Abstract
The gut microbiota has a great impact on the host immune systems. Recent evidence suggests that the maternal gut microbiota affects the immune systems of offspring. Metabolites produced by the gut microbiota play crucial roles in the immune system. Previous studies have also revealed that metabolites such as short-chain fatty acids (SCFAs) and the aryl hydrocarbon receptor (AhR) ligands are involved in host health and diseases. Great progress has been made in understanding the roles of diet-derived SCFAs in the offspring’s immune system. The findings to date raise the possibility that maternal dietary soluble fiber intake may play a role in the development of the offspring’s systemic immune response. In this review, we summarize the present knowledge and discuss future therapeutic possibilities for using dietary soluble fiber intake against inflammatory diseases.
Keywords: maternal diet, gut microbiota, offspring’s immune systems, metabolites, short-chain fatty acids, aryl hydrocarbon receptor ligands, early-life critical window
INTRODUCTION
Hundreds of bacterial species and trillions of commensal bacteria make up the gut microbiota of the intestinal tract. The gut microbiota and its metabolites are involved in host homeostasis and the developing immune system in the host’s intestinal tract [1, 2]. Recent studies have shown that the maternal gut microbiota strongly affects the offspring’s immune system development [3, 4]. However, the detailed mechanisms of this process remain unclear.
The components of the gut microbiota are closely linked to the host’s diet. Cross-talk between the diet and the gut microbiota impacts the development of the immune system and the development of many diseases [5,6,7]. The diet shapes the gut microbiota’s composition and function. As part of the diet, dietary fat and dietary fibers are important nutrients that affect the gut immune system. Dietary fiber can be either soluble or insoluble [8, 9]. Soluble dietary fiber is fermented into short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate, by specific microbes in the gut [10]. SCFAs are end-products of microbial fermentation and influence the host’s physiology [10]. Previous studies have shown that consuming soluble dietary fiber increases SCFA levels in the gut [11, 12], while low-fiber diets and high-fat diets decrease these levels [13]. SCFAs provide energy to intestinal epithelial cells and affect the gut immune system by inducing regulatory T (Treg) cells in the gut [14, 15].
The aryl hydrocarbon receptor (AhR) is a transcriptional factor that regulates the host immune system. AhR signals regulate the number of intraepithelial lymphocytes (IELs) and innate lymphoid cells (ILCs) [16, 17]. AhR activity is essential for the anti-inflammatory response that induces production of IL-22 in ILCs.
Endogenous ligands of AhR are contained in dietary components such as broccoli, cauliflower, Brussel sprouts, and cabbage [18]. These dietary components are converted into AhR ligands such as indole and indole derivatives by the gut microbiota in the gut.
The metabolism of tryptophan (Trp) is also a physiological source of AhR ligands. Trp is an essential amino acid absorbed by dietary protein, and Trp is converted into AhR ligands [19].
Recently, some studies demonstrated that metabolites derived from the maternal gut microbiota, including SCFAs and AhR ligands, can benefit the immune responses of the offspring [11, 20,21,22]. Increased maternal SCFAs affect the immune systems of their offspring; however, how the maternal gut microbiota impacts the offspring’s immune system remains uncertain [20]. Based on these reports, this review examines recent evidence regarding the maternal diet’s effect on the offspring’s immune system and the possible therapeutic effects of the maternal diet and gut microbiota against allergic diseases.
METABOLITES DERIVED FROM GUT MICROBIOTA
Maternal metabolites during pregnancy
SCFAs, such as acetate, propionate, and butyrate, in the gut content and in the plasma can be measured by gas chromatography-mass spectrometry (GC-MS), 1H-nuclear magnetic resonance (NMR) spectroscopy, and liquid chromatography-mass spectrometry (LC-MS), and capillary electrophoresis [23]. The SCFA levels in the gut and plasma depend largely on the dietary fiber intake and the gut microbiota components. For example, De Fillipo et al. showed that African people living in a Burkina Faso village, the diets of which contain a lot of high-fiber components, had increased fecal SCFAs levels [13]. There are not so many reports about plasma SCFAs, but Vetrani et al. showed that 12 weeks of consumption of a diet rich in whole-grain products (mainly wheat) increases fasting propionate plasma levels in humans [24]. Physiologically, SCFAs are increased by the consumption of a soluble high-fiber diet (sHFD) and decreased by consuming a no-fiber diet (NFD) or a high-fat diet [11, 12, 22]. At the phylum level, the relative abundance of Bacteroidetes in the gut is decreased after NFD intake compared with that after sHFD intake. Consuming an sHFD increases the Bacteroidetes population and reduces Firmicutes at the phylum level, thus increasing SCFA levels under normal conditions [12].
