕ 6 (El Omari et al., 2013). It is interesting to note that VP6 has no homologues displaying a unique sequence to BTV and as such could plausibly present a new structural class of helicase.
The use of recently developed RG for BTV has allowed further in vivo characterisation of VP6. It was shown to be an essential component of the primary replication complex which is the minimum genes required to stimulate efficient rescue upon subsequent transfection of ten ssRNA genome segments (Matsuo and Roy, 2009, 2013). Thus, VP6 is key to preliminary transcription of assembled replicase particles, alluding to its role as a helicase. Additionally, virus grown in complementary cell lines that are defective in this protein produce empty particles upon infection of native BSR cells and is unable to replicate (Matsuo and Roy, 2013). This supports the essential nature of this protein and suggests a potential role in genome packaging.
Using RG this property of VP6 has been exploited to develop replication deficient viruses that are only viable in trans complementary cell lines (Matsuo and Roy, 2009). This has enabled the development of the first disabled infectious single cycle (DISC) vaccines for a dsRNA virus (Matsuo et al., 2011). These next generation vaccines elicit a robust immune response due to their single infectivity. The segmented nature of the BTV genome allows for the rapid production of serotype specific vaccines using this system (Celma et al., 2013) which can be achieved with relative ease in comparison to VLP technology.
6. Expression of viral proteins
The ten ssRNA transcripts synthesised by the core particle encode the viral proteins and are translated by the host cell machinery. These proteins are essential to viral assembly maturation and release. Twelve proteins are expressed upon translation of viral ssRNA, seven of which are structural.
Transfection of mammalian cells with in vitro derived BTV transcripts also leads to generation of viral proteins and infectious virus particles as demonstrated by the passage of infectivity in BSR cells (Boyce and Roy, 2007). These findings have allowed the development of a helper virus-independent RG system for BTV and other orbiviruses and for the generation of targeted mutant viruses (Boyce et al., 2008; Kaname et al., 2013; Matsuo et al., 2010).
Each segment encodes a single protein, with the exceptions of segment 9 which encodes two proteins (VP6 and NS4) each in different reading frames (Belhouchet et al., 2011; Ratinier et al., 2011) and segment 10 which encodes two isoforms of the same protein (NS3 and NS3A) through alternative translation start sites (French et al., 1989; Mertens et al., 1984). Table 1 outlines the proteins encoded by each segment.
Table 1. The proteins encoded by BTV segments and their functions (sizes for BTV serotype 10, modified from Roy, 2001).
| Segment (size in base pairs | Protein/s encoded (size in kilo Daltons) | Protein function |
|---|---|---|
| S1 (3944) | VP1 (150kDa) | RNA dependent RNA polymerase |
| S2 (2926) | VP2 (111 kDa) | Receptor binding and cell entry |
| S3 (2772) | VP3 (103 kDa) | Sub-core structural protein, localises viral polymerase complex |
| S4 (2011) | VP4 (76 kDa) | Capping enzyme |
| S5 (1769) | NS1 (64 kDa) | Viral protein translation enhancer |
| S6 (1638) | VP5 (59 kDa) | Membrane permeabilization protein |
| S7 (1156) | VP7 (38 kDa) | Core structural protein and receptor binding protein for Culicoides cells |
| S8 (1124) | NS2 (42 kDa) | Concentrator of core components, viral inclusion body formation |
| S9 (1046) | VP6 (36 kDa) NS4 (17 kDa) |
Viral fitness to interferon response |
| S 10 (822) | NS3 (26 kDa) NS3A (25 kDa) |
Adaptor protein facilitating egress |
BTV-specific proteins are detectable within 2 to 4 hrs post infection and the rate of protein synthesis increases rapidly until 11 to 13 hrs post infection (Eaton et al., 1990; Verwoerd et al., 1972). Infection induces apoptosis via multiple signalling pathways (Mortola et al., 2004; Stewart and Roy, 2010) and morphological changes in host cell cytosol are apparent over this period, with the formation of tubules and dense globular structures which have been attributed to the non-structural proteins NS1 and NS2 respectively (Brookes et al., 1993; Eaton et al., 1990; Huismans and Els, 1979).
6.1. NS1 and tubule structures in infected cells
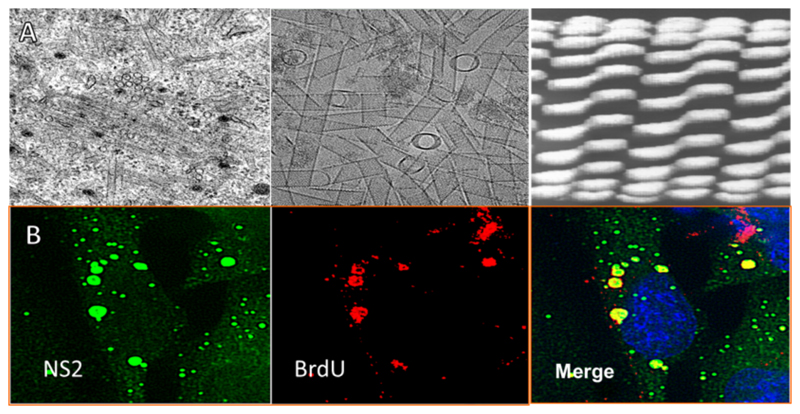
NS1 is a highly conserved 64 kDa tubule forming protein which is synthesised in the infected cell cytoplasm (Fig. 5A, left panel) from very early stage of virus replication at a high level (Roy, 1989). The expression of recombinant protein in Sf9 cells has confirmed the multimeric nature of this protein (Fig. 5A middle panel), forming large tubule structures in the cytosol (Urakawa and Roy, 1988). Cryo-EM reveals a structure comprised of coils of NS1 dimers (Hewat et al., 1992b) (Fig. 5A, right panel).
Fig. 5. Cellular morphogenesis by non-structural proteins.
(A) NS1 forms tubules. Transmission electron micrographs show the morphology of NS1. The left panel shows BSR cells 24 hrs post BTV infection, tubular structures are clearly visible in the cytosol. The middle panel displays purified NS1 tubules expressed using a recombinant baculovirus system. The right panel shows the 40 Å Cryo-EM structure of NS1 tubules, which illustrate a coiled tube of dimers. (B) NS2 forms viral inclusion bodies. The left panel shows cells 17 hrs post infection stained for NS2 (green). The middle panel displays the same cells stained for bromo-deoxyuridine (BrDU) (red). The right panel represents the merged image. Globular structures with peri-nuclear localisation are seen with the co-localisation of BrDU staining. (Adapted from Kar et al., 2007).
Initially the function of NS1 during viral replication remained largely unknown with evidence pointing to a role in viral morphogenesis, due to its association with virions both during purification and intracellularly (Eaton et al., 1988; Owens et al., 2004). However, more recent studies showed that NS1 is able to specifically promote the expression of BTV encoded proteins, acting as a positive regulator of translation (Boyce et al., 2012). A luciferase reporter segment exhibited specific up regulation of expression upon co-expression of NS1, and it appears NS1 enhances BTV protein synthesis by specifically interacting with the ACUUAC hexanucleotides of 3′ untranslated region (UTR) of BTV ssRNA transcripts (Boyce et al., 2012). Further, it has been shown that NS1 is one of the components of primary replication of the bi-phased replication cycle of BTV (Matsuo and Roy, 2013).
Together these data suggest NS1 plays a crucial role in regulation of viral gene expression allowing preferential expression of BTV transcripts by interacting with conserved sequence at their 3′ terminus. Thus NS1 could act analogously to host polyA binding protein which has been demonstrated for NSP3 of closely related Rotavirus (Vende et al., 2000), however this function is ambiguous as another report indicates it is not required for protein synthesis (Montero et al., 2006). Since BTV has been demonstrated to elicit substantial interferon response and by which, may induce host cell shutoff of protein synthesis via PKR activation (Jameson et al., 1978; Ruscanu et al., 2012). NS1 could act to enable BTV transcripts to overcome this response by specifically promoting their expression in a host cell senescent background.
If this model is the case, the functional unit of NS1 is likely to be the soluble dimer form and not that of the tubular multi- mer. The role tubules play in infection is not apparent. They could act as a repository of NS1, through sequestration via multimerisation and thereby controlling the level of soluble NS1.This may be relevant to orchestrate controlled regulation of translation. How- ever an unrelated role of this NS1 structural form cannot be ruled out.
6.2. NS2 and inclusion bodies
The second cellular morphological change upon BTV infection presents with the formation of punctate densities, which localise to form large perinuclear globules (Brookes et al., 1993; Eaton et al., 1987, 1990) (Fig. 5B, left & right panels). These have been termed viral inclusion bodies (VIBs) and have been viewed as the site of viral assembly (Brookes et al., 1993; Kar et al., 2007; Modrof et al., 2005).
The non-structural protein NS2 is both necessary and sufficient for VIB formation in both Sf9 cell and mammalian cell expression, forming VIBs with similar morphology to BTV infection when singly expressed (Kar et al., 2007; Thomas et al., 1990). NS2 can recruit proteins required for core assembly to the VIB. In culture NS2 colocalises with VP1, VP3, VP4, and VP6 when co-expressed. The core surface protein VP7, however, required the co-expression of VP3 to be recruited (Kar et al., 2007). It is plausible that the attachment of VP7 to formed sub-cores may act as a mechanism to modulate their release from VIB.
NS2 RNA binding: NS2 is a 42 kDa protein, it is highly charged and forms multimers (Fukusho et al., 1989) and possesses both ssRNA binding and NTPase activity (Horscroft and Roy, 2000; Taraporewala et al., 2001; Thomas et al., 1990). Dissection of the ssRNA binding regions using deletion analysis has revealed three sites of ssRNA interaction, an N-terminal region of residues 1–7, a middle region residues 153–166 and a C-terminal region residues 274–286 (Fillmore et al., 2002). Initial evidence suggested this binding was non-specific, however competition assays show a higher affinity for BTV derived ssRNA (Lymperopoulos et al., 2003, 2006; Modrof et al., 2005). This could be due to different binding modes of NS2 for ssRNA species as a result of multiple binding regions. Indeed BTV ssRNA must be specifically recognised in order to be recruited for packaging.
