Abstract
Chondrosarcoma is the second most common primary bone sarcoma. Treatment of chondrosarcoma is limited to surgery due to radiation and chemotherapy resistance of this cancer. An ideal treatment for chondrosarcoma would be a well-tolerated, minimally invasive local or systemic treatment modality to halt or slow tumor growth prior to resection of local, unresectable local, or metastatic disease. Palovarotene, an agonist of nuclear retinoic acid receptor gamma (RARγ) has shown therapeutic actions for heterotopic ossification and osteochondroma without serious adverse effects in animal models. We hypothesized that selective agonists of RARγ would have an inhibitory effect on chondrosarcoma. All human chondrosarcoma specimens expressed RARγ as determined by immunohistochemical staining. The HCS-2/8 chondrosarcoma cell line, established from a low-grade human chondrosarcoma, was used to examine the actions of RARγ agonists. In HCS2/8 pellet cultures, RARγ agonist treatment reduced the mass size and significantly decreased total glycosaminoglycan, protein amounts, and gene expression levels of cartilage matrix molecules when compared to control groups. Systemic treatment with RARγ agonists significantly inhibited growth of HCS-2/8 cell transplants in vivo. Furthermore, local injection of the RARγ agonist-loaded poly-lactic acid (PLA) nanoparticles induced regression of the mass size of the transplants. Histologic analysis demonstrated that RARγ agonist treatment inhibited cell proliferation activity and stimulated encapsulation of the tumor. These findings indicate that RARγ agonists, including palovarotene, may have an anti-tumor effect on low-grade chondrosarcomas.
Keywords: Chondrosarcoma, Palovarotene, retinoic acid receptor agonist, nanoparticles
Introduction
Chondrosarcoma is the second most common primary bone sarcoma in adults and has been demonstrated to account for approximately 20–30% of primary malignant neoplasms of bone.1–3 The approximate incidence of chondrosarcoma is 1 in 200,000 or 1,600 new cases of chondrosarcoma each year in the United States.4 While there have been many attempts to improve survival and patient outcomes of this disease, large database studies have demonstrated that survival of chondrosarcoma has not changed in the past 30 years.4 Several molecular pathways have been identified as potential therapeutic targets,5–8 however the mainstay treatment of chondrosarcoma is surgical as this disease is generally considered to be radiation and chemotherapy resistant.9, 10 Once a chondrosarcoma metastasizes it is considered essentially incurable because of a lack of effective systemic therapy, however there is retrospective evidence that demonstrates a survival benefit with resection of the primary tumor in the setting of metastatic disease.11 While surgery alone is relatively effective for localized disease and even oligometastatic disease, the need for an effective treatment modality to address both locally recurrent as well as metastatic chondrosarcoma is apparent.
Retinoic acid (RA) has been known to alter chondrocyte growth and matrix production.12 Additionally, RA has been demonstrated previously to inhibit growth of chondrosarcoma cells in vitro13 and in animal studies.14 These findings suggest potential of RA as a therapeutic agent for skeletal disorders of cartilage, however use of this drug is not without side effects.15 Palovarotene, an agonist of RARγ, a nuclear retinoic acid receptor, has effects on cartilage biology and has been demonstrated to play a critical role in the inhibition of heterotopic ossification.16 Furthermore, preclinical studies have demonstrated that Palovarotene is a potent inhibitor of ectopic cartilage formation in rodent models of both acquired and genetic heterotopic ossification16–18 and have shown inhibition of growth of benign cartilage tumors in an osteochondroma animal model.19 Notably, Palovarotene is currently being investigated in phase 3 clinical trials for treatment of fibrodysplasia ossificans progressiva (FOP) (Clinicaltrials.gov registration NCT02190747, phase 3) and multiple osteochondroma ( NCT03442985, phase 2). Considering previous findings of both retinoic acid and Palovarotene on cartilage biology in the setting of multiple pathologic conditions and the fact that it is well tolerated in clinical trials20, we hypothesized that RARγ agonists including Palovarotene would have an in vitro and in vivo effect on the a human chondrosarcoma cell line, HCS-2/8, which has been established from a low-grade human chondrosarcoma cell line.21 The findings obtained in this study suggest that selective RARγ agonists including palovarotene may provide an anti-tumor affect on low-grade human chondrosarcoma cells.
Methods
All animal experiments were performed following institutional guidelines and were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Maryland, Baltimore.