The gut microbiota composition is altered during pregnancy [4, 25, 26]. Although the detailed mechanisms of the microbiota alterations are unclear, many factors, such as metabolic changes and increased hormone secretion, may be involved [4, 27]. Additionally, SCFA levels in the gut and plasma tend to be elevated in pregnant mice compared with those in nonpregnant mice. Acetate and propionate levels in cecum metabolites are also increased in pregnant mice compared with those in nonpregnant mice [28]. At the plasma level, SCFA levels are similar between pregnant and nonpregnant mice at the beginning of pregnancy; however, acetate and butyrate levels are significantly increased during the late stage of pregnancy [11, 29]. Further studies are warranted to clarify the mechanism underlying how SCFAs are increased during pregnancy.
Gut metabolite levels in offspring
Importantly, whether increased SCFAs in maternal plasma are transferred to offspring during pregnancy and lactation remains unclear. An embryo in utero during pregnancy resides in a nearly sterile environment. Neonates are exposed to maternal commensal bacteria during delivery. Coprophagy is the eating of feces and is believed to be important for development of the microbiota and the gut immune system in mice [30, 31]. Coprophagy might be one of the possible mechanisms of maternal transfer of the gut microbiota and metabolites from mother to offspring.
The ratio of the relative abundance of bacterial species in feces in neonates at 1 day after birth is relatively small. Additionally, 16S rRNA sequencing revealed that the gut microbiota compositions of neonate mice differed greatly from those of adult mice [11]. The neonatal intestine at birth is an aerobic environment in which Enterobacteriaceae can grow [32]. Furthermore, the Bacteroidetes-to-Firmicutes ratio is minimal at 1 day after birth [11]. Despite the SCFA sources in neonatal mice being limited, SCFAs can be detected in the plasma by GC-MS at embryonic day 18 and at day 1 after birth [11, 12, 33]. Prentice et al. demonstrated that SCFAs such as butyrate, acetate, and formic acid can be detected in human milk by NMR and GC-MS [34]. In their study, they suggested that SCFAs in human milk play beneficial roles with respect to weight gain and adiposity during infancy [34]. Based on the evidence that SCFAs are largely produced from the gut microbiota, SCFAs detected in maternal milk might be derived from the maternal gut microbiota. This evidence suggests that SCFAs are transferred from mother to offspring during pregnancy and lactation.
The abovementioned findings regarding SCFA levels in offspring just after birth provide evidence that the maternal diet during pregnancy and lactation influences the gut microbiota composition. Thus, plasma SCFA levels in the offspring might reflect the maternal SCFA levels.
FUNCTIONS OF SCFAS IN THE IMMUNE SYSTEMS OF OFFSPRING
SCFAs derived from the gut microbiota have emerged as major contributors to the host immune response. Many studies have been performed on SCFAs to understand the mechanisms underlying how the microbiota modulates the host immune system [35]. Two SCFA properties may modulate the host immune system. The first property is that SCFA signals are transmitted through G protein-coupled receptors (GPCRs) [36]. GPCRs, such as GPR41, GPR43, and GPR109A, are SCFA receptors that modulate the gut homeostasis and regulate inflammatory responses [37]. GPR41, also known as free fatty acid receptor (FFAR)3, and GPR43, also known as FFAR2, have been identified as SCFA receptors. Both GPR41 and GPR43 recognize acetate, propionate, and butyrate, but with different affinities. For example, GPR43 has higher affinity for acetate than GPR41 [38]. Both are expressed in tissue-specific cells such as colon epithelial cells, adipocytes, and peripheral blood mononuclear cells and are activated by SCFAs [39]. GPCRs mediate the interaction of host cells and the gut microbiota and are associated with chronic inflammatory diseases such as colitis, asthma, and arthritis.
The second property is that SCFAs inhibit histone deacetylase (HDAC) [40, 41], which affects the expression of genes such as Forkhead box p3 (Foxp3). The expression of Foxp3 in Treg cells is involved in the development and functions of Treg cells as described below.
