Abstract
Recently many researchers have revealed that certain lactic acid bacteria (LAB) have beneficial effects on the immune system. Understanding the mechanisms of how certain LAB induce immunomodulatory functions is important for the development of food ingredients that improve our health. Lactobacillus plantarum OLL2712 has been shown to induce production of interleukin (IL)-10, an anti-inflammatory cytokine, by murine in vitro-induced dendritic cells (DCs) and peritoneal macrophages. However, it is probable that in vitro-induced DCs have different properties compared with intestinal DCs, and the effects of the LAB on intestinal DCs are not fully understood. In this report, we investigated whether L. plantarum OLL2712 had efficacy for inducing intestinal DCs to produce IL-10 in vitro and whether oral administration of the bacteria induced the same effect. Co-culture of L. plantarum OLL2712 with purified DCs from the mesenteric lymph node (MLN) or Peyer’s patch (PP) elevated IL-10 mRNA expression and protein production by both kinds of DCs. Addition of the LAB enhanced IL-10 production by T cells during antigen-specific responses in co-culture of MLN or PP DCs and T cells. Oral administration of L. plantarum OLL2712 for 6 days increased IL-10 gene expression in MLN DCs, and upregulated IL-10 gene expression in PP DCs was observed 12 hr after oral administration of the LAB. Our results suggested that L. plantarum OLL2712 could modulate immune responses by enhancing IL-10 production from intestinal DCs.
Keywords: lactic acid bacteria, IL-10, dendritic cells, Lactobacillus plantarum, intestine
INTRODUCTION
Recently many researchers have revealed that certain lactic acid bacteria (LAB) have beneficial effects on the immune system [1, 2]. Several LAB strains have been reported to have anti-inflammatory effects and can be a preventive or therapeutic for chronic inflammation, such as metabolic disorders [3]. Understanding the mechanism by which LAB suppresses inflammation is important for the development of food ingredients that have more effective immunomodulatory functions.
A previous study showed that a LAB strain, Lactobacillus plantarum OLL2712, possessed strong activity to induce the production of IL-10, an anti-inflammatory cytokine, from bone marrow-derived dendritic cells (DCs) and peritoneal macrophages [4]. Furthermore, L. plantarum OLL2712 improved the metabolic parameters of type 2 diabetes model mice, and improved insulin resistance and inflammatory cytokine levels were observed in prediabetic individuals [4,5,6].
Several LAB strains have been reported to induce IL-10 production by DCs. In most studies, bone marrow or peripheral blood monocyte-derived DCs were used to evaluate the nature of LAB strains. Previous studies reported that DCs in Peyer’s patch (PP) captured microbes in the lumen and migrated to the mesenteric lymph node (MLN) [7] to present antigens to T cells and that they induced appropriate immune responses [8]. So it is important to understand the response of intestinal DCs to LAB. However, it is probable that in vitro-induced DCs have different properties compared with gastrointestinal DCs. In the intestine, TGF-β and IL-10 are more abundant than other secondary lymphoid tissues, and tolerogenic DCs are induced more frequently. Intestinal DCs have a high ability to produce retinoic acid, a vitamin A metabolite, and are more efficient in inducing regulatory T cells (Treg) and IgA production than DCs in other tissues or in vitro-induced DCs [9, 10]. In our previous study, it was found that DCs in the MLN consist of four subsets that express different levels of CD11b, CD103, and PD-L1 with distinct functions, and it was revealed that the CD11b-CD103+PD-L1high population has an especially high ability to induce Treg [11]. To our knowledge, there are few studies on IL-10-inducing LAB evaluated using intestinal DCs. In this study, we purified DCs from gastrointestinal lymph tissue and evaluated the IL-10 induction properties of L. plantarum OLL2712 using both in vitro and in vivo systems.
MATERIALS AND METHODS
Animals
Male 8- to 12-week-old BALB/c mice were purchased from Charles River Laboratories Japan (Yokohama, Japan), and age-matched male DO11.10 [12] mice were obtained from Sankyo Labo Service (Tokyo, Japan). These mice were fed CE-2 diet (CLEA Japan, Shizuoka, Japan) and distilled deionized water ad lib and maintained in a specific pathogen-free environment in a temperature-controlled room, using a 12-hr light-dark cycle. All experiments were performed in accordance with guidelines for the care and use of laboratory animals of the University of Tokyo.