Cells:
Human chondrosarcoma cell line, HCS-2/8, established from low-grade chondrosarcoma (72yr, male)21 were maintained in high-glucose DMEM containing 15–20% FBS. Cultures were passaged biweekly at a 1:2–1:3 ratio.
Mice:
NOD/Shi-scid IL2rgamma(null) (NOG) female mice (7–8 weeks old) were purchased from the Animal Service at University of Maryland, Baltimore.
Tumor cell transplantation and measurement of tumors:
HCS-2/8 Cells (2 million cells in 100μl) were inoculated subcutaneously in NOD/Shi-scid IL2rgamma(null) (NOG) mice. Tumor size was measured weekly by a caliper, and the tumor volume (WxWxLx1/2) was calculated by two independent researchers.22 At the endpoint, the harvested tumors were weighed by two independent researchers and fixed with 4% paraformaldehyde.
Drug and Drug treatment:
Palovarotene (CAS410528-02-8)23 and NRX20464716 were synthesized at Atomax Chemicals Company Limited (Shenzhen, China). Drug quality and activity were confirmed by mass spectrometry and the reporter assay using the RARγ Reporter Cellular Assay Pack (BPS Bioscience, San Diego, CA). NRX204647 was loaded into biodegradable nanoparticles (NPs) using poly(D,L-lactide) (PLA), herein called RARγ-NP.24 The release of active NRX204647 from the nanoparticles was validated by the reporter assay using the RARγ Reporter Cellular Assay Pack (BPS Bioscience). After randomization of the mice that received HCS-2/8 cell transplantation, Palovarotene (2.5 mg/kg) or the same volume of vehicle (1:9, DMSO:corn oil) were given to mice via gavage 3 times/week, starting 1 week after tumor inoculation when tumor masses were viable (n=10). Seven days after tumor inoculation, mice received subcutaneous injections of PLA nanoparticles loaded with NRX204647 (5 μg/tumor) or the same volume of control nanoparticles (n=10) twice a week.
Histology:
Human chondrosarcoma specimens were obtained from the University of Maryland Pathology Biorepository Shared Service (PBBS) and subjected to immunostaining for RARγ. Tumor sections were graded according to the American Joint Committee on Cancer with support from PBBS. The staining of RARγ was evaluated by two independent researchers and scored as negative, positive, and strongly positive. The HCS-2/8 cell transplants were sectioned (4 μm) and stained with hematoxylin and eosin (HE), alcian blue, or picrosirius red. The images of the HE and alcian blue-stained sections were captured and analyzed using BZX-700 (Keyence, Itasca, IL). Picrosirius red-stained images were captured under a polarized filter and analyzed using BZX-700.
For RARγ immunohistochemical staining, sections were incubated in a 10mM sodium citrate buffer solution, pH 6.0, for 8 minutes at 95°C in 3% hydrogen peroxide, methanol for 10 minutes at room temperature, and blocked with blocking buffer, 5% BSA with 1% goat serum, for 30 minutes at room temperature. Sections were then incubated with polyclonal antibodies for RARγ (1:100, #8965, Cell signaling, Danvers MA; 1:300, HPA05388, Sigma, St. Louis, MO) at 4°C overnight. Antibodies were visualized by incubation with biotinylated anti-rabbit IgG 1:200 (Vector Laboratories, Burlingame, CA) followed by color detection using ImmPACT DAB peroxidase substrate (Vector Laboratories). Counter staining was performed with methyl green. Two independent researchers observed the immunoreactivity in nuclei of sections (3 fields/section/specimen). Cells with RARγ antibody nuclei staining were identified as positive while nuclei found to be negative to RARγ antibody staining, but positive to methyl green counter staining were evaluated as negative. Results were scored as weak, modest, and strong groups (−/+, + and ++). The weak group had less than 20% positive cells to the immunoreactivity. The modest and strong groups had 20–70% and more than 70% positive cells, respectively. For Ki67 immunofluorescence staining, sections were incubated with 10 μg/ml protease K and stained with the polyclonal antibody for Ki67 (1:300, #16667) (Abcam, Cambridge, MA) overnight at 4°C followed by visualization with goat anti-rabbit IgG, Alexa Fluor 488 (1:200, Thermo Fisher Scientific) and DAPI staining. The Ki67 and DAPI images were captured and analyzed in BZX-700. The positive ratio of Ki67 to the DAPI-positive nuclei was calculated. Three fields per section, three sections per sample and three samples per group were analyzed.
