Abstract
Type I interferons (IFNs) have diverse effects on innate and adaptive immune cells during infection with viruses, bacteria, parasites and fungi, directly and/or through the induction of other mediators. Type I IFNs are important for host defence against viruses. However, more recently, they have been shown to cause immune pathology in some acute viral infections, such as influenza virus, and, conversely, they can lead to immune suppression during chronic viral infections, such as lymphocytic choriomeningitis virus. During bacterial infections, type I IFNs may be required early and at low levels to initiate cell-mediated immune responses. High concentrations of type I IFNs may block B cell responses or lead to the production of immunosuppressive molecules and also reduce the responsiveness of macrophages to activation by IFNγ, as shown for infections with Listeria monocytogenes and Mycobacterium tuberculosis. Recent studies in experimental models of tuberculosis have demonstrated that prostaglandin E2 and interleukin-1 inhibit type I IFN expression and the downstream effects, demonstrating the cross-regulatory network of cytokines that operates during infectious diseases to provide protection with minimum host damage.
There are three distinct interferon (IFN) families. The type I IFN family is a multigene cytokine family, consisting of 13 (in humans, 14 in mice) partially homologous IFNα subtypes, a single IFNβ, as well as several other poorly defined single gene products including IFNε, IFNτ, IFNκ, IFNω, IFNδ and IFNζ1. The type II IFN family consists of the single gene product, IFNγ, that is predominantly produced by T cells and natural killer (NK) cells and can act on a broad range of cell types that express the IFNγ receptor (IFNγR) 2. The type III IFN family comprises IFNλ1, λ2 and λ3 (also known as IL-29, IL-28A, and IL-28B, respectively) and the recently identified IFNλ4 3,4, which have similar functions to cytokines of the type I IFN family but restricted activity as expression of their receptor is largely restricted to epithelial cell surfaces5; indeed, immune cells are largely unresponsive to IFNλ (reviewed by 5, 6). This Review focuses on IFNα and IFNβ, which are the most well-defined and broadly expressed type I IFNs, hereafter referred to as IFNα/β. These cytokines are best known for their ability to induce an antiviral state in both infected cells and uninfected bystander cells, through the induction of a programme of gene transcription that results in the interference of multiple stages of the viral replication cycle through various mechanisms 7. However, IFNα/β have numerous additional functions that influence innate and adaptive immune responses not only to viruses but also to bacterial and other pathogens. The outcome of the IFNα/β response during infectious disease is highly context dependent. Different conditions that are induced during specific infections affect when and where IFNα/β signals are delivered, as well as the signalling pathways that are triggered downstream of the type I IFN receptor (IFNAR). This in turn influences the IFN-stimulated genes (ISGs) that are activated or repressed. This can lead to beneficial or detrimental outcomes for the host. In this Review, we discuss IFNα/β-mediated effects on the host response during various infectious diseases and the mechanisms involved in conferring these effects.
Type I IFN production and signalling
Induction of IFNα/β production
Almost all cells in the body can produce IFNα/β, usually in response to stimulation by microbial products of receptors known as pathogen recognition receptors (PRR), located on the cell surface, in the cytosol or in endosomal compartments. These receptors recognize foreign and self nucleic acids (which are generally not found in the cytosol) as well as a limited number of other non-nucleic acid pathogen-associated molecular patterns (PAMPs). The RNA helicases retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) are the main cytosolic receptors that are responsible for the recognition of RNA, and may also recognize certain AT-rich DNA motifs, although this is controversial (reviewed in 8), and are highly associated with the induction of type I IFNs (Figure 1). Other classes of DNA in the cytosol can be recognized by various receptors, including the DNA-dependent activator of IFN regulatory factors (DAI; also known as ZBP1), the DEAD box and DEAH (DExD/H box) helicases and the recently described cytosolic GAMP synthase (cGAS) (reviewed in 8 and 9), all of which are highly associated with the induction of type I IFN production. Finally, the molecular sensors nucleotide-binding oligomerization domain containing protein 1 (NOD1) and NOD2 are expressed by various cell types and recognize both non-nucleic acid and nucleic acid ligands that can lead to IFNα/β production 10–12(reviewed in 13).
Figure 1. Pathways of type I interferon induction and receptor signalling.
Recognition of microbial products by a range of cell surface and intracellular pattern recognition receptors including TLRs and RIG-I can lead to type I IFN induction in cells, mediated by a number of distinct signalling pathways. Upon binding of type I IFNs to the IFNAR (IFNαβ receptor) multiple downstream signalling pathways can be induced, leading to a diverse range of biological effects. The canonical STAT1/STAT2/IRF9 (also known as the ISGF3 complex) signalling complex binds to ISRE elements in gene promoters, leading to induction of a large number of IFN-stimulated genes (ISGs). Type I IFNs can also signal through STAT1 homodimers, more commonly associated with the IFNγ signalling pathway. Other STAT heterodimers and homodimers may also be activated downstream, including STAT3, STAT4 and STAT5. Other signalling pathways that do not rely on JAK/STAT activity may also be activated, including mitogen activated protein kinases (MAPKs) and the phosphoinositide 3-kinase (PI3K) pathway, that leads to diverse effects on the cell.
In addition to these cytosolic receptors, several Toll-like receptors (TLRs) activate pathways that lead to IFNα/β production. Of the cell surface TLRs, TLR4 recognizing lipopolysaccharide (LPS) from bacteria is the most potent type I IFN inducer, signalling through the adaptor protein TIR-domain-containing adaptor protein inducing IFNβ (TRIF). In endosomal compartments, TLR3, TLR7 and TLR9 respond to double-stranded RNA, single-stranded RNA and unmethylated CpG DNA, respectively 14.
Diverse pathways downstream of all these receptors transduce signals that converge on a few key molecules, such as the IFN regulatory factor (IRF) family of transcription factors, that activate the transcription of genes encoding IFNα/β. In most cases, IRF3 and IRF7 are the fundamental IRFs required, although others such as IRF1, IRF5 and IRF8 can also induce IFN gene transcription. The central tenet of IFNα/β induction is that IFNB and IFNA4 genes are induced in an initial wave of transcription that relies on IRF3. This initial IFN burst triggers the transcription of IRF7 that then mediates a positive-feedback loop, leading to the induction of a second wave of transcription of genes including additional IFNα subtypes 15,16. Nuclear factor-κB (NF-κB) is also required as a co-factor, although there is some disagreement regarding the importance of this pathway in IFNα/β induction 15. Immediately upstream of IRFs, the kinases IκB kinase-ε (IKKε) and TANK-binding kinase 1 (TBK1) are responsible for the phosphorylation of IRF3 and IRF7. The cytosolic RNA sensors RIG-I and MDA5 rely on the adaptor mitochondrial antiviral signalling (MAVS; also known as IPS1 or VISA) to activate TBK1, whereas stimulator of IFN genes (STING) is an important mediator for much of the response to cytosolic DNA 9. TLR3 and TLR4 use the adaptor molecule TRIF, which associates with TBK1, leading to the activation of IRF3. TLR7 and TLR9 are preferentially expressed by plasmacytoid dendritic cells (pDCs) and transduce signals for IFNα/β induction through myeloid differentiation primary-response protein 88 (MyD88) rather than TRIF, and in pDCs, the potent production of IFNα/β is due to constitutively expressed IRF7 and retention of the MyD88–IRF7 complex in endosomes 14,16.
Type I IFN signalling and the induction of ISGs
IFNβ and all IFNα subtypes bind and signal through a heterodimeric transmembrane receptor composed of the IFNAR1 and IFNAR2 subunits. Ligation of the IFNAR activates the receptor associated protein tyrosine kinases Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2). In the canonical pathway of IFNα/β signalling, activated JAK1 and TYK2 phosphorylate signal transducer and activator of transcription 1 (STAT1) and STAT2 present in the cytosol, leading to their dimerization, nuclear translocation and binding to IRF9 to form IFN-stimulated gene factor 3 (ISGF3). The ISGF3 complex then binds to IFN-stimulated response elements (ISREs) upstream of ISGs, leading to the activation of their transcription (reviewed in 17). In this manner, IFNα/β induces the expression of several hundred ISGs, a large number of which act to induce an antiviral state within the cell.
However, IFNα/β signalling is not limited to this canonical pathway. In addition to signalling through STAT1–STAT2 heterodimers, IFNα/β can signal through STAT1 homodimers, which are more commonly associated with IFNγ signalling, that bind to γ-activated sequences (GAS) in gene promoters 17. IFNα/β can also signal through other STATs that are usually associated with other cytokine signalling pathways, including STAT3, STAT4 and STAT5. The phosphoinositide 3-kinase (PI3K)–mammalian target of rapamycin (mTOR) pathway and multiple mitogen-activated protein kinase (MAPK) pathways can also be activated downstream of the IFNAR. This diversity of signalling pathways may in part explain the broad effects of IFNα/β signalling, as it allows for the transcription of a broad range of genes beyond those dedicated to viral restriction (reviewed in 17). This includes genes that encode cytokines and chemokines, antibacterial effectors, pro- and anti-apoptotic molecules and molecules involved in metabolic processes18 (Figure 1).
Protective effects in viral infection
Virus restriction in vitro
IFNs were named for their ability to restrict viral replication (“interference”) in vertebrate cells, which has now been shown for many viruses both in human and mouse cells and cell lines7. The ability of IFNα/β to restrict viral replication is largely attributed to the induction of ISGs. These genes are either expressed constitutively in cells in response to low levels of IFNα/β in the microenvironment, or, more often, in response to IFNα/β induced by infection, where their induction promotes an antiviral state in bystander cells and restricts the viral replicative cycle in already infected cells 7. The fact that most viruses devote a part of their limited genome to mechanisms that perturb IFNα/β production and/or IFNα/β signalling to stop these ISGs being induced illustrates the importance of this cytokine family in host cell protection from viral infection 19.
The mechanisms of action of many of these ISGs have been described, some of the most well-known are myxovirus resistance 1 (MX1), IFN-inducible dsRNA-dependent protein kinase (PKR), 2’,5’-oligoadenylate synthetase (OAS), IFN-induced transmembrane protein (IFITM), apolipoprotein B mRNA-editing enzyme, catalytic polypeptide 1 (APOBEC1) 7 and the tripartite motif-containing (TRIM) family of molecules 7,20. These ISGs have been reviewed in great detail elsewhere, therefore are not discussed further here 7. However, it is worth noting interesting recent work aimed at understanding this acute ISG response at a broader level by defining the programmes of ISGs that are induced by different viruses (see 21). These studies reveal that specific sets of ISGs induced are effective in different viral infections.
Virus restriction in vivo
The study of IFNAR1-deficient mice provided definitive proof that IFNα/β mediates potent protection from viruses in vivo22, although previous studies in which exogenous IFN was experimentally used to treat viral infections were also strongly suggestive 23. Ifnar1-/- mice proved to be susceptible to infection with four different viruses — vesicular stomatitis virus (VSV), Semliki Forest virus, vaccinia virus and lymphocytic choriomeningitis virus (LCMV) — a list that interestingly did not include influenza virus. Subsequently, Stat1-/- mice were shown to be highly susceptible to influenza virus whereas the role of the IFNAR1 was less clear24–27.
This discrepancy was solved when IFNAR1/IFNλR double deficient mice were shown to be unable to control influenza virus, whereas the singly deficient mice had a mild phenotype28,29. This suggests that there is redundancy between the type I and type III IFN systems, which both require STAT1 downstream of their respective receptors. Only STAT1-deficient or IFNAR1/IFNλR double deficient mice lack all IFN responsiveness in both haematopoietic and epithelial cells, whereas IFNAR1 single knockout mice retain type III IFN signalling in the epithelium and are able to control influenza virus infection to a degree 29. However, it is only when both Type I and Type III IFN signalling in epithelial cells is deficient that mice succumb to influenza infection30.
Naturally occurring mutations in JAK and STAT genes in humans provided further evidence for the importance of IFNs in host protection from viruses, as well as other types of pathogen, although the relative contribution of type I versus type III IFNs has been unclear, given that these mutations affect signalling downstream of both receptors 31,32. That IFNα/β and IFNλ pathways often intersect in antiviral responses is supported by studies of patients with hepatitis C virus (HCV), in which single nucleotide polymorphisms (SNPs) in the IL28 locus (encoding IFNλ genes) are predictive of a successful response to treatment with IFNα (or the drug ribavirin), associated with a sustained virological response and clearance of the virus 33–36. Recently, a new type III IFN (IFNλ4) has been identified and associated with impaired spontaneous clearance of HCV3,4.
It has recently been shown in rhesus macaques during simian immunodeficiency virus (SIV) transmission and acute infection that blockade of IFNAR signalling caused reduced antiviral gene expression, increased SIV reservoir size and accelerated CD4+ T cell depletion with progression to AIDS despite decreased T cell activation37. Conversely, IFNα2a administration initially upregulated the expression of antiviral genes and prevented systemic infection in these animals, although continued IFNα2a treatment induced desensitization to IFNα/β and decreased antiviral gene expression, enabling infection with increased SIV reservoir size and accelerated CD4+ T cell loss. This study emphasizes that the timing of IFN-induced innate responses in acute SIV infection profoundly affects the overall disease course and outweighs the detrimental consequences of increased immune activation37, which is likely to be the case with most infections.