BTV genome segments possess conserved features, most prominently and common to other members of the Reoviridae, hexanucleotide sequences are present at extreme 5′ and 3′ termini (Fukusho et al., 1989). It is logical to hypothesise that these are determinants for specific VIB recruitment, however deletion analysis proves these unnecessary for NS2 interaction, with transcripts lacking these features displaying similar affinity as wild-type (WT) BTV transcripts by electro mobility shift assay (EMSA) (Markotter et al., 2004; Theron and Nel, 1997).
In the absence of sequence specificity it is therefore likely that distinct secondary structural elements are required to instill specific recruitment. Inverted repeat sequences present at 5′ and 3′ termini are an additional conserved feature of BTV RNA segments. Removal of these regions significantly influences EMSA NS2 inter- action in vitro (Markotter et al., 2004), however the predicted RNA secondary structures formed by these deletions are drastically different from that of WT RNA segments and as such more supporting data is required.
Further studies demonstrate a specific stem-loop secondary structure present in the coding region of S10 (99–170) mediates a strong interaction with NS2 as well as various fragments of S10 possessing this structural motif (Lymperopoulos et al., 2003). Chemical foot printing confirmed this predicted structure in vitro and mutagenesis revealed this structure to be important in facilitating S10–NS2 interaction (Lymperopoulos et al., 2003, 2006). Additional ssRNA transcripts (S6, S8 and S9) were dissected for NS2 interaction regions in this manner. This study demonstrated that (i) ssRNAs form secondary structures within coding regions that mediate high affinity interactions with NS2 and that (ii) these regions display secondary structure unique to each segment (Lymperopoulos et al., 2006). Furthermore, competition experiments with S9 and S10 suggest that at least for these ssRNA segments NS2 possesses differential binding sites (Lymperopoulos et al., 2006).
Taken together, the emerging model presented by this data indicates that NS2 consists of different independent binding modes to unique secondary structures within BTV ssRNA segments. Whether this is true for all ten RNA segments remains to be validated. Nevertheless, given the small size of the protein it is unlikely that even an oligomeric form could present ten specific binding sites, and it may be that certain RNA segments initially interact with NS2 and nucleate interactions with the remainder by forming RNA–RNA interactions.
In spite of the exact mechanism of ssRNA binding, this activity suggests this protein is able to bind BTV ssRNA transcripts concentrating them within the VIB and, additionally, may couple this to energy transduction via NTPase activity (Horscroft and Roy, 2000; Taraporewala et al., 2001). The role of NTPase activity is not certain however it may modulate ssRNA binding and release.
6.3. NS2 structure
A crystal structure for the N-terminal region 1–182 has been solved, revealing a β-sandwich fold, which forms a spiral helix of dimers in the crystal form (Butan et al., 2004). It is proposed that this is the mode of oligomerisation forming a helical channel with ten monomers per turn lined by residues binding ssRNA with the missing C-terminus accommodated on the helical surface (Butan and Tucker, 2010; Mumtsidu et al., 2007).
Whilst appealing in vivo data validating this model is lacking. Complete structures of related P9-1 (Rice black streak dwarf virus) and NSP2 (Rotavirus) VIB proteins of other members of the family have been solved (Akita et al., 2012; Jayaram et al., 2002). Interestingly despite low sequence homology they also demonstrate oligomeric ring-like structures; however rings are closed, not helical and composed of octamers. Thus the interpretation of the NS2 crystal structure may be un-valid, as the oligomeric state may be an artefact of crystal packing constraints and may not faithfully represent full-length protein. Similarly it cannot be ruled out that NS2 diverges from the structural architecture presented by these two proteins due to a differential mechanism of action, as indicated by its singular role in VIB formation unlike that of NSP2 (Fabbretti et al., 1999).
NS2 phosphorylation: NS2 has been shown to be phosphorylated in vivo and is the only BTV protein to do so (Modrof et al., 2005; Thomas et al., 1990). Alanine substitution mutagenesis maps this modification to serines 249 and 259, which are substrates for phosphorylation by casein kinase II (Modrof et al., 2005). The phosphorylation state of these residues is key in controlling VIB morphogenesis, single and double alanine substitutions at these positions failed to form larger aggregates characteristic of VIBs, demonstrating a dispersed granular appearance in culture (Modrof et al., 2005). However a mutant with aspartic acid substations at these positions demonstrated wild type morphology. This indicates that it is the charge at these positions that modulates VIB formation. Indeed immunoprecipitation showed the phosphorylation inhibited double alanine mutant forms smaller aggregates in vivo but was not affected in its ssRNA binding capacity by EMSA (Modrof et al., 2005).
Phosphorylation could act as a molecular switch controlling the stability of interaction of the NS2 matrix. Speculatively, the region of serines 249 and 259 could lie at an interface between NS2 monomers with charge modulation regulating the strength of surface interaction. It is plausible that dephosphorylating of NS2 within VIB facilitates the exit of assembled cores by forming channels of disaggregated NS2 as observed by TEM (Kar et al., 2007). The manner in which this process is controlled remains elusive as the process of NS2 dephosphorylation remains unresolved.
In summary, NS2 acts to form a VIB viral assembly matrix, by recruiting BTV core proteins and the ten ssRNA transcripts and possible host factors. Thus, NS2 plays a vital role in modulating core assembly in vivo and releasing the assembled cores into the cytosol.
6.4. NS3 and NS3A
BTV encodes a third non-structural protein, which occurs in two isoforms, full length NS3 (229 aa) and N-terminally truncated NS3A (216 aa) arising from an alternative translation start site. These proteins are produced at a relatively minor amount in comparison to NS1 and NS2, however, both proteins play an essential role in intracellular trafficking and host cell egress of the mature virions, which is discussed in detail in the section of virus egress.
6.5. NS4
An additional non-structural protein, NS4, has recently been identified for BTV. It is produced from an alternative reading frame in segment 9, interestingly for a group III virus, this protein has a nucleolar localisation, and associates with the cell lipid droplet (Belhouchet et al., 2011; Ratinier et al., 2011). Whilst at present a conclusive role for this protein has yet to be established initial studies demonstrate it may play a role in viral fitness to the interferon response (Ratinier et al., 2011).
7. Assembly
NS2 recruits and concentrates the components required for assembly of cores, however these components must assemble into the complex layered structure of BTV cores, encapsidating the ten dsRNA segments. The process by which this occurs has been studied using recombinant protein expression although much still remains to be clarified in detail. It is noteworthy that in absence of NS2, co-expression of structural proteins can enable assembly into core-like (CLPs) and virus-like (VLPs) particles which lack an encapsidated genome, demonstrating that these proteins have an intrinsic self-assembly pathway (French and Roy, 1990; French et al., 1990; Loudon and Roy, 1991). Utilising this system mutagenesis has revealed finer stages of the assembly process.
The X-ray crystal structure of the BTV core reveals the architecture of VP3, which exhibits three domains named as apical, carapace, and dimerization domains (Grimes et al., 1998). Two structural isoforms of VP3 exist A and B which differ in their folding primarily in the dimerization domain. This enables the formation of AB VP3 dimers which constitute a pseudo-triangular face an icosahedral lattice (Fig. 2D, orange outline). These AB dimers are observed to form decamers which comprise each fivefold axis in the crystal structure (Grimes et al., 1998) (Fig. 2D, white outline). A deletion mutant lacking the dimerization domain of VP3 abolishes sub-core formation but generates decamer and dimer species when expressed in insect cells (Kar et al., 2004). This suggests an assembly pathway in which VP3 dimers form decamer intermediates and that decamer–decamer interactions via the dimerization domain induce the assembly of the complete VP3 sub-core. Furthermore, unlike WT VP3 this deletion mutant failed to bind BTV dsRNA, indicating that this property is encoded within the dimerization domain as predicted from the crystal structure (Gouet et al., 1999).
The internal proteins VP1 and VP4, however, can directly inter- act with VP3 independently and are encapsidated in CLPs (Loudon and Roy, 1991; Ramadevi et al., 1998b). The exact method of encapsidation of VP6, which has strong RNA binding affinity, remains elusive as it fails to incorporate into CLPs, and it may be that this protein is recruited by the newly synthesised ssRNAs during assembly. This data proposes a model in which VP3 decamers bind VP1 and VP4 which recruit ssRNAs and VP6, this RNA incorporation and replication to dsRNA could drive sub-core formation through allosteric interaction with dimerization domains.
The assembled sub-core must be coated with 260 trimers of VP7 to form mature cores which exit the VIB. VP7 coating and core assembly depends on trimer formation (Limn et al., 2000). Structure-based mutational analysis demonstrates that the VP7 coat is dependent on both VP3 interactions with the trimer base through helix 2 and on lateral association of VP7 trimers mediated by helix 9 (Limn and Roy, 2003). It has been observed that CLPs lack the P trimer at the fivefold axis (Nason et al., 2004) suggesting that weak interactions occur at this localisation (Limn and Roy, 2003). This is presumably due to the symmetry mismatch between the VP3 (T = 2) and VP7 layers (T = 13) causing localised non-equivalent interactions (Grimes et al., 1998). A likely pathway of core assembly is that a number of strong VP7 trimer VP3 contacts act as multiple equivalent initiation sites that nucleate a second set of pseudo equivalent weaker interactions that complete the outer layer of the core (sequentially T up to P), as illustrated in Fig. 6B.
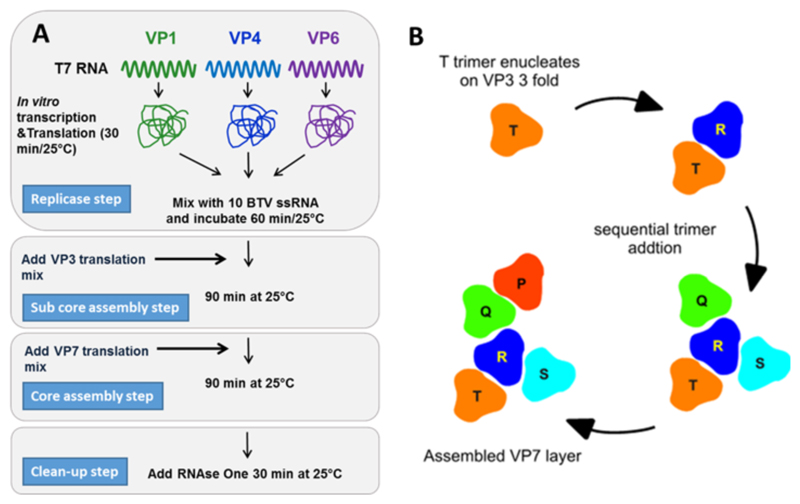
Fig. 6. The assembly pathway of the core particle.