Media
RPMI 1640 (Nissui Pharmaceutical, Tokyo, Japan) containing 100 U/mL Penicillin G potassium (Meiji Seika Pharma, Tokyo, Japan), 100 µg/mL Streptomycin sulfate (Meiji Seika Pharma), 50 μM 2-mercaptoethanol (Tokyo Chemical Industry, Tokyo, Japan), 0.03% l-glutamine (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan), and 0.2% sodium hydrogen carbonate (FUJIFILM Wako Pure Chemical Corporation) was prepared with 10% heat-inactivated fetal calf serum.
Bacteria
Heat-killed preparations of LAB strains, L. plantarum OLL2712, L. amylovorus MEP222812, L. brevis MEP222815, L. crispatus MEP222805, and L. plantarum ATCC14917T, were provided by Meiji Co., Ltd. Preparation of samples was performed as previously described [3].
Oral administration of L. plantarum OLL2712
BALB/c mice were divided into two groups. On each day, mice in the OLL2712 treatment group (n=3–6) were intragastrically administered 0.2 mL of distilled water containing heat-killed OLL2712 (4 mg). Mice in the control group were administered 0.2 mL distilled water. Four hours after the 6th feeding, the mice were euthanized. Other mice were intragastrically administered OLL2712 or distilled water once and were euthanized 12 hr later. The DCs were purified and used for analysis of mRNA expression.
Immune cells preparation
For DC isolation, MLNs and PPs obtained from BALB/c mice were digested in 10% FCS-RPMI containing 0.5 mg/mL collagenase (FUJIFILM Wako Pure Chemical Corporation) with 10 µg/mL DNase I (Roche Diagnostics GmbH, Mannheim, Germany), and a cell suspension was obtained by filtering the digestion. From the obtained whole cells, CD11c+ cells were magnetically purified with a MACS cell separation system (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. CD11c+ purification with MACS was conducted twice to achieve high purity, and CD11c+ cells were used as DCs. For CD4+ T cell isolation, splenocytes were obtained from DO11.10 mice. CD4+ cells were separated from whole splenocytes with the MACS system. Cells were isolated from multiple mice and pooled to obtain the required number of cells.
Cell culture
For analysis of DC mRNA expression, 1 × 105 cells of DCs were cultured with or without heat-killed bacteria in a 96-well flat bottom plate (Corning, New York, NY, USA), and cells were collected after 18 hr. For analysis of IL-10 production of from DCs, 2 × 105 DCs were cultured, and the supernatant was collected after 72 hr. For co-culture of DCs and CD4+ T cells, 2 × 104 DCs and 2 × 105 CD4+ T cells were co-cultured with or without bacteria and ovalbumin (OVA) 323-339 residue peptide. For measurement of IL-10 production, the supernatant was collected after 72 hr of co-culture.
qPCR
Total RNA was extracted from the cells with an RNeasy Mini Kit (QIAGEN, Hilden, Germany). cDNA was synthesized with SuperScript VILO MasterMix (Thermo Fisher Sciences, Waltham, MA, USA). Subsequently, real-time PCR was performed to measure relative gene expression with a QuantiTect SYBR Green PCR Kit (QIAGEN) and LightCycler 96 (Roche Diagnostics GmbH). The relative gene expression was calculated assuming that the targeted cDNA was doubled at one cycle. Results were normalized to Gapdh gene expression as the internal control. The following primers were used: 5′-TGTCCGTCGTGGATCTGAC-3′, forward, and 5′-CCTGCTTCACCACCTTCTTG-3′, reverse, for Gapdh; 5′-CCCAGAAATCAAGGAGCATTTG -3′, forward, and 5′-CATGTATGCTTCTATGCAGTTG -3′, reverse, for Il10; 5′-TGGCTACTAGAGAGACTTCTTCCACAA -3′, forward, and 5′-GCACAGGGTCATCAAAGA -3′, reverse, for Il12a; and 5′-AGGTGCGTTCCTCGTAGAGA -3′, forward, and 5′-AAAGCCAACCAAGCAGAAGA -3′, reverse, for Il12b.
Measurement of IL-10 protein
IL-10 protein in the supernatants was measured and analyzed by immunoassay using a MESO® QuickPlex SQ 120 (MESO Scale Diagnostics, Rockville, MD, USA) or quantified by sandwich ELISA with purified rat anti-mouse IL-10 (JES5-2A5, BD Biosciences, San Jose, CA, USA), biotin rat anti-mouse IL-10 (JES5-16E3, BD Biosciences), avidin-horseradish peroxidase (BD Biosciences), and TMB Substrate Reagent A (BD Biosciences). Absorbance was measured with a Model 680 Microplate Reader (Bio-Rad, Hercules, CA, USA), and data were analyzed using Microplate Manager III (Bio-Rad).