To date, relatively few downstream effector ISGs (that is, molecules downstream of but not involved in the IFN signalling cascade) have been shown to control viral infection in humans. However, recent studies 38,39 found that the ISG IFITM3 controls influenza virus infection in mice in vivo and that an allele of IFITM3 that renders the molecule ineffective at restricting the virus in cells in vitro was over-represented in patients requiring hospitalization due to influenza virus infection38 and among patients suffering severe pandemic influenza virus39. The ISG MX1 (also known as MXA in humans) also has important antiviral functions in influenza virus infection. Most inbred mouse strains have deletions or point mutations in Mx140, and reintroduction of a functional gene into deficient mouse strains markedly increases their resistance to influenza virus 41. In keeping with this, it has been shown that type I IFNs can provide protection against wild-type influenza virus in the presence of Mx126. However, it should be noted that the strongest phenotype of susceptibility to influenza virus infection is observed in mice carrying deletions in both the type I and type III IFN receptors 29. Human MX1 has antiviral effects in vitro, but polymorphisms in the MX1 gene have not been investigated for their impact on influenza virus susceptibility within the human population 42.
Enhanced action of dendritic cells and monocytes
The effects of IFNα/β on the host response to infection are not limited to the acute cell-intrinsic antiviral response described above. IFNα/β have effects on both the innate and adaptive cellular immune response, in contrast to type III IFNs, which largely limit their effects to non-haematopoietic cells through restricted expression of the IFNλR. The action of IFNα/β includes effects on myeloid cells, B cells, T cells and natural killer (NK) cells that act to enhance the immune response, more effectively resolve viral infection and improve the generation of memory responses for reacting to future viral challenge.
Myriad studies in both human and mouse systems indicate that IFNα/β are involved at various stages in the activation of adaptive immune cell responses by dendritic cells (DCs), either promoting or restricting them depending on the context. IFNα/β variously inhibits or promotes the differentiation of precursors into DCs 43–46 and some viruses, such as measles virus and LCMV, can exploit this property to reduce the DC pool47. However, IFNα/β seems to have an activating effect on immature but committed DCs, enhancing their cell surface expression of MHC molecules and co-stimulatory molecules, such as CD80 and CD86, which is associated with increased ability to stimulate T cells47–49. It has also been observed that IFNα/β enhance the ability of DCs to cross-present antigens during viral infections, such as vaccinia virus and LCMV infections50–52. IFNα/β may also enhance the migration of DCs to lymph nodes through their upregulation of chemokine receptor expression, thus promoting T cell activation 53,54.
DCs are potent producers of IL-12, which is crucial for driving T helper 1 (Th1)-type responses during some bacterial and viral infections and important for IFNγ production by T cells and NK cells. In some settings, IFNα/β signalling has been shown to be necessary for IL-12 production by DCs following PRR stimulation 55. However, high but physiological levels of IFNα/β strongly inhibit IL-12 production during murine cytomegalovirus (MCMV) and LCMV infections56,57. This suppression of IL-12 may have developed to favour optimal antiviral T and NK cell cytotoxic responses, while limiting the pathological effects of excessive IL-12 production56–59. However, in other situations in which IL-12 production is crucial to the host response, such as during infection with intracellular bacteria, certain pathogens may be able to exploit the IL-12 suppressive function of IFNα/β for their own benefit (discussed below).
Promotion of CD4+ and CD8+ T cell responses
In addition to effects on DCs that drive or inhibit T cell activation as a downstream consequence, IFNα/β can act directly on both CD4+ and CD8+ T cells to influence their function. Both inhibitory and stimulatory effects on T cell survival and proliferation, cytokine (IFNγ) production, cytotoxic function and memory formation by IFNα/β have all been described. Detailed dissection of these effects has revealed that differential levels and activation of STAT molecules downstream of the IFNAR controls these diverse outcomes.
In CD4+ T cells, IFNα/β enhance their ability to help B cells 60, their survival and thus their expansion in response to viral (LCMV) but not bacterial infection 61, and in human T cells IFNα/β promote the differentiation of IFNγ-producing Th1 cells 62. In LCMV infection, depletion of CD4+ T cells prevented lethality in LCMV-infected STAT1-deficient mice and was associated with a reduction in tissue immune pathology63. In West Nile virus (WNV) infection, IFNAR signalling controls CD4+ regulatory T cell differentiation, which suggests further effects on CD4+ T cell differentiation and function64. In addition, lymphocyte responses by type I IFNs may be reduced as it has been shown that type I IFNs can inhibit lymphocyte egress during LCMV infection65.
Although IFNα/β can promote growth inhibitory signals in CD8+ T cells 66–68, in line with the known, STAT1-dependent, anti-proliferative effects of IFNα/β 69–71, in activated CD8+ T cells and during viral (LCMV and VSV) infection, IFNα/β can also promote the survival and expansion of the CD8+ T cell pool 72–76. A possible explanation for these opposing findings may relate to differential STAT signalling downstream of the IFNAR, as in STAT1-deficient T cells, IFNα/β provide pro-survival and mitogenic signals, possibly through STAT3 and STAT5, rather than the anti-proliferative effects of signalling through STAT1 71,77. Furthermore, activated CD8+ T cells ‘escape’ the antiproliferative effects of IFNα/β during viral (LCMV) infection by expressing lower total levels of STAT1 78. With regard to CD8+ T cell function, cytotoxicity is positively regulated by IFNα/β 75,79,80, and IFNγ production is both positively 81,82 and negatively affected by IFNα/β 83. This dichotomous outcome depends on relative levels of STATs, with dominant STAT1 driving inhibition of IFNγ production but STAT4 activation promoting IFNγ production82,83. Therefore, the levels of IFNα/β expressed during a specific infection, the relative strength of signalling pathways induced and the kinetics of this signalling seem to determine the nature of the CD8+ T cell response that develops 76,84. Indeed, it is likely that both the quantity and the timing of type I IFN delivery may be crucial for consequent adaptive immune responses to infection, as previously reported85.
IFNα/β also influence the differentiation and function of memory CD8+ T cells. By affecting the initial expansion of the T cell pool following infection with viruses, such as vaccinia virus, VSV and LCMV, IFNα/β also determine the downstream memory T cell pool 74,84,86. Furthermore, IFNα/β support memory T cell effector function and trafficking upon secondary infection, including driving cytotoxicity of circulating memory T cells that are recruited to the lungs during respiratory infection with Sendai virus 87; promoting chemokine production for correct trafficking of central memory T cells during recall responses to LCMV88; and driving inflammatory monocytes to produce factors such as IL-15 and IL-18 that support memory CD8+ T cell survival and function in infections including MCMV 89. Finally, two recent studies indicate that type I IFNs can protect T cells from NK cell-mediated killing, through the induction of expression of inhibitory NK cell receptor ligands on the target T cells90,91.
Enhancement of NK cell responses
Similarly to T cells, IFNα/β promote the function and survival of NK cells, through both direct and indirect means. The inflammatory conditions induced by specific viral infections seem to dictate the degree to which direct or indirect effects of IFNα/β modulate NK cell function and which NK cell function is affected. During both influenza virus92 and vaccinia virus infections93, the direct action of IFNα/β on NK cells is required for their activation and expression of cytolytic effector functions and IFNγ production. By contrast, in MCMV infection, IFNα/β signalling through STAT1 is required for NK cell accumulation and cytolytic function but not for IFNγ production 94. These effects were also described to be mediated indirectly through IL-15, with similar findings in TLR-stimulated mice95, although others reported no IL-15 requirement96. A recent study investigating the transcriptional response of NK cells and DCs during MCMV infection supports a largely IL-15-dependent role for IFNα/β in this infection97. In this study, the NK cell transcriptional response revealed a relatively weak IFNα/β responsive profile but a distinct and prolific IL-15-dependent response, whereas DCs displayed high levels of IFNα/β-inducible gene expression97.
As with T cells, the ability of IFNα/β to induce or restrict NK cell IFNγ production is related to differential STAT1 and STAT4 signalling. High levels of STAT1-dependent signalling inhibits NK cell IFNγ production, whereas high basal levels of STAT4 prime NK cells for IFNγ production83,98. Accordingly, the kinetics and levels of IFNα/β production and signalling during infection with viruses such as LCMV and MCMV will modulate the NK cell IFNγ response99.
Enhancement of B cell responses
B cells have an important role in the resolution of many viral infections, largely through the production of neutralizing antibodies. Whereas some studies100–102 indicate that IFNα/β may impair the survival and development of precursor and immature B cells, committed B cells seem to benefit from the presence of IFNα/β for various functions.
Similarly to findings with viral protein antigens60,103,104, IFNs can promote B cell activation and antibody responses, including class switching, during viral infection. Within the first 48 hours following influenza viral infection, early activation of B cells was mediated by IFNAR signalling, resulting in the upregulation of activation markers and alteration of the transcriptional response105–107. This response involved only the respiratory tract B cells and not the systemic B cells105,106 and affected both the magnitude and quality of the antibody response105. IFNα/β were also reported to ‘fine-tune’ B cell antibody class switching between IgG subtypes during influenza virus infection108. Interestingly, although IFNα/β appear to be beneficial for the antibody response early in infection, at least one study found that at late time-points post-influenza virus infection antibody titres were higher in IFNAR-deficient mice than in wild-type mice, although the biology underlying this was not explored27.
Similarly to influenza virus infection, IFNα/β are important for early B cell responses during VSV infection109 and for class switching110. Likewise, during WNV infection, IFNα/β are required for B cell activation in the lymph nodes but not in the spleen of infected animals111. Moreover, recent work in VSV infection shows that rather than acting as targets of IFNα/β, B cells in the lymph nodes produced lymphotoxin to drive a protective macrophage phenotype. In the absence of this, host protective IFNα/β were not produced and mice succumbed to VSV infection112.
Detrimental effects in viral infection
Chronic viral infection
As described, IFNs contribute to antiviral protection through induction of an ISG-based cellular antiviral programme and through enhancing immune responses for efficient termination of infection. However, there is an increasing appreciation that IFNα/β can also be harmful in virus infection, either by immunosuppressive effects that impede viral control 113 or by triggering inflammation and tissue damage that aggravate disease 114.
Comparisons of SIV infection in primate species that develop AIDS and those without disease symptoms indicate that strong IFNα/β responses only occurred in pathogenic infection in macaques, whereas natural SIV hosts without disease progression had weaker IFN responses115,116. Similar findings were made in HIV patients: rapid progressors show stronger IFN signatures than viraemic non-progressors117. These studies suggest a link between sustained IFNα/β levels and disease progression, yet the mechanisms involved are as yet unclear. One possibility is that IFN-induced chronic inflammation and activation of the immune system facilitate the recruitment of target CD4+ T cells and thereby the spread of HIV. Another possibility is that the immunosuppressive effect of IFNα/β 113 reduces T cell expansion (through STAT1 signalling) and their ability to restrict HIV. The negative effects of IFNα/β on CD8+ T cell proliferation may depend on the timing of the IFN exposure. Exposure prior to antigenic stimulus is reported to be suppressive, whereas simultaneous exposure is stimulatory68. It was also demonstrated in mice that transfer of antigen-specific CD8+ T cells or treatment with polyinosinic–polycytidylic acid (polyI:C) cause IFNα/β-dependent apoptosis and thus attrition of bystander CD8+ T cells 118. A similar type I and type III IFN-dependent suppression was shown in vitro for human CD4+ T cells co-cultured with RSV-infected monocyte-derived DCs119. Whereas the signalling mechanisms that control whether T cell expansion is limited following exposure to IFNα/β are relatively well described (see references 78,120 as examples), the outcomes for viral infection of this IFNα/β-mediated suppression require more investigation.
TNF-related apoptosis-inducing ligand (TRAIL) and its receptor death receptor 5 (DR5) have been suggested as candidate molecules linking high IFN levels to lymphocyte death. For example, in HIV-infected individuals, IFNα/β expression by pDCs and the expression levels of TRAIL and DR5 in tonsil tissue were higher in progressors than non-progressors 121. Similarly, an in vitro study showed that HIV caused IFNα/β-mediated upregulation of TRAIL expression by pDCs, enabling them to induce TRAIL-dependent CD4+ T cell apoptosis 122. Expression of DR5 was found to be increased on CD4+ T cells in the blood of HIV patients123, and also B cells undergo apoptosis in a TRAIL-dependent manner in HIV infection 124. In another chronic viral infection (HCV), it was shown in the human hepatoma cell line Huh-7 that caspase 8, DR5 or TRAIL act alone or in concert to increase apoptosis in response to exogenously added type I IFNs125–127. However, the extent to which these mechanisms are mediating immune suppression and/or pathology in patients with hepatitis will require further investigation.
Two recent in vivo studies have identified suppressive mechanisms involved in the harmful IFNα/β effects in chronic viral infection128,129. Blocking of IFN signalling by antibodies or by receptor deficiency improved CD4+ T cell-mediated virus control in chronic infection with the LCMV clone 13. Furthermore, IFNα/β were shown to reduce T cell responses through the induction of immunosuppressive genes such as IL-10 and programmed cell death 1 ligand 1 (PDL1).
Acute viral infection
As discussed, both type I and type III IFNs contribute to protection against influenza virus. The disease-promoting effects of IFNα/β in acute infection such as influenza virus infection were more recently discovered and were perhaps more surprising given their well-established antiviral activities. It was shown that severe influenza virus infection is associated with TRAIL-mediated epithelial cell damage 130 and that IFNα/β can induce TRAIL expression by inflammatory monocytes131. Similarly, exposure to influenza virus induces TRAIL expression by human pDCs in vitro 132 but the involvement of IFNα/β was not assessed.