Cell culture and treatment:
HCS-2/8 cells were plated in monolayer (300,000/well in 12 well plate) or in pellet cultures (1,000,000/tube) and cultured in high glucose DMEM containing 20% FBS. Two independent experiments were performed using HCS-2/8 cultures (n=3/group/individual experiment). Similar results were obtained. HCS-2/8 cells were treated with 300nM Palovarotene or the same volume of ethanol (vehicle) for 4 or 7 days (n=3, two independent experiments). Culture medium was changed every other day. Protein, DNA and glycosaminoglycan (GAG) contents of the pellets were measured by the Pirece BCA Protein assay kit (Thermo Fisher Scientific, Waltherm, MA), Qbit quantification kit (Thermo Fisher Scientific) and the DMMB (dimethylmethylene blue) method25, respectively. For gene expression analysis, HCS-2/8 cells were treated with Palovarotene at the concentration of 300 nM or vehicle for 4 days. Total RNAs were isolated using RNeasy Mini Kit (Qiagen, Germantown, MD) and subjected to qPCR using the validated PrimeTime qPCR primers (Integrated DNA Technology, Coralville, IA): ACTB, Hs.PT.39a.22214847; ACAN, Hs.PT.56a.742783; COL2A1, Hs.PT.58.4107778HAS2; COL9A1, Hs.PT.58.38805503; HAS2, Hs.PT.58.40275566; 38517191MMP13, Hs.PT.58.4073501; TGM2, Hs.PT.58.3966447; CYP26B1, Hs.PT.58.38517191. qPCR was performed with an Applied Biosystems 7900HT Sequence Detection Systems running SDS 2.1 software using SYBR green reagents (Applied Biosystems, Foster City, CA). Average threshold cycle value (Ct value) was calculated from quadruplicate reactions. Standard curves were generated using 10-fold serial dilutions of cDNA of each gene with a correlation coefficient of >0.98. Relative expression levels were calculated based on a standard curve and normalized to beta-Actin (ACTB).
Statistical Methods:
Statistical analysis was performed using unpaired t test or one-way analysis of variance (ANOVA) and Tukey’s multiple comparison tests using Prism 6 (GraphPad Software, La Jolla, CA).
Results
RARγ expression in human chondrosarcomas
Eleven independent human chondrosarcoma specimens were subjected to immunohistochemical examination of RARγ. Immunoreactivity to RARγ antibodies was observed in both chondrosarcoma cells and the surrounding connective tissue. All examined human chondrosarcoma specimens, including 7 low grade chondrosarcomas, showed immunoreactivity to the RARγ antibodies (Fig. 1 and Table 1), suggesting that human chondrosarcoma cells express RARγ in at least low and intermediate grade chondrosarcomas.
Figure 1. RARγ expression in human chondrosarcomas.
The human chondrosarcoma specimens were obtained from the Pathology Biorepository Shared Service (PBSS) at University of Maryland, Baltimore. The sections were subjected to HE staining (A, D and G) and immunohistochemical staining for RARγ (B, C, E, F, H and I). A-C are sections from P0595. D-F are sections from P0597. G-I are sections from P0599. A, D and G are serial sections of B, E, H, respectively. C, F and I are high magnified images of the box areas of B, E and H, respectively. The bar is 50 μm for A, B, D, E, G and H, and 25 μm for C, F and I. The staining image of the section (P0595) with non-immune rabbit IgG is shown in inset of C.
Table 1.
Expression of RARg in human chondrosarcomas
| ID | Grade | Staining |
|---|---|---|
| P0593 | Grade 3 | ++ |
| P0594 | Grade 3 | ++ |
| P0595 | Grade 1 | ++ |
| P0596 | Grade 2 | + |
| P0597 | Grade 1 | ++ |
| P0598 | Grade 2–3 | + |
| P0599 | Grade 1 | ++ |
| P0997 | Grade 1 | + |
| P0999 | Grade 1 | ++ |
| P1000 | Grade 1 | ++ |
| P1002 | Grade 1 | + |
The human chondrosarcoma specimens were obtained from the Pathology Biorepository Shared Service (PBSS) at University of Maryland, Baltimore. The sections were subjected to immunohistochemical staining for RARγ. The results were evaluated by 2 independent researchers.