Statistical analysis
Results are shown as the mean ± SD. Statistical analysis was performed using Student’s t-test, Dunnett’s test, or Tukey’s HSD test. Differences were considered significant at p<0.05.
RESULTS
L. plantarum OLL2712 induces IL-10 production by intestinal DCs
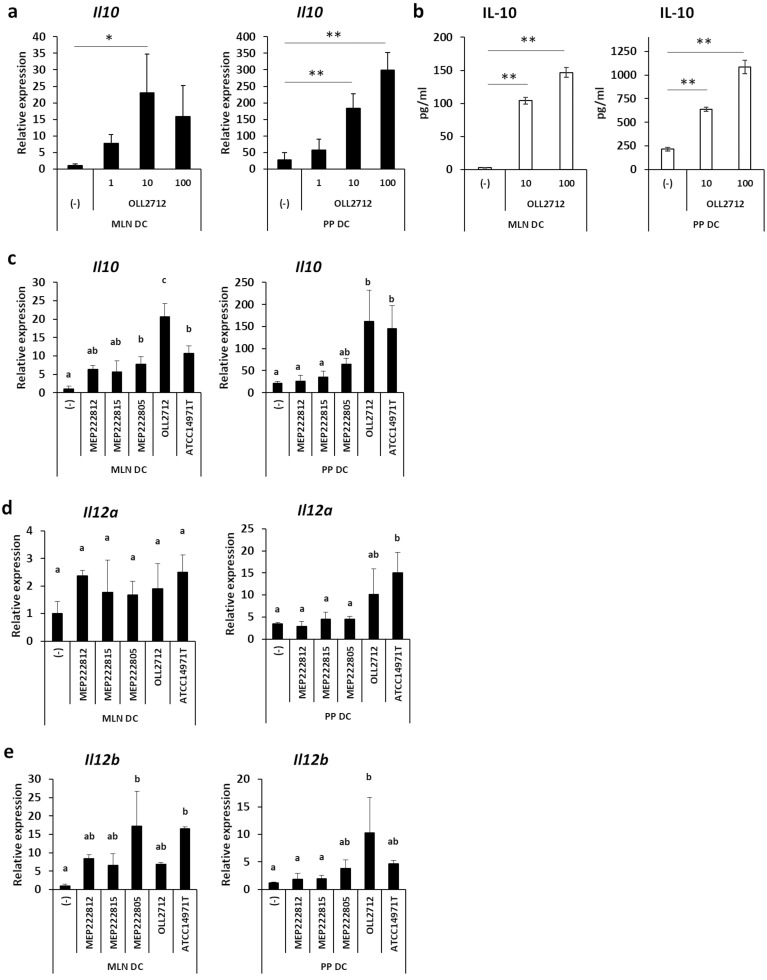
We investigated whether intestinal DCs, namely MLN DCs and PP DCs, produced IL-10 in the presence of L. plantarum OLL2712. Co-culture with the bacteria induced significantly higher IL-10 mRNA expression in MLN and PP DCs than without the bacteria (Fig. 1a). Consistent with this result, OLL2712 induced IL-10 protein production by MLN and PP DCs (Fig. 1b).
Fig. 1.
Effects of L. plantarum OLL2712 on intestinal dendritic cells (DCs).
(a) Mesenteric lymph node (MLN) or Peyer’s patch (PP) DCs (1 × 105 cells) were incubated with heat-killed L. plantarum OLL2712 (1 µg/mL, 10 µg/mL, 100 µg/mL) for 18 hr. The cells were pooled, and relative IL-10 mRNA expression was measured by qPCR. Data are shown as the mean ± SD of 3 independent experiments. (b) MLN or PP DCs (2 × 105 cells) were incubated with heat-killed L. plantarum OLL2712 (10 µg/mL, 100 µg/mL) for 72 hr. The IL-10 protein in the supernatants was measured by immunoassay. The plot shows representative data from one experiment. Data are shown as the mean ± SD of cultured wells (n=3). (c) MLN or PP DCs (1 × 105 cells) were incubated with heat-killed lactic acid bacteria (LAB) (10 µg/mL) for 18 hr. The cells were collected, and relative IL-10 mRNA expression was measured by qPCR. The plot shows representative data from one experiment. Data are shown as the mean ± SD of cultured wells (n=3). (d, e) MLN or PP DCs (1 × 105 cells) were incubated with heat-killed LAB (10 µg/mL) for 18 hr. The cells were collected, and relative Il12a (IL-12p35) (d) and Il12b (IL-12p40) (e) mRNA expression levels were measured by qPCR. The plot shows representative data from one experiment. Data are shown as the mean ± SD of cultured wells (n=3). (a, c, d, e) Relative expression was calculated as the ratio to expression of non-stimulated MLN DCs. (a, b) Two to three independent experiments were performed. Statistical analysis was performed by Dunnett’s test. *p<0.05; **p<0.01. (c–e) Two independent experiments were performed. Statistical analysis was performed by Tukey’s HSD test. Values not sharing a common letter are significantly different (p<0.05).