(A) Theinvitroassemblyofcoreparticles.VP1,VP4 andVP6 are translated and mixed with ten BTV ssRNA transcripts.VP3 is then added followed by VP7. Note no non-structural proteins are required. (Adapted from Lourenco and Roy, 2011 (B)). The model of VP7 layer assembly. The T trimer sits on the icosahedral three fold axis where strong interactions with the sub-core layer a present. This trimer nucleates the assembly of trimer positions R to P through lateral association, which bind through weaker quasi-equivalent local interactions with the VP3 sub-core. (Adapted from Limn and Roy, 2003).
An in vitro cell free assembly (CFA) system has recently been developed. This major breakthrough has allowed exquisite dis- section of the assembly pathway (Lourenco and Roy, 2011). BTV structural proteins were expressed in vitro using wheat germ extract in the presence of the ten positive strand ssRNA segments. This reaction was fractionated on a sucrose gradient and was demonstrated to contain infectious particles (Lourenco and Roy, 2011). In this assay minor proteins VP1, VP4 and VP6 where incubated with the ten genomic ssRNAs followed by incubation with VP3 then VP7 (see Fig. 6A), this was sufficient to create viable particles which strongly supports this order of assembly process. Furthermore, it was found that the sub-core prior to encapsidation by VP7 was sensitive to RNase treatment which abrogated the formation of putative sub-cores when analysed on a gradient. This sensitivity was mitigated by the addition of VP7 (Lourenco and Roy, 2011).
These results indicate that the sub-core is partially stable and binding of VP7 trimers strengthens the overall structure. These findings were further supported by baculovirus expression system, which demonstrates that unlike CLPs, sub-cores of VP3 alone are unstable when singly expressed (Loudon and Roy, 1991). Importantly this CFA system demonstrates that BTV structural proteins and RNA segments alone can assemble to form infectious particles without the aid of the non-structural proteins, including NS2. Thus NS2 may play a passive role in vivo as a condenser of the necessary components required for assembly, however a more active role cannot be ruled out as the efficiency of CFA is relatively poor.
Genomic RNAs must contain specific packaging signals that facilitate their selective packaging over those of the host cell. Deletion mutants encoding a GFP reporter gene demonstrate that that the packaging signal of segment 9 is encoded in both 5′ and 3′ termini, present within 276 nt at the 5′ end and within 93 nt to 393 nt at the 3′ end (Matsuo and Roy, 2009). More recent studies have developed an in vivo packaging system, using infection of cells pre-transfected with purified in vitro transcribed segments, to address this hypothesis. This delineation of the process of packaging from other RNA properties such as transcription and translation signals has gained insight into the sequence and structure required to enable this. Initial studies have shown that secondary structure in the 3′ UTR but not 5′ UTR of segments is a major contributing factor to packaging (Burkhardt et al., submitted for publication).
Following the assembly of the core particle the viral outer capsid proteins, VP2 and VP5, are added to form mature virions. Cryo-EM experiments provide some insight into the nature of interaction of outer capsid proteins with the VP7 core outer layer (Nason et al., 2004; Zhang et al., 2010). It is of note that while neither VP2 nor VP5 are recruited by VIBs, instead each interacts independently with host cell machinery. For example VP5 interacts with SNARE regulatory protein synaptotagmin I of the exocytosis pathway (Bhattacharya and Roy, 2008) and VP2 with vimentin (Bhattacharya et al., 2007), a major component of intermediate filaments and cell cytoskeleton. Due to their lack of association with VIBs and the characterised host cell interactions these proteins may be acquired by newly assembled core upon VIB exit during host cell egress.
Addition of VP2 and VP5 may consequently abolish transcription activity, as such the assembly of the outer capsid must be highly regulated in order to prevent premature shut-off of transcription. Baculovirus expression of cores with VP5 and VP2 leads to assembly of a VLP mimicking authentic virions (French et al., 1990; Hewat et al., 1992a), indicating intrinsic self-assembly. Each triskelion VP2 trimer interacts with four VP7 trimers at its under- side and via the hub which connects to the crystallographic position Q VP7 trimer by a wall of density (Zhang et al., 2010). The globular- shaped VP5 trimers fill the gaps created by the three legs of the VP2 triskelion and sit right above the type II and III channels of the core. VP5 trimers make relatively few, weak interactions with adjacent VP7 trimers possibly to permit conformational changes of VP5 during the penetration process (Zhang et al., 2010). Owing to the weak interaction network of VP7 with VP2 and VP5, these proteins may be added proximally to virion egress to ensure maintenance of complete virion architecture upon cellular release.
The introduction of mutations in the replicating viral genome by RG system developed recently has opened up a new opportunity to dissect the minimal components required to rescue BTV infection. This system indicates the four sub-core proteins together with NS2 along with ten ssRNA transcripts are the sole components required to rescue infectivity, however, the inclusion of VP7 and NS1 significantly increases efficiency (Matsuo and Roy, 2013).
This data suggests that the assembled sub-core is sufficient to constitute a ‘replicase’ particle with the addition of VP7 stabilising such a structure, as also indicated in CFA (Lourenco and Roy, 2011). Thus, assembled sub-cores are transcriptionally active within VIBs allowing exponential viral growth. Indeed cell biology is indicative of this. BTV infected cells treated first with actinomycin D, to arrest the host transcription, followed by bromo-deoxyuridne transfection demonstrated newly synthesised labelled transcripts localised within the VIBs (Kar et al., 2007) (Fig. 5B).
8. Virus egress
Although core particles are assembled in VIBs, the addition of the outer VP2 and VP5 capsid onto the core to form mature infectious virus particles does not occur within these structures (Kar et al., 2007). Floatation experiments have confirmed that the outer capsid proteins VP2 and VP5, the minor core protein VP6 and the nonstructural glycosylated NS3 protein, all interact with lipid raft domains (Bhattacharya and Roy, 2010) indicating the importance of these membrane micro domains in BTV virion maturation and egress.
8.1. Role of lipid raft
Lipid rafts occur in both internal multi vesicular body (MVB) membranes and the plasma membrane of cells. These cholesterol and sphingolipid rich membrane micro domains act to concentrate specific proteins such as signalling molecules, glycosylphosphatidylinositol (GPI) anchored proteins and membrane trafficking proteins (Helms and Zurzolo, 2004; Lingwood and Simons, 2010). Lipid rafts have been implicated in the assembly of enveloped viruses such as HIV, Influenza virus and Ebola virus (Chazal and Gerlier, 2003; Waheed and Freed, 2009). Interestingly, their disruption with methyl β cyclodextrin alters the distribution of VP5 and NS3 and decreases the relative BTV viral titre without altering BTV protein synthesis (Bhattacharya and Roy, 2008).
VP5 possesses a highly conserved WHXL sequence that is present in the SNARE regulatory protein synaptotagmin I (Fukuda et al., 2000). Disruption of this sequence by alanine scan mutagenesis perturbed the association of VP5 with lipid rafts in mammalian cells (Bhattacharya and Roy, 2008).
The SNARE domains of cellular proteins have been shown to interact with the negatively charged lipid phosphatidylinositol (4,5) bisphosphate [PI(4,5)P2] present in membrane rafts (James et al., 2008). Depletion of cellular PI(4,5)P2, by expression of phosphatase 5ptaseIV or its relocation to endosomal-like structures by Arf6/Q67L expression, inhibited normal BTV maturation without altering BTV protein expression levels (Bhattacharya and Roy, 2013).
TEM showed the attachment of viral particles to the outer surface of vesicle-like structures in the cytoplasm, which were absent in PI(4,5)P2 depleted cells (Bhattacharya and Roy, 2013). PI(4,5)P2 plays a key role in vesicular trafficking, mediating the formation of endo- and exocytotic intra-cytoplasmic vesicles and in organising the association of these with the cytoskeleton via actin recruitment and polymerisation (Caroni, 2001; Di Paolo et al., 2004; Martin, 2001). Perturbation of PI(4,5)P2 homeostasis through depletion thus inhibits intracellular vesicle formation and consequently trafficking of newly synthesised BTV particles.
8.2. Role of NS3/NS3A
TEM data, the co-localisation of NS3 with PI(4,5)P2 and lipid rafts along with its ability to interact with both outer capsid proteins (Bhattacharya and Roy, 2008; Bhattacharya et al., 2007; Celma and Roy, 2011) suggest that NS3 might bring the newly assembled BTV cores and outer capsid proteins together to form mature virions.
Our current understanding is that this process takes place on the lipid raft domains of exocytotic vesicles leading to com- bined particle maturation and export, a process that is common to other viral infections (Domitrovic et al., 2013; Meng and Lever, 2013). Indeed BTV infected cells display virus particles associated with the vimentin intermediate filaments of the cytoskeleton and the disruption of this association prevents normal virus release (Bhattacharya et al., 2007). This is presumably due to interruption of vesicular transport to the cytoplasmic membrane.
Subsequent to outer capsid assembly and the virion transport to the cytoplasmic membrane, the virus must release from the cell into the extracellular medium in order to continue cell transmission. It is canonically believed that non-enveloped viruses release from infected cells by cellular lysis. However, strong evidence is mounting indicating these viruses can utilise budding pathways for cellular egress (Feng et al., 2013).
In the case of BTV both mechanisms appear to act with the utilisation of differential release mechanism in specific host cell backgrounds. A predominantly lytic infection occurs in mammalian cell infection characterised by extensive cytopathic effect (CPE), however essentially no CPE is observed during infection of vector insect cells in which the virus establishes a persistent non-lytic infection (Celma and Roy, 2011; Hyatt et al., 1989; Wechsler and McHolland, 1988).