Recent evidence indicating that colonic Treg cells are induced by SCFAs in the gut has greatly impacted our understanding of the mechanism by which SCFAs promote anti-inflammatory responses [14, 15, 42]. Treg cells are a specific T cell subset with potential roles in inhibiting inflammation and maintaining homeostasis [43]. Butyrate inhibits HDACs to enhance histone H3 acetylation on the Foxp3 gene locus and induce Treg cells in the gut [14, 15]. Propionate is involved in GPR43 dependently inducing colonic Treg cells, which promotes gut homeostasis and prevents gut inflammation [42]. In addition to colonic Treg cells, thymic Treg (tTreg) cells are influenced by fiber diets and gut SCFAs. One study found that the tTreg cells were increased in offspring born to sHFD-fed mother [11] and decreased in offspring born to NFD-fed mothers [11]. The thymus is a lymphoid organ that begins developing in the neonatal stage and generates T cells, including Treg cells. Treg cells may have roles in preventing autoimmune reactions against self-components that induce allergies and autoimmune diseases. Previous studies found that autoimmune regulator (Aire) expression in medullary thymic epithelial cells (mTECs) is essential for inducing Treg cells in the thymus [44, 45]. Because maternal sHFD intake might increase the plasma SCFA levels in offspring during pregnancy and lactation, increased SCFAs in offspring born to sHFD-fed mothers yielded increased numbers of tTreg cells in the offspring. Butyrate increases tTreg cells through GPR41-mediated Aire induction in mTECs [11]. Notably, recent evidence suggested that Aire-dependent tTreg cells in neonates can provide long-term protection against autoimmune diseases [46]. Consistent with this finding, maternal sHFD or acetate intake attenuates allergic airway diseases (AADs) in offspring in the long term because acetate inhibits HDACs, which results in enhancement of Foxp3 gene expression and production of Treg cells in the lungs [22].
Collectively, these findings suggest that SCFAs promote Treg induction in the thymus and peripheral organs through different mechanisms and pathways, including GPCRs and HDAC inhibitors, and thereby provide protection against allergies and inflammation.
AHR LIGANDS AND THE OTHER METABOLITES THAT MAY AFFECT OFFSPRING IMMUNE RESPONSES
The gut microbiota has crucial roles with respect to the production of many AhR ligands. Tryptophan is an essential amino acid that can be converted to AhR ligands such as indole and indole derivatives by certain gut microbiota. Indole and indole derivatives such as tryptamine and indole-3-acetic acid (IAA) activate AhR signaling that is involved in immune responses [47]. The agonists of AhR may play a role in increasing the number of NKp46+ ILC3 cells, which are ILCs [20]. NKp46+ ILC3 cells contribute to the mucosal barrier and play crucial roles in protecting against infections by producing IL-22 [48]. Maternal AhR ligands may be transferred from mother to offspring, thus increasing ILC3 in the offspring [20]. Additionally, ILC3 cells in utero are regulated by retinoic acid signaling, and maternal dietary retinoic acids control the size of secondary lymphoid organs and the abundance of ILC3 [21]. This evidence suggests that the maternal diet influences the number and function of ILC3 cells in offspring via retinoic acid and AhR ligands.
Several other gut microbiota-derived metabolites are reported to modulate the immune response. Polysaccharide A (PSA) and peptidoglycan (PGN) are also possible candidates for host immune modulators [1]. PSA is derived from Bacteroides fragilis and contributes to maintaining Th1/Th2 balance. It stimulates toll-like receptor (TLR) 2 signaling and IL-12 production by dendritic cells [49]. PGN is a component of the bacterial outer membrane and is a ligand of nucleotide oligomerization domains (NODs) 1 and 2 [50]. PGN derived from the gut microbiota contributes to immune responses via NOD1 and NOD2 signaling [50]. Notably, circulating PGN regulates the immune system systemically, thereby influencing expression of the Aire gene in mTECs via NOD1 signaling [51, 52]. Although the effects of maternal PSA or PGN on offspring are unclear, these metabolites may affect immune system development in offspring in the same manner as SCFAs and AhR ligands.
Most vitamins must be supplied by the diet and the gut microbiota. Vitamin D deficiency is reported to be associated with asthma and allergic airway diseases [53, 54]. A recent study demonstrated that maternal vitamin C is required for proper DNA methylation in female fetal germ cells in a mouse model [55]. Thus, maternal vitamins are crucial for development and preventing allergic and inflammatory diseases in offspring [56, 57].