We compared the ability of L. plantarum OLL2712 to induce cytokine production from intestinal DCs with 4 other LAB strains, L. amylovorus MEP222812, L. brevis MEP222815, L. crispatus MEP222805, and L. plantarum ATCC14917T. The balance of cytokine production between IL-10 and IL-12 is one of the good indicators representing the anti-inflammatory properties of LAB strains [13]. L. plantarum OLL2712 showed significantly higher IL-10 mRNA expression in MLN DCs (Fig. 1c). In PP DCs, both L. plantarum OLL2712 and ATCC14917T, the standard strain of L. plantarum, showed significantly high IL-10 mRNA expression (Fig. 1c). L. plantarum OLL2712 did not induce high Il12a (IL-12p35) or Il12b (IL-12 p40) mRNA expression in MLN DCs (Fig. 1d, e). It also did not show Il12a induction in PP DCs, although the strain induced Il12b mRNA expression that was higher than in two other strains, L. amylovorus MEP222812 and L. brevis MEP222815, and was comparable to the levels induced by L. plantarum OLL2712 and ATCC14917T (Fig. 1d, e). These results indicated that L. plantarum OLL2712 had higher potential to induce IL-10 production, as compared with IL-12, than other strains.
Oral administration of L. plantarum OLL2712 induces IL-10 expression by intestinal DCs
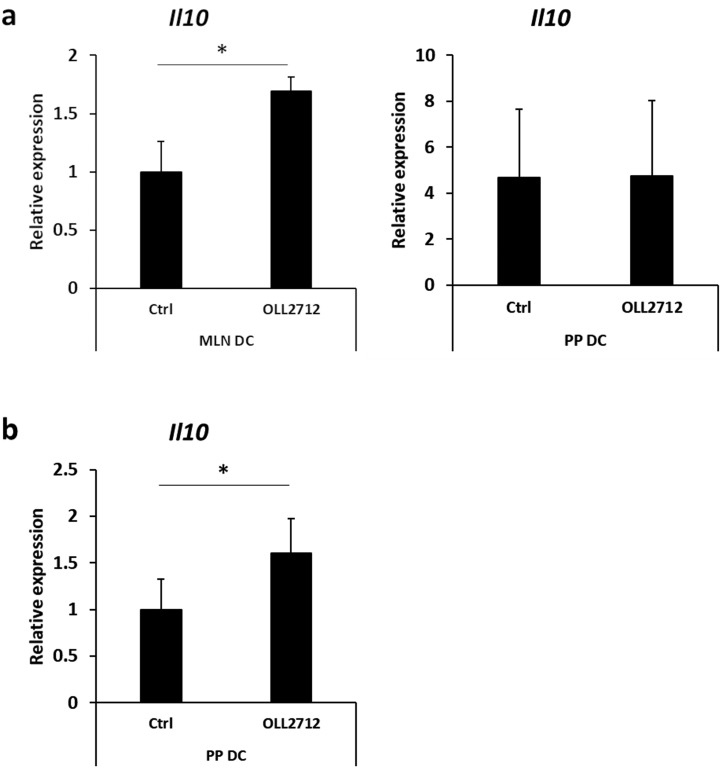
We further investigated the effect of the bacteria in an in vivo model. We orally administered 4 mg of L. plantarum OLL2712 to a treatment group once a day for 6 days and distilled water to a control group for the same period. At the 6th day, we examined intestinal DCs for IL-10 mRNA expression. Although PP DCs showed no significant increase, L. plantarum OLL2712 administration enhanced IL-10 mRNA expression by MLN DCs (Fig. 2a). As we could not detect the effect of OLL2712 on PP DCs, we hypothesized that PP DCs responded to the bacteria in a shorter period. Consistent with this expectation, elevated expression of IL-10 mRNA was observed in PP DCs at 12 hr after oral administration of the bacteria (Fig. 2b). These results suggested that L. plantarum OLL2712 stimulated intestinal DCs to produce IL-10 in vivo.
