Abstract
In the present study, we investigated the glucose-decreasing action of lactic acid bacteria (LAB). The finding of this study could be helpful for people in controlling their blood sugar levels. The LAB candidate was isolated from a Japanese fermented food and identified as Pediococcus pentosaceus by an analysis of its genome sequence. Postprandial blood glucose elevation was investigated using oral starch tolerance tests in mice. Normal mice were fed starch and lyophilized cells of P. pentosaceus QU 19 at the same time. Even without pre-administration of P. pentosaceus QU 19, elevation of the blood glucose level was significantly suppressed by the intake of P. pentosaceus QU 19 at the same time as oral administration of starch. According to the results for its survival in simulated digestive juice and the reduction of blood glucose level in mice, P. pentosaceus QU 19 has potential hypoglycemic activity. In vitro measurements revealed that the glucose-decreasing action of P. pentosaceus QU 19 is probably caused by the glucose assimilation of the strain, not the inhibition of carbohydrate-splitting enzymes which has been reported for other LABs previously. These findings indicate that specific strains of LAB, especially P. pentosaceus QU 19, and foods fermented by LAB may be beneficial for people who must manage glucose ingestion.
Keywords: lactic acid bacteria, functional foods, fermented foods, mechanism of action, diabetes
INTRODUCTION
Lactic acid bacteria (LAB) microorganisms, most of which are generally regarded as safe, have been involved in human diets for a long time. They belong to the phylum Firmicutes and are characterized by the production of lactic acid as the main end product of carbohydrate metabolism. LAB microorganisms have been used extensively for long-term preservation. For example, milk has been made into cheese or yogurt by using Lactococcus strains and preserved for a long-term. Lactococcus strains are used in the production of cheese and yogurt. Fermented vegetables such as kimchi and nukazuke are prepared using Lactobacillus and Pediococcus strains.
Functions of LAB in the human body are not limited to intestine conditioning [1,2,3]. According to the literature, LAB also has functions such as immunostimulation [4,5,6], cholesterol reduction [7, 8], reduction of high blood pressure [9, 10], and reduction of carcinogenic risks [11,12,13].
Changing cultural habits have led to an increase in the incidence of diabetes mellitus. Diabetes is classified into two main forms. Type 1 diabetes mellitus (T1DM) results from destruction of the insulin-producing beta cells. Type 2 diabetes mellitus (T2DM) involves abnormally high blood glucose levels and is one of the most common chronic diseases. Almost 90% of diabetic patients suffer from T2DM [14]. Blood sugar control is recommended for T2DM patients and pre-T2DM people.
Several studies have reported that LAB lowers the blood sugar level, but the mechanisms are poorly understood. Tabuchi et al. [15] reported that the continuous administration of Lactobacillus rhamnosus strain GG ATCC53103 (L. rhamnosus GG) cells lowered the blood HbA1C levels and improved glucose tolerance in T2DM rats. Viable L. rhamnosus GG cells were also found to improve hyperinsulinemia in T2DM mice and decrease the blood glucose levels of normal and T2DM mice [16]. Dahi, an Indian yogurt containing Lactobacillus acidophilus and Lactobacillus casei, was reported to significantly delay the progression of diabetes in high fructose-induced T2DM rats [17]. Yun et al. [18] reported that Lactobacillus gasseri BNR17 decreased blood glucose levels gradually in T2DM mice. Lactobacillus strains were also found to have inhibitory activity against carbohydrate-splitting enzymes and to show anti-diabetic effects in T2DM mice [19] and normal SD rats [20]. Maintaining blood glucose levels is effective for not only treating diabetic patients but also preventing healthy people from developing diabetes.
Although there have been reports that the symptoms of diabetes are improved by ingesting LABs as probiotics, further studies are needed to identify the LAB strains involved and to determine the underlying mechanisms [21].
In this study, we focused on the use of LAB to prevent healthy people from developing diabetes. The LAB were isolated from a traditional fermented pickled turnip (Tsuda-Kabu Zuke), from which the cholesterol-lowering probiotic Lactobacillus brevis has previously been obtained [22]. After survival tests of the isolate in simulated digestive juices, the strain was administered to normal mice, with the aim of searching for new LAB that can suppress the rise in postprandial blood glucose level without requiring long-term administration. We investigated the mechanism of action under the conditions in which a significant difference was obtained.
MATERIALS AND METHODS
Isolation and identification of LAB strains
LAB candidates were isolated from a traditional fermented pickled turnip, Tsuda-Kabu Zuke, which is a specialty product of Shimane Prefecture, Japan. It is made by pickling turnips in rice-bran paste and salt for one week at room temperature in late fall and early winter. A diluted solution of the pickle juice was spread on MRS agar (BD, Franklin Lakes, NJ, USA) containing 5 ppm sodium azide and 50 ppm cycloheximide. A single colony was isolated and investigated using Gram staining, catalase, and oxidase tests. The isolate was identified to genus level using 16S-rDNA (1,600 bp), and DNA sequencing analysis was performed by FASMAC Corporation (Kanagawa, Japan).
Preparation of lyophilized cells
Each strain was cultured in MRS broth (BD) and incubated for 18 hr at 30°C. After cultivation, the cells were harvested by centrifugation at 18,000 g for 10 min and washed twice with distilled water. The cells were lyophilized at −40°C overnight.
Survival in simulated digestive juice
The experiments were performed as described in a previous report with minor modifications [23,24,25].
The simulated gastric juice tolerance test was performed as follows: MRS broth was transferred into sterile tubes, and the broth pH was adjusted to 3.0 using 1N-HCl. After the addition of 0.04% pepsin from the porcine stomach mucosa (10,000 U/mg, FUJIFILM Wako Pure Chemical, Osaka, Japan), we added 1% (v/v) of lyophilized cells of each strain. The cells were incubated at 37°C for 2 hr, and the viable cell count was measured over time.
To evaluate the simulated intestinal juice tolerance, cells treated for 2 hr with acid-pepsin as previously described were added to MRS medium containing a 3% bile concentration using oxgall powder (BD). The pH was adjusted to 7.0 using 5N-NaOH. The cells were cultured at 37°C under anaerobic conditions for 18 hr. The growth rate of viable cells was calculated from the increase in the number of viable bacteria after the two-hour treatment with acid-pepsin.