OFFSPRING IMMUNE DEVELOPMENT DURING THE EARLY-LIFE CRITICAL WINDOW
The gut microbiota affects the host’s immune system as well as immune system development in the offspring. The early-life microbiome is critical for developing the host’s immune system and maintaining future health. In humans, recent studies proposed that the early-life critical window is within 100 days after birth and is crucial to preventing allergic and metabolic diseases in the long term [58]. Maternal nutrition influences development of multiple organs in offspring, such as the gastrointestinal tract, lungs, and central nervous system, in addition to tTreg cells in the thymus during the early-life critical window [11, 20,21,22].
One study found that maternal microbiota exposure increased the number of ILCs in the early-life critical window [20]. In mice, the microbiota shapes the number of NKp46+ ILC3s and F/480+CD11c+ intestinal mononuclear cells (iMNCs) in offspring born to mothers under gestation-only colonization conditions [20]. These cells promote production of IL-22, a cytokine that enhances epithelial intensity and infection resistance. The number of NKp46+ ILC3s and F/480+CD11c+ iMNCs was maximized between postnatal days 14 and 21 and persisted until 8 weeks of age in mice. This evidence suggests that NKp46+ ILC3s and F/480+CD11c+ iMNCs increase during the early-life critical window and may play roles in preventing future infections.
Recent studies have shown that the maternal microbiota affects brain and behavioral development in the offspring. Autism spectrum disorder (ASD) is a neurodevelopment disorder, and a maternal high-fat diet induces ASD via dysbiosis of maternal microbiota; this effect is referred to as the gut-brain axis [59]. The gut microbiota also modulates respiratory immune responses. Maternal intake of sHFDs and treatment of SCFAs attenuated AADs such as asthma in offspring. This modulation is referred to as the gut-lung axis [12, 22]. Recent evidence suggests that the maternal microbiome during pregnancy and lactation influences tTreg cell differentiation in offspring in a GPR41-mediated Aire-dependent manner [11]. As described above, Aire gene expression during the prenatal stage is crucial for Treg cell production in the thymus [11, 46], and tTreg cells produced in the early-life critical window are indispensable for long-term prevention of autoimmune diseases [46]. Thus, the correlation of Aire gene expression in the thymus suggests that the gut-thymus axis emerges to develop the immune system early in life. This suggests that bacterial metabolic effects might be observed in multiple other organs. Metabolites such as SCFAs mediate interactions between the gut microbiota and the organs. The axis between the gut microbiota and other organs provides a clue to understanding the mechanism by which the gut microbiota affects the host immune system.
THERAPEUTIC POSSIBILITIES OF MATERNAL METABOLITES
Dysbiosis of the maternal microbiota increased the risk of allergic and metabolic diseases, such as asthma, obesity, and type 2 diabetes mellitus (T2DM), in offspring [60]. The maternal diet during pregnancy and lactation affects allergic diseases and asthma in offspring [61, 62]. Thus, nutrition during pregnancy and lactation represents a potential therapeutic target for improving the offspring’s long-term health [63].
Consumption of Western-type diets during pregnancy induces dysbiosis and decreases SCFA production in offspring. Conversely, consumption of high-fiber diets improves glucose control via SCFA production in patients with T2DM [64]. Metabolites derived from the gut microbiota, such as SCFAs and AhR ligands, are potential candidates for preventing metabolic diseases and allergies. Recent studies suggested that maternal SCFA supplementation prevents type 1 diabetes mellitus [65]. Maternal sHFD intake during pregnancy prevented allergic airway inflammation in a mouse model [22]. AhR activation through dietary elements promotes Treg responses and protects against AADs [66]. Treatment with SCFAs and AhR ligands during pregnancy and the early-life critical window might play a potential role in preventing allergic and metabolic diseases in offspring in the long-term because SCFAs and AhR ligands may modulate Treg and ILC biology as described above [11, 20,21,22]. In addition, probiotics are also effective materials. Maternal supplementation with probiotics during pregnancy prevents allergic diseases and metabolic diseases such as asthma, diabetes mellitus, and obesity. Supplementation of short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides in pregnant mice reduced allergic asthma [67]. Maternal supplementation with probiotics can be widely applied for preventing or ameliorating diseases in offspring later in life. Recent studies suggested that maternal probiotic supplementation during pregnancy and lactation effectively prevents obesity, hypertension, and allergic diseases in offspring [68,69,70,71,72,73].
In summary, evidence suggests that improving maternal nutrition during pregnancy and lactation affects the immune system development and prevents allergic and metabolic diseases in offspring (Table 1). Although several clinical studies have been performed, further studies are needed to clarify the significant association between probiotic supplementation during pregnancy and allergic diseases such as asthma in human offspring (Table 2).