Fig. 2.
Effects of oral administration of L. plantarum OLL2712 on IL-10 gene expression by intestinal dendritic cells (DCs). (a) BALB/c mice were intragastrically administered heat-killed L. plantarum OLL2712 or distilled water. After 6 days of feeding, mesenteric lymph node (MLN) DCs or Peyer’s patch (PP) DCs were analyzed for IL-10 mRNA expression by qPCR. Data are shown as the mean ± SD of 3 independent experiments. Relative expression was calculated as the ratio to the expression of MLN DCs from control mice. Statistical analysis was performed by Student’s t-test. *p<0.05. (b) BALB/c mice were intragastrically administered heat-killed L. plantarum OLL2712 or distilled water. Twelve hours after feeding, PP DCs were analyzed for IL-10 mRNA expression by qPCR. Data are shown as the mean ± SD of independent mice. Relative expression was calculated as the ratio to the expression of PP DCs from control mice. Statistical analysis was performed by Student’s t-test. *p<0.05.
L. plantarum OLL2712 induces IL-10 production in a DC-T cell co-culture
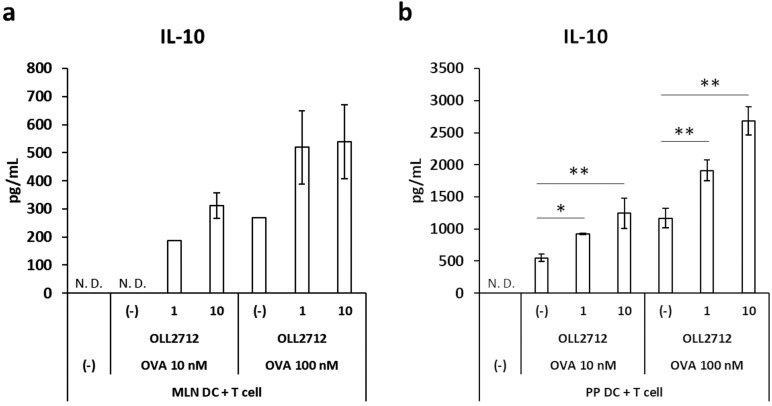
DCs are involved in T cell differentiation and immune responses. We hypothesized that DCs stimulated by the LAB promote IL-10 production by T cells. We therefore co-cultured DCs and T cells in the presence of L. plantarum OLL2712 and measured IL-10 production. In this experiment, we used DO11.10 mice, which possess T cells specific to the antigenic peptide corresponding to 323-339 residues of OVA (OVA 323-339), to evaluate the effect of the bacteria on antigen-specific T cell response. Addition of L. plantarum OLL2712 showed a tendency to elevate production of IL-10 in co-culture of MLN DCs with T cells (Fig. 3a), and a significant increase was observed in co-culture of PP DCs with T cells (Fig. 3b). These results suggested that L. plantarum OLL2712 enhanced IL-10 production by T cells.
Fig. 3.
Effects of L. plantarum OLL2712 on IL-10 production in a DC-T cell co-culture.
Spleen CD4+ T cells from DO11.10 mice were co-cultured with MLN DCs (a) or PP DCs (b) in the presence of OVA 323-339 residue peptide (10 nM, 100 nM) and heat-killed L. plantarum OLL2712 (1 µg/mL, 10 µg/mL) for 72 hr. The IL-10 protein in the supernatants was measured by ELISA. The plot shows representative data from one experiment. Data are shown as the mean ± SD of cultured wells (n=3). Two independent experiments were performed. Statistical analysis was performed by Dunnett’s test. *p<0.05; **p<0.01. N.D.: not detected.
DISCUSSION
In the present study, we demonstrated that L. plantarum OLL2712 induced IL-10 production by DCs in gastrointestinal lymph tissue. Moreover, L. plantarum OLL2712 enhanced IL-10 production by T cells. Finally, oral administration of the bacteria resulted in increased levels of IL-10 expression by MLN and PP DCs.