In the survival test, six strains were examined. A Pediococcus strain (P. pentosaceus QU 19) and Weissella strain were isolated from the Tsuda-Kabu pickle. Pediococcus pentosaceus NBRC107768T, Pediococcus acidilactici NBRC109619T, and Weissella confusa NBRC106469T were used as the type strains for comparisons with the above strains. Also, Lactococcus lactis subsp. lactis NBRC100933T was selected as a control, since it is often used well for lactic acid fermentation.
Species identification of the Pediococcus strain
The species identification of the Pediococcus strain from the 16S rDNA was conducted using an API 50 CHL test kit (bioMérieux, Marcy l´Etoile, France) according to the manufacturer’s instructions. Next, we used whole-genomic sequence analysis, which was carried out by Takara Bio Inc. (Shiga, Japan). Briefly, the sequence was analyzed by using a PacBio RS II (Pacific Biosciences, Menlo Park, CA, USA), and the obtained reads were assembled by a genome assemble algorithm, SMRT Analysis v2.3.0 (Pacific Biosciences, Menlo Park, CA, USA). The obtained contig sequences including the 16S rRNA gene sequence were further analyzed by NCBI BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Single administration of the carbohydrate tolerance test in normal mice
Approximately 100 six-week-old male ICR normal mice (SLC Japan, Shizuoka, Japan) were obtained for the animal tests. The mice were housed in a special room with constant temperature conditions (23 ± 3°C) and 55 ± 5% humidity on a 12 hr light and dark cycle. Drinking water and a standard rodent maintenance diet (Labo MR Stock, Nosan Corp., Yokohama, Japan) were freely available to the mice.
After a week of acclimatization, the mice could be used in testing without any difficulties. Mice that had been fasted overnight for 20 hr were divided into two groups (n=10), a control group and a lyophilized cell group. The control group was administrated 0.2 mL of carbohydrate solution (2 g/kg body weight), and the lyophilized cells group was administrated 0.2 mL of a mixture of a carbohydrate (2 g/kg body weight) and lyophilized cells. Soluble starch was used as the carbohydrate. Lyophilized cells were fed at 0 (control), 62.5, 125, 250, or 500 mg/kg body weight. The effect of heat-treated cells was tested in the same way as lyophilized cells. Heat-treated cells were made from viable cells that were heated at 80°C for 10 min. Blood samples were collected from the tail vein at 0, 30, 60, 90, and 120 min after administration, and the glucose levels were determined by a glucose analyzer (DKK-TOA, Tokyo, Japan).
Differences were considered significant at p<0.01 and p<0.05, compared with the control group, by Dunnett’s test. The area under the curve for glucose (AUCglucose) was determined using the trapezoidal rule.
This study was performed in strict accordance with the ethics code for animal experiments at the Tama Laboratory of Japan Food Research Laboratories (JFRL, Tokyo, Japan), which is based on The Standard for Care and Management of Laboratory Animals and Alleviation of Pain (Notice No. 88 of Ministry of the Environment, 2006). All animal experiments were approved by the Animal Experiments Ethics Committee of Tama Laboratory, JFRL, Japan.
In vitro measurement of α-glucosidase and α-amylase inhibition
The inhibitory activity on α-glucosidase was assessed by the amount of glucose released through maltose hydrolysis. It is known that Caco-2 cells derived from human colon carcinoma differentiate into intestinal epithelium-like cultures and express α-glucosidase. Using such features, the assay was carried out using the enzymes expressed on cells, as previously described [26, 27]. In addition to the viable cells of the isolated strain, Guava Tea Leaf Extract (GTLEx), which is known to be a glucosidase inhibitor [28], was used as a positive control. Caco-2 cells were seeded in 96-well plates and cultured for 15 days.
The inhibitory effect was investigated in detail using lyophilized cells of the isolated strain. The lyophilized cells were suspended in 0.025 M sodium phosphate buffer (pH 6.86) and added to the wells at final concentrations of 250, 125, and 62.5 µg/mL. Buffer solution without cells was used as a control. Maltose was added to the wells at a final concentration of 20 mmol/L and incubated at 37°C for 2 hr. The cell supernatant was then separated. The glucose concentration in the cell supernatant was measured using CII-Test Wako (Wako Pure Chemical Industries, Tokyo, Japan). Respective inhibitory activities were expressed relative to the control.
The maltose concentration of the substrate was measured before and after the α-glucosidase inhibitory activity test. Three hundred microliters of cell suspension (6 mg/mL) was prepared with the lyophilized LAB and 0.025 M sodium phosphate buffer (pH 6.86). The cell suspension was supplemented with 300 µL of rat intestinal acetone powder extract containing α-glucosidase and 300 µL of 2% maltose and incubated at 37°C for 60 min. Part of the reaction solution was removed, inactivated by treatment at 105°C for 10 min, and centrifuged at 18,000 g for 10 min. The supernatant was analyzed using a glucose analyzer (DKK-TOA). Subsequently, the maltose concentration in the supernatant was analyzed using a high-performance liquid chromatography system (Shimadzu, Kyoto, Japan) with a KS-802 column (Showa Denko Tokyo, Japan) and RID-10A differential refractometer (Shimadzu) under the following conditions: column temperature, 80°C; mobile phase, distilled water; and mobile phase flow rate, 1.0 mL/min. On the same day, viable LAB cells were counted to be used for the measurement of inhibition.
Determination of the activity of α-amylase inhibition was carried out according to a previously published previous method [29, 30] based on starch-iodine color changes, with minor modifications [31, 32]. One hundred and fifty microliters of α-amylase suspension (final concentration at 500 U/L in 0.025 M sodium phosphate buffer, pH 6.86) was added to a mixture of 150 µL of 1.5% soluble starch solution and 150 µL of 6 g/L LAB suspension. The enzyme reaction was performed at 37°C for 60 min. The same conditions were used for α-amylase blank determinations. One normal HCl was added to inactivate the enzyme. The enzyme mixture was centrifuged at 18,000 g for 10 min, after addition of iodine solution. Starch amylolysis was calculated at an absorbance of 595 nm before and after the reaction.