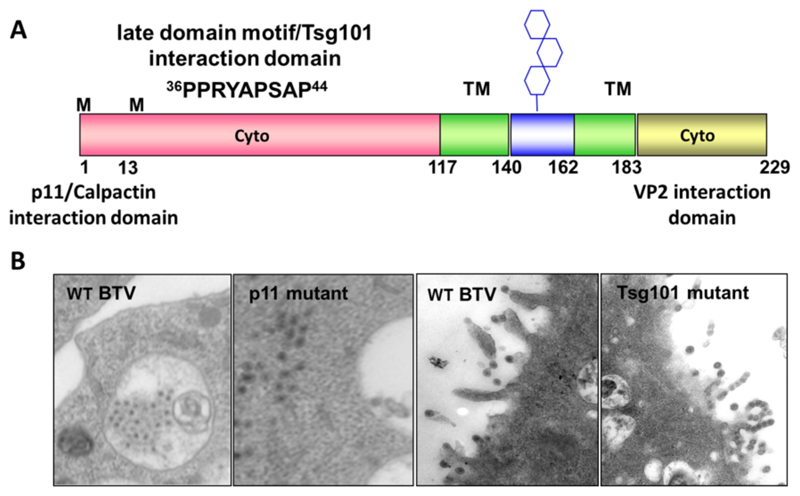
The only membrane proteins that are encoded by BTV are NS3 (229 aa) and its shorter form, produced from an alternative translation start site which lacks the N-terminal 13 amino acids, NS3A (216 aa) (French et al., 1989; Lee and Roy, 1986). Both proteins have been found localised with smooth-surfaced intracellular vesicles under TEM (Hyatt et al., 1993). Fig. 7A illustrates the features of NS3/NS3A. These proteins comprise of long N-terminal and a shorter C-terminal cytoplasmic domains which are linked by two transmembrane domains connected by a short extracellular loop (Bansal et al., 1998; Wu et al., 1992). This loop contains a glycosylation signal at asparagine 150 and the protein exists in both non-glycosylated and glycosylated forms in vivo (Bansal et al., 1998; Beaton et al., 2002). NS3 is responsible for virus release, co- expression of NS3 and NS3A with baculovirus-expressed VLPs stimulates VLPs, which are normally intracellular, to be released from cells with NS3 observed at the site of release (Hyatt et al., 1993).
Fig. 7. NS3 interaction with host cell factors and effect on particle trafficking.
(A) A schematic representing the structure of NS3 the longer N-terminal cytoplasmic domain (pink) contains interaction motifs for host cell factors. Residues 1–14 interact with calpactin/p11 and residues 36–44 with the cellular ESCRT machinery. Two transmembrane (TM) domains are shown in green. The loop connecting the two TM domains (blue) contains a glycosylation site represented by hexagons on a stick. The C-terminal cytoplasmic domain (gold) interacts with VP2. (B) Transmission electron micrographs of BSR cells infected with wt BTV or mutant virus that lacks interaction with either calpactin/p11 or Tsg101/ESCRT. (Adapted from Celma and Roy 2009, 2011).
8.3. Calpactin interaction
Cell biology, in conjunction with biochemical data, has gained insight to the molecular mechanism by which NS3 mediates BTV egress by interaction with the host cell machinery. Using the yeast two-hybrid analysis, residues 1–14 of the N-terminal cytoplasmic domain of NS3 have been identified to specifically interact with S100A10/p11 (Beaton et al., 2002). S100A10/p11 is a Ca2+ insensitive member of the S100 family that forms a heterotetrameric complex with two heavy chains of annexin II (also known as annexin A2) to form the calpactin complex in eukaryotic cells, which is involved in trafficking of proteins and membrane targeting (Rescher and Gerke, 2008). The C-terminus interacts specifically with the BTV outer capsid protein VP2 suggesting that NS3 acts as a bridging molecule facilitating virus engagement with host cell membrane trafficking machinery (Bhattacharya et al., 2007; Celma and Roy, 2011).
Direct site specific mutagenesis in the replicating genome demonstrated that a mutant virus synthesising NS3 but not NS3A was able to propagate in and release from mammalian cells efficiently (Celma and Roy, 2011). However, growth of a mutant virus expressing only NS3A was severely attenuated despite protein expression, dsRNA synthesis and particle assembly remaining unaffected. Two single amino acid substitutions in the N-terminal 13 residues of NS3 that interact with S100A10/p11 exhibited phenotypically similar effects.
In insect cells virus replication was apparent for mutant viruses lacking either NS3 or NS3A expression, however, titre was significantly reduced. Additionally TEM indicated that WT particles were found predominantly in intra-cytoplasmic vesicles, whilst mutant viruses were not vesicular confined, scattered throughout the cytoplasm (Celma and Roy, 2011) (Fig. 7B). Thus the extreme amino terminus of NS3 is required for trafficking in mammalian cells via interaction with S100A10/p11 while both NS3 and NS3A were required for efficient BTV growth in insect cells supporting a differential role for NS3/NS3A in varying host backgrounds.
8.4. The late domain
NS3 also possesses late domain motifs PSAP and PPRY similar to those characterised in budding enveloped viruses such as Ebola virus and HIV (Celma and Roy, 2009; Martin-Serrano et al., 2001; Wirblich et al., 2006). Late domains engage with the endosomal sorting complex required for trafficking (ESCRT) of the vacuolar protein sorting pathway, a cellular budding network that gives rise to MVB (Hurley, 2008; Schmidt and Teis, 2012).
NS3 interacts with human tumour-susceptibility gene 101 protein (Tsg101, a component of the pathway) and both NS3 and NS3A of BTV bind in vitro with to human Tsg101 and to its Drosophila homologue mediated by the PSAP motif (Wirblich et al., 2006). Mutations of the PSAP motif to inhibit Tsg101 interaction of infectious virus led to nascent BTV particles to be tethered to the cytosolic membrane (Celma and Roy, 2009), suggesting a role of this protein interaction in viral bud scission (Fig. 7B).
In addition to PSAP, NS3 possesses a second PPRY motif, which bears homology to the PPXY late domain motif, implicated in interaction with NEDD4-like ubiquitin ligases in several viruses (Bieniasz, 2006; Wirblich et al., 2006). The presence of the arginine at position three renders this motif less effective, at budding HIV gag derived VLPs bearing this motif, than the native PPPY found in Retro- and Filoviridae (Wirblich et al., 2006). It is plausible that BTV has modulated its affinity with the ESCRT machinery in order to strike a beneficial equilibrium between egress by budding or lysis.
Whilst the budding pathway has been partly understood molecularly, the process of lysis remains predominantly unknown. The NS3 proteins of BTV are cytotoxic when expressed in mammalian or insect cells (French et al., 1989; Staden et al., 1995) and BTV NS3 has been demonstrated to possess viroporin like properties (Han and Harty, 2004). NS3 was shown to homo-oligomerize when expressed in culture and cells expressing NS3 were sensitive to translation inhibition by hygromycin B an activity that tracked to the first transmembrane region of NS3 (Han and Harty, 2004).
Speculatively such an activity may facilitate virus release through lysis by inducing membrane permeabilization, causing cell rupture by osmosis. In any case, should the mechanism of cell lysis be controlled by NS3, the method by which BTV regulates the dynamic between budding and rupture of the cytosolic membrane utilising the same protein will be of great insight in understanding the difference in infection outcome between mammalian and insect cells. It may be that in mammalian cells lysis is simply induced by a concentration effect of NS3 with a threshold level inducing sufficient cell permeabilization to cause osmotic lysis.
All together the accumulating data suggest that BTV morphogenesis uses multipleegress pathways, with the utilisation of differential pathways in mammalian host and insect vector cells. NS3 initially mediates virus trafficking via S100A10/p11 and VP2 interaction, which then allows engagement with ESCRT pathway via Tsg101 to facilitate virus release by vesicle formation at the cytoplasmic membrane. The pathway of lysis process in mammalian cells and its inhibition in insect vector cells, remain to be conclusively established.
9. Conclusion
Significant progress has been made in understanding the structure of BTV proteins and particles as well as the biochemistry of the BTV replication cycle. The function of BTV proteins as enzymes in the transcription complex, as structural proteins in capsid assembly and as mediators of host–virus interaction especially in virus entry, egress and pathogenicity have all been described. BTV is thus very well characterised both genetically and structurally and it is reasonable to suppose that other orbiviruses have similar basic functions.
The recent development of a reverse genetics system for the virus is currently allowing these molecular functions to be con- firmed and refined in the context of a replicating virus. From this knowledge it is possible to further develop precisely engineered attenuated vaccines able to induce a vigorous immune response capable of preventing infection while excluding the possibility of reversion or reassortments that limit current live vaccines. Thus, the applications of the basic understanding of BTV virology have already resulted in the pathway of improvement to disease prevention and management. Other significant challenges, however, still remain. The studies of certain aspects of virus replication have been impeded for lack of appropriate tools and assay systems. However, recent advancement in technologies now make it possible to obtain new information in these critical areas.
The majority of our understanding of BTV and related orbiviruses is focused on the replication in mammalian cells, principally in tissue culture as these are the standard tools of molecular biology that have been solidly substantiated. The replication and spread of BTV in whole animals and in Culicoides sp, however, present challenging fields for which the experimental paradigms require establishment and as such have been sparingly studied. These should improve with the development of a tractable small animal model for the former and the whole genome sequence of Culicoides for the latter. The identification of host cell factors critical to BTV replication may be important here as they could inform the construction of a suitable transgenic model and indicate why certain species of Culicoides are preferred for transmission. Assuming developments in these areas, the whole biology of BTV infection including pathogenesis, breed susceptibility and transmission should become as well understood as the molecular aspects described.
When they do, the mechanisms of virus evolution and emergence, and the roles played by each host in the process should become apparent. As for the application of basic virology, these developments will also result in better control of Orbivirus disease in the future.