The effects of RARγ agonists on HCS-2/8 cells
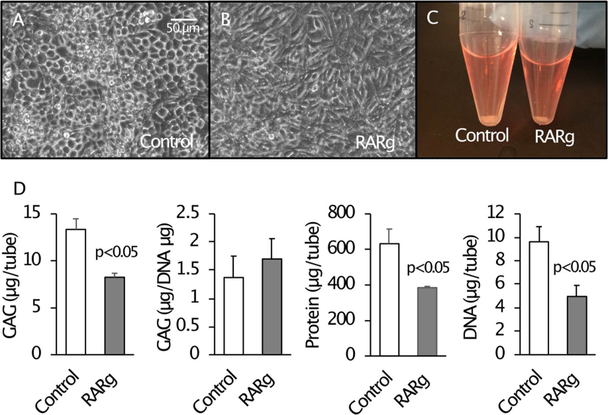
HCS-2/8 cells were seeded in pellet culture for 7 days and then treated with Palovarotene for 7 days. The pellet size was smaller in the Palovarotene-treated culture (Fig. 2C). The drug-treated culture also had lower amounts of GAG, protein, and DNA compared to the vehicle-treated control culture (Fig. 2D). However, the GAG amount when normalized to the total DNA content in the drug-treated culture was similar (Fig. 2D). This suggests that decreases in the GAG in Palovarotene-treated cultures is due to a decrease in cell number. Reduction in mass size, however, may also be a result of decreasing other extracellular matrix components. Gene expression was examined 4 days after Palovarotene treatment because a change at a gene expression level precedes a change at a protein level. Palovarotene treatment inhibited expression of ACAN, COL2A1, COL9A1, and HAS2 (Hyaluronan synthase 2) (Fig. 3). The response to RARγ agonists in HCS-2/8 cells and chondrocytes were confirmed by up-regulation of the target molecules, TGM2 and CYP26B1 (Fig. 3, TGM2 and CYP26B1).
Figure 2. The effects of Palovarotene on HCS-2/8 cells in vitro.
HCS-2/8 cells were cultured in monolayer (A and B) or pellet (C and D). Cultured cells (n=3) were treated with 300 nM Palovarotene (RARg) or vehicle (0.1% EtOH) for 4 days (A and B) or 7 days (C and D). Palovarotene-treated cells showed fibroblastic morphology (B), a smaller pellet mass (C), and reduced total and relative amounts of glycosaminoglycan, total protein, and total DNA contents (D).
Figure 3. RARγ decreased gene expression level of cartilage matrix-related molecules in HCS-2/8 cells.
HCS-2/8 cells were cultured in monolayer and treated with 300 nM NRX204647 (RARγ agonist) for 4 days (n=3). Gene expression levels of ACAN, COL2A1, COL9A1, HAS, MMP13, TGM2, and Cyp26B1 were examined by qPCR and calculated as a relative ratio to ACTB (beta-Actin). The values are average and standard deviation (SD).
Response of chondrosarcoma tumors to systemic treatment
HCS-2/8 cells were subcutaneously transplanted on the backs of NOG mice. One week after transplantation and tumors were found to be viable, systemic administration of Palovarotene was started 3 times per week by oral gavage, and the tumor size was measured until 8 weeks after cell transplantation (Fig. 4A). An increase in the tumor size was significantly inhibited by Palovarotene treatment 6 weeks after treatment initiation (Fig. 4B). Tumor weights of the treated group was found to be approximately 50% of the control group at the endpoint (Fig. 4C).
Figure 4. Systemic treatment with Palovarotene inhibited tumor mass growth of HCS-2/8 cells.
HCS-2/8 Cells (2 million cells in 100μl) were inoculated subcutaneously in NOD/Shi-scid IL2rgamma(null) (NOG) mice. A, One week after cell transplantation, Palovarotene (2.5 mg/kg) or vehicle was administrated by gavage 3 times/week. B, Tumor size was measured weekly by a caliper, and the tumor volume (WxWxLx1/2) was calculated. C, 7 weeks after the administration started (Week 8), the tumors were harvested and weighed. Values are average and SD. *, p<0.05.