Glucose assimilation by LAB
Examination of glucose assimilation was performed in accordance with a previously published method [33], with minor modifications. LAB (5 mg/mL) was added to phosphate buffer containing 0.5% glucose, incubated at 37°C for 30 min, and then heat sterilized. After centrifugation at 18,000 g for 10 min, residual sugar was measured with a glucose analyzer. Specific glucose consumption was calculated with viable LAB cells (g/L•CFU). In the glucose assimilation assay, six strains were examined. Five strains, including P. pentosaceus QU 19, P. pentosaceus NBRC107768T, P. acidilactici NBRC109619T, W. confusa NBRC106469T, and L. lactis subsp. lactis NBRC100933T, were the same as described earlier in Survival in simulated digestive juice. Another one, Lactobacillus rhamnosus GG ATCC53103, was also selected, as it had been reported to suppress the rise in postprandial blood glucose levels in animal tests [16].
RESULTS
Isolation and identification of LAB strains
Many bacterial isolates were obtained from Tsuda-Kabu Zuke, and some of them were catalase negative on the MRS agar plate. From the catalase negative isolates, six colonies, including three white ones and three gray ones, were picked. White colonies were oxidase negative, and gray ones were oxidase positive. Both white and gray colonies were found to be Gram positive.
The white colonies showed more than 99.9% identity to Pediococcus acidilactici and Pediococcus pentosaceus using 16S rDNA (1,600 bp), while the strain comprising the gray colonies showed more than 99.4% identity to Weissella confusa.
Survival in simulated digestive juice
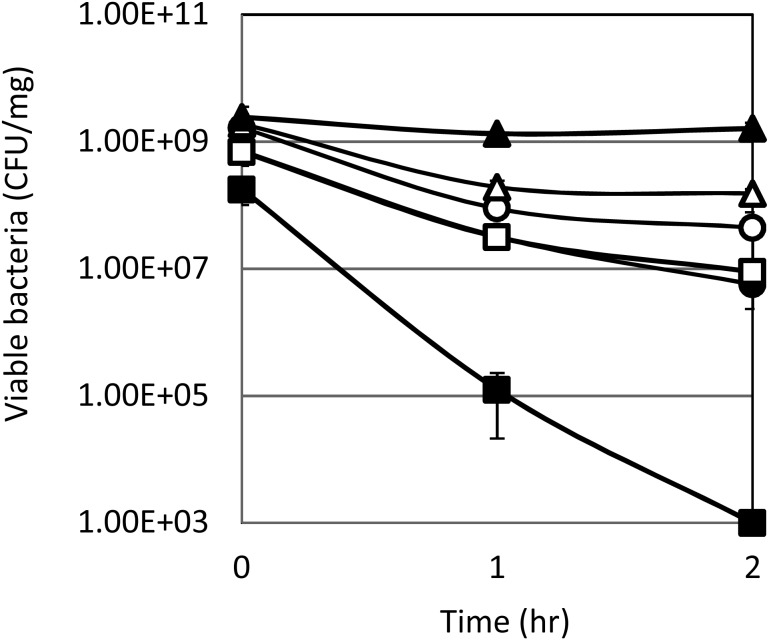
To understand the behavior of cells in the stomach and intestine, growth assays using simulated digestive juices were conducted. Human gastric juice was simulated using MRS broth containing 0.04% pepsin at pH 3.0. As shown in Fig. 1, the viable cells of most strains decreased to the same level, approximately 1–10%, at the end of the assay, while Lactococcus lactis subsp. lactis NBRC100933T decreased to less than 0.01%.
Fig. 1.
Survival of cells at pH 3.0 in MRS broth containing 0.04% pepsin.
MRS broth was transferred into sterile tubes, and the broth was adjusted to pH 3.0 using 1N-HCl. After the addition of 0.04% pepsin from the porcine stomach mucosa (10,000 U/mg, FUJIFILM Wako Pure Chemical, Osaka, Japan), 1% (v/v) lyophilized cells of each strain was added. The cells were incubated at 37°C for 2 hr, and the viable cell count was measured over time.
○, Pediococcus strain (later identified as P. pentosaceus QU 19); ●, Weissella strain; Δ, Pediococcus pentosaceus NBRC107768T; ▲, Pediococcus acidilactici NBRC109619T; □, Weissella confusa NBRC106469T; ■, L. lactis NBRC100933T.
Each value represents the mean ± SD of triplicate determinations.
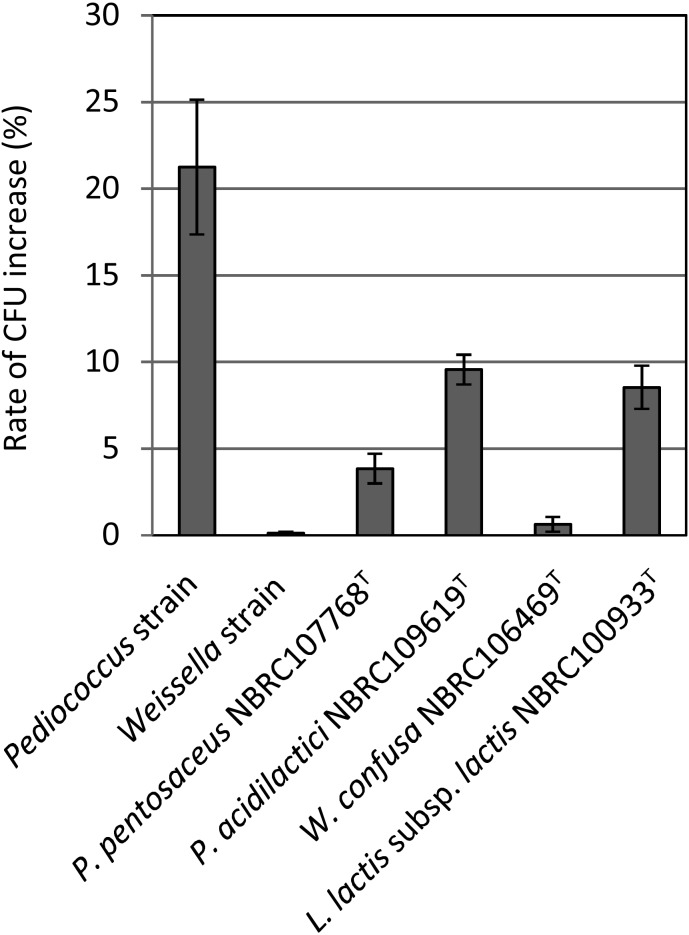
Human intestinal juice was simulated using MRS broth containing 0.3% of bile powder at pH 7.0. As shown in Fig. 2, the growths of the Weissella strain and Weissella confusa NBRC106469T were lower than those of the Pediococcus strain, Pediococcus pentosaceus NBRC107768T, and Pediococcus acidilactici NBRC109619T. In particular, there was a significant difference in the growth of the Weissella strain and the Pediococcus strain. The growth of the Pediococcus strain was the highest, at approximately 21%, while that of Weissella strain was the lowest, at approximately 0.1%.