Table 1. The relationship between maternal gut metabolites and effects of the immune system in offspring.
| Maternal gut metabolites derived from gut microbiota | Effect of immune system in offspring | References |
|---|---|---|
| Short-chain fatty acids (SCFAs) | Ameliorate allergic airway diseases | [22] |
| Ameliorate type 1 diabetes | [65] | |
| Increase the number of tTreg | [11] | |
| Aryl hydrocarbon receptor (AhR) ligands | Increase the number of NKp46 ILC3 cells | [20] |
| Vitamin C | DNA methylation of female fetal germ cells | [55] |
| Vitamin D and E | Decrease risk of childhood asthma | [56, 57] |
| Retinoic acid | Increase the number of ILC3 cells | [21] |
Table 2. Effects of probiotics during pregnancy to the immune system in offspring.
| Probiotics | Effect | References |
|---|---|---|
| Oligosaccharides | Reduce allergic asthma | [67] |
| Fructose | Prevent hypertension | [68] |
| Oligofructose | Prevent obesity | [69] |
| Fructo-oligosaccharide | Attenuate acute allergic skin response | [70] |
| Fructo-oligosaccharide | Intestinal immune function | [71] |
| L. rhamnosus, B. animalis, L. acidophilus | Decrease risk of atopic dermatitis | [72] |
CONCLUSION
The gut microbiota is a unique group of organisms that benefit the host’s immune system. Cross-talk between gut microbiota metabolites and the host immune system greatly affects host homeostasis. Moreover, accumulating evidence suggests that maternal-host gut interactions during pregnancy and lactation play crucial roles in the offspring’s immune system development.
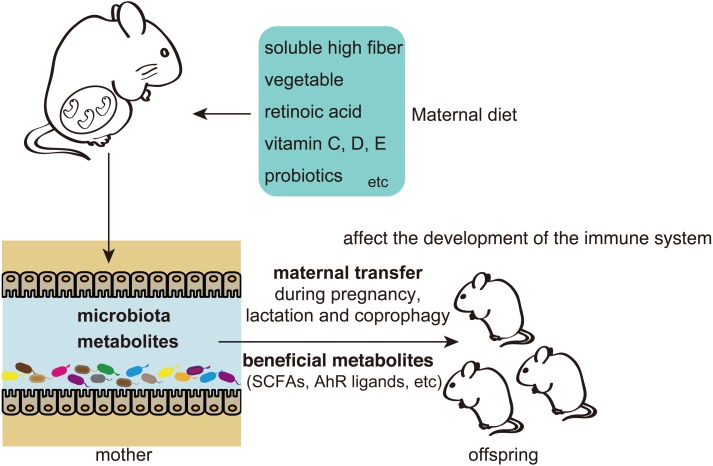
In this review, we described the effects of maternal gut metabolites on the offspring’s immune system development and the therapeutic possibilities for preventing allergic and metabolic diseases in a mouse model (Fig. 1). SCFAs may play roles in the development of the immune system by mediating various pathways. Future research should clarify SCFA signaling in GPCR pathways and HDAC inhibitors.
Fig. 1.
Short-chain fatty acids (SCFAs) and other metabolites derived from the maternal gut microbiota affect offspring immune system development, thus preventing allergies and metabolic diseases. Maternal intake of a soluble high fiber that includes vegetable, retinoic acid, vitamins, and probiotics promotes the production of beneficial metabolites in the maternal gut and affects offspring immune system development in a mouse model. Maternal milk and coprophagy are the possible mechanisms underlying the involvement of maternal transfer and affect the composition of the microbiota and the immune system of offspring in mice. Effects of the maternal gut microbiota on offspring during the early-life critical window may impact the future health of the offspring.
The numbers of allergic and metabolic patients are increasing in developed countries. Consumption of sHFDs that include vegetables, mushrooms, and seaweed during pregnancy and lactation may be a long-term and effective means of preventing allergies and metabolic diseases in offspring.