When mouse strains (MX1 deficient) were ranked by susceptibility to influenza virus and IFN levels were assessed, a stronger and more sustained IFNα/β signal was found in susceptible strains compared to resistant strains, even at early time points where no difference in virus titres were detected 114. Higher pDC numbers and high levels of pro-inflammatory cytokines were found in susceptible strains compared with resistant strains, and blocking the IFNα/β signal by receptor deficiency or by removing pDCs in susceptible strains reduced inflammation and lung damage, resulting in improved survival114. The pathogenic mechanism downstream of type I IFNs was the upregulation of expression of TRAIL by monocytes and of DR5 by epithelial cells 114. Thus, excessive levels of IFNα/β can contribute to immunopathology in severe influenza virus infection, mainly by inducing immune cell-mediated tissue damage, although it remains to be seen what occurs in MX1-sufficient mice.
Besides TRAIL, expression of the apoptosis-inducing ligand CD95 ligand (also known as FASL) was shown to be upregulated in severe influenza virus infection in an IFN-dependent manner, and blocking the CD95–CD95L interaction or a functional mutation in the CD95L gene reduced mortality after high-dose influenza virus infection 133. In contrast to the effects in chronic viral infection, it seems that most of the disease-promoting effects of IFNα/β in acute influenza virus infection involve the induction of immunopathology rather than the suppression of the antiviral adaptive immune response, as virus titres are mostly unaffected. However, it has been shown134 that IFNα/β-dependent PDL1 expression by influenza virus-infected airway epithelial cells suppresses PD1+ T cell function. Similarly, influenza virus-induced TRAIL expression by mouse CD8+ T cells controls the magnitude of the CD8+ T cell response135 (although the role of IFNs in this mechanism was not assessed), indicating that immunosuppressive pathways similar to those in chronic viral infection are also active in acute infections such as influenza virus.
In conclusion, a theme emerges of IFNα/β-mediated upregulation of expression of apoptosis-inducing proteins that, if expressed by non-haematopoietic body cells, mediates tissue damage. The same molecules, when induced by IFNα/β on immune cells, can contribute to immunosuppression in a similar manner to PDL1 and IL-10. Therefore, depending on the pathogen, the host and the context, type I IFNs may have protective effects in viral infection or contribute to immunosuppression or immunopathology (Figure 2).
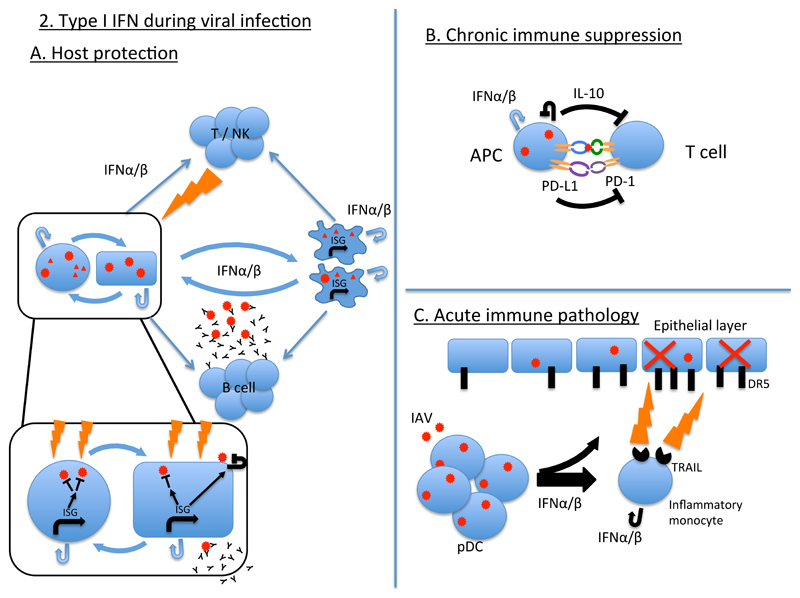
Figure 2. Type I interferons during viral infection.
A. Infected cells of the vertebrate body produce type I IFNs in response to viral infection and/or contacting viral products (box, left side). Feedback of type I IFNs onto infected and bystander cells leads to the induction of IFN-stimulated genes (ISGs) that act to block the viral replicative cycle (inset box). Type I IFNs are also produced by, and act on, innate immune cells including professional antigen presenting cells (APCs), in response to viral infection and viral products. Type I IFNs acting on APCs can enhance their antigen presenting function as well as enhancing the antiviral function of adaptive cells including B cells, T cells and NK cells, which act to restrict viral infection through the production of antibody (B cells) and cytotoxic responses (T and NK cells). B. During chronic viral infection, type I IFNs can induce the production of immunosuppressive cytokines such as interleukin-10 (IL-10), together with expression on APCs of ligands, such as PDL1, for T cell inhibitory receptors such as PD1, leading to the suppression of T cell function and failure to clear infection. C. During acute viral infections such as influenza virus, type I IFN production by myeloid cells, such as plasmacytoid dendritic cells (pDCs) and inflammatory monocytes, leads to the upregulation of expression of the death receptor TRAIL on inflammatory monocytes and the TRAIL ligand DR5 on epithelial cells. TRAIL-expressing inflammatory monocytes then induce immune pathology and host morbidity and/or mortality through killing of epithelial cells.
Protective effects in bacterial infection
As seen in viral infection, IFNα/β can be protective or detrimental to the host during bacterial infection, in an infection-specific manner, although less is known about their role in bacterial infections than viral infections136. Immunity to intracellular bacteria relies on Th1 cell responses, which activate macrophages and other phagocytic cells to kill intracellular bacteria. By contrast, immunity to extracellular bacteria typically requires a combination of antibody responses, activation of phagocytic cells, such as neutrophils, and Th17 cell responses.
Many of the cytokines and chemokines responsible for orchestrating these responses are IFN inducible (mainly through IFNγ), as are many of the antibacterial effector molecules, such as indoleamine 2,3-dioxygenase (IDO), inducible nitric oxide synthase (iNOS), immunoresponsive genes and guanylate-binding proteins136. Conversely, under different conditions IFNα/β can inhibit many of these host antibacterial effector mechanisms, chemokines and pro-inflammatory cytokines. The mechanisms by which IFNα/β promotes host protection or susceptibility to bacterial pathogens are as yet not well defined, nor is it fully understood what determines whether a response will be protective or pathogenic.
Some of the earliest reports of a protective role of IFN were described in infection with Chlamydial species. Treatment of mice with exogenous IFN or IFN-inducing agents such as polyI:C protected mice from Chlamydia trachomatis infection137 and inhibited intracellular C. trachomatis replication in various human and mouse cell types138. This protection resulted from IDO-mediated depletion of intracellular L-tryptophan, thereby reducing its availability to intracellular pathogens for their survival139. IFNα/β may also be involved in protection from C. pneumonia, through co-operative interaction with IFNγ to induce antimicrobial effectors that suppress bacterial survival140,141. However, IFNα/β are not universally protective against Chlamydial species, as Ifnar1-/- mice are protected from C. muridarum infection, showing better survival and reduced bacterial loads compared with wild-type controls 142.
IFNα/β also protect macrophages and lung epithelial cells in vitro from infection with Legionella pneumophila, the causative agent of Legionnaire’s disease143–145. Ifnar1-/- macrophages had higher bacterial loads compared with wild-type cells144 and treatment of both cell types with IFNα/β restricted intracellular bacterial growth143–145. The mechanisms behind this were not fully elucidated but were STAT1, STAT2 and STAT3 independent in macrophages and were associated with polarization towards a classically activated M1 macrophage phenotype and induction of iNOS expression144. Similar inhibition of bacterial growth was observed in IFNαβ-treated human macrophages infected with Bacillus anthracis, suggesting a protective role for IFNα/β in anthrax146.
In addition to promoting the restriction of bacterial growth and bacterial killing within cells, IFNα/β may prevent or reduce cellular invasion by invasive gut bacteria, such as Shigella flexneri and Salmonella typhimurium, in which treatment of mice with IFN increased survival and reduced invasion of intestinal epithelial cells in vivo and fibroblasts in vitro147,148.
A protective role for IFNα/β has also been reported in mouse models of Group B Streptococcal (GBS), S. pneumonia, E. coli, H. pylori and S. pyogenes infections 12,149–151, as well as in a model of caecal ligation and puncture 152. In all of these infections, Ifnar1-/- mice had reduced survival compared with wild-type controls and/or increased bacterial growth. By contrast, type I IFNs have been shown to have adverse effects in a colon ascendens stent peritonitis model of peritoneal sepsis153.
In the case of the immune response to GBS, E. coli and S. pneumoniae, it was found that IFNα/β signalling contributed to the optimal activation of macrophages, in terms of their ability to produce TNF and nitric oxide (NO), although plasma TNF and IL-6 levels during in vivo infection were much higher in Ifnar1-/- mice, which may reflect greater inflammation due to the greater bacterial burden in the knockout mice or multiple effects of IFNα/β at the systemic versus local level 149. IFNα/β may also contribute to the production of host protective cytokines during S. typhimurium infection, inducing strong IFNγ production in an IL-12-independent manner, although the direct contribution of this response to host protection was not established 154.
Watanabe et al. defined a role for IFNα/β signalling downstream of NOD1 signalling in intestinal epithelial cells for protection from H. pylori. Although the mechanism of protection was not fully elucidated, impairment of chemokine and IFNγ production in the absence of IFNα/β signalling was implicated12. The importance of correct recruitment of host protective phagocytic cells by IFNα/β-dependent chemokine production was highlighted by results from a caecal ligation and puncture model of infection152. Ifnar1-/- mice in this model have decreased survival and elevated bacteraemia. This was associated with decreased levels of CXC-chemokine ligand 10 (CXCL10) and reduced neutrophil numbers and function. Treatment of Ifnar1-/- mice with recombinant CXCL10 rescued them from fatal infection and restored neutrophil function. Conversely, during subcutaneous S. pyogenes infection, Ifnar1-/- mice had increased tissue damage and reduced survival post-infection and this was associated with uncontrolled neutrophilia at the site of disease, although whether neutrophils had a detrimental role in this case was not confirmed152.
So, the induction of cell-intrinsic immunity for bacterial killing or prevention of bacterial invasion, regulation of chemokines, pro-inflammatory cytokines and phagocytic cells are all implicated as mechanisms by which IFNα/β suppresses bacterial infection, with the exact mechanisms involved being dependent on the pathogen.
Detrimental effects in bacterial infection
Perhaps the two best-described examples of a harmful role for IFNα/β are in infections with L. monocytogenes and M. tuberculosis. Both of which are intracellular pathogens that preferentially infect macrophages and require broadly similar immune responses for their control.
L. monocytogenes
Three groups initially described that Ifnar1-/- mice were resistant to L. monocytogenes infection 155–157, having better survival and lower spleen and liver bacterial loads post-infection compared with wild-type mice. The main mechanism attributed to this was reduced apoptotic cell death, particularly of lymphocytes, with IFNα/β sensitizing these cells to the L. monocytogenes virulence factor listeriolysin O (LLO) and resultant cell death in wild-type mice 156–158. This reduced cell death was also associated with lower levels of expression of IFN-inducible apoptosis-associated genes, such as TRAIL, p53 and death domain-associated protein 6, in infected Ifnar1-/- mice 157. Subsequent induction of immune suppressive cytokines, particularly IL-10, following this large scale apoptosis of lymphoid cells was suggested as the mechanism to explain how lymphocyte apoptosis led to the IFNα/β-dependent increase in susceptibility to infection158.
Increased expression of pro-apoptotic genes was also reported in infected Ifnar1-/- bone marrow-derived macrophages compared with wild-type cells157. Several other reports also suggest that macrophages are targets of IFNα/β-induced cell death following L. monocytogenes infection159–161. This can take the form of apoptosis that is STAT1 dependent but iNOS and PKR independent 159, or necrotic cell death that is iNOS dependent but TRAIL and PKR independent 160,161 and is related to STAT1-dependent breakdown of the cell plasma membrane 160. The death of myeloid cells may be involved in pathology in vivo, as Auerbuch et al. reported increased levels of host protective TNF- and iNOS-producing DCs (Tip-DCs) in Ifnar1-/- mice following L. monocytogenes infection155, and a role for Tip-DCs was suggested based on mice that selectively produce IFNβ162. Interestingly, CD11b+ DCs seem to be one of the main IFNβ-producing cells during L. monocytogenes infection163. This might suggest that the IFNα/β production is a method of self-regulation by immune cells that in this case is subverted by L. monocytogenes for its own advantage. However, whether Tip-DCs as well as CD11b+ DCs are themselves the targets of IFNα/β-induced cell death remains unclear.
A second important mechanism of host immune suppression by IFNα/β was elucidated in later studies. During infection with pathogens such as L. monocytogenes, activation of macrophages by T cell- and/or NK cell-derived IFNγ is crucial for inducing antimicrobial pathways and subsequent eradication of the intracellular bacteria136. Although IFNα/β can induce some of these antimicrobial pathways in particular circumstances, it has now been shown that during infection with L. monocytogenes, IFNα/β potently inhibit these pathways by blocking responsiveness of macrophages to IFNγ 164. This block in responsiveness results from downregulation of IFNγ receptor expression by macrophages164 owing to silencing of new Ifngr1 transcription by repressive transcriptional regulators 165.