References
- Akita F, Higashiura A, Shimizu T, Pu Y, Suzuki M, Uehara-Ichiki T, Sasaya T, Kanamaru S, Arisaka F, Tsukihara T, Nakagawa A, et al. Crystallographic analysis reveals octamerization of viroplasm matrix protein P 9-1 of Rice black streaked dwarf virus. J Virol. 2012;86:746–756. doi: 10.1128/JVI.00826-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal OB, Stokes A, Bansal A, Bishop D, Roy P. Membrane organization of bluetongue virus nonstructural glycoprotein NS3. J Virol. 1998;72:3362–3369. doi: 10.1128/jvi.72.4.3362-3369.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt-Boyes SM, MacLachlan NJ. Dynamics of viral spread in Bluetongue virus infected calves. Vet Microbiol. 1994;40:361–371. doi: 10.1016/0378-1135(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Beaton AR, Rodriguez J, Reddy YK, Roy P. The membrane trafficking protein calpactin forms a complex with bluetongue virus protein NS3 and mediates virus release. Proc Natl Acad Sci U S A. 2002;99:13154–13159. doi: 10.1073/pnas.192432299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhouchet M, Mohd Jaafar F, Firth AE, Grimes JM, Mertens PPC, Attoui H. Detection of a fourth orbivirus non-structural protein. PLoS One. 2011;6:e25697. doi: 10.1371/journal.pone.0025697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya B, Roy P. Bluetongue virus outer capsid protein VP5 interacts with membrane lipid rafts via a SNARE domain. J Virol. 2008;82:10600–10612. doi: 10.1128/JVI.01274-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya B, Roy P. Role of lipids on entry and exit of bluetongue virus, a complex non-enveloped virus. Viruses. 2010;2:1218–1235. doi: 10.3390/v2051218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya B, Roy P. Cellular phosphoinositides and the maturation of bluetongue virus, a non-enveloped capsid virus. Virol J. 2013;10:73. doi: 10.1186/1743-422X-10-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya B, Noad RJ, Roy P. Interaction between Bluetongue virus outer capsid protein VP2 and vimentin is necessary for virus egress. Virol J. 2007;4:7. doi: 10.1186/1743-422X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieniasz PD. Late budding domains and host proteins in enveloped virus release. Virology. 2006;344:55–63. doi: 10.1016/j.virol.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Boyce M, Roy P. Recovery of infectious bluetongue virus from RNA. J Virol. 2007;81:2179–2186. doi: 10.1128/JVI.01819-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Wehrfritz J, Noad R, Roy P. Purified recombinant bluetongue virus VP1 exhibits RNA replicase activity. J Virol. 2004;78:3994–4002. doi: 10.1128/JVI.78.8.3994-4002.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Celma CCP, Roy P. Development of reverse genetics systems for bluetongue virus: recovery of infectious virus from synthetic RNA transcripts. J Virol. 2008;82:8339–8348. doi: 10.1128/JVI.00808-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Celma CCP, Roy P. Bluetongue virus non-structural protein 1 is a positive regulator of viral protein synthesis. Virol J. 2012;9:178. doi: 10.1186/1743-422X-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes SM, Hyatt AD, Eaton BT. Characterization of virus inclusion bodies in bluetongue virus-infected cells. J Gen Virol. 1993;74(Pt 3):525–530. doi: 10.1099/0022-1317-74-3-525. [DOI] [PubMed] [Google Scholar]
- Butan C, Tucker P. Insights into the role of the non-structural protein 2 (NS2) in Bluetongue virus morphogenesis. Virus Res. 2010;151:109–117. doi: 10.1016/j.virusres.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Butan C, Van Der Zandt H, Tucker P. Structure and assembly of the RNA binding domain of bluetongue virus non-structural protein 2. J Biol Chem. 2004;279:37613–37621. doi: 10.1074/jbc.M400502200. [DOI] [PubMed] [Google Scholar]
- Caroni P. New EMBO members’ review: actin cytoskeleton regulation through modulation of PI(4,5)P(2) rafts. EMBO J. 2001;20:4332–4336. doi: 10.1093/emboj/20.16.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celma CCP, Roy P. A viral nonstructural protein regulates bluetongue virus trafficking and release. J Virol. 2009;83:6806–6816. doi: 10.1128/JVI.00263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celma CCP, Roy P. Interaction of calpactin light chain (S100A10/p11) and a viral NS protein is essential for intracellular trafficking of nonenveloped blue-tongue virus. J Virol. 2011;85:4783–4791. doi: 10.1128/JVI.02352-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celma CCP, Boyce M, van Rijn PA, Eschbaumer M, Wernike K, Hoffmann B, Beer M, Haegeman A, De Clercq K, Roy P. Rapid generation of replication-deficient monovalent and multivalent vaccines for bluetongue virus: protection against virulent virus challenge in cattle and sheep. J Virol. 2013;87:9856–9864. doi: 10.1128/JVI.01514-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC, Fass D, Berger JM, Kim PS. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- Chazal N, Gerlier D. Virus entry, assembly, budding, and membrane rafts. Microbiol Mol Biol Rev. 2003;67:226–237. doi: 10.1128/MMBR.67.2.226-237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Patton JT. De novo synthesis of minus strand RNA by the rotavirus RNA polymerase in a cell-free system involves a novel mechanism of initiation. RNA. 2000;6:1455–1467. doi: 10.1017/s1355838200001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman PM, Lawrence MC. The structural biology of type I viral membrane fusion. Nat Rev Mol Cell Biol. 2003;4:309–319. doi: 10.1038/nrm1076. [DOI] [PubMed] [Google Scholar]
- Darpel KE, Langner KFA, Nimtz M, Anthony SJ, Brownlie J, Takamatsu H-H, Mellor PS, Mertens PPC. Saliva proteins of vector Culicoides modify structure and infectivity of bluetongue virus particles. PLoS One. 2011;6:e17545. doi: 10.1371/journal.pone.0017545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan Ta, De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- Diprose JM, Burroughs JN, Sutton GC, Goldsmith A, Gouet P, Malby R, Overton I, Ziéntara S, Mertens PP, Stuart DI, Grimes JM. Translocation portals for the substrates and products of a viral transcription complex: the bluetongue virus core. EMBO J. 2001;20:7229–7239. doi: 10.1093/emboj/20.24.7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domitrovic T, Movahed N, Bothner B, Matsui T, Wang Q, Doerschuk PC, Johnson JE. Virus assembly and maturation: auto-regulation through allosteric molecular switches. J Mol Biol. 2013;425:1488–1496. doi: 10.1016/j.jmb.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormitzer PR, Sun Z-YJ, Wagner G, Harrison SC. The rhesus rotavirus VP4 sialic acid binding domain has a galectin fold with a novel carbohydrate binding site. EMBO J. 2002;21:885–897. doi: 10.1093/emboj/21.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton BT, Crameri GS. The site of bluetongue virus attachment to glycophorins from a number of animal erythrocytes. J Gen Virol. 1989;70:3347–3353. doi: 10.1099/0022-1317-70-12-3347. [DOI] [PubMed] [Google Scholar]
- Eaton BT, Hyatt AD, White JR. Association of bluetongue virus with the cytoskeleton. Virology. 1987;157:107–116. doi: 10.1016/0042-6822(87)90319-9. [DOI] [PubMed] [Google Scholar]
- Eaton BT, Hyatt AD, White JR. Localization of the nonstructural protein NS1 in bluetongue virus-infected cells and its presence in virus particles. Virology. 1988;163:527–537. doi: 10.1016/0042-6822(88)90293-0. [DOI] [PubMed] [Google Scholar]
- Eaton BT, Hyatt AD, Brookes SM. The replication of bluetongue virus. Curr Top Microbiol Immunol. 1990;162:89–118. doi: 10.1007/978-3-642-75247-6_4. [DOI] [PubMed] [Google Scholar]
- El Omari K, Meier C, Kainov D, Sutton G, Grimes JM, Poranen MM, Bamford DH, Tuma R, Stuart DI, Mancini EJ. Tracking in atomic detail the functional specializations in viral RecA helicases that occur during evolution. Nucleic Acids Res. 2013:1–15. doi: 10.1093/nar/gkt713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbretti E, Afrikanova I, Vascotto F, Burrone O. Two non-structural rotavirus proteins, NSP2 and NSP5, form viroplasm-like structures in vivo. J Gen Virol. 1999;80:333–339. doi: 10.1099/0022-1317-80-2-333. [DOI] [PubMed] [Google Scholar]
- Fabrega C, Hausmann S, Shen V, Shuman S, Lima CD. Structure and mechanism of mRNA cap (guanine-N7) methyltransferase. Mol Cell. 2004;13:77–89. doi: 10.1016/s1097-2765(03)00522-7. [DOI] [PubMed] [Google Scholar]
- Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong S-H, Walker C, Lanford RE, Lemon SM. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496:1–6. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore GC, Lin H, Li JK. Localization of the single-stranded RNA-binding domains of bluetongue virus nonstructural protein NS2. J Virol. 2002;76:499–506. doi: 10.1128/JVI.76.2.499-506.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forzan M, Wirblich C, Roy P. A capsid protein of nonenveloped Blue-tongue virus exhibits membrane fusion activity. Proc Natl Acad Sci U S A. 2004;101:2100–2105. doi: 10.1073/pnas.0306448101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forzan M, Marsh M, Roy P. Bluetongue virus entry into cells. J Virol. 2007;81:4819–4827. doi: 10.1128/JVI.02284-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G, Parry NR, Barnett P, McGinn V, Rowlands B, Brown DJF. The cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid) J Gen Virol. 1989;70:625–637. doi: 10.1099/0022-1317-70-3-625. [DOI] [PubMed] [Google Scholar]