AUTHOR CONTRIBUTIONS
A. Nakajima conceived the project and wrote the draft of the protocol. A. Nakajima and S. Habu wrote the manuscript. All authors interpreted and discussed the results.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research (C) No. 15K08533 to A. Nakajima from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Honda K, Littman DR. 2012. The microbiome in infectious disease and inflammation. Annu Rev Immunol 30: 759–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rooks MG, Garrett WS. 2016. Gut microbiota, metabolites and host immunity. Nat Rev Immunol 16: 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stiemsma LT, Michels KB. 2018. The role of the microbiome in the developmental origins of health and disease. Pediatrics 141: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, Bäckhed F, Isolauri E, Salminen S, Ley RE. 2012. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150: 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKenzie C, Tan J, Macia L, Mackay CR. 2017. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol Rev 278: 277–295. [DOI] [PubMed] [Google Scholar]
- 6.Macpherson AJ, de Agüero MG, Ganal-Vonarburg SC. 2017. How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol 17: 508–517. [DOI] [PubMed] [Google Scholar]
- 7.Maslowski KM, Mackay CR. 2011. Diet, gut microbiota and immune responses. Nat Immunol 12: 5–9. [DOI] [PubMed] [Google Scholar]
- 8.Dhingra D, Michael M, Rajput H, Patil RT. 2012. Dietary fibre in foods: a review. J Food Sci Technol 49: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai FJ, Chau CF. 2017. Classification and regulatory perspectives of dietary fiber. J Food Drug Anal 25: 37–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. 2013. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakajima A, Kaga N, Nakanishi Y, Ohno H, Miyamoto J, Kimura I, Hori S, Sasaki T, Hiramatsu K, Okumura K, Miyake S, Habu S, Watanabe S. 2017. Maternal high fiber diet during pregnancy and lactation influences regulatory T cell differentiation in offspring in mice. J Immunol 199: 3516–3524. [DOI] [PubMed] [Google Scholar]
- 12.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. 2014. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20: 159–166. [DOI] [PubMed] [Google Scholar]
- 13.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107: 14691–14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450. [DOI] [PubMed] [Google Scholar]
- 15.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, Wilhelm C, Veldhoen M. 2011. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147: 629–640. [DOI] [PubMed] [Google Scholar]
- 17.Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. 2011. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334: 1561–1565. [DOI] [PubMed] [Google Scholar]
- 18.Gutiérrez-Vázquez C, Quintana FJ. 2018. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity 48: 19–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roager HM, Licht TR. 2018. Microbial tryptophan catabolites in health and disease. Nat Commun 9: 3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, McCoy KD, Macpherson AJ. 2016. The maternal microbiota drives early postnatal innate immune development. Science 351: 1296–1302. [DOI] [PubMed] [Google Scholar]
- 21.van de Pavert SA, Ferreira M, Domingues RG, Ribeiro H, Molenaar R, Moreira-Santos L, Almeida FF, Ibiza S, Barbosa I, Goverse G, Labão-Almeida C, Godinho-Silva C, Konijn T, Schooneman D, O’Toole T, Mizee MR, Habani Y, Haak E, Santori FR, Littman DR, Schulte-Merker S, Dzierzak E, Simas JP, Mebius RE, Veiga-Fernandes H. 2014. Maternal retinoids control type 3 innate lymphoid cells and set the offspring immunity. Nature 508: 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorburn AN, McKenzie CI, Shen S, Stanley D, Macia L, Mason LJ, Roberts LK, Wong CH, Shim R, Robert R, Chevalier N, Tan JK, Mariño E, Moore RJ, Wong L, McConville MJ, Tull DL, Wood LG, Murphy VE, Mattes J, Gibson PG, Mackay CR. 2015. Evidence that asthma is a developmental origin disease influenced by maternal diet and bacterial metabolites. Nat Commun 6: 7320. [DOI] [PubMed] [Google Scholar]
- 23.Primec M, Mičetić-Turk D, Langerholc T. 2017. Analysis of short-chain fatty acids in human feces: a scoping review. Anal Biochem 526: 9–21. [DOI] [PubMed] [Google Scholar]
- 24.Vetrani C, Costabile G, Luongo D, Naviglio D, Rivellese AA, Riccardi G, Giacco R. 2016. Effects of whole-grain cereal foods on plasma short chain fatty acid concentrations in individuals with the metabolic syndrome. Nutrition 32: 217–221. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Hou C, Li N, Zhang X, Zhang G, Yang F, Zeng X, Liu Z, Qiao S. 2019. Microbial and metabolic alterations in gut microbiota of sows during pregnancy and lactation. FASEB J 33: 4490–4501. [DOI] [PubMed] [Google Scholar]