Mycobacterium tuberculosis
Studies performed in patients and mouse models of infection collectively point to a detrimental role for IFNα/β during tuberculosis. Several papers reported decreased bacterial load and/or improved host survival in the absence of IFN signalling166–169, although this was not universally observed170 and there was not always concordance regarding bacterial load and survival data between different studies. It is likely that the differences between studies result from experimental protocol and the genetics of the host and M. tuberculosis strain used.
The importance of type I IFNs as a potentially detrimental factor during tuberculosis was revealed by studies of patient cohorts from the UK and South Africa171. Patients with active tuberculosis had a prominent whole blood IFNα/β transcriptional profile that correlated with the extent of radiographic disease and was diminished upon successful treatment171. Several other studies have since verified these findings in additional patient cohorts from Africa172,173 and Indonesia174, suggesting this IFN-inducible signature is broadly applicable to the human population.
IFNα/β overexpression during M. tuberculosis infection in experimental mouse models has provided additional robust evidence for the detrimental effects of the IFNα/β system during tuberculosis. Studies of infection with hyper-virulent M .tuberculosis strains showed a correlation between increased levels of IFNα/β and increased virulence166,167,169. Direct instillation of IFNα/β into the lungs during infection was also injurious to the host169. Similarly, enhanced induction of IFNα/β expression during M. tuberculosis infection via administration of a TLR3 ligand derivative led to increased severity of infection175,176. Likewise, deletion of a negative regulator of IFNα/β, mitogen-activated protein kinase kinase kinase 8 (MAP3K8; also known as TPL2), that acts downstream of TLRs led to increased levels of IFNα/β production and increased bacterial burdens177, which were abrogated in Map3k8-/-Ifnar1-/- double knockout mice during either M. tuberculosis or L. monocytogenes infection. Control of bacterial load in the Map3k8-/-Ifnar1-/- mice was correlated with reduced IL-10 and increased IL-12 levels in the serum. Finally, concurrent co-infection of mice with influenza A virus and M. tuberculosis results in increased bacterial loads in an IFNα/β-dependent manner 178 as seen in other scenarios outlined in Box 1.
Box 1. Type I interferon-mediated exacerbation of bacterial infection by viruses.
Bacterial infection subsequent to or together with viral infection have long been known to be a significant cause of mortality and morbidity in humans, particularly following influenza virus infection 225. Intensive research has therefore gone into understanding how viral infection sensitizes the host to bacterial infection. Perhaps unsurprisingly, IFNα/β have emerged as important players in this phenomenon. Influenza virus-infected Ifnar1-/- mice survive secondary infection with Streptococcus pneumoniae better than wild-type controls, with increased bacterial clearance226–228. This was attributed to increased production of the neutrophil chemoattractants CXC-chemokine ligand 1 (CXCL1) and CXCL2228, increased production of the macrophage chemoattractant CC-chemokine ligand 2 (CCL2)227 and to enhancement of the γδ T cell response226. Similar results were reported with Staphylococcus aureus secondary infection when polyI:C was administered as a surrogate for viral infection229. Likewise, influenza virus infection has a harmful effect on the host response to Mycobacterium tuberculosis, in an IFNα/β-dependent manner, although the underlying mechanism is currently unclear178. Negative effects on granulocyte generation in bone marrow were also implicated in a model of lymphocytic choriomeningitis virus-L. monocytogenes super-infection 230. Finally, viral infection or polyI:C administration with E. coli or M. tuberculosis super-infection leads to enhanced lethality in mice owing to excessive inflammation in a IFNα/β- and NOD1/NOD2-dependent manner 231 175. Thus, IFNα/β contribute to viral priming of the host for increased susceptibility to bacterial assault. Interestingly, in this scenario IFNα/β are damaging to the host in response to infections where they are normally protective during a primary infection (for example, with S. pneumoniae and E. coli). Again, this supports the idea that the circumstances of IFNα/β induction and action are crucial in determining host protection versus pathogenesis and highlights the opposing role in inflammation during viral versus certain bacterial infections.
The mechanisms that mediate IFNα/β-driven disease exacerbation are not fully understood but appear to be multifactorial. Data from investigations of hyper-virulent strains initially suggested that suppression of pro-inflammatory cytokines and Th1-type immunity are important 166,167,169 and there is good evidence both in human cells and in mouse models that IFNα/β suppress the production of host-protective cytokines following M. tuberculosis infection. Production of IL-1α and IL-1β, which are crucial for host defence against M. tuberculosis 179, is inhibited by IFNα/β in vitro in infected human and mouse cells and in vivo in mouse models 176,180,181,182. This is in line with a previous study using lipopolysaccharide that showed that IFNα/β could potently inhibit the NLRP1 and NLRP3 inflammasomes, which are responsible for post-translational maturation of IL-1β183.
In addition, cell-intrinsic type I IFN signals negatively regulated iNOS production by pulmonary myeloid cells, particularly Tip-DCs176. The production of other pro-inflammatory cytokines such as TNF and IL-12 are also negatively affected (177,180, 182 Mayer-Barber, Sher, unpublished observations;). The induction of immune suppressive IL-10 and IL-1 receptor antagonist by IFNα/β seems to have an important role in this suppression of pro-inflammatory cytokines (176,177,180,182).
Conversely, IL-1α and IL-1β have recently been shown to inhibit type I IFN induction in mouse and human macrophages, and when IL-1 was present in type I IFN-treated cultures, it also suppressed the pro-bacterial effects downstream of IFNβ. Interestingly, IL-1-induced prostaglandin E2 (PGE2) was also able to potently inhibit type I IFNs in this context 184, as observed previously in the context of LPS-induced type IFN responses 185 and more recently during influenza virus infection 186. Moreover, targeting PGE2 during M. tuberculosis infection, either via direct administration of the prostanoid or enhancement by 5-lipoxygenase blockade with Zileuton, reversed polyI:C-mediated type I IFN-driven mortality184.
Similarly to the findings in L. monocytogenes infection, repression of innate cell responsiveness to IFNγ is emerging as an important mechanism of IFNα/β immune suppression during mycobacterial infection 180,182,187. However, direct downregulation of IFNγR expression may not be the central mechanism of type I IFN effects on IFNγ activity176. Rather, in both mouse and humans cells, it has been shown that IFNα/β potently suppress the ability of macrophages to upregulate antimycobacterial effector molecules and to restrict bacterial growth in response to both Mycobacterium leprae and M. tuberculosis (McNab, Ewbank, O’Garra, unpublished observations; Mayer-Barber, unpublished observations) 180,187. The importance of this mechanism of action by IFNα/β is further suggested by experiments using Ifngr-/-Ifnar1-/- mice, in which IFNα/β are suggested to contribute to host protection in the absence of the IFNγ pathway188. Furthermore, the observation of naturally occurring mutations in host protective ISG15 in humans suggests that IFNα/β can induce host protective responses to mycobacterial infection, although it is not clear under what circumstances IFNα/β induce this gene during M. tuberculosis infection 189.
Additionally, the production of innate cytokines such as IL-12p70 were also suppressed by IFNα/β during M. tuberculosis infection (Mayer-Barber, unpublished observations) 180,182,187. This could result from IL-10 activity, downregulation of IFNγR and induction of negative regulators of IFN signalling such as protein arginine N-methyltransferase 1 (PRMT1) 180,187,182. Finally, type I IFNs, possibly by influencing chemokine expression, have been shown to be involved in the generation and trafficking of M. tuberculosis-permissive innate cells to the lungs in a mouse model, thus contributing to exacerbated infection175,190.
Francisella tularensis and F. novicida
The facultative intracellular bacterium F. tularensis and the subspecies F. novicida which is highly pathogenic in mice, have been investigated for a possible role of IFNα/β in the immune response 191–193. Two studies found that type I IFNs were necessary for activation of the inflammasome during F. novicida 192 and F. tularensis 191 infections and that, in turn, the AIM2 inflammasome was necessary for host protection against F. tularensis 191. This is in contrast to other studies showing that IFNα/β inhibit inflammasomes (see above) or where type I IFN-dependent AIM2 inflammasomes were engaged in vitro during mycobacterial infection but their role is unclear in vivo 194, suggesting that IFNα/β may have differential effects on inflammasome activity, depending on the type of inflammasome involved.
Similarly to infection with L. monocytogenes, IFNα/β were shown to be involved in apoptosis of macrophages during F. novicida infection192, although this was not correlated with outcome for the host. Despite these data indicating that IFNα/β may mediate some host protective mechanisms during these infections, a comparison of wild-type and Ifnar1-/- mice infected with F. novicida revealed that IFNα/β are detrimental to the host, restricting the development of a protective IL-17-producing γδ T cell response193.
Other bacterial infections
A limited range of studies further implicate IFNα/β in enhancing susceptibility to various other bacterial agents. IFNα/β have been suggested as a detrimental factor during Whipple’s disease (caused by Tropheryma whipplei), diverting macrophages to an alternatively polarized, permissive state and further, promoting macrophage apoptosis195.
IFNα/β are also detrimental during Brucella abortus infection, with Ifnar1-/- mice having reduced bacterial loads compared with wild-type controls. Bacterial control in these mice correlated with increased IFNγ and NO production and reduced TRAIL expression and apoptosis 196. Ifnar1-/- mice are also reportedly more resistant to infection with the plague agent Yersinia pestis. This resistance was associated with increased levels of neutrophils and enhanced function of phagocytic cells197. In contrast to earlier reports147,154, Robinson et al. 198 found that IFNα/β were harmful for the host during S. typhimurium infection. Protection was associated with macrophage resistance to necroptosis, rather than alterations in cytokine production or inflammasome activation.
IFNα/β have also been implicated in mediating deleterious inflammation during infection with a large range of Gram-negative bacteria through activation of caspase 11, leading to IL-1β and IL-18 production and caspase 1-independent cell death 199. A second paper also found a role for IFNα/β in inducing caspase 11 activation during S. typhimurium, which resulted in macrophage cell death that was injurious to the host, but only in the absence of caspase 1, which was required for the antibacterial function of neutrophils 200.
Martin et al. 201 found that IFNα/β are detrimental for Staphylococcus aureus infection, with Ifnar1-/- mice surviving intranasal infection better than wild-type mice. Protection correlated with increased proportions of CD11c+ cells in the airways and reduced pro-inflammatory cytokine production in the lungs.
To conclude, IFNαβ may contribute to host protection against bacterial infection by upregulation of antimicrobial effectors, such and IDO, iNOS and pro-inflammatory cytokines but conversely may impair the response to bacteria through the production of IL-10 and IL-1RA, suppression of pro-inflammatory cytokines, induction of immune cell death, including apoptosis, and restriction of host responses to IFNγ (Figure 3).
Figure 3. Positive and negative effects of type I interferons during bacterial infection.
Low level autocrine IFNα/β signalling primes the production of interleukin-10 (IL-10), pro-inflammatory cytokines and antimicrobial effector mechanisms. 2b: type I IFN induces IL-1Rα, which in turn inhibits IL-1 signaling. IL-10 mediates a negative feedback loop, suppressing production of pro-inflammatory cytokines including IL-12, TNFα and IL-1α/β. Upon infection high levels of IFNα/β affecting myeloid cells can be contributed by autocrine production as well as from exocrine cellular sources. IFNα/β can also suppress pro-inflammatory cytokine production in an IL-10 independent manner. A major suppressive mechanism of type I IFN is down-regulation of the IFNγR, thus abrogating IFNγ dependent host protective immune responses. IFNα/β signalling can promote high levels of IL-10 production as well as the induction of pro-apoptotic factors. IL-1α/β induces COX-2 dependent PGE2. PGE2 and IL-1 inhibit type I IFN expression and downstream effects
Effects in parasitic and fungal infection
Analyses of the effects of IFNα/β on the course of disease during parasitic and fungal infections have been relatively limited, with most work done in Leishmania, Trypanosome and Plasmodium models of parasite infection and Candida (yeast) models of fungal infection.
Parasitic infections
Work conducted during the late 1990s and early 2000s elucidated an important role for IFNα/β in inducing iNOS expression during Leishmania major infection202–204. Interestingly, it was noted that high levels of IFNα/β actually impaired iNOS induction, implicating levels of IFN as important in determining a host protective or pathogenic role for IFNα/β203,204. More recent work with different strains of Leishmania suggest a detrimental role for IFNα/β, through inhibition of macrophage function and regulation of neutrophil number and function205,206.
During malaria, IFNα/β can have either a host protective or detrimental effect depending on both the stage of infection and the species of infecting Plasmodium. For blood stages of the mouse malaria parasites Plasmodium berghei and P. chabaudi, IFNα/β enhance infection through inhibition of CD4+ T cell function207. By contrast, studies on P. yoelii infection indicate a protective role for IFNαβ, possibly through inhibition of reticulocytosis, a condition in which immature red blood cells accumulate208. Treatment with recombinant IFNα also reportedly protects mice from cerebral malaria induced by P. berghei ANKA, in part through enhancing the Th1 cell response 209. However, using Ifnar1-/- mice, others report only a minor influence of IFNα/β during acute P. chabaudi infection210. An interesting recent report has shown that P. berghei induces an IFNα/β response during the liver stage of infection that is essential for host protection211. This protection, mediated through cytosolic recognition of parasite RNA by the pattern recognition receptor MDA5, was associated with IFN-dependent recruitment of leukocytes to infectious foci. It remains to be seen whether this host resistance promoting function of type I IFNs in malaria liver stages is also parasite species specific and occurs in human malaria infection.