Fig. 2.
Growth of viable cells in artificial intestinal juice.
Cells treated for 2 hr with acid-pepsin as previously described were added to MRS medium containing 0.3% oxgall powder. The pH was adjusted to 7.0 using 5N-NaOH. The cells were cultured at 37°C under anaerobic conditions for 18 hr. The growth rate of viable cells was calculated from the increase in the number of viable bacteria after 2 hr treatment with acid-pepsin. Straight lines above bars indicate the standard deviation (n=3). Pediococcus strain was later identified as P. pentosaceus QU 19.
Species identification of the Pediococcus strain
Various identification tests were performed on the Pediococcus sp. strains that were highly resistant to digestive fluids. According to the identification using API50, the isolated strain was predicted to be Pediococcus subsp. (data not shown). In addition, whole-genome sequence analysis revealed that the bit score against the complete genome of P. pentosaceus strain wikim 20 was the highest (Table 1). As shown in Fig. 3, the strain formed pairs or tetrads. Considering all these characteristics, the strain was determined to be P. pentosaceus and named P. pentosaceus QU 19 (NTE No. BP-02595).
Table 1. Genome information and bit scores of Pediococcus strains against strain QU 19.
| Rank | Accession number | Definition | Bit score |
|---|---|---|---|
| 1 | CP015918 | Pediococcus pentosaceus strain wikim 20, complete genome | 432,100 |
| 2 | CP006854 | Pediococcus pentosaceus strain SL4, complete genome | 414,500 |
| 3 | CP000422 | Pediococcus pentosaceus strain ATCC25745, complete genome | 369,300 |
| 4 | CP018763 | Pediococcus acidilactici strain BCC1, complete genome | 18,750 |
| 5 | CP015206 | Pediococcus acidilactici strain ZPA017, complete genome | 18,660 |
The chromosome of each strain was deposited in NCBI/GenBank under the accession number indicated.
Fig. 3.
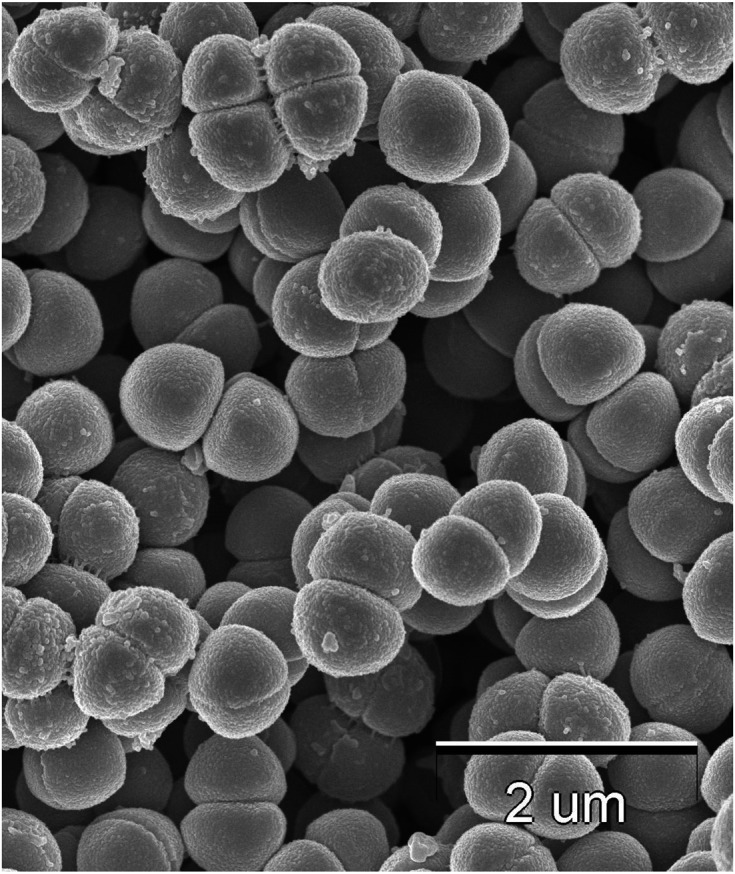
Scanning electron microscope image of Pediococcus pentosaceus QU 19.
Single administration of the carbohydrate tolerance tests in normal mice
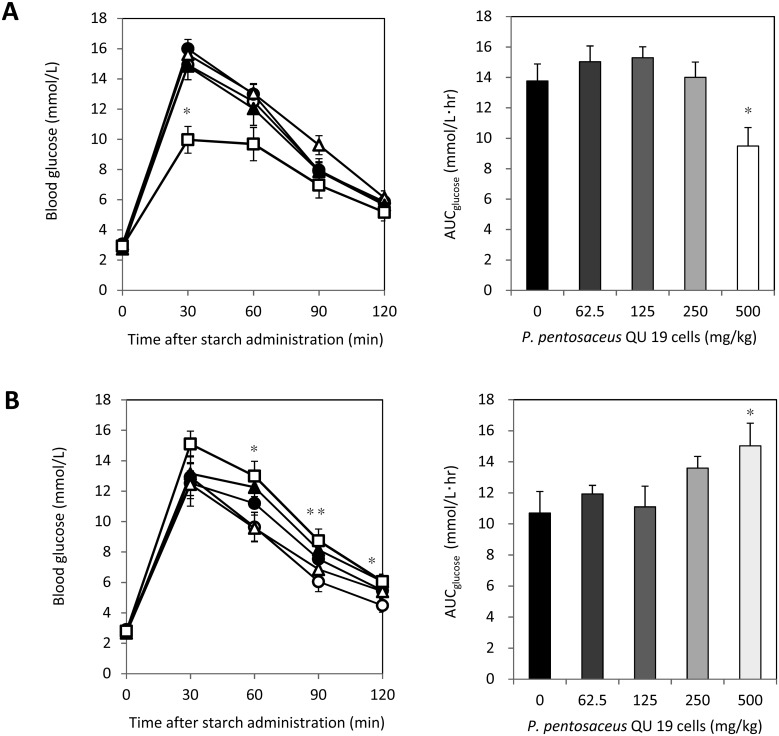
The results of the starch tolerance test are shown in Fig. 4. The blood glucose level at 30 min and the AUCglucose of the group administered 500 mg/kg of viable cells were markedly lower than those of the control group, whereas there was no significant difference for the values of the lower dosage groups at any time point in the time course or for AUCglucose (Fig. 4A). On the other hand, heat-treated cells tended to elevate the blood glucose levels, particularly after 60 min at the higher dose, and the group administered 500 mg/kg showed significantly higher AUCglucose than the control group (Fig. 4B, p<0.01).