Several studies have reported that LAB strains induce IL-10 production by DCs. Ratajczak et al. showed that L. casei ATCC393 increased IL-10 and IL-12 production by human blood monocyte-derived DCs [14]. Konstantinov et al. reported that L. acidophilus NCFM induced IL-10 production by monocyte-derived DCs [15]. Gad et al. demonstrated that L. salivarius Ls-33 and Bifidobacterium infantis 35624 induced production of IL-10 preferentially to IL-12p70 by peripheral blood mononuclear cell-derived DCs [16]. Matsuzaki et al. reported that exopolysaccharide produced by Leuconostoc mesenteroides strain NTM048 enhanced expression of IL-10 mRNA by murine bone marrow DCs [17]. In many studies, in vitro-induced DCs are obtained by culturing monocyte precursors in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-4 [18]. However, DC generation and maturation are regulated by many factors [19]. Previous studies indicated that DC precursors are stimulated to mature by Toll-like receptor (TLR) ligands or soluble factors like retinoic acid, a metabolite of vitamin A [20, 21]. It is reported that intestinal DCs have unique characteristics compared with DCs in other sites [9]. To our knowledge, we have investigated for the first time the IL-10-inducing properties of LAB using intestinal DCs, not in vitro-induced DCs.
LAB treatment can induce the production of various kinds of cytokines by immune cells. The cytokine induction ratio of IL-10 to the Th1 cytokine IL-12 is considered to be an important anti-inflammatory property [13]. We showed that L. plantarum OLL2712 was a high inducer of IL-10 production from intestinal DCs, although it did not greatly enhance IL-12 expression. This characteristic should be important with respect to the anti-inflammatory effect of the bacteria in the intestine. Toshimitsu et al. showed that L. plantarum OLL2712 did not induce IL-10 secretion by Tlr2−/− bone marrow DCs [5]. Bacterial cell components are known as typical TLR2 ligands, and it was reported that teichoic acids, one of the cell wall components, are involved in IL-10 induction [13]. Teichoic acids in the L. plantarum OLL2712 cell wall may possibly stimulate TLR2 and enhance IL-10 production [5].
After DCs in PPs or the lamina propria (LP) capture antigens in the lumen, they migrate into lymph nodes [22], such as the MLN, to present antigen to T cells. Therefore, orally administered L. plantarum OLL2712 may influence T cell functions. Consistent with this hypothesis, co-culture with L. plantarum OLL2712 accelerated IL-10 protein production in the antigen-specific T cell response. In this culture model, DO11.10 mouse-derived CD4+ T cells were OVA 323-339 residue-peptide specific, and they would not respond to the presented L. plantarum OLL2712. Although IL-10 protein was produced by both DCs and T cells in this model, the results showing that the amount of IL-10 in the supernatant increased in an OVA-peptide concentration-dependent manner suggested that L. plantarum OLL2712 affected IL-10 production by T cells. The enhancement of IL-10 production from T cells is likely to be mediated by DCs; however, direct stimulation of T cells by the bacteria cannot be denied. It may also be possible that activated T cells stimulated DCs to secrete more IL-10. Previous reports showed that IL-10-producing CD4+ T cells consisted of at least two subpopulations, Foxp3+ Treg and Foxp3- T regulatory type 1 (Tr1) cells [23]. We investigated whether L. plantarum OLL2712 induced Treg in the co-culture model; however, a significant increase of Foxp3+ Treg was not observed (data not shown). This result suggested that the LAB promoted Tr1 cell differentiation and enhanced IL-10 production. Several factors that induce Tr1 differentiation were identified. In previous studies, IL-10 and IL-27 were reported to have generated Tr1 cells [24, 25]. In this study, it is considered that DCs stimulated by L. plantarum OLL2712 produced IL-10 and that the secreted IL-10 promoted the differentiation of naïve CD4 T cells into Tr1 cells. Further investigation is needed to determine whether L. plantarum OLL2712 induces IL-27 expression by DCs or innate immune cells.
Based on the results observed in our in vitro experiment, 6 days of oral administration of L. plantarum OLL2712 promoted MLN DC IL-10 gene expression. This result suggested that DCs that migrated into the MLN were affected by ingested L. plantarum OLL2712. Unexpectedly, 6 days of treatment with the bacteria did not affect IL-10 gene expression in PP DCs. However, IL-10 gene expression in PP DCs was observed 12 hr after oral administration of the bacteria. A possible explanation is as follows: After PP DCs were stimulated by the LAB, IL-10 gene expression was elevated within at least 12 hr. PP DCs that were stimulated by the LAB started to migrate to MLNs. This hypothesis is supported by the previous findings showing that although few DCs carrying the orally administrated Enterobacter cloacae reached MLNs at 5 hr after bacterial gavage, after 12 hr, a considerable number of DCs carrying the bacteria reached MLNs [7]. New DCs or DC precursors will be supplied; however, if the balance between supply of DCs in PPs and migration to MLNs is biased toward migration, upregulated IL-10 gene expression will not be detected in PPs. A previous study showed that CD11b+ DCs in the PP produce high levels of IL-10 [26]. CD11b+ migrating DCs may be involved in upregulating IL-10 gene expression in MLNs. Our results suggested that L. plantarum OLL2712 affected migrating DCs in a relatively short time and modulated IL-10 production in intestinal lymph tissue.