- 26.Nuriel-Ohayon M, Neuman H, Koren O. 2016. Microbial changes during pregnancy, birth, and infancy. Front Microbiol 7: 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M, Group ST, SPRING Trial Group.2016. Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes 65: 2214–2223. [DOI] [PubMed] [Google Scholar]
- 28.Fuller M, Priyadarshini M, Gibbons SM, Angueira AR, Brodsky M, Hayes MG, Kovatcheva-Datchary P, Bäckhed F, Gilbert JA, Lowe WL, Jr, Layden BT. 2015. The short-chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis. Am J Physiol Endocrinol Metab 309: E840–E851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG. 2015. The infant microbiome development: mom matters. Trends Mol Med 21: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hugenholtz F, de Vos WM. 2018. Mouse models for human intestinal microbiota research: a critical evaluation. Cell Mol Life Sci 75: 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen TL, Vieira-Silva S, Liston A, Raes J. 2015. How informative is the mouse for human gut microbiota research? Dis Model Mech 8: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B. 2014. The intestinal microbiome in early life: health and disease. Front Immunol 5: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thum C, Cookson AL, Otter DE, McNabb WC, Hodgkinson AJ, Dyer J, Roy NC. 2012. Can nutritional modulation of maternal intestinal microbiota influence the development of the infant gastrointestinal tract? J Nutr 142: 1921–1928. [DOI] [PubMed] [Google Scholar]
- 34.Prentice PM, Schoemaker MH, Vervoort J, Hettinga K, Lambers TT, van Tol EAF, Acerini CL, Olga L, Petry CJ, Hughes IA, Koulman A, Ong KK, Dunger DB. 2019. Human milk short-chain fatty acid composition is associated with adiposity outcomes in infants. J Nutr 149: 716–722. [DOI] [PubMed] [Google Scholar]
- 35.Brestoff JR, Artis D. 2013. Commensal bacteria at the interface of host metabolism and the immune system. Nat Immunol 14: 676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koh A, De Vadder F, Kovatcheva-Datchary P, Bäckhed F. 2016. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165: 1332–1345. [DOI] [PubMed] [Google Scholar]
- 37.Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I. 2015. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 7: 2839–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Husted AS, Trauelsen M, Rudenko O, Hjorth SA, Schwartz TW. 2017. GPCR-mediated signaling of metabolites. Cell Metab 25: 777–796. [DOI] [PubMed] [Google Scholar]
- 39.Ang Z, Ding JL. 2016. GPR41 and GPR43 in obesity and inflammation—protective or causative? Front Immunol 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan JK, McKenzie C, Mariño E, Macia L, Mackay CR. 2017. Metabolite-sensing G protein-coupled receptors-facilitators of diet-related immune regulation. Annu Rev Immunol 35: 371–402. [DOI] [PubMed] [Google Scholar]
- 41.Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, Macia L, Mackay CR. 2016. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Reports 15: 2809–2824. [DOI] [PubMed] [Google Scholar]
- 42.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, Glickman JN, Garrett WS. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanoue T, Atarashi K, Honda K. 2016. Development and maintenance of intestinal regulatory T cells. Nat Rev Immunol 16: 295–309. [DOI] [PubMed] [Google Scholar]
- 44.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. 2007. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol 8: 351–358. [DOI] [PubMed] [Google Scholar]
- 45.Lin J, Yang L, Silva HM, Trzeciak A, Choi Y, Schwab SR, Dustin ML, Lafaille JJ. 2016. Increased generation of Foxp3(+) regulatory T cells by manipulating antigen presentation in the thymus. Nat Commun 7: 10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang S, Fujikado N, Kolodin D, Benoist C, Mathis D. 2015. Immune tolerance. Regulatory T cells generated early in life play a distinct role in maintaining self-tolerance. Science 348: 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hubbard TD, Murray IA, Perdew GH. 2015. Indole and tryptophan metabolism: endogenous and dietary routes to Ah receptor activation. Drug Metab Dispos 43: 1522–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L. 2012. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36: 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Troy EB, Kasper DL. 2010. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front Biosci (Landmark Ed) 15: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamada N, Seo SU, Chen GY, Núñez G. 2013. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol 13: 321–335. [DOI] [PubMed] [Google Scholar]