Fig. 4.
Effects of viable P. pentosaceus QU 19 cells (A) and heat-treated cells (B) on the oral starch tolerance test and AUCglucose (mean ± SEM).
Blood samples were taken at 0, 30, 60, 90, and 120 min after the starch and cell loading. Symbols: ○, control; ●, 62.5 mg/kg; Δ, 125 mg/kg; ▲, 250 mg/kg; □, 500 mg/kg. Values with asterisks are significantly different from those of the control (0 mg/kg) group by Dunnett’s test (n=8). *p<0.01; **p<0.05. The area under the curve for glucose (AUCglucose) was determined using the trapezoidal rule.
In vitro measurement of α-glucosidase and α-amylase inhibition
Based on the results of the α-glucosidase inhibition assay using human Caco-2 cells, half maximal inhibitory concentration (IC50) values were calculated. The IC50 values revealed that the inhibitory effect of viable cells of P. pentosaceus QU 19 on the α-glucosidase activity (IC50 of 138.0 ± 16.8 mg/L) was significantly higher than that of GTLEx (IC50 of 200.0 ± 9.2 mg/L).
As shown in Table 2, the glucose concentration of the test solution without lyophilized cells of P. pentosaceus QU 19 was 0.148 mg/mL, whereas that of the solution with the cells was 0 mg/mL. However, Table 2 also shows that the maltose concentration of the test solution decreased to about 35% after incubation for 60 min, regardless of the addition of P. pentosaceus QU 19. In addition, after incubation, the pH of the solution with QU 19 was 4.75, lower than that without the cells (6.95). It had shifted largely to being acidic. Glucosidase inhibition was dependent on the number of viable cells in the lyophilized QU 19 sample (Table 3).
Table 2. α-Glucosidase inhibitory activities of viable P. pentosaceus QU 19 cells.
| Strain | Reaction time (min) |
Glucose (mg/mL) |
Maltose (mg/mL) |
Maltose hydrolysis (%) |
pH after reaction |
|---|---|---|---|---|---|
| QU 19 | 0 | 0.007 | 0.751 | ND | 6.86 |
| QU 19 | 60 | 0.000 | 0.496 | 34.0 | 4.75 |
| − | 0 | 0.006 | 0.746 | ND | 6.96 |
| − | 60 | 0.248 | 0.480 | 35.6 | 6.95 |
The inhibitory effect of viable P. pentosaceus QU 19 cells (2 mg/mL) on maltose hydrolysis was determined with or without strain QU 19 added to the corresponding enzyme reaction mixture. Maltose and glucose concentrations were measured by HPLC. The hydrolysis rate of maltose was calculated by the decrease in maltose concentration. ND: not detected.
Table 3. Relation between α-glucosidase inhibitory activity and the number of viable cells.
| Lot | α-Glucosidase inhibitory activity (%) | Viable cells (CFU/mg) |
|---|---|---|
| A batch | 47 | 1.40E + 08 |
| B batch | 24 | 3.00E + 07 |
| C batch | 97 | 4.00E + 08 |
Inhibitory activities are expressed as relative to those without the substance, which was taken as 100%. On the same day, viable LAB cells were counted and used for the measurement of inhibition.
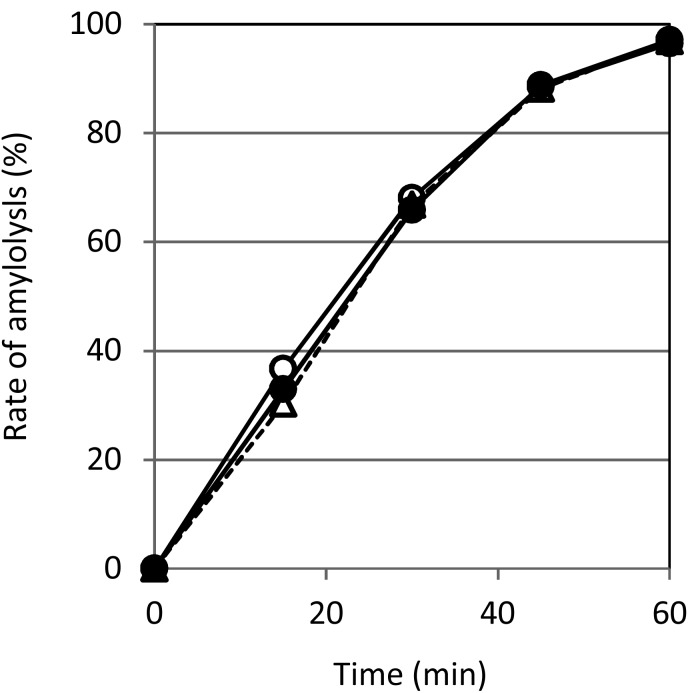
In the assay of α-amylase inhibition, there was no significant difference in amylolysis rates between the solutions with viable cells, heat-treated cells, and no cells (Fig. 5).
Fig. 5.
Effects of P. pentosaceus QU 19 cells on amylolysis.
The rates of soluble starch hydrolysis by α-amylase and P. pentosaceus QU 19 cells were calculated from the absorbance (595 nm) of the solutions before and after the reaction. Symbols: ○, control; ●, viable cells; Δ, heat-treated cells.
Glucose assimilation by LAB
Table 4 shows the glucose assimilation ability of LAB. L. rhamnosus GG, which has been reported to be a strain capable of reducing postprandial blood glucose levels [33] and was used for comparison in this study. P. pentosaceus QU 19 showed higher glucose assimilation ability than the other LAB, including L. rhamnosus GG.