- French TJ, Roy P. Synthesis of bluetongue virus (BTV) corelike particles by a recombinant baculovirus expressing the two major structural core proteins of BTV. J Virol. 1990;64:1530–1536. doi: 10.1128/jvi.64.4.1530-1536.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French TJ, Inumaru S, Roy P. Expression of two related nonstructural proteins of bluetongue virus (BTV) type 10 in insect cells by a recombinant baculovirus: production of polyclonal ascitic fluid and characterization of the gene product in BTV-infected BHK cells. J Virol. 1989;63:3270–3278. doi: 10.1128/jvi.63.8.3270-3278.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French TJ, Marshall JJ, Roy P. Assembly of double-shelled, virus like particles of bluetongue virus by the simultaneous expression of four structural proteins. J Virol. 1990;64:5695–5700. doi: 10.1128/jvi.64.12.5695-5700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry EE, Lea SM, Jackson T, Newman JW, Ellard FM, Blakemore WE, AbuGhazaleh R, Samuel a, King aM, Stuart DI. The structure and function of a foot-and-mouth disease virus-oligosaccharide receptor complex. EMBO J. 1999;18:543–554. doi: 10.1093/emboj/18.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Moreira JE, Liu V, Sugimori M, Mikoshiba K, Llinás RR. Role of the conserved WHXL motif in the C terminus of synaptotagmin in synaptic vesicle docking. Proc Natl Acad Sci U S A. 2000;97:14715–14719. doi: 10.1073/pnas.260491197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukusho A, Yu Y, Yamaguchi S, Roy P. Completion of the sequence of blue- tongue virus serotype 10 by the characterization of a structural protein, VP6, and a non-structural protein, NS2. J Gen Virol. 1989;70:1677–1689. doi: 10.1099/0022-1317-70-7-1677. [DOI] [PubMed] [Google Scholar]
- Ghiasi H, Fukusho A, Eshita Y, Roy P. Identification and characterization of conserved and variable regions in the neutralization VP2 gene of bluetongue virus. Virology. 1987;160:100–109. doi: 10.1016/0042-6822(87)90050-x. [DOI] [PubMed] [Google Scholar]
- Gouet P, Diprose JM, Grimes JM, Malby R, Burroughs JN, Zientara S, Stuart DI, Mertens PP. The highly ordered double-stranded RNA genome of bluetongue virus revealed by crystallography. Cell. 1999;97:481–490. doi: 10.1016/s0092-8674(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Jakana J, Ghosh M, Basak AK, Roy P, Chiu W, Stuart DI, Prasad BV. An atomic model of the outer layer of the bluetongue virus core derived from X-ray crystallography and electron cryomicroscopy. Structure. 1997;5:885–893. doi: 10.1016/s0969-2126(97)00243-8. [DOI] [PubMed] [Google Scholar]
- Grimes JM, Burroughs JN, Gouet P, Diprose JM, Malby R, Ziéntara S, Mertens PP, Stuart DI. The atomic structure of the bluetongue virus core. Nature. 1998;395:470–478. doi: 10.1038/26694. [DOI] [PubMed] [Google Scholar]
- Guinea R, Carrasco L. Requirement for vacuolar proton-ATPase activity during entry of influenza virus into cells. J Virol. 1995;69:2306–2312. doi: 10.1128/jvi.69.4.2306-2312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Harty RN. The NS3 protein of bluetongue virus exhibits viroporin-like properties. J Biol Chem. 2004;279:43092–43097. doi: 10.1074/jbc.M403663200. [DOI] [PubMed] [Google Scholar]
- Harber J, Bernhardt G, Lu HH, Sgro JY, Wimmer E. Canyon rim residues, including antigenic determinants, modulate serotype-specific binding of polioviruses to mutants of the poliovirus receptor. Virology. 1995;214:559–570. doi: 10.1006/viro.1995.0067. [DOI] [PubMed] [Google Scholar]
- Hassan SS, Roy P. Expression and functional characterization of bluetongue virus VP2 protein: role in cell entry. J Virol. 1999;73:9832–9842. doi: 10.1128/jvi.73.12.9832-9842.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan SH, Wirblich C, Forzan M. Expression and functional characterization of bluetongue virus VP5 protein: role in cellular permeabilization. J Virol. 2001;75:8356–8367. doi: 10.1128/JVI.75.18.8356-8367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms JB, Zurzolo C. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic. 2004;5:247–254. doi: 10.1111/j.1600-0854.2004.0181.x. [DOI] [PubMed] [Google Scholar]
- Hewat EA, Booth TF, Roy P. Structure of bluetongue virus particles by cryoelectron microscopy. J Struct Biol. 1992a;109:61–69. doi: 10.1016/1047-8477(92)90068-l. [DOI] [PubMed] [Google Scholar]
- Hewat EA, Booth TF, Wade RH, Roy P. 3-D reconstruction of bluetongue virus tubules using cryoelectron microscopy. J Struct Biol. 1992b;108:35–48. doi: 10.1016/1047-8477(92)90005-u. [DOI] [PubMed] [Google Scholar]
- Hewat EA, Booth TF, Roy P. Structure of correctly self-assembled blue-tongue virus-like particles. J Struct Biol. 1994;112:183–191. doi: 10.1006/jsbi.1994.1019. [DOI] [PubMed] [Google Scholar]
- Hodel aE, Gershon PD, Shi X, Quiocho Fa. The 1.85 A ° structure of vaccinia protein VP39: a bifunctional enzyme that participates in the modification of both mRNA ends. Cell. 1996;85:247–256. doi: 10.1016/s0092-8674(00)81101-0. [DOI] [PubMed] [Google Scholar]
- Horscroft NJ, Roy P. NTP binding and phosphohydrolase activity associated with purified bluetongue virus non-structural protein NS2. J Gen Virol. 2000;81:1961–1965. doi: 10.1099/0022-1317-81-8-1961. [DOI] [PubMed] [Google Scholar]
- Huismans H, Els HJ. Characterization of the tubules associated with the replication of three different orbiviruses. Virology. 1979;92:397–406. doi: 10.1016/0042-6822(79)90144-2. [DOI] [PubMed] [Google Scholar]
- Huismans H, van Der Walt NTT, Cloete M, Erasmus BJJ. Isolation of a capsid protein of bluetongue virus that induces a protective immune response in sheep. Virology. 1987a;157:172–179. doi: 10.1016/0042-6822(87)90326-6. [DOI] [PubMed] [Google Scholar]
- Huismans Hendrik, van Dijk AA, Els HJ. Uncoating of parental bluetongue virus to core and subcore particles in infected L cells. Virology. 1987b;157:180–188. doi: 10.1016/0042-6822(87)90327-8. [DOI] [PubMed] [Google Scholar]
- Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol. 2008;20:4–11. doi: 10.1016/j.ceb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt AD, Eaton BT, Brookes SM. The release of bluetongue virus from infected cells and their superinfection by progeny virus. Virology. 1989;173:21–34. doi: 10.1016/0042-6822(89)90218-3. [DOI] [PubMed] [Google Scholar]
- Hyatt AD, Zhao Y, Roy P. Release of bluetongue virus-like particles from insect cells is mediated by BTV nonstructural protein NS3/NS3A. Virology. 1993;193:592–603. doi: 10.1006/viro.1993.1167. [DOI] [PubMed] [Google Scholar]
- Inumaru S, Roy P. Production and characterization of the neutralization antigen VP2 of bluetongue virus serotype 10 using a baculovirus expression vector. Virology. 1987;157:472–479. doi: 10.1016/0042-6822(87)90289-3. [DOI] [PubMed] [Google Scholar]
- Isa P, López S, Segovia L, Arias CF, Lo S. Functional and structural analysis of the sialic acid-binding domain of rotaviruses. J Virol. 1997;71:6749–6756. doi: 10.1128/jvi.71.9.6749-6756.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovic T, Agosto MA, Zhang L, Chandran K, Harrison SC, Nibert ML. Peptides released fromreovirus outer capsid form membrane pores that recruit virus particles. EMBO J. 2008;27:1289–1298. doi: 10.1038/emboj.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DJ, Khodthong C, Kowalchyk JA, Martin TFJ. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol. 2008;182:355–366. doi: 10.1083/jcb.200801056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson P, Schoenherr CK, Grossberg SE. Bluetongue virus, an exceptionally potent interferon inducer in mice. Infect Immun. 1978;20:321–323. doi: 10.1128/iai.20.1.321-323.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram H, Taraporewala Z, Patton JT, Prasad BVV. Rotavirus protein involved in genome replication and packaging exhibits a HIT-like fold. Nature. 2002;417:311–315. doi: 10.1038/417311a. [DOI] [PubMed] [Google Scholar]
- Kaname Y, Celma CCP, Kanai Y, Roy P. Recovery of African horse sickness virus from synthetic RNA. J Gen Virol. 2013;94:2259–2265. doi: 10.1099/vir.0.055905-0. [DOI] [PubMed] [Google Scholar]
- Kar AK, Roy P. Definingthe structure-functionrelationships of bluetongue virus helicase protein VP6. J Virol. 2003;77:11347–11356. doi: 10.1128/JVI.77.21.11347-11356.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar AK, Ghosh M, Roy P. Mapping the assembly pathway of Bluetongue virus scaffolding protein VP3. Virology. 2004;324:387–399. doi: 10.1016/j.virol.2004.04.018. [DOI] [PubMed] [Google Scholar]
- Kar AK, Bhattacharya B, Roy P. Bluetongue virus RNA binding protein NS2 is a modulator of viral replication and assembly. BMC Mol Biol. 2007;8:4. doi: 10.1186/1471-2199-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M, Rey FA. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat Rev Microbiol. 2006;4:67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov MM, Chernomordik LV. The protein coat in membrane fusion: lessons from fission. Traffic. 2002;3:256–267. doi: 10.1034/j.1600-0854.2002.030403.x. [DOI] [PubMed] [Google Scholar]
- Kraschnefski MJ, Bugarcic A, Fleming FE, Yu X, von Itzstein M, Coulson BS, Blanchard H. Effects on sialic acid recognition of amino acid mutations in the carbohydrate-binding cleft of the rotavirus spike protein. Glycobiology. 2009;19:194–200. doi: 10.1093/glycob/cwn119. [DOI] [PubMed] [Google Scholar]
- Lawton JA, Estes MK, Prasad BVV. Three-dimensional visualization of mRNA release from actively transcribing rotavirus particles. Nat Struct Biol. 1997;4:118–121. doi: 10.1038/nsb0297-118. [DOI] [PubMed] [Google Scholar]
- Lawton JA, Estes MK, Prasad BV. Comparative structural analysis of transcriptionally competent and incompetent rotavirus-antibody complexes. Proc Natl Acad Sci U S A. 1999;96:5428–5433. doi: 10.1073/pnas.96.10.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Roy P. Nucleotide sequence of a cDNA clone of RNA segment 10 of bluetongue virus (serotype 10) J Gen Virol. 1986;67:2833–2837. doi: 10.1099/0022-1317-67-12-2833. [DOI] [PubMed] [Google Scholar]