In previous studies, oral administration of L. plantarum OLL2712 improved the metabolic parameters of type 2 diabetes model mice, and improved insulin resistance and inflammatory cytokine levels were observed in prediabetic individuals [4,5,6]. However, the induction of IL-10 production was not observed in this in vivo model. One explanation for this is that inflammatory conditions resulted in elevated levels of IL-10 to counter inflammation [27], and this might prevent clarification of the mechanism of effects of L. plantarum. For this reason, we evaluated the immunomodulatory effect of L. plantarum OLL2712 in inducing IL-10 expression using noninflammatory wild-type mice. Increased IL-10 production by intestinal DCs in wild-type mice suggested that L. plantarum OLL2712 may modulate metabolic function by inducing IL-10 production in type 2 diabetic model mice or prediabetic individuals.
Toshimitsu et al. reported that L. plantarum OLL2712 treatment suppressed inflammatory cytokine expression in visceral adipose tissue [4, 5]; however, the mechanism has not been revealed. We further investigated whether OLL2712 also induced IL-10 mRNA expression in adipose tissue-derived stromal vascular fraction (SVF), which contains adipose tissue immune cells. Elevated IL-10 mRNA expression was observed by co-culture with OLL2712 in epididymal fat and mesenteric fat-derived SVF (Supplemental Fig). These results suggested the possibility that OLL2712-stimulated DCs may alleviate inflammation in adipose tissue after accessing the adipose tissues, probably via IL-10 production. Further investigation will be needed to determine whether DCs stimulated by OLL2712 can migrate into adipose tissue in a model in which we can track movement of DCs.
In the present report, we showed using murine derived intestinal MLN and PP DCs that one strain of LABs, L. plantarum OLL2712, possessed IL-10 induction activity in DCs. These results suggested that supplementation of food ingredients, such as with LAB, could be used to regulate and ameliorate inflammatory status. By clarifying the mechanism by which LAB induce IL-10 in intestinal DCs, the mechanism can be applied to the development of an effective food ingredient for the treatment and prevention of inflammatory diseases.
Supplementary
Acknowledgments
We thank Takayuki Toshimitsu (Meiji Co., Ltd.) for assistance in preparing the bacterial strains.
REFERENCES
- 1.Tsai YT, Cheng PC, Pan TM. 2012. The immunomodulatory effects of lactic acid bacteria for improving immune functions and benefits. Appl Microbiol Biotechnol 96: 853–862. [DOI] [PubMed] [Google Scholar]
- 2.Hachimura S, Totsuka M, Hosono A. 2018. Immunomodulation by food: impact on gut immunity and immune cell function. Biosci Biotechnol Biochem 82: 584–599. [DOI] [PubMed] [Google Scholar]
- 3.Tsai YT, Cheng PC, Pan TM. 2014. Anti-obesity effects of gut microbiota are associated with lactic acid bacteria. Appl Microbiol Biotechnol 98: 1–10. [DOI] [PubMed] [Google Scholar]
- 4.Toshimitsu T, Mochizuki J, Ikegami S, Itou H. 2016. Identification of a Lactobacillus plantarum strain that ameliorates chronic inflammation and metabolic disorders in obese and type 2 diabetic mice. J Dairy Sci 99: 933–946. [DOI] [PubMed] [Google Scholar]
- 5.Toshimitsu T, Ozaki S, Mochizuki J, Furuichi K, Asami Y. 2017. Effects of Lactobacillus plantarum strain OLL2712 culture conditions on the anti-inflammatory activities for murine immune cells and obese and type 2 diabetic mice. Appl Environ Microbiol 83: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toshimitsu T, Gotou A, Furuichi K, Hachimura S, Asami Y. 2019. Effects of 12-wk Lactobacillus plantarum OLL2712 treatment on glucose metabolism and chronic inflammation in prediabetic individuals: a single-arm pilot study. Nutrition 58: 175–180. [DOI] [PubMed] [Google Scholar]
- 7.Macpherson AJ, Uhr T. 2004. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science 303: 1662–1665. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez D, Vollmann EH, von Andrian UH. 2008. Mechanisms and consequences of dendritic cell migration. Immunity 29: 325–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coombes JL, Siddiqui KRR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. 2007. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204: 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. 2006. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 314: 1157–1160. [DOI] [PubMed] [Google Scholar]