Table 4. Specific glucose consumption by viable LAB cells.
| Specific glucose consumption (g/L•CFU) | |
|---|---|
| Pediococcus pentosaceus QU 19 | 5.46E–10 |
| Pediococcus pentosaceus NBRC107768T | 7.96E–11 |
| Pediococcus acidilactici NBRC109619T | 3.29E–10 |
| Wissella confusa NBRC106469T | 4.34E–10 |
| Lactococcus lactis subsp. lactis NBRC100933T | 3.26E–10 |
| Lactobacillus rhamnosus strain GG ATCC53103 | 1.92E–10 |
One milliliter of 0.5% glucose solution was incubated with various LAB at 37°C for 30 min. The residual sugar concentration was measured with a glucose meter, and the specific glucose consumption was calculated (g/L•CFU). The number of viable cells was counted using the plate count method.
DISCUSSION
In this study, P. pentosaceus QU 19, which was able to grow in the simulated digestive juice, was isolated from a Japanese Tsuda-Kabu pickle. We found a reduction in the blood glucose level of mice after a meal with viable P. pentosaceus QU 19, without requiring long-term administration. Thus, it was suggested that a single administration of P. pentosaceus QU 19 after a meal can reduce the blood glucose level.
Several previous studies have focused on preventing or treating T2DM and its complications using LAB [15,16,17,18]. There are several theories concerning the mechanism of action of LAB with regard to the reduction of blood glucose after a meal, and one of them is the concept of a carbohydrate-splitting enzyme inhibitor produced by LAB [18]. To confirm whether the addition of P. pentosaceus QU 19 causes inhibition of carbohydrate-splitting enzymes, we evaluated the inhibitory effect of the strain toward α-amylase and α-glucosidase. In the assay of α-amylase inhibition, the addition of P. pentosaceus QU 19 cells did not delay starch degradation, regardless of whether the cells were viable or not (Fig. 5), indicating that P. pentosaceus QU 19 had no inhibitory activity toward α-amylase. In contrast, in the assay of α-glucosidase inhibition, there was a significant difference in the concentration of glucose between the solutions with and without viable P. pentosaceus QU 19 cells (Table 2). Since a certain amount of glucose remained in the solution without viable cells, but not in the solution with viable cells, it seemed that the addition of the viable cells delayed maltose hydrolysis by α-glucosidase. However, the maltose concentration was almost the same regardless of whether viable cells were added or not (Table 2). The degree of glucosidase inhibition was dependent on the number of viable P. pentosaceus QU 19 cells (Table 3). As P. pentosaceus QU 19 showed no ability to assimilate maltose (data not shown), it was suggested that the addition of the viable cells did not cause inhibitory activity toward α-glucosidase and α-amylase. With viable cells added to the solution, the pH was largely shifted to being acidic after 60 min (Table 2). Actually, lactic acid was detected in the solution (data not shown). These observations suggested that P. pentosaceus QU 19 had assimilated glucose to produce lactic acid but did not inhibit carbohydrate-splitting enzymes. It appears that P. pentosaceus QU 19 assimilated glucose, the final product of carbohydrate hydrolysis, in mouse gastrointestinal tracts immediately after a meal. As a result, glucose absorption from the intestine of mice was decreased in mice, which suppressed the elevation of the blood glucose level.
If the hypothesis that the reduction of blood glucose is caused by glucose assimilation by P. pentosaceus QU 19 is true, the reduction occurs only when the cells are viable. To confirm whether the life or death of the cells affects the changes in the blood glucose level, we administered viable or heat-treated P. pentosaceus QU 19 cells to fasted mice simultaneously with a carbohydrate solution. In previous studies, a monosaccharide such as glucose was often administered to mice when conducting the tolerance test. However, we employed a starch solution in this study because it is more similar to the actual meals we eat than monosaccharide. The heat-killed cells showed a trend of AUCglucose elevation at the higher dose (Fig. 4B). Some components of P. pentosaceus QU 19 cells may promote glucose transfer into the blood and increase AUCglucose, although the mechanism is unclear. Nevertheless, despite the counter action, the viable P. pentosaceus QU 19 cells clearly suppressed the rise in blood glucose level at the high dose.
To investigate the detailed mechanisms of P. pentosaceus QU 19 in the suppression of the rise in the blood glucose level, we conducted survival tests in simulated digestive juices. Generally, the pH in the fasted human stomach is maintained at 1.0–2.0 by gastric acid and is highly bactericidal. However, after ingesting 180 mL of milk, the pH rises to 3.0–4.0, and in the case of ingesting a normal meal, such as meat and vegetables, it can rise to 4.0–5.0. In addition, since it usually takes about 2 hr for digested food to be completely transferred to the duodenum from the stomach, the treatment time was limited to 2 hr in the present study [24]. Furthermore, the survival test in artificial gastric juice was examined under conditions of pH 3.0. In the human intestine, the amount of bile secretion is dependent on the type of food ingested, and the concentration of bile varies according to the part of the intestinal tract. Generally, it is difficult to estimate the bile concentration in the intestinal tract. However, a previous study reported that the maximum bile concentration in the intestinal tract is 2% [34]. Thus, the survival test was conducted under concentrations of 3% bile (1.5 times the maximum concentration in the intestinal tract) in this study.
In the survival tests, P. pentosaceus QU 19 showed good tolerance both in the artificial gastric juice and the artificial intestinal juice. In the artificial gastric juice, the viable cells of most strains decreased to the same level, approximately 1–10% at the end of the assay (Fig. 1). P. pentosaceus QU 19 grew faster in the artificial intestinal juice than any other strains, including P. pentosaceus NBRC107768T, other strains of the same species, and Pediococcus acidilactici NBRC109619T, which is known for its acid tolerance (Fig. 2). These results suggested that P. pentosaceus QU 19 survived passage through the stomach to reach the intestine in a viable condition at the same time as foods and assimilated glucose rapidly inside the animal body, moderating glucose absorption from the intestine and the subsequent rise in blood glucose level. The growth of the Weissella strain was very poor in the artificial intestinal juice despite the fact that its origin was the same as that of P. pentosaceus QU 19 (Fig. 2). Animal tests were also conducted with the Weissella strain, but as expected, there was no significant difference from the results of the control group (data not shown), which were fed without lyophilized cells.