- Liemann S, Chandran K, Baker TS, Nibert ML, Harrison SC. Structure of the reovirus membrane-penetration protein, Mu1, in a complex with is protector protein, Sigma3. Cell. 2002;108:283–295. doi: 10.1016/s0092-8674(02)00612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limn C, Roy P. Intermolecular interactions in a two-layered viral capsid that requires a complex symmetry mismatch. J Virol. 2003;77:11114–11124. doi: 10.1128/JVI.77.20.11114-11124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limn C, Staeuber N, Gouet P, Roy P, Monastyrskaya K. Functional dissection of the major structural protein of bluetongue virus: identification of key residues within VP7 essential for capsid assembly. J Virol. 2000;74:8658–8669. doi: 10.1128/jvi.74.18.8658-8669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- Loudon PT, Roy P. Assembly of five bluetongue virus proteins expressed by recombinant baculoviruses: inclusion of the largest protein VP1 in the core and virus-like particles. Virology. 1991;180:798–802. doi: 10.1016/0042-6822(91)90094-r. [DOI] [PubMed] [Google Scholar]
- Lourenco S, Roy P. In vitro reconstitution of Bluetongue virus infectious cores. Proc Natl Acad Sci U S A. 2011;108:13746–13751. doi: 10.1073/pnas.1108667108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymperopoulos K, Wirblich C, Brierley I, Roy P. Sequence specificity in the interaction of Bluetongue virus non-structural protein 2 (NS2) with viral RNA. J Biol Chem. 2003;278:31722–31730. doi: 10.1074/jbc.M301072200. [DOI] [PubMed] [Google Scholar]
- Lymperopoulos K, Noad R, Tosi S, Nethisinghe S, Brierley I, Roy P. Specific binding of Bluetongue virus NS2 to different viral plus-strand RNAs. Virology. 2006;353:17–26. doi: 10.1016/j.virol.2006.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maan S, Maan NS, Samuel AR, Rao S, Attoui H, Mertens PPC. Analysis and phylogenetic comparisons of full-length VP2 genes of the 24 bluetongue virus serotypes. J Gen Virol. 2007;88:621–630. doi: 10.1099/vir.0.82456-0. [DOI] [PubMed] [Google Scholar]
- Maan S, Maan NS, Nomikou K, Veronesi E, Bachanek-Bankowska K, Belaganahalli MN, Attoui H, Mertens PPC. Complete genome characterisation of a novel 26th bluetongue virus serotype from Kuwait. PLoS One. 2011;6:e26147. doi: 10.1371/journal.pone.0026147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi PR, Rawlings P, Burroughs JN, Wellby M, Mertens PP, Mellor PS, Wade-Evans aM. Proteolytic cleavage of VP2, an outer capsid protein of African horse sickness virus, by species-specific serum proteases enhances infectivity in Culicoides. J Gen Virol. 1995;76:2607–2611. doi: 10.1099/0022-1317-76-10-2607. [DOI] [PubMed] [Google Scholar]
- Markotter W, Theron J, Nel LH. Segment specific inverted repeat sequences in bluetongue virus mRNA are required for interaction with the virus non structural protein NS2. Virus Res. 2004;105:1–9. doi: 10.1016/j.virusres.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TF. PI(4,5)P(2) regulation of surface membrane traffic. Curr Opin Cell Biol. 2001;13:493–499. doi: 10.1016/s0955-0674(00)00241-6. [DOI] [PubMed] [Google Scholar]
- Martin SA, Zweerink HJ. Isolation and characterization of two types of bluetongue virus particles. Virology. 1972;50:495–506. doi: 10.1016/0042-6822(72)90400-x. [DOI] [PubMed] [Google Scholar]
- Martinez-Costas J, Sutton G, Ramadevi N, Roy P. Guanylyltransferase and RNA 5'-triphosphatase activities of the purified expressed VP4 protein of bluetongue virus. J Mol Biol. 1998;280:859–866. doi: 10.1006/jmbi.1998.1926. [DOI] [PubMed] [Google Scholar]
- Martin-Serrano J, Zang T, Bieniasz PD. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat Med. 2001;7:1313–1319. doi: 10.1038/nm1201-1313. [DOI] [PubMed] [Google Scholar]
- Mason PW, Rieder E, Baxt B. RGD sequence of foot-and-mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody-dependent enhancement pathway. Proc Natl Acad Sci USA. 1994;91:1932–1936. doi: 10.1073/pnas.91.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo E, Roy P. Bluetongue virus VP6 acts early in the replication cycle and can form the basis of chimeric virus formation. J Virol. 2009;83:8842–8848. doi: 10.1128/JVI.00465-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo E, Roy P. Bluetongue virus VP1 polymerase activity in vitro: template dependency, dinucleotide priming and cap dependency. PLoS One. 2011;6:e27702. doi: 10.1371/journal.pone.0027702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo E, Roy P. Minimum requirements for bluetongue virus primary replication in vivo. J Virol. 2013;87:882–889. doi: 10.1128/JVI.02363-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo E, Celma CCP, Roy P. A reverse genetics system of African horse sickness virus reveals existence of primary replication. FEBS Lett. 2010;584:3386–3391. doi: 10.1016/j.febslet.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Matsuo E, Celma CCP, Boyce M, Viarouge C, Sailleau C, Dubois E, Bréard E, Thiéry R, Zientara S, Roy P. Generation of replication-defective virus-based vaccines that confer full protection in sheep against virulent bluetongue virus challenge. J Virol. 2011;85:10213–10221. doi: 10.1128/JVI.05412-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor PS, Boorman J, Baylis M. Culicoides biting midges: their role as arbovirus vectors. Annu Rev Entomol. 2000;45:307–340. doi: 10.1146/annurev.ento.45.1.307. [DOI] [PubMed] [Google Scholar]
- Meng B, Lever AM. Wrapping up the bad news: HIV assembly and release. Retrovirology. 2013;10:5. doi: 10.1186/1742-4690-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens PP, Brown F, Sangar DV. Assignment of the genome segments of bluetongue virus type 1 to the proteins which they encode. Virology. 1984;135:207–217. doi: 10.1016/0042-6822(84)90131-4. [DOI] [PubMed] [Google Scholar]
- Mertens PP, Burroughs JN, Walton A, Wellby MP, Fu H, O’Hara RS, Brookes SM, Mellor PS. Enhanced infectivity of modified bluetongue virus particles for two insect cell lines and for two Culicoides vector species. Virology. 1996;217:582–593. doi: 10.1006/viro.1996.0153. [DOI] [PubMed] [Google Scholar]
- Modrof J, Lymperopoulos K, Roy P. Phosphorylation of bluetongue virus nonstructural protein 2 is essential for formation of viral inclusion bodies. J Virol. 2005;79:10023–10031. doi: 10.1128/JVI.79.15.10023-10031.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero H, Arias CF, Lopez S. Rotavirus nonstructural protein NSP3 is not required for viral protein synthesis. J Virol. 2006;80:9031–9038. doi: 10.1128/JVI.00437-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortola E, Noad R, Roy P. Bluetongue virus outer capsid proteins are sufficient to trigger apoptosis in mammalian cells. J Virol. 2004;78:2875–2883. doi: 10.1128/JVI.78.6.2875-2883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtsidu E, Makhov AM, Roessle M, Bathke A, Tucker PA. Structural features of the Bluetongue virus NS2 protein. J Struct Biol. 2007;160:157–167. doi: 10.1016/j.jsb.2007.07.013. [DOI] [PubMed] [Google Scholar]
- Nason EL, Rothagel R, Mukherjee SK, Kar AK, Forzan M, Prasad BVV, Roy P. Interactions between the inner and outer capsids of bluetongue virus. J Virol. 2004;78:8059–8067. doi: 10.1128/JVI.78.15.8059-8067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens RJ, Limn C, Roy P. Role of an arbovirus nonstructural protein in cellular pathogenesis and virus release. J Virol. 2004;78:6649–6656. doi: 10.1128/JVI.78.12.6649-6656.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pager CT, Dutch RE. Cathepsin L is involved in proteolytic processing of the hendra virus fusion protein. J Virol. 2005;79:12714–12720. doi: 10.1128/JVI.79.20.12714-12720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pager CT, Craft WW, Patch J, Dutch RE. A mature and fusogenic form of the Nipah virus fusion protein requires proteolytic processing by cathepsin L. Virology. 2006;346:251–257. doi: 10.1016/j.virol.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JT, Jones MT, Kalbach AN, He YW, Xiaobo J, Patton JohnT, Jones MelindaT, Kalbach AnneN. Rotavirus RNA polymerase requires the core shell protein to synthesize the double-stranded RNA genome. J Virol. 1997;71:9618–9626. doi: 10.1128/jvi.71.12.9618-9626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periz J, Celma C, Jing B, Pinkney JNM, Roy P, Kapanidis AN. Rotavirus mRNAS are released by transcript-specific channels in the double-layered viral capsid. Proc Natl Acad Sci USA. 2013;110:12042–12047. doi: 10.1073/pnas.1220345110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesavento JB, Lawton JA, Estes ME, Venkataram Prasad BV. The reversible condensation and expansion of the rotavirus genome. Proc Natl Acad Sci USA. 2001;98:1381–1386. doi: 10.1073/pnas.98.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BV, Yamaguchi S, Roy P. Three-dimensional structure of single-shelled bluetongue virus. J Virol. 1992;66:2135–2142. doi: 10.1128/jvi.66.4.2135-2142.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad BV, Rothnagel R, Zeng CQ, Jakana J, Lawton JA, Chiu W, Estes MK. Visualization of ordered genomic RNA and localization of transcriptional complexes in rotavirus. Nature. 1996;382:471–473. doi: 10.1038/382471a0. [DOI] [PubMed] [Google Scholar]
- Ramadevi N, Roy P. Bluetongue virus core protein VP4 has nucleoside triphosphate phosphohydrolase activity. J Gen Virol. 1998;79:2475–2480. doi: 10.1099/0022-1317-79-10-2475. [DOI] [PubMed] [Google Scholar]