Infection studies with the protozoan parasite Trypanosoma cruzi show variously positive effects 212–214, negative effects 215 or no difference 216 to host immunity. The reasons for these differences are not fully understood but may relate to the route of infections, as studies showing a positive role for IFNα/β used the intraperitoneal route 212–214, whereas those showing a negative role used intradermal infection 215. The levels of IFNα/β signalling induced may also be crucial, as Ifnar1-/- mice reportedly succumbed earlier than wild-type mice, yet mice lacking UBP43, which are hyper-responsive to IFNα/β, were also more susceptible than wild-type mice214. Finally, the relative balance between effects on the innate versus adaptive response seem to be important, as in the absence of the innate signalling molecules MyD88 and/or TRIF, IFNα/β are important for host protection213 and additionally for NO generation 212 yet IFNα/β also inhibit the production of host protective IFNγ during T. cruzi infection215, and this is most likely from T cells, since NK cells reportedly do not require IFNα/β for IFNγ production in this infection 216.
Fungal infections
Studies of IFNα/β during fungal infection have generated conflicting results. Several findings suggest a host protective contribution of IFNα/β to immunity to C. albicans, Saccharomyces cerevisiae and Cryptococcus neoformans217–219. IFNα/β signalling was found to be required variously for induction of reactive oxygen intermediates that are necessary for killing of C. albicans by phagocytic cells218, for maintenance of a Th1-like (high IFNγ, TNF, iNOS and CXCL10) immune response against C. neoformans217, and attraction of leukocytes, particularly neutrophils, to the site of disease during C. albicans infection 219. Interestingly, another study of C. albicans infection of wild-type and Ifnar1-/- mice found a similar requirement for IFN signalling in attracting neutrophils and inflammatory monocytes to the site of disease, yet in this study these cells had no effect on fungal burden but rather caused lethal immunopathology 220. The reason for these opposing findings is unclear, but given the very similar infection protocols used, it is possible that it is due to differences in the microbiota from different animal facilities. IFNα/β were also found to mediate polyI:C sensitization of mice to C. albicans through suppression of IL-1β183. IFNα/β have also been implicated in sensitizing the host in model infections of Candida glabrata and Histoplasma capsulatum, although no mechanism was investigated in these cases221,222.
Studies of humans with inborn errors in immune signalling components may provide the strongest clues to the role of IFNα/β in fungal infections. Whole-exome sequencing and genome wide association studies looking for genetic aetiologies for chronic mucocutaneous candidiasis identified mutations in STAT1 in some patients 223,224(reviewed in 31). The same STAT1 mutations were also found in patients with disseminated disease caused by other fungal pathogens such as H. capsulatum31. Interestingly, these mutations are gain of function and dominant, suggesting a potentially detrimental role for IFNα/β in the response to fungal infection, possibly through suppression of Th17 cell responses223. However, other cytokines that depend on STAT1 for signalling, such as IFNγ and IL-27, may also be responsible.(Figure 4)
Figure 4. Mechanisms of interferon action in non-viral infections.
The diagram indicates the mechanistic processes that are influenced by IFNα/β during bacterial infections. Arrows indicate whether IFNα/β promote, suppress or have variable, context dependent, effects on the associated process. For each process, infections in which IFN-mediated effects may be manifest are shown. In green are those infections where IFNα/β are thought to be protective and in red those where IFNα/β have host detrimental effects. For processes in which IFNs have variable effects the relevant effect is shown for each described disease by an arrow indicating promotion or suppression. For example, IFNα/β have variable effects on chemokine production and cell migration. In S. pyogenes infection (where IFNα/β is protective), IFNα/β have promoting effects on chemokine production and cell migration.
Closing remarks
Type I IFNs are among the first cytokines induced by a plethora of cells during infection. Due to the broad distribution of expression of IFNAR, type I IFNs have wide-ranging effects on epithelial cells and innate and adaptive immune cells. The net effect of type I IFNs towards protection or pathogenesis during infection is determined by the type and dose of pathogen, as well as by the genetic background of the host and possibly the microbiota (Box 2). Progress is needed to better understand, first, the precise regulation of the induction of type I IFNs at the transcriptional and post-transcriptional level, and second, the factors determining responsiveness to type I IFNs. Such knowledge will uncover mechanisms to harness the immune response for maximum protection with minimum host damage.
Box 2. Commensal microbiota and the type I interferon response.
Interest in the ability of the resident microbial flora to influence the homeostasis and function of the host immune system has attracted growing attention in recent years232. At least three studies published in the last two years define a role for IFNα/β either as mediators of host-microbiota interactions and/or as downstream targets of these interactions, leading to further effects on immune system function. Both Ganal et al.233 and Abt et al.234 describe that in mice lacking commensal microorganisms, either through antibiotic treatment or being bred germ-free, the IFN-inducible and inflammatory transcriptional response was greatly reduced. In both cases antiviral immunity was severely compromised as mononuclear phagocytes had a defective response to viral challenge, abrogating their ability both to limit viral replication and also to prime other aspects of the antiviral response such as natural killer cell activation. A recent study suggests that an absence of IFNAR1 signalling in intestinal epithelial cells leads to Paneth cell proliferation and a consequential alteration in intestinal microbiota composition235. Microbiota-induced production of IFNβ by dendritic cells (DCs) in the intestine has also recently been shown to protect mice from dextran-sulphate sodium-induced colitis236. In this model, Toll-like receptor 3 activation by double-stranded RNA allowed DCs to discriminate between non-pathogenic commensal bacteria and harmful pathogens, with only non-pathogenic bacteria inducing protective IFNβ production236. These findings expand our understanding of IFNα/β as important homeostatic factors for the immune response and may explain the putative role of constitutive type I IFN production in modulating basal STAT expression 17,237.
Glossary.
Cytosolic GAMP synthase
(cGAS). A cytosolic DNA sensor that catalyses the production of the second messenger cyclic-di-GMP-AMP (cGAMP) in response to DNA which is then recognized by the sensor and signalling intermediate STING (stimulator of IFN genes) to trigger type 1 interferon production.
Plasmacytoid dendritic cell
(pDC). Immature DCs with a morphology that resembles that of plasma cells. On a per cell basis, pDCs are the main producers of type I interferons in response to viral infections or Toll-like receptor stimulation.
Ribavirin
A drug that interferes with RNA metabolism and blocks viral replication. Ribavirin is used in combination with interferon-α to treat hepatitis C viral infection.
M1 macrophage
A pro-inflammatory or 'classically activated' subset of macrophages, which are characterized by phagocytic activity and expression of particular inflammatory cytokines (such as tumour necrosis factor) and inflammatory mediators (such as inducible nitric oxide synthase).
References
- 1.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 2.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien TR, Prokunina-Olsson L, Donnelly RP. IFN-lambda4: The Paradoxical New Member of the Interferon Lambda Family. J Interferon Cytokine Res. 2014 doi: 10.1089/jir.2013.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prokunina-Olsson L, et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45:164–171. doi: 10.1038/ng.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Witte K, Witte E, Sabat R, Wolk K. IL-28A, IL-28B, and IL-29: promising cytokines with type I interferon-like properties. Cytokine Growth Factor Rev. 2010;21:237–251. doi: 10.1016/j.cytogfr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Durbin RK, Kotenko SV, Durbin JE. Interferon induction and function at the mucosal surface. Immunol Rev. 2013;255:25–39. doi: 10.1111/imr.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan N, Chen ZJ. Intrinsic antiviral immunity. Nat Immunol. 2012;13:214–222. doi: 10.1038/ni.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goubau D, Deddouche S, Reis e Sousa C. Cytosolic sensing of viruses. Immunity. 2013;38:855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity. 2013;38:870–880. doi: 10.1016/j.immuni.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leber JH, et al. Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog. 2008;4:e6. doi: 10.1371/journal.ppat.0040006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey AK, et al. NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000500. doi: 10.1371/journal.ppat.1000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe T, et al. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J Clin Invest. 2010;120:1645–1662. doi: 10.1172/JCI39481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreira LO, Zamboni DS. NOD1 and NOD2 Signaling in Infection and Inflammation. Front Immunol. 2012;3:328. doi: 10.3389/fimmu.2012.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moynagh PN. TLR signalling and activation of IRFs: revisiting old friends from the NF-kappaB pathway. Trends Immunol. 2005;26:469–476. doi: 10.1016/j.it.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 15.Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity. 2006;25:349–360. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Tamura T, Yanai H, Savitsky D, Taniguchi T. The IRF family transcription factors in immunity and oncogenesis. Annu Rev Immunol. 2008;26:535–584. doi: 10.1146/annurev.immunol.26.021607.090400. [DOI] [PubMed] [Google Scholar]
- 17.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14:36–49. doi: 10.1038/nri3581. This review is a perfect prelude to the present review which quotes it significantly as describing in more detail the molecular mechanisms of regulation of Type I IFN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rauch I, Muller M, Decker T. The regulation of inflammation by interferons and their STATs. Jak-Stat. 2013;2:e23820. doi: 10.4161/jkst.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Versteeg GA, Garcia-Sastre A. Viral tricks to grid-lock the type I interferon system. Curr Opin Microbiol. 2010;13:508–516. doi: 10.1016/j.mib.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNab FW, Rajsbaum R, Stoye JP, O'Garra A. Tripartite-motif proteins and innate immune regulation. Curr Opin Immunol. 2011;23:46–56. doi: 10.1016/j.coi.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 21.Diamond MS, Schoggins JW. Host restriction factor screening: let the virus do the work. Cell Host Microbe. 2013;14:229–231. doi: 10.1016/j.chom.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller U, et al. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 23.Haller O, Arnheiter H, Gresser I, Lindenmann J. Virus-specific interferon action. Protection of newborn Mx carriers against lethal infection with influenza virus. J Exp Med. 1981;154:199–203. doi: 10.1084/jem.154.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Durbin JE, et al. Type I IFN modulates innate and specific antiviral immunity. J Immunol. 2000;164:4220–4228. doi: 10.4049/jimmunol.164.8.4220. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Sastre A, et al. The role of interferon in influenza virus tissue tropism. Journal of virology. 1998;72:8550–8558. doi: 10.1128/jvi.72.11.8550-8558.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koerner I, Kochs G, Kalinke U, Weiss S, Staeheli P. Protective role of beta interferon in host defense against influenza A virus. Journal of virology. 2007;81:2025–2030. doi: 10.1128/JVI.01718-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Price GE, Gaszewska-Mastarlarz A, Moskophidis D. The role of alpha/beta and gamma interferons in development of immunity to influenza A virus in mice. Journal of virology. 2000;74:3996–4003. doi: 10.1128/jvi.74.9.3996-4003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mordstein M, et al. Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections. Journal of virology. 2010;84:5670–5677. doi: 10.1128/JVI.00272-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mordstein M, et al. Interferon-lambda contributes to innate immunity of mice against influenza A virus but not against hepatotropic viruses. PLoS Pathog. 2008;4:e1000151. doi: 10.1371/journal.ppat.1000151. This study importantly demonstrates the redundant roles of Type I and Type III IFNs in the anti-influenza response clarifying the confusion from earlier literature on observations that Type I IFN cannot account for the requirement of STAT1 signaling in protection against influenza. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crotta S, et al. Type I and type III interferons drive redundant amplification loops to induce a transcriptional signature in influenza-infected airway epithelia. PLoS Pathog. 2013;9:e1003773. doi: 10.1371/journal.ppat.1003773. This study importantly demonstrates the redundant roles of Type I and Type III IFN signaling in epithelial cells in the anti-influenza response clarifying the confusion from earlier literature in protection against influenza. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casanova JL, Holland SM, Notarangelo LD. Inborn errors of human JAKs and STATs. Immunity. 2012;36:515–528. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang SY, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunol Rev. 2008;226:29–40. doi: 10.1111/j.1600-065X.2008.00698.x. [DOI] [PubMed] [Google Scholar]
- 33.Suppiah V, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100–1104. doi: 10.1038/ng.447. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka Y, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–1109. doi: 10.1038/ng.449. [DOI] [PubMed] [Google Scholar]