- 51.Nakajima A, Negishi N, Tsurui H, Kadowaki-Ohtsuji N, Maeda K, Nanno M, Yamaguchi Y, Shimizu N, Yagita H, Okumura K, Habu S. 2014. Commensal bacteria regulate thymic Aire expression. PLoS One 9: e105904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. 2010. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 16: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ali NS, Nanji K. 2017. A review on the role of vitamin D in asthma. Cureus 9: e1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paul G, Brehm JM, Alcorn JF, Holguín F, Aujla SJ, Celedón JC. 2012. Vitamin D and asthma. Am J Respir Crit Care Med 185: 124–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.DiTroia SP, Percharde M, Guerquin MJ, Wall E, Collignon E, Ebata KT, Mesh K, Mahesula S, Agathocleous M, Laird DJ, Livera G, Ramalho-Santos M. 2019. Maternal vitamin C regulates reprogramming of DNA methylation and germline development. Nature 573: 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allan KM, Prabhu N, Craig LC, McNeill G, Kirby B, McLay J, Helms PJ, Ayres JG, Seaton A, Turner SW, Devereux G. 2015. Maternal vitamin D and E intakes during pregnancy are associated with asthma in children. Eur Respir J 45: 1027–1036. [DOI] [PubMed] [Google Scholar]
- 57.Devereux G, Craig L, Seaton A, Turner S. 2019. Maternal vitamin D and E intakes in pregnancy and asthma to age 15 years: a cohort study. Pediatr Pulmonol 54: 11–19. [DOI] [PubMed] [Google Scholar]
- 58.Stiemsma LT, Turvey SE. 2017. Asthma and the microbiome: defining the critical window in early life. Allergy Asthma Clin Immunol 13: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buffington SA, Di Prisco GV, Auchtung TA, Ajami NJ, Petrosino JF, Costa-Mattioli M. 2016. Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165: 1762–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Durack J, Lynch SV. 2019. The gut microbiome: relationships with disease and opportunities for therapy. J Exp Med 216: 20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vuillermin PJ, Macia L, Nanan R, Tang ML, Collier F, Brix S. 2017. The maternal microbiome during pregnancy and allergic disease in the offspring. Semin Immunopathol 39: 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gray LE, O’Hely M, Ranganathan S, Sly PD, Vuillermin P. 2017. The maternal diet, gut bacteria, and bacterial metabolites during pregnancy influence offspring asthma. Front Immunol 8: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chu DM, Meyer KM, Prince AL, Aagaard KM. 2016. Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function. Gut Microbes 7: 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Wang X, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C. 2018. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359: 1151–1156. [DOI] [PubMed] [Google Scholar]
- 65.Needell JC, Ir D, Robertson CE, Kroehl ME, Frank DN, Zipris D. 2017. Maternal treatment with short-chain fatty acids modulates the intestinal microbiota and immunity and ameliorates type 1 diabetes in the offspring. PLoS One 12: e0183786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mulero-Navarro S, Fernandez-Salguero PM. 2016. New trends in aryl hydrocarbon receptor biology. Front Cell Dev Biol 4: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hogenkamp A, Thijssen S, van Vlies N, Garssen J. 2015. Supplementing pregnant mice with a specific mixture of nondigestible oligosaccharides reduces symptoms of allergic asthma in male offspring. J Nutr 145: 640–646. [DOI] [PubMed] [Google Scholar]
- 68.Hsu CN, Lin YJ, Hou CY, Tain YL. 2018. Maternal administration of probiotic or prebiotic prevents male adult rat offspring against developmental programming of hypertension induced by high fructose consumption in pregnancy and lactation. Nutrients 10: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paul HA, Bomhof MR, Vogel HJ, Reimer RA. 2016. Diet-induced changes in maternal gut microbiota and metabolomic profiles influence programming of offspring obesity risk in rats. Sci Rep 6: 20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fujiwara R, Takemura N, Watanabe J, Sonoyama K. 2010. Maternal consumption of fructo-oligosaccharide diminishes the severity of skin inflammation in offspring of NC/Nga mice. Br J Nutr 103: 530–538. [DOI] [PubMed] [Google Scholar]
- 71.Hogenkamp A, Knippels LM, Garssen J, van Esch BC. 2015. Supplementation of mice with specific nondigestible oligosaccharides during pregnancy or lactation leads to diminished sensitization and allergy in the female offspring. J Nutr 145: 996–1002. [DOI] [PubMed] [Google Scholar]
- 72.Le Bourgot C, Ferret-Bernard S, Le Normand L, Savary G, Menendez-Aparicio E, Blat S, Appert-Bossard E, Respondek F, Le Huërou-Luron I. 2014. Maternal short-chain fructooligosaccharide supplementation influences intestinal immune system maturation in piglets. PLoS One 9: e107508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rø ADB, Simpson MR, Rø TB, Storrø O, Johnsen R, Videm V, Øien T. 2017. Reduced Th22 cell proportion and prevention of atopic dermatitis in infants following maternal probiotic supplementation. Clin Exp Allergy 47: 1014–1021. [DOI] [PubMed] [Google Scholar]