In a previous study, it was reported that L. rhamnosus GG suppressed the rise in postprandial blood glucose levels in animal tests [16]. There is also a report indicating that continuous administration of lyophilized cells of L. rhamnosus GG suppressed the rise in blood glucose level, which suggested glucose assimilation by the strain [33]. However, these studies did not include actual data from in vitro tests showing a decrease in the glucose concentration of the test solution with the cells. We compared the glucose assimilation ability of P. pentosaceus QU 19 with that of other LAB, including L. rhamnosus GG. The glucose assimilation rate of P. pentosaceus QU 19 was the highest among all six LAB evaluated (Table 4). P. pentosaceus QU 19 has a greater ability to assimilate glucose and is expected to suppress the rise in blood glucose level more rapidly than L. rhamnosus GG. Since P. pentosaceus QU 19 grows well in the intestine, it is not necessary to administer the cells continuously. The lyophilized L. rhamnosus GG cells needed to be administered continuously for 14 days to suppress the rise in the blood glucose level [33]. P. pentosaceus QU 19 is a spherical LAB, not a rod-shaped one like L. rhamnosus GG. The present study suggests that even spherical LAB may show a suppression effect on the rise of blood glucose level based on carbohydrate assimilation if they are tolerant to the digestive juices.
Whole-genome sequence analysis revealed that the number of genes related to sugar metabolism in the P. pentosaceus QU 19 genome is larger than that in other sequenced Pediococcus strains (data not shown). Therefore, P. pentosaceus QU 19 appears to be a novel strain, showing a superior ability to assimilate sugars, especially glucose.
In this study, we successfully isolated a novel LAB, P. pentosaceus QU 19, from a Japanese Tsuda-Kabu pickle. Administering viable cells with a meal showed a suppressive effect on the rise of blood glucose in mice. It is notable that even a single administration of the strain can reduce blood glucose, since P. pentosaceus QU 19 is tolerant of digestive juices and able to grow by assimilating glucose rapidly in the intestine. Although in vivo tests were conducted using mice, the same effect is expected in the human body based on the results of the survival tests in artificial digestive juices. Thus, P. pentosaceus QU 19 has potential as an effective means of maintaining blood glucose levels for preventing diabetes in healthy people. This study has potential limitations. Although the effects in the model were estimated based on previous studies, the data for metabolites during glucose assimilation was lacking. The number of viable P. pentosaceus QU 19 cells in the intestine should be determined. The mechanism of the rise in glucose level induced by the administration of a small amount of viable P. pentosaceus QU 19 cells also needs to be studied with reference to the data above. To demonstrate the effect of lowering the blood glucose level via P. pentosaceus QU 19 for not only healthy subjects but also for diabetics, further testing using a diabetic model mouse should be conducted. We believe that further study of this strain will contribute to human health in the future.
CONFLICTS OF INTEREST
There is no conflict of interest declared.
Acknowledgments
The authors would like to thank Yuji Aso (Kyoto Institute of Technology, Japan) for technical assistance with the experiments. We also thank Syuuichi Mihashi and Takeshi Uemura (JXTG Nippon Oil & Energy Corp., Japan) for discussions which greatly assisted the research.
REFERENCES
- 1.Vanderhoof JA, Whitney DB, Antonson DL, Hanner TL, Lupo JV, Young RJ. 1999. Lactobacillus GG in the prevention of antibiotic-associated diarrhea in children. J Pediatr 135: 564–568. [DOI] [PubMed] [Google Scholar]
- 2.Xiao SD, Zhang DZ, Lu H, Jiang SH, Liu HY, Wang GS, Xu GM, Zhang ZB, Lin GJ, Wang GL. 2003. Multicenter, randomized, controlled trial of heat-killed Lactobacillus acidophilus LB in patients with chronic diarrhea. Adv Ther 20: 253–260. [DOI] [PubMed] [Google Scholar]
- 3.Olivares M, Díaz-Ropero MA, Gómez N, Lara-Villoslada F, Sierra S, Maldonado JA, Martín R, López-Huertas E, Rodríguez JM, Xaus J. 2006. Oral administration of two probiotic strains, Lactobacillus gasseri CECT5714 and Lactobacillus coryniformis CECT5711, enhances the intestinal function of healthy adults. Int J Food Microbiol 107: 104–111. [DOI] [PubMed] [Google Scholar]
- 4.Shida K, Makino K, Morishita A, Takamizawa K, Hachimura S, Ametani A, Sato T, Kumagai Y, Habu S, Kaminogawa S. 1998. Lactobacillus casei inhibits antigen-induced IgE secretion through regulation of cytokine production in murine splenocyte cultures. Int Arch Allergy Immunol 115: 278–287. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara D, Inoue S, Wakabayashi H, Fujii T. 2004. The anti-allergic effects of lactic acid bacteria are strain dependent and mediated by effects on both Th1/Th2 cytokine expression and balance. Int Arch Allergy Immunol 135: 205–215. [DOI] [PubMed] [Google Scholar]
- 6.Kalliomäki MA, Isolauri E. 2004. Probiotics and down-regulation of the allergic response. Immunol Allergy Clin North Am 24: 739–752, viii. [DOI] [PubMed] [Google Scholar]
- 7.Pereira DI, McCartney AL, Gibson GR. 2003. An in vitro study of the probiotic potential of a bile-salt-hydrolyzing Lactobacillus fermentum strain, and determination of its cholesterol-lowering properties. Appl Environ Microbiol 69: 4743–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen TD, Kang JH, Lee MS. 2007. Characterization of Lactobacillus plantarum PH04, a potential probiotic bacterium with cholesterol-lowering effects. Int J Food Microbiol 113: 358–361. [DOI] [PubMed] [Google Scholar]