- 35.Ge D, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 36.Thomas DL, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandler NG, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature. 2014;511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everitt AR, et al. IFITM3 restricts the morbidity and mortality associated with influenza. Nature. 2012;484:519–523. doi: 10.1038/nature10921. The first evidence for host genetics (IFITM3) contributing to susceptibility to influenza in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang YH, et al. Interferon-induced transmembrane protein-3 genetic variant rs12252-C is associated with severe influenza in Chinese individuals. Nat Commun. 2013;4:1418. doi: 10.1038/ncomms2433. Follow up study showing that IFITM3 variants contributing to severity to influenza are predominant in the Chinese population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staeheli P, Grob R, Meier E, Sutcliffe JG, Haller O. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol Cell Biol. 1988;8:4518–4523. doi: 10.1128/mcb.8.10.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horisberger MA, Staeheli P, Haller O. Interferon induces a unique protein in mouse cells bearing a gene for resistance to influenza virus. Proc Natl Acad Sci U S A. 1983;80:1910–1914. doi: 10.1073/pnas.80.7.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horby P, Nguyen NY, Dunstan SJ, Baillie JK. The role of host genetics in susceptibility to influenza: a systematic review. PLoS One. 2012;7:e33180. doi: 10.1371/journal.pone.0033180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dauer M, et al. Interferon-alpha disables dendritic cell precursors: dendritic cells derived from interferon-alpha-treated monocytes are defective in maturation and T-cell stimulation. Immunology. 2003;110:38–47. doi: 10.1046/j.1365-2567.2003.01702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lapenta C, et al. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-alpha. J Exp Med. 2003;198:361–367. doi: 10.1084/jem.20021924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santini SM, et al. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santodonato L, et al. Monocyte-derived dendritic cells generated after a short-term culture with IFN-alpha and granulocyte-macrophage colony-stimulating factor stimulate a potent Epstein-Barr virus-specific CD8+ T cell response. J Immunol. 2003;170:5195–5202. doi: 10.4049/jimmunol.170.10.5195. [DOI] [PubMed] [Google Scholar]
- 47.Hahm B, Trifilo MJ, Zuniga EI, Oldstone MB. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity. 2005;22:247–257. doi: 10.1016/j.immuni.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Ito T, et al. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–2969. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 49.Montoya M, et al. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–3271. doi: 10.1182/blood.v99.9.3263. [DOI] [PubMed] [Google Scholar]
- 50.Le Bon A, et al. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat Immunol. 2003;4:1009–1015. doi: 10.1038/ni978. [DOI] [PubMed] [Google Scholar]
- 51.Le Bon A, et al. Direct stimulation of T cells by type I IFN enhances the CD8+ T cell response during cross-priming. J Immunol. 2006;176:4682–4689. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- 52.Spadaro F, et al. IFN-alpha enhances cross-presentation in human dendritic cells by modulating antigen survival, endocytic routing, and processing. Blood. 2012;119:1407–1417. doi: 10.1182/blood-2011-06-363564. [DOI] [PubMed] [Google Scholar]
- 53.Parlato S, et al. Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98:3022–3029. doi: 10.1182/blood.v98.10.3022. [DOI] [PubMed] [Google Scholar]
- 54.Rouzaut A, et al. Dendritic cells adhere to and transmigrate across lymphatic endothelium in response to IFN-alpha. Eur J Immunol. 2010;40:3054–3063. doi: 10.1002/eji.201040523. [DOI] [PubMed] [Google Scholar]
- 55.Gautier G, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med. 2005;201:1435–1446. doi: 10.1084/jem.20041964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cousens LP, Orange JS, Su HC, Biron CA. Interferon-alpha/beta inhibition of interleukin 12 and interferon-gamma production in vitro and endogenously during viral infection. Proc Natl Acad Sci U S A. 1997;94:634–639. doi: 10.1073/pnas.94.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dalod M, et al. Interferon alpha/beta and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J Exp Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Orange JS, Wolf SF, Biron CA. Effects of IL-12 on the response and susceptibility to experimental viral infections. J Immunol. 1994;152:1253–1264. [PubMed] [Google Scholar]
- 59.Orange JS, et al. Mechanism of interleukin 12-mediated toxicities during experimental viral infections: role of tumor necrosis factor and glucocorticoids. J Exp Med. 1995;181:901–914. doi: 10.1084/jem.181.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Bon A, et al. Cutting edge: enhancement of antibody responses through direct stimulation of B and T cells by type I IFN. J Immunol. 2006;176:2074–2078. doi: 10.4049/jimmunol.176.4.2074. [DOI] [PubMed] [Google Scholar]
- 61.Havenar-Daughton C, Kolumam GA, Murali-Krishna K. Cutting Edge: The direct action of type I IFN on CD4 T cells is critical for sustaining clonal expansion in response to a viral but not a bacterial infection. J Immunol. 2006;176:3315–3319. doi: 10.4049/jimmunol.176.6.3315. [DOI] [PubMed] [Google Scholar]
- 62.Brinkmann V, Geiger T, Alkan S, Heusser CH. Interferon alpha increases the frequency of interferon gamma-producing human CD4+ T cells. J Exp Med. 1993;178:1655–1663. doi: 10.1084/jem.178.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hofer MJ, et al. Mice deficient in STAT1 but not STAT2 or IRF9 develop a lethal CD4+ T-cell-mediated disease following infection with lymphocytic choriomeningitis virus. Journal of virology. 2012;86:6932–6946. doi: 10.1128/JVI.07147-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lazear HM, Pinto AK, Vogt MR, Gale M, Jr, Diamond MS. Beta interferon controls West Nile virus infection and pathogenesis in mice. Journal of virology. 2011;85:7186–7194. doi: 10.1128/JVI.00396-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shiow LR, et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 66.Petricoin EF, et al. Antiproliferative action of interferon-alpha requires components of T-cell-receptor signalling. Nature. 1997;390:629–632. doi: 10.1038/37648. [DOI] [PubMed] [Google Scholar]
- 67.Kaser A, Nagata S, Tilg H. Interferon alpha augments activation-induced T cell death by upregulation of Fas (CD95/APO-1) and Fas ligand expression. Cytokine. 1999;11:736–743. doi: 10.1006/cyto.1998.0484. [DOI] [PubMed] [Google Scholar]
- 68.Marshall HD, Urban SL, Welsh RM. Virus-induced transient immune suppression and the inhibition of T cell proliferation by type I interferon. Journal of virology. 2011;85:5929–5939. doi: 10.1128/JVI.02516-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bromberg JF, Horvath CM, Wen Z, Schreiber RD, Darnell JE., Jr Transcriptionally active Stat1 is required for the antiproliferative effects of both interferon alpha and interferon gamma. Proc Natl Acad Sci U S A. 1996;93:7673–7678. doi: 10.1073/pnas.93.15.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee CK, Smith E, Gimeno R, Gertner R, Levy DE. STAT1 affects lymphocyte survival and proliferation partially independent of its role downstream of IFN-gamma. J Immunol. 2000;164:1286–1292. doi: 10.4049/jimmunol.164.3.1286. [DOI] [PubMed] [Google Scholar]
- 71.Tanabe Y, et al. Cutting edge: role of STAT1, STAT3, and STAT5 in IFN-alpha beta responses in T lymphocytes. J Immunol. 2005;174:609–613. doi: 10.4049/jimmunol.174.2.609. [DOI] [PubMed] [Google Scholar]
- 72.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aichele P, et al. CD8 T cells specific for lymphocytic choriomeningitis virus require type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 74.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 76.Keppler SJ, Rosenits K, Koegl T, Vucikuja S, Aichele P. Signal 3 cytokines as modulators of primary immune responses during infections: the interplay of type I IFN and IL-12 in CD8 T cell responses. PLoS One. 2012;7:e40865. doi: 10.1371/journal.pone.0040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gimeno R, Lee CK, Schindler C, Levy DE. Stat1 and Stat2 but not Stat3 arbitrate contradictory growth signals elicited by alpha/beta interferon in T lymphocytes. Mol Cell Biol. 2005;25:5456–5465. doi: 10.1128/MCB.25.13.5456-5465.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gil MP, Salomon R, Louten J, Biron CA. Modulation of STAT1 protein levels: a mechanism shaping CD8 T-cell responses in vivo. Blood. 2006;107:987–993. doi: 10.1182/blood-2005-07-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agarwal P, et al. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183:1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marshall HD, Prince AL, Berg LJ, Welsh RM. IFN-alpha beta and self-MHC divert CD8 T cells into a distinct differentiation pathway characterized by rapid acquisition of effector functions. J Immunol. 2010;185:1419–1428. doi: 10.4049/jimmunol.1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cousens LP, et al. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nguyen KB, et al. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297:2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 83.Nguyen KB, et al. Interferon alpha/beta-mediated inhibition and promotion of interferon gamma: STAT1 resolves a paradox. Nat Immunol. 2000;1:70–76. doi: 10.1038/76940. [DOI] [PubMed] [Google Scholar]
- 84.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 85.Pinto AK, et al. A temporal role of type I interferon signaling in CD8+ T cell maturation during acute West Nile virus infection. PLoS Pathog. 2011;7:e1002407. doi: 10.1371/journal.ppat.1002407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramos HJ, et al. Reciprocal responsiveness to interleukin-12 and interferon-alpha specifies human CD8+ effector versus central memory T-cell fates. Blood. 2009;113:5516–5525. doi: 10.1182/blood-2008-11-188458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 2010;33:96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sung JH, et al. Chemokine guidance of central memory T cells is critical for antiviral recall responses in lymph nodes. Cell. 2012;150:1249–1263. doi: 10.1016/j.cell.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crouse J, et al. Type I Interferons Protect T Cells against NK Cell Attack Mediated by the Activating Receptor NCR1. Immunity. 2014;40:961–973. doi: 10.1016/j.immuni.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 91.Xu HC, et al. Type I interferon protects antiviral CD8(+) T cells from NK cell cytotoxicity. Immunity. 2014;40:949–960. doi: 10.1016/j.immuni.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 92.Hwang I, et al. Activation mechanisms of natural killer cells during influenza virus infection. PLoS One. 2012;7:e51858. doi: 10.1371/journal.pone.0051858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martinez J, Huang X, Yang Y. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J Immunol. 2008;180:1592–1597. doi: 10.4049/jimmunol.180.3.1592. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen KB, et al. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 95.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun JC, Ma A, Lanier LL. Cutting edge: IL-15-independent NK cell response to mouse cytomegalovirus infection. J Immunol. 2009;183:2911–2914. doi: 10.4049/jimmunol.0901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baranek T, et al. Differential responses of immune cells to type I interferon contribute to host resistance to viral infection. Cell Host Microbe. 2012;12:571–584. doi: 10.1016/j.chom.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 98.Miyagi T, et al. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J Exp Med. 2007;204:2383–2396. doi: 10.1084/jem.20070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mack EA, Kallal LE, Demers DA, Biron CA. Type 1 interferon induction of natural killer cell gamma interferon production for defense during lymphocytic choriomeningitis virus infection. MBio. 2011;2 doi: 10.1128/mBio.00169-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang J, Lin Q, Langston H, Cooper MD. Resident bone marrow macrophages produce type 1 interferons that can selectively inhibit interleukin-7-driven growth of B lineage cells. Immunity. 1995;3:475–484. doi: 10.1016/1074-7613(95)90176-0. [DOI] [PubMed] [Google Scholar]
- 101.Lin Q, Dong C, Cooper MD. Impairment of T and B cell development by treatment with a type I interferon. J Exp Med. 1998;187:79–87. doi: 10.1084/jem.187.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bosio E, Cluning CL, Beilharz MW. Low-dose orally administered type I interferon reduces splenic B cell numbers in mice. J Interferon Cytokine Res. 2001;21:721–728. doi: 10.1089/107999001753124453. [DOI] [PubMed] [Google Scholar]
- 103.Le Bon A, et al. Type i interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 2001;14:461–470. doi: 10.1016/s1074-7613(01)00126-1. [DOI] [PubMed] [Google Scholar]
- 104.Swanson CL, et al. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J Exp Med. 2010;207:1485–1500. doi: 10.1084/jem.20092695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coro ES, Chang WL, Baumgarth N. Type I IFN receptor signals directly stimulate local B cells early following influenza virus infection. J Immunol. 2006;176:4343–4351. doi: 10.4049/jimmunol.176.7.4343. [DOI] [PubMed] [Google Scholar]
- 106.Chang WL, et al. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J Immunol. 2007;178:1457–1467. doi: 10.4049/jimmunol.178.3.1457. [DOI] [PubMed] [Google Scholar]
- 107.Rau FC, Dieter J, Luo Z, Priest SO, Baumgarth N. B7-1/2 (CD80/CD86) direct signaling to B cells enhances IgG secretion. J Immunol. 2009;183:7661–7671. doi: 10.4049/jimmunol.0803783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Heer AK, et al. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 109.Fink K, et al. Early type I interferon-mediated signals on B cells specifically enhance antiviral humoral responses. Eur J Immunol. 2006;36:2094–2105. doi: 10.1002/eji.200635993. [DOI] [PubMed] [Google Scholar]
- 110.Bach P, et al. Vesicular stomatitis virus glycoprotein displaying retrovirus-like particles induce a type I IFN receptor-dependent switch to neutralizing IgG antibodies. J Immunol. 2007;178:5839–5847. doi: 10.4049/jimmunol.178.9.5839. [DOI] [PubMed] [Google Scholar]