- Ramadevi N, Burroughs NJ, Mertens PP, Jones IM, Roy P. Capping and methylation of mRNA by purified recombinant VP4 protein of bluetongue virus. Proc Natl Acad Sci USA. 1998a;95:13537–13542. doi: 10.1073/pnas.95.23.13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadevi N, Rodriguez J, Roy P. A leucine zipper-like domain is essential for dimerization and encapsidation of bluetongue virus nucleocapsid protein VP4. J Virol. 1998b;72:2983–2990. doi: 10.1128/jvi.72.4.2983-2990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratinier M, Caporale M, Golder M, Franzoni G, Allan K, Nunes SF, Armezzani A, Bayoumy A, Rixon F, Shaw A, Palmarini M. Identification and characterization of a novel non-structural protein of bluetongue virus. PLoS Pathog. 2011;7:e1002477. doi: 10.1371/journal.ppat.1002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescher U, Gerke V. S100A10/p11: family, friends and functions. Pflugers Arch. 2008;455:575–582. doi: 10.1007/s00424-007-0313-4. [DOI] [PubMed] [Google Scholar]
- Rossmann M. The canyon hypothesis. Hiding the host cell receptor attachment site on a viral surface from immune surveillance. J Biol Chem. 1989;264:14587–14590. [PubMed] [Google Scholar]
- Roy P. Bluetongue virus genetics and genome structure. Virus Res. 1989;13:179–206. doi: 10.1016/0168-1702(89)90015-4. [DOI] [PubMed] [Google Scholar]
- Roy P. Orbiviruses and their replication. In: Fields BN, editor. Fields Virology. 4th. Vol. 2. Lippincott-Raven Publishers: Philadelphia,PA: 2001. pp. 1835–1869. [Google Scholar]
- Roy P. Bluetongue virus: dissection of the polymerase complex. J Gen Virol. 2008;89:1789–1804. doi: 10.1099/vir.0.2008/002089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscanu S, Pascale F, Bourge M, Hemati B, Elhmouzi-Younes J, Urien C, Bonneau M, Takamatsu H, Hope J, Mertens P, Meyer G, et al. The double-stranded RNA bluetongue virus induces type I interferon in plasmacytoid dendritic cells via a MYD88-dependent TLR7/8-independent signaling pathway. J Virol. 2012;86:5817–5828. doi: 10.1128/JVI.06716-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rust MJ, Lakadamyali M, Zhang F, Zhuang X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat Struct Mol Biol. 2004;11:567–573. doi: 10.1038/nsmb769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt O, Teis D. The ESCRT machinery. Curr Biol. 2012;22:116–120. doi: 10.1016/j.cub.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schornberg K, Matsuyama S, Kabsch K, Delos S, Bouton A, White J. Role of endosomal cathepsins in entry mediated by the ebola virus glycoprotein. J Virol. 2006;80:4174–4178. doi: 10.1128/JVI.80.8.4174-4178.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settembre EC, Chen JZ, Dormitzer PR, Grigorieff N, Harrison SC. Atomic model of an infectious rotavirus particle. EMBO J. 2011;30:408–416. doi: 10.1038/emboj.2010.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton MR, Sawaya MR, Ellenberger T, Wigley DB. Crystal structure of T7 gene 4 ring helicase indicates a mechanism for sequential hydrolysis of nucleotides. Cell. 2000;101:589–600. doi: 10.1016/s0092-8674(00)80871-5. [DOI] [PubMed] [Google Scholar]
- Spillmann D. Heparan sulfate: anchor for viral intruders? Biochimie. 2001;83:811–817. doi: 10.1016/s0300-9084(01)01290-1. [DOI] [PubMed] [Google Scholar]
- Staden V, Stoltz MA, Huismans H. Expression of nonstructural protein NS3 of African horsesickness virus (AHSV): evidence for a cytotoxic effect of NS3 in insect cells, and characterization of the gene products in AHSV infected Vero cells. Arch Virol. 1995;140:289–306. doi: 10.1007/BF01309863. [DOI] [PubMed] [Google Scholar]
- Stäuber N, Sutton G, Monastyrskaya K, Monastyrskaya Katherine Roy P. Bluetongue virus VP6 protein binds ATP and exhibits an RNA-dependent ATPase function and a helicase activity that catalyze the unwinding of double-stranded RNA substrates. J Virol. 1997;71:7220–7226. doi: 10.1128/jvi.71.10.7220-7226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart ME, Roy P. Role of cellular caspases, nuclear factor-kappa B and interferon regulatory factors in Bluetongue virus infection and cell fate. Virol J. 2010;7:362. doi: 10.1186/1743-422X-7-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton G, Grimes JM, Stuart DI, Roy P. Bluetongue virus VP4 is an RNA-capping assembly line. Nat Struct Mol Biol. 2007;14:449–451. doi: 10.1038/nsmb1225. [DOI] [PubMed] [Google Scholar]
- Tan B, Nason E, Staeuber N, Monastryrskaya K, Roy P, Jiang W. RGD tripeptide of bluetongue virus VP7 protein is responsible for core attachment to culicoides cells. J Virol. 2001;75:3937–3947. doi: 10.1128/JVI.75.8.3937-3947.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Farsetta DL, Nibert ML, Harrison SC. RNA synthesis in a cage - structural studies of reovirus polymerase lambda3. Cell. 2002;111:733–745. doi: 10.1016/s0092-8674(02)01110-8. [DOI] [PubMed] [Google Scholar]
- Taraporewala ZF, Chen D, Patton JT. Multimers of the bluetongue virus nonstructural protein, NS2, possess nucleotidyl phosphatase activity: similarities between NS2 and rotavirus NSP2. Virology. 2001;280:221–231. doi: 10.1006/viro.2000.0764. [DOI] [PubMed] [Google Scholar]
- Theron J, Nel LH. Stable protein-RNA interaction involves the terminal domains of bluetongue virus mRNA, but not the terminally conserved sequences. Virology. 1997;229:134–142. doi: 10.1006/viro.1996.8389. [DOI] [PubMed] [Google Scholar]
- Thomas CP, Booth TF, Roy P. Synthesis of bluetongue virus-encoded phosphoprotein and formation of inclusion bodies by recombinant baculovirus in insect cells: it binds the single-stranded RNA species. J Gen Virol. 1990;71:2073–2083. doi: 10.1099/0022-1317-71-9-2073. [DOI] [PubMed] [Google Scholar]
- Urakawa T, Roy P. Bluetongue virus tubules made in insect cells by recombinant baculoviruses: expression of the NS1 gene of bluetongue virus serotype 10. J Virol. 1988;62:3919–3927. doi: 10.1128/jvi.62.11.3919-3927.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa T, Ritter DG, Roy P. Expression of targets RNA segment and synthesis of VP1 protein of bluetongue virus in insect cells by recombinant baculovirus: association of VP1 protein with RNA polymerase activity. Nucleic Acids Res. 1989;17:7395–7401. doi: 10.1093/nar/17.18.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk AA, Huismans H. The in vitro activation and further characterization of the bluetongue virus-associated transcriptase. Virology. 1980;104:347–356. doi: 10.1016/0042-6822(80)90339-6. [DOI] [PubMed] [Google Scholar]
- Van Dijk AA, Huismans H. In vitro transcription and translation of bluetongue virus mRNA. J Gen Virol. 1988;69(Pt 3):573–581. doi: 10.1099/0022-1317-69-3-573. [DOI] [PubMed] [Google Scholar]
- Vende P, Piron M, Castagné N, Castagne N. Efficient translation of rotavirus mRNA requires simultaneous interaction of NSP3 with the eukaryotic translation initiation factor eIF4G and the mRNA 3′ end. J Virol. 2000;74:7064–7071. doi: 10.1128/jvi.74.15.7064-7071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan S, Gershowitz A, Moss B. Modification of the 5′ end of mRNA. J Biol Chem. 1980;255:903–908. [PubMed] [Google Scholar]
- Verwoerd DW, Els HJ, De Villiers E-M, Huismans H. Structure of the bluetongue virus capsid. J Virol. 1972;10:783–794. doi: 10.1128/jvi.10.4.783-794.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed AA, Freed EO. Lipids and membrane microdomains in HIV-1 replication. Virus Res. 2009;143:162–176. doi: 10.1016/j.virusres.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler SJ, McHolland LE. Susceptibilities of 14 cell lines to bluetongue virus infection. J Clin Microbiol. 1988;26:2324–2327. doi: 10.1128/jcm.26.11.2324-2327.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrfritz J, Boyce M, Mirza S, Roy P. Reconstitution of bluetongue virus polymerase activity from isolated domains based on a three-dimensional structural model. Biopolymers. 2007;86:83–94. doi: 10.1002/bip.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenhorn W, Hinz A, Gaudin Y. Virus membrane fusion. FEBS Lett. 2007;581:2150–2155. doi: 10.1016/j.febslet.2007.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel R, Schweizer J, Stahl H. Simian virus 40 T-antigen DNA helicase is a hexamer which forms a binary complex during bidirectional unwinding from the viral origin of DNA replication. J Virol. 1992;66:804–815. doi: 10.1128/jvi.66.2.804-815.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Delos SE, Brecher M, Schornberg K. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit Rev Biochem Mol Biol. 2008;43:189–219. doi: 10.1080/10409230802058320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirblich C, Bhattacharya B, Roy P. Nonstructural protein 3 of bluetongue virus assists virus release by recruiting ESCRT-I protein Tsg101. J Virol. 2006;80:460–473. doi: 10.1128/JVI.80.1.460-473.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Chen SY, Iwata H, Compans RW, Roy P. Multiple glycoproteins synthesized by the smallest RNA segment (S10) of bluetongue virus. J Virol. 1992;66:7104–7112. doi: 10.1128/jvi.66.12.7104-7112.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder JD, Trask SD, Vo TP, Binka M, Feng N, Harrison SC, Greenberg HB, Dormitzer PR. VP5* rearranges when rotavirus uncoats. J Virol. 2009;83:11372–11377. doi: 10.1128/JVI.01228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XX, Boyce M, Bhattacharya B, Zhang XX, Schein S, Roy P, Zhou ZH. Bluetongue virus coat protein VP2 contains sialic acid-binding domains, and VP5 resembles enveloped virus fusion proteins. Proc Natl Acad Sci U S A. 2010;107:6292–6297. doi: 10.1073/pnas.0913403107. [DOI] [PMC free article] [PubMed] [Google Scholar]