- 9.Meisel H, Bockelmann W. 1999. Bioactive peptides encrypted in milk proteins: proteolytic activation and thropho-functional properties. Antonie van Leeuwenhoek 76: 207–215. [PubMed] [Google Scholar]
- 10.Minervini F, Algaron F, Rizzello CG, Fox PF, Monnet V, Gobbetti M. 2003. Angiotensin I-converting-enzyme-inhibitory and antibacterial peptides from Lactobacillus helveticus PR4 proteinase-hydrolyzed caseins of milk from six species. Appl Environ Microbiol 69: 5297–5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rafter JJ. 1995. The role of lactic acid bacteria in colon cancer prevention. Scand J Gastroenterol 30: 497–502. [DOI] [PubMed] [Google Scholar]
- 12.Pool-Zobel BL, Neudecker C, Domizlaff I, Ji S, Schillinger U, Rumney C, Moretti M, Vilarini I, Scassellati-Sforzolini R, Rowland I. 1996. Lactobacillus- and bifidobacterium-mediated antigenotoxicity in the colon of rats. Nutr Cancer 26: 365–380. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi Y, Nakai S, Tsukamoto T, Masumori N, Akaza H, Miyanaga N, Kitamura T, Kawabe K, Kotake T, Kuroda M, Naito S, Koga H, Saito Y, Nomata K, Kitagawa M, Aso Y. 2002. Habitual intake of lactic acid bacteria and risk reduction of bladder cancer. Urol Int 68: 273–280. [DOI] [PubMed] [Google Scholar]
- 14.Zimmet P, Alberti KG, Shaw J. 2001. Global and societal implications of the diabetes epidemic. Nature 414: 782–787. [DOI] [PubMed] [Google Scholar]
- 15.Tabuchi M, Ozaki M, Tamura A, Yamada N, Ishida T, Hosoda M, Hosono A. 2003. Antidiabetic effect of Lactobacillus GG in streptozotocin-induced diabetic rats. Biosci Biotechnol Biochem 67: 1421–1424. [DOI] [PubMed] [Google Scholar]
- 16.Honda K, Moto M, Uchida N, He F, Hashizume N. 2012. Anti-diabetic effects of lactic acid bacteria in normal and type 2 diabetic mice. J Clin Biochem Nutr 51: 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yadav H, Jain S, Sinha PR. 2007. Antidiabetic effect of probiotic dahi containing Lactobacillus acidophilus and Lactobacillus casei in high fructose fed rats. Nutrition 23: 62–68. [DOI] [PubMed] [Google Scholar]
- 18.Yun SI, Park HO, Kang JH. 2009. Effect of Lactobacillus gasseri BNR17 on blood glucose levels and body weight in a mouse model of type 2 diabetes. J Appl Microbiol 107: 1681–1686. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Wang N, Yin B, Fang D, Jiang T, Fang S, Zhao J, Zhang H, Wang G, Chen W. 2016. Effects of Lactobacillus plantarum CCFM0236 on hyperglycaemia and insulin resistance in high-fat and streptozotocin-induced type 2 diabetic mice. J Appl Microbiol 121: 1727–1736. [DOI] [PubMed] [Google Scholar]
- 20.Panwar H, Calderwood D, Grant IR, Grover S, Green BD. 2014. Lactobacillus strains isolated from infant faeces possess potent inhibitory activity against intestinal alpha- and beta-glucosidases suggesting anti-diabetic potential. Eur J Nutr 53: 1465–1474. [DOI] [PubMed] [Google Scholar]
- 21.Evivie SE, Huo GC, Igene JO, Bian X. 2017. Some current applications, limitations and future perspectives of lactic acid bacteria as probiotics. Food Nutr Res 61: 1318034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe S, Katsube T, Sonomoto K. 2012. Cholesterol-lowering effects of Lactobacillus brevis isolated from turnip “tsuda kabu”. Food Sci Technol Res 18: 825–834. [Google Scholar]
- 23.Takiguchi R, Suzuki Y. 2000. Survival of lactic acid bacteria in simulated digestive juice. J Intestinal Microbiol 14: 11–18 (in Japanese). [Google Scholar]
- 24.Azuma Y, Ito K, Sato M. 2001. Simulated digestive juice tolerance and inhibitory effect on harmful intestinal bacteria of Lactobacillus gasseri NY0509 and Lactobacillus casei NY1301. J Jpn Soc Food Sci and Tec 48: 656–663 (in Japanese). [Google Scholar]
- 25.Huang Y, Adams MC. 2004. In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int J Food Microbiol 91: 253–260. [DOI] [PubMed] [Google Scholar]
- 26.Han J, Inoue S, Isoda H. 2007. Effects of silkworm powder on glucose absorption by human intestinal epithelial cell line Caco-2. J Nat Med 61: 387–390. [Google Scholar]
- 27.Chang TC, Huang SF, Yang TC, Chan FN, Lin HC, Chang WL. 2007. Effect of ginsenosides on glucose uptake in human Caco-2 cells is mediated through altered Na+/glucose cotransporter 1 expression. J Agric Food Chem 55: 1993–1998. [DOI] [PubMed] [Google Scholar]
- 28.Deguchi Y, Miyazaki K. 2010. Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr Metab (Lond) 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao WW, Kinsella JE. 1981. Amylase activity in banana fruit: properties and changes in activity with ripening. J Food Sci 46: 1400–1403, 1409. [Google Scholar]
- 30.Kotowaroo MI, Mahomoodally MF, Gurib-Fakim A, Subratty AH. 2006. Screening of traditional antidiabetic medicinal plants of Mauritius for possible alpha-amylase inhibitory effects in vitro. Phytother Res 20: 228–231. [DOI] [PubMed] [Google Scholar]
- 31.Deguchi Y, Osada K, Uchida K, Kimura H, Yoshikawa M, Kudo T, Yasui H, Watanuki M. 1998. Effects of extract of guava leaves on the development of diabetes in the db/db mouse and on the postprandial blood glucose of human subjects. Nippon Nogeikagaku Kaishi 72: 923–931 (in Japanese). [Google Scholar]
- 32.Xiao Z, Storms R, Tsang A. 2006. A quantitative starch-iodine method for measuring alpha-amylase and glucoamylase activities. Anal Biochem 351: 146–148. [DOI] [PubMed] [Google Scholar]
- 33.Tabuchi M, Morita H, He F, Hosoda M, Yamada N, Ishida T. 2005. Effect of administration of Lactobacillus GG on postprandial blood glucose levels in rats. Milk Science 54: 17–21. [Google Scholar]
- 34.Davenport HW. 2006. Physiology of the Digestive Tract 4th edition, Johnson L, Barrett K, Ghishan F, Merchant J, Said H, Wood J (eds), Academic Press, Cambridge, pp. 232. [Google Scholar]