- 111.Purtha WE, Chachu KA, Virgin HWt, Diamond MS. Early B-cell activation after West Nile virus infection requires alpha/beta interferon but not antigen receptor signaling. Journal of virology. 2008;82:10964–10974. doi: 10.1128/JVI.01646-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moseman EA, et al. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity. 2012;36:415–426. doi: 10.1016/j.immuni.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Biron CA. Interferons alpha and beta as immune regulators--a new look. Immunity. 2001;14:661–664. doi: 10.1016/s1074-7613(01)00154-6. [DOI] [PubMed] [Google Scholar]
- 114.Davidson S, Crotta S, McCabe TM, Wack A. Pathogenic potential of interferon alphabeta in acute influenza infection. Nat Commun. 2014;5 doi: 10.1038/ncomms4864. 3864. Seminal publication showing that contrary to dogma Type I IFNs can cause morbidity and mortality as opposed protection during influenza infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mandl JN, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nature medicine. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 116.Jacquelin B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rotger M, et al. Comparative transcriptomics of extreme phenotypes of human HIV-1 infection and SIV infection in sooty mangabey and rhesus macaque. J Clin Invest. 2011;121:2391–2400. doi: 10.1172/JCI45235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.McNally JM, et al. Attrition of bystander CD8 T cells during virus-induced T-cell and interferon responses. Journal of virology. 2001;75:5965–5976. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chi B, et al. Alpha and lambda interferon together mediate suppression of CD4 T cells induced by respiratory syncytial virus. Journal of virology. 2006;80:5032–5040. doi: 10.1128/JVI.80.10.5032-5040.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gil MP, et al. Regulating type 1 IFN effects in CD8 T cells during viral infections: changing STAT4 and STAT1 expression for function. Blood. 2012;120:3718–3728. doi: 10.1182/blood-2012-05-428672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Herbeuval JP, et al. Differential expression of IFN-alpha and TRAIL/DR5 in lymphoid tissue of progressor versus nonprogressor HIV-1-infected patients. Proc Natl Acad Sci U S A. 2006;103:7000–7005. doi: 10.1073/pnas.0600363103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hardy AW, Graham DR, Shearer GM, Herbeuval JP. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc Natl Acad Sci U S A. 2007;104:17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Herbeuval JP, et al. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood. 2005;106:3524–3531. doi: 10.1182/blood-2005-03-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Grevenynghe J, et al. Loss of memory B cells during chronic HIV infection is driven by Foxo3a- and TRAIL-mediated apoptosis. J Clin Invest. 2011;121:3877–3888. doi: 10.1172/JCI59211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liedtke C, Groger N, Manns MP, Trautwein C. Interferon-alpha enhances TRAIL-mediated apoptosis by up-regulating caspase-8 transcription in human hepatoma cells. Journal of hepatology. 2006;44:342–349. doi: 10.1016/j.jhep.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 126.Shigeno M, et al. Interferon-alpha sensitizes human hepatoma cells to TRAIL-induced apoptosis through DR5 upregulation and NF-kappa B inactivation. Oncogene. 2003;22:1653–1662. doi: 10.1038/sj.onc.1206139. [DOI] [PubMed] [Google Scholar]
- 127.Toomey NL, et al. Induction of a TRAIL-mediated suicide program by interferon alpha in primary effusion lymphoma. Oncogene. 2001;20:7029–7040. doi: 10.1038/sj.onc.1204895. [DOI] [PubMed] [Google Scholar]
- 128.Teijaro JR, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science. 2013;340:207–211. doi: 10.1126/science.1235214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wilson EB, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science. 2013;340:202–207. doi: 10.1126/science.1235208. References 128 and 129 were first to show that Type I IFN contributes to pathogenesis by induction of suppressive mechanisms in chronic LCMV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Herold S, et al. Lung epithelial apoptosis in influenza virus pneumonia: the role of macrophage-expressed TNF-related apoptosis-inducing ligand. J Exp Med. 2008;205:3065–3077. doi: 10.1084/jem.20080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hogner K, et al. Macrophage-expressed IFN-beta contributes to apoptotic alveolar epithelial cell injury in severe influenza virus pneumonia. PLoS Pathog. 2013;9:e1003188. doi: 10.1371/journal.ppat.1003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chaperot L, et al. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol. 2006;176:248–255. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- 133.Fujikura D, et al. Type-I interferon is critical for FasL expression on lung cells to determine the severity of influenza. PLoS One. 2013;8:e55321. doi: 10.1371/journal.pone.0055321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McNally B, Ye F, Willette M, Flano E. Local blockade of epithelial PDL-1 in the airways enhances T cell function and viral clearance during influenza virus infection. Journal of virology. 2013;87:12916–12924. doi: 10.1128/JVI.02423-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Brincks EL, et al. The magnitude of the T cell response to a clinically significant dose of influenza virus is regulated by TRAIL. J Immunol. 2011;187:4581–4588. doi: 10.4049/jimmunol.1002241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.MacMicking JD. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat Rev Immunol. 2012;12:367–382. doi: 10.1038/nri3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kazar J, Gillmore JD, Gordon FB. Effect of Interferon and Interferon Inducers on Infections with a Nonviral Intracellular Microorganism, Chlamydia trachomatis. Infect Immun. 1971;3:825–832. doi: 10.1128/iai.3.6.825-832.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de la Maza LM, Peterson EM, Goebel JM, Fennie CW, Czarniecki CW. Interferon-induced inhibition of Chlamydia trachomatis: dissociation from antiviral and antiproliferative effects. Infect Immun. 1985;47:719–722. doi: 10.1128/iai.47.3.719-722.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ishihara T, et al. Inhibition of chlamydia trachomatis growth by human interferon-alpha: mechanisms and synergistic effect with interferon-gamma and tumor necrosis factor-alpha. Biomed Res. 2005;26:179–185. doi: 10.2220/biomedres.26.179. [DOI] [PubMed] [Google Scholar]
- 140.Rothfuchs AG, et al. IFN-alpha beta-dependent, IFN-gamma secretion by bone marrow-derived macrophages controls an intracellular bacterial infection. J Immunol. 2001;167:6453–6461. doi: 10.4049/jimmunol.167.11.6453. [DOI] [PubMed] [Google Scholar]
- 141.Rothfuchs AG, et al. STAT1 regulates IFN-alpha beta- and IFN-gamma-dependent control of infection with Chlamydia pneumoniae by nonhemopoietic cells. J Immunol. 2006;176:6982–6990. doi: 10.4049/jimmunol.176.11.6982. [DOI] [PubMed] [Google Scholar]
- 142.Qiu H, et al. Type I IFNs enhance susceptibility to Chlamydia muridarum lung infection by enhancing apoptosis of local macrophages. J Immunol. 2008;181:2092–2102. doi: 10.4049/jimmunol.181.3.2092. [DOI] [PubMed] [Google Scholar]
- 143.Opitz B, et al. Legionella pneumophila induces IFNbeta in lung epithelial cells via IPS-1 and IRF3, which also control bacterial replication. J Biol Chem. 2006;281:36173–36179. doi: 10.1074/jbc.M604638200. [DOI] [PubMed] [Google Scholar]
- 144.Plumlee CR, et al. Interferons direct an effective innate response to Legionella pneumophila infection. J Biol Chem. 2009;284:30058–30066. doi: 10.1074/jbc.M109.018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schiavoni G, et al. Type I IFN protects permissive macrophages from Legionella pneumophila infection through an IFN-gamma-independent pathway. J Immunol. 2004;173:1266–1275. doi: 10.4049/jimmunol.173.2.1266. [DOI] [PubMed] [Google Scholar]
- 146.Gold JA, et al. Exogenous gamma and alpha/beta interferon rescues human macrophages from cell death induced by Bacillus anthracis. Infect Immun. 2004;72:1291–1297. doi: 10.1128/IAI.72.3.1291-1297.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bukholm G, Berdal BP, Haug C, Degre M. Mouse fibroblast interferon modifies Salmonella typhimurium infection in infant mice. Infect Immun. 1984;45:62–66. doi: 10.1128/iai.45.1.62-66.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Niesel DW, Hess CB, Cho YJ, Klimpel KD, Klimpel GR. Natural and recombinant interferons inhibit epithelial cell invasion by Shigella spp. Infect Immun. 1986;52:828–833. doi: 10.1128/iai.52.3.828-833.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mancuso G, et al. Type I IFN signaling is crucial for host resistance against different species of pathogenic bacteria. J Immunol. 2007;178:3126–3133. doi: 10.4049/jimmunol.178.5.3126. [DOI] [PubMed] [Google Scholar]
- 150.Parker D, et al. Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. MBio. 2011;2:e00016–00011. doi: 10.1128/mBio.00016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Weigent DA, Huff TL, Peterson JW, Stanton GJ, Baron S. Role of interferon in streptococcal infection in the mouse. Microb Pathog. 1986;1:399–407. doi: 10.1016/0882-4010(86)90071-9. [DOI] [PubMed] [Google Scholar]
- 152.Kelly-Scumpia KM, et al. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J Exp Med. 2010;207:319–326. doi: 10.1084/jem.20091959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Weighardt H, et al. Type I IFN modulates host defense and late hyperinflammation in septic peritonitis. J Immunol. 2006;177:5623–5630. doi: 10.4049/jimmunol.177.8.5623. [DOI] [PubMed] [Google Scholar]
- 154.Freudenberg MA, et al. Cutting edge: a murine, IL-12-independent pathway of IFN-gamma induction by gram-negative bacteria based on STAT4 activation by Type I IFN and IL-18 signaling. J Immunol. 2002;169:1665–1668. doi: 10.4049/jimmunol.169.4.1665. [DOI] [PubMed] [Google Scholar]
- 155.Auerbuch V, Brockstedt DG, Meyer-Morse N, O'Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Carrero JA, Calderon B, Unanue ER. Type I interferon sensitizes lymphocytes to apoptosis and reduces resistance to Listeria infection. J Exp Med. 2004;200:535–540. doi: 10.1084/jem.20040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.O'Connell RM, et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. J Exp Med. 2004;200:437–445. doi: 10.1084/jem.20040712. References 155 - 157 are the first publications demonstrating an adverse effect of Type I IFN in Listeria intracellular bacterial infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carrero JA, Calderon B, Unanue ER. Lymphocytes are detrimental during the early innate immune response against Listeria monocytogenes. J Exp Med. 2006;203:933–940. doi: 10.1084/jem.20060045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stockinger S, et al. Production of type I IFN sensitizes macrophages to cell death induced by Listeria monocytogenes. J Immunol. 2002;169:6522–6529. doi: 10.4049/jimmunol.169.11.6522. [DOI] [PubMed] [Google Scholar]
- 160.Zwaferink H, Stockinger S, Hazemi P, Lemmens-Gruber R, Decker T. IFN-beta increases listeriolysin O-induced membrane permeabilization and death of macrophages. J Immunol. 2008;180:4116–4123. doi: 10.4049/jimmunol.180.6.4116. [DOI] [PubMed] [Google Scholar]
- 161.Zwaferink H, Stockinger S, Reipert S, Decker T. Stimulation of inducible nitric oxide synthase expression by beta interferon increases necrotic death of macrophages upon Listeria monocytogenes infection. Infect Immun. 2008;76:1649–1656. doi: 10.1128/IAI.01251-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dresing P, Borkens S, Kocur M, Kropp S, Scheu S. A fluorescence reporter model defines “Tip-DCs” as the cellular source of interferon beta in murine listeriosis. PLoS One. 2010;5:e15567. doi: 10.1371/journal.pone.0015567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stockinger S, et al. Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes. PLoS Pathog. 2009;5:e1000355. doi: 10.1371/journal.ppat.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rayamajhi M, Humann J, Penheiter K, Andreasen K, Lenz LL. Induction of IFN-alphabeta enables Listeria monocytogenes to suppress macrophage activation by IFN-gamma. J Exp Med. 2010;207:327–337. doi: 10.1084/jem.20091746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kearney SJ, et al. Type I IFNs downregulate myeloid cell IFN-gamma receptor by inducing recruitment of an early growth response 3/NGFI-A binding protein 1 complex that silences ifngr1 transcription. J Immunol. 2013;191:3384–3392. doi: 10.4049/jimmunol.1203510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Manca C, et al. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 2005;25:694–701. doi: 10.1089/jir.2005.25.694. [DOI] [PubMed] [Google Scholar]
- 167.Ordway D, et al. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J Immunol. 2007;179:522–531. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- 168.Stanley SA, Johndrow JE, Manzanillo P, Cox JS. The Type I IFN response to infection with Mycobacterium tuberculosis requires ESX-1-mediated secretion and contributes to pathogenesis. J Immunol. 2007;178:3143–3152. doi: 10.4049/jimmunol.178.5.3143. [DOI] [PubMed] [Google Scholar]
- 169.Manca C, et al. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha /beta. Proc Natl Acad Sci U S A. 2001;98:5752–5757. doi: 10.1073/pnas.091096998. First demonstration that Type I IFN contributes to exacerbation of tuberculosis in experimental mouse models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cooper AM, Pearl JE, Brooks JV, Ehlers S, Orme IM. Expression of the nitric oxide synthase 2 gene is not essential for early control of Mycobacterium tuberculosis in the murine lung. Infect Immun. 2000;68:6879–6882. doi: 10.1128/iai.68.12.6879-6882.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Berry MP, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. First demonstration that Type I IFN signaling is associated with active tuberculosis in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cliff JM, et al. Distinct phases of blood gene expression pattern through tuberculosis treatment reflect modulation of the humoral immune response. J Infect Dis. 2013;207:18–29. doi: 10.1093/infdis/jis499. [DOI] [PubMed] [Google Scholar]
- 173.Maertzdorf J, et al. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 2011;12:15–22. doi: 10.1038/gene.2010.51. [DOI] [PubMed] [Google Scholar]