- 11.Shiokawa A, Kotaki R, Takano T, Nakajima-Adachi H, Hachimura S. 2017. Mesenteric lymph node CD11b– CD103+ PD-L1High dendritic cells highly induce regulatory T cells. Immunology 152: 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy KM, Heimberger AB, Loh DY. 1990. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science 250: 1720–1723. [DOI] [PubMed] [Google Scholar]
- 13.Kaji R, Kiyoshima-Shibata J, Nagaoka M, Nanno M, Shida K. 2010. Bacterial teichoic acids reverse predominant IL-12 production induced by certain Lactobacillus strains into predominant IL-10 production via TLR2-dependent ERK activation in macrophages. J Immunol 184: 3505–3513. [DOI] [PubMed] [Google Scholar]
- 14.Ratajczak C, Duez C, Grangette C, Pochard P, Tonnel AB, Pestel J. 2007. Impact of lactic acid bacteria on dendritic cells from allergic patients in an experimental model of intestinal epithelium. J Biomed Biotechnol 2007: 71921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Konstantinov SR, Smidt H, de Vos WM, Bruijns SCM, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer TR, van Kooyk Y. 2008. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cell and T cell functions. Proc Natl Acad Sci USA 105: 19474–19479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gad M, Ravn P, Søborg DA, Lund-Jensen K, Ouwehand AC, Jensen SS. 2011. Regulation of the IL-10/IL-12 axis in human dendritic cells with probiotic bacteria. FEMS Immunol Med Microbiol 63: 93–107. [DOI] [PubMed] [Google Scholar]
- 17.Matsuzaki C, Takagaki C, Higashimura Y, Nakashima Y, Hosomi K, Kunisawa J, Yamamoto K, Hisa K. 2018. Immunostimulatory effect on dendritic cells of the adjuvant-active exopolysaccharide from Leuconostoc mesenteroides strain NTM048. Biosci Biotechnol Biochem 82: 1647–1651. [DOI] [PubMed] [Google Scholar]
- 18.Son YI, Egawa S, Tatsumi T, Redlinger RE, Jr, Kalinski P, Kanto T. 2002. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J Immunol Methods 262: 145–157. [DOI] [PubMed] [Google Scholar]
- 19.Dalod M, Chelbi R, Malissen B, Lawrence T. 2014. Dendritic cell maturation: functional specialization through signaling specificity and transcriptional programming. EMBO J 33: 1104–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beijer MR, Kraal G, den Haan JMM. 2014. Vitamin A and dendritic cell differentiation. Immunology 142: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng T, Cong Y, Qin H, Benveniste EN, Elson CO. 2010. Generation of mucosal dendritic cells from bone marrow reveals a critical role of retinoic acid. J Immunol 185: 5915–5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houston SA, Cerovic V, Thomson C, Brewer J, Mowat AM, Milling S. 2016. The lymph nodes draining the small intestine and colon are anatomically separate and immunologically distinct. Mucosal Immunol 9: 468–478. [DOI] [PubMed] [Google Scholar]
- 23.Gregori S, Roncarolo MG. 2018. Engineered T regulatory type 1 cells for clinical application. Front Immunol 9: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brockmann L, Gagliani N, Steglich B, Giannou AD, Kempski J, Pelczar P, Geffken M, Mfarrej B, Huber F, Herkel J, Wan YY, Esplugues E, Battaglia M, Krebs CF, Flavell RA, Huber S. 2017. IL-10 receptor signaling is essential for TR1 cell function in vivo. J Immunol 198: 1130–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. 2009. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol 183: 2435–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiokawa A, Tanabe K, Tsuji NM, Sato R, Hachimura S. 2009. IL-10 and IL-27 producing dendritic cells capable of enhancing IL-10 production of T cells are induced in oral tolerance. Immunol Lett 125: 7–14. [DOI] [PubMed] [Google Scholar]
- 27.Cintra DE, Pauli JR, Araújo EP, Moraes JC, de Souza CT, Milanski M, Morari J, Gambero A, Saad MJ, Velloso LA. 2008. Interleukin-10 is a protective factor against diet-induced insulin resistance in liver. J Hepatol 48: 628–637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.