- 174.Ottenhoff TH, et al. Genome-wide expression profiling identifies type 1 interferon response pathways in active tuberculosis. PLoS One. 2012;7:e45839. doi: 10.1371/journal.pone.0045839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Antonelli LR, et al. Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest. 2010;120:1674–1682. doi: 10.1172/JCI40817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mayer-Barber KD, et al. Innate and adaptive interferons suppress IL-1alpha and IL-1beta production by distinct pulmonary myeloid subsets during Mycobacterium tuberculosis infection. Immunity. 2011;35:1023–1034. doi: 10.1016/j.immuni.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.McNab FW, et al. TPL-2-ERK1/2 signaling promotes host resistance against intracellular bacterial infection by negative regulation of type I IFN production. J Immunol. 2013;191:1732–1743. doi: 10.4049/jimmunol.1300146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Redford PS, et al. Influenza A virus impairs control of Mycobacterium tuberculosis coinfection through a type I interferon receptor-dependent pathway. J Infect Dis. 2014;209:270–274. doi: 10.1093/infdis/jit424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mayer-Barber KD, et al. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol. 2010;184:3326–3330. doi: 10.4049/jimmunol.0904189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.de Paus RA, et al. Inhibition of the type I immune responses of human monocytes by IFN-alpha and IFN-beta. Cytokine. 2013;61:645–655. doi: 10.1016/j.cyto.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 181.Novikov A, et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1beta production in human macrophages. J Immunol. 2011;187:2540–2547. doi: 10.4049/jimmunol.1100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McNab FW, et al. Type I IFN Induces IL-10 Production in an IL-27-Independent Manner and Blocks Responsiveness to IFN-gamma for Production of IL-12 and Bacterial Killing in Mycobacterium tuberculosis-Infected Macrophages. J Immunol. 2014;193:3600–3612. doi: 10.4049/jimmunol.1401088. Key study demonstrating mechanisms for Type I IFN adverse effects in tuberculosis, including blocking of protective Type II IFN action, IL-12, IL-1 and TNF production, in part through IL-10 induction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Guarda G, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. First study demonstrating inhibition of the inflammasome by Type I IFN. [DOI] [PubMed] [Google Scholar]
- 184.Mayer-Barber KD, et al. Host-directed therapy of tuberculosis based on interleukin-1 and type I interferon crosstalk. Nature. 2014;511:99–103. doi: 10.1038/nature13489. Seminal study showing counter regulatory function of IL-1 and type I IFNs in the control of the outcome of M. tuberculosis infection via eicosanoids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Xu XJ, Reichner JS, Mastrofrancesco B, Henry WL Jr, Albina JE. Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. J Immunol. 2008;180:2125–2131. doi: 10.4049/jimmunol.180.4.2125. First demonstration that Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Coulombe F, et al. Targeted prostaglandin E2 inhibition enhances antiviral immunity through induction of type I interferon and apoptosis in macrophages. Immunity. 2014;40:554–568. doi: 10.1016/j.immuni.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 187.Teles RM, et al. Type I interferon suppresses type II interferon-triggered human anti-mycobacterial responses. Science. 2013;339:1448–1453. doi: 10.1126/science.1233665. Key study demonstrating a mechanism for Type I IFN blocking the protective role of Type II IFN in tuberculosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Desvignes L, Wolf AJ, Ernst JD. Dynamic roles of type I and type II IFNs in early infection with Mycobacterium tuberculosis. J Immunol. 2012;188:6205–6215. doi: 10.4049/jimmunol.1200255. Important study showing that Type I IFN contributes to protection against M. tuberculosis when Type II IFN signaling is aberrant. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bogunovic D, et al. Mycobacterial disease and impaired IFN-gamma immunity in humans with inherited ISG15 deficiency. Science. 2012;337:1684–1688. doi: 10.1126/science.1224026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mariotti S, et al. Mycobacterium tuberculosis diverts alpha interferon-induced monocyte differentiation from dendritic cells into immunoprivileged macrophage-like host cells. Infect Immun. 2004;72:4385–4392. doi: 10.1128/IAI.72.8.4385-4392.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fernandes-Alnemri T, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat Immunol. 2010;11:385–393. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Henry T, Brotcke A, Weiss DS, Thompson LJ, Monack DM. Type I interferon signaling is required for activation of the inflammasome during Francisella infection. J Exp Med. 2007;204:987–994. doi: 10.1084/jem.20062665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Henry T, et al. Type I IFN signaling constrains IL-17A/F secretion by gammadelta T cells during bacterial infections. J Immunol. 2010;184:3755–3767. doi: 10.4049/jimmunol.0902065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shah S, et al. Cutting edge: Mycobacterium tuberculosis but not nonvirulent mycobacteria inhibits IFN-beta and AIM2 inflammasome-dependent IL-1beta production via its ESX-1 secretion system. J Immunol. 2013;191:3514–3518. doi: 10.4049/jimmunol.1301331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Al Moussawi K, et al. Type I interferon induction is detrimental during infection with the Whipple's disease bacterium, Tropheryma whipplei. PLoS Pathog. 2010;6:e1000722. doi: 10.1371/journal.ppat.1000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.de Almeida LA, et al. MyD88 and STING signaling pathways are required for IRF3-mediated IFN-beta induction in response to Brucella abortus infection. PLoS One. 2011;6:e23135. doi: 10.1371/journal.pone.0023135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Patel AA, Lee-Lewis H, Hughes-Hanks J, Lewis CA, Anderson DM. Opposing roles for interferon regulatory factor-3 (IRF-3) and type I interferon signaling during plague. PLoS Pathog. 2012;8:e1002817. doi: 10.1371/journal.ppat.1002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Robinson N, et al. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rathinam VA, et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell. 2012;150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Broz P, et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Martin FJ, et al. Staphylococcus aureus activates type I IFN signaling in mice and humans through the Xr repeated sequences of protein A. J Clin Invest. 2009;119:1931–1939. doi: 10.1172/JCI35879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Diefenbach A, et al. Type 1 interferon (IFNalpha/beta) and type 2 nitric oxide synthase regulate the innate immune response to a protozoan parasite. Immunity. 1998;8:77–87. doi: 10.1016/s1074-7613(00)80460-4. [DOI] [PubMed] [Google Scholar]
- 203.Mattner J, et al. Regulation of type 2 nitric oxide synthase by type 1 interferons in macrophages infected with Leishmania major. Eur J Immunol. 2000;30:2257–2267. doi: 10.1002/1521-4141(2000)30:8<2257::AID-IMMU2257>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 204.Mattner J, et al. Protection against progressive leishmaniasis by IFN-beta. J Immunol. 2004;172:7574–7582. doi: 10.4049/jimmunol.172.12.7574. [DOI] [PubMed] [Google Scholar]
- 205.Khouri R, et al. IFN-beta impairs superoxide-dependent parasite killing in human macrophages: evidence for a deleterious role of SOD1 in cutaneous leishmaniasis. J Immunol. 2009;182:2525–2531. doi: 10.4049/jimmunol.0802860. [DOI] [PubMed] [Google Scholar]
- 206.Xin L, et al. Type I IFN receptor regulates neutrophil functions and innate immunity to Leishmania parasites. J Immunol. 2010;184:7047–7056. doi: 10.4049/jimmunol.0903273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Haque A, et al. Type I interferons suppress CD4(+) T-cell-dependent parasite control during blood-stage Plasmodium infection. Eur J Immunol. 2011;41:2688–2698. doi: 10.1002/eji.201141539. [DOI] [PubMed] [Google Scholar]
- 208.Vigario AM, et al. Inhibition of Plasmodium yoelii blood-stage malaria by interferon alpha through the inhibition of the production of its target cell, the reticulocyte. Blood. 2001;97:3966–3971. doi: 10.1182/blood.v97.12.3966. [DOI] [PubMed] [Google Scholar]
- 209.Vigario AM, et al. Recombinant human IFN-alpha inhibits cerebral malaria and reduces parasite burden in mice. J Immunol. 2007;178:6416–6425. doi: 10.4049/jimmunol.178.10.6416. [DOI] [PubMed] [Google Scholar]
- 210.Voisine C, Mastelic B, Sponaas AM, Langhorne J. Classical CD11c+ dendritic cells, not plasmacytoid dendritic cells, induce T cell responses to Plasmodium chabaudi malaria. Int J Parasitol. 2010;40:711–719. doi: 10.1016/j.ijpara.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 211.Liehl P, et al. Host-cell sensors for Plasmodium activate innate immunity against liver-stage infection. Nature medicine. 2014;20:47–53. doi: 10.1038/nm.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Costa VM, et al. Type I IFNs stimulate nitric oxide production and resistance to Trypanosoma cruzi infection. J Immunol. 2006;177:3193–3200. doi: 10.4049/jimmunol.177.5.3193. [DOI] [PubMed] [Google Scholar]
- 213.Koga R, et al. TLR-dependent induction of IFN-beta mediates host defense against Trypanosoma cruzi. J Immunol. 2006;177:7059–7066. doi: 10.4049/jimmunol.177.10.7059. [DOI] [PubMed] [Google Scholar]
- 214.Lopez R, Demick KP, Mansfield JM, Paulnock DM. Type I IFNs play a role in early resistance, but subsequent susceptibility, to the African trypanosomes. J Immunol. 2008;181:4908–4917. doi: 10.4049/jimmunol.181.7.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Chessler AD, Caradonna KL, Da'dara A, Burleigh BA. Type I interferons increase host susceptibility to Trypanosoma cruzi infection. Infect Immun. 2011;79:2112–2119. doi: 10.1128/IAI.01176-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Une C, Andersson J, Orn A. Role of IFN-alpha/beta and IL-12 in the activation of natural killer cells and interferon-gamma production during experimental infection with Trypanosoma cruzi. Clin Exp Immunol. 2003;134:195–201. doi: 10.1046/j.1365-2249.2003.02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Biondo C, et al. IFN-alpha/beta signaling is required for polarization of cytokine responses toward a protective type 1 pattern during experimental cryptococcosis. J Immunol. 2008;181:566–573. doi: 10.4049/jimmunol.181.1.566. [DOI] [PubMed] [Google Scholar]
- 218.Biondo C, et al. Recognition of yeast nucleic acids triggers a host-protective type I interferon response. Eur J Immunol. 2011;41:1969–1979. doi: 10.1002/eji.201141490. [DOI] [PubMed] [Google Scholar]
- 219.del Fresno C, et al. Interferon-beta production via Dectin-1-Syk-IRF5 signaling in dendritic cells is crucial for immunity to C. albicans Immunity. 2013;38:1176–1186. doi: 10.1016/j.immuni.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 220.Majer O, et al. Type I interferons promote fatal immunopathology by regulating inflammatory monocytes and neutrophils during Candida infections. PLoS Pathog. 2012;8:e1002811. doi: 10.1371/journal.ppat.1002811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Bourgeois C, et al. Conventional dendritic cells mount a type I IFN response against Candida spp. requiring novel phagosomal TLR7-mediated IFN-beta signaling. J Immunol. 2011;186:3104–3112. doi: 10.4049/jimmunol.1002599. [DOI] [PubMed] [Google Scholar]
- 222.Inglis DO, Berkes CA, Hocking Murray DR, Sil A. Conidia but not yeast cells of the fungal pathogen Histoplasma capsulatum trigger a type I interferon innate immune response in murine macrophages. Infect Immun. 2010;78:3871–3882. doi: 10.1128/IAI.00204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu L, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. 2011;208:1635–1648. doi: 10.1084/jem.20110958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.van de Veerdonk FL, et al. STAT1 mutations in autosomal dominant chronic mucocutaneous candidiasis. N Engl J Med. 2011;365:54–61. doi: 10.1056/NEJMoa1100102. [DOI] [PubMed] [Google Scholar]
- 225.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Li W, Moltedo B, Moran TM. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. Journal of virology. 2012;86:12304–12312. doi: 10.1128/JVI.01269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nakamura S, Davis KM, Weiser JN. Synergistic stimulation of type I interferons during influenza virus coinfection promotes Streptococcus pneumoniae colonization in mice. J Clin Invest. 2011;121:3657–3665. doi: 10.1172/JCI57762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shahangian A, et al. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest. 2009;119:1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tian X, et al. Poly I:C enhances susceptibility to secondary pulmonary infections by gram-positive bacteria. PLoS One. 2012;7:e41879. doi: 10.1371/journal.pone.0041879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Navarini AA, et al. Increased susceptibility to bacterial superinfection as a consequence of innate antiviral responses. Proc Natl Acad Sci U S A. 2006;103:15535–15539. doi: 10.1073/pnas.0607325103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kim YG, et al. Viral infection augments Nod1/2 signaling to potentiate lethality associated with secondary bacterial infections. Cell Host Microbe. 2011;9:496–507. doi: 10.1016/j.chom.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Belkaid Y, Hand TW. Role of the Microbiota in Immunity and Inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ganal SC, et al. Priming of natural killer cells by nonmucosal mononuclear phagocytes requires instructive signals from commensal microbiota. Immunity. 2012;37:171–186. doi: 10.1016/j.immuni.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 234.Abt MC, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37:158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tschurtschenthaler M, et al. Type I interferon signalling in the intestinal epithelium affects Paneth cells, microbial ecology and epithelial regeneration. Gut. 2014;63:1921–1931. doi: 10.1136/gutjnl-2013-305863. [DOI] [PubMed] [Google Scholar]
- 236.Kawashima T, et al. Double-stranded RNA of intestinal commensal but not pathogenic bacteria triggers production of protective interferon-beta. Immunity. 2013;38:1187–1197. doi: 10.1016/j.immuni.2013.02.024. This study shows that microbiota contribute to initail production of protective Type I IFN. References 235 and 236 collectively demonstrate a novel interplay between microbiota, Type I IFN and consequent protection against pathogens. [DOI] [PubMed] [Google Scholar]
- 237.Gough DJ, Messina NL, Clarke CJ, Johnstone RW, Levy DE. Constitutive type I interferon modulates homeostatic balance through tonic signaling. Immunity. 2012;36:166–174. doi: 10.1016/j.immuni.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]