Response of chondrosarcoma tumors to local treatment
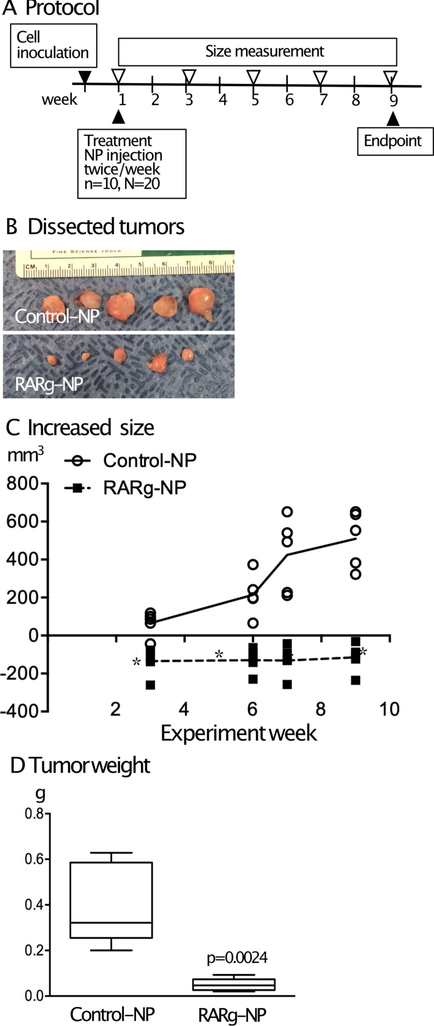
RARγ-NPs were injected adjacent to HCS-2/8 transplants twice per week starting one week after cell transplantation (Fig. 5A). NRX204647 was chosen as it is an RARγ agonist that is chemically well-suited for loading into nanoparticles and it exhibits an approximately 100-fold higher affinity to RARγ compared to other RAR isoforms. Additionally, the median effective dose (ED50) is 3–10 ng/ml as determined by biologic activity in cultured chondrocytes.16 Tumor size decreased from the initial size in the RARγ-NP groups (Fig. 5B and Fig. 5C, squares) while the tumor size increased in the control group mice that received injections of control nanoparticles (Fig. 5B and Fig. 5C, circles). Tumor weight was 10% of the control at the endpoint Fig. 5D. Histological examination showed that both the drug treated HCS-2/8 tumors and the control HCS-2/8 cells accumulated proteoglycans (Figs. 6A and 6B). Immunostaining for RARγ showed that HCS-2/8 cells express RARγ (Fig. 6C). The results of Ki67 staining indicated that drug-treated HCS-2/8 cells had less proliferating activity (Figs. 6D and 6E). Furthermore, the drug treated HCS-2/8 tumors were encapsulated by dense collagen fibers as determined by picrosirius red staining (Fig. 6F).
Figure 5. Local treatment using nanoparticle delivery of RARγ agonists inhibited tumor growth of HCS-2/8 cells.
HCS-2/8 Cells (2 million cells in 100μl) were inoculated subcutaneously in NOD/Shi-scid IL2rgamma(null) (NOG) mice. A, One week after tumor transplantation, mice received subcutaneous injections of PLA nanoparticles loaded with NRX204647 (5 μg/tumor) (RARγ-NP) or the same volume of control nanoparticles (n=10) twice a week. C, Tumor size was weekly measured by a caliper, and the tumor volume (WxWxLx1/2) was calculated. Values are average and SD. *, p<0.05. B and D, 8 weeks after the drug treatment started (Week 9), the tumors were harvested (B) and weighed (D). Values are average and SD.
Figure 6. RARg agonists inhibited cell proliferation and induced encapsulation of the tumors of HCS-2/8 cells.
HCS-2/8 Cells (2 million cells in 100μl) were inoculated subcutaneously in NOD/Shi-scid IL2rgamma(null) (NOG) mice. One week after tumor transplantation, mice received subcutaneous injections of PLA nanoparticles loaded with NRX204647 (5 μg/tumor) (RARg-NP) or the same volume of control nanoparticles. Transplants were harvested 8 weeks after drug treatment started (Week 9) and subjected to histological analysis. A and B, The control-NP (A) and RARγ-NP-treated (B) masses contained matrix positive for alcian blue although their size was smaller compared to the vehicle-treated masses. C, Immunostaining of RARγ in control transplants. D, Ki67 staining. The RARγ-treated masses contained fewer cells positive to Ki67 cells compared to control. E, Quantitative analysis of Ki67 staining. Values are average and SD (n=4). F, Picrosirus red staining. The RARγ-treated masses were surrounded thicker collagen-rich tissues, which were visualized using a polarizing filter. The images in square were magnified (right). The bars are 800 μm for A, B and F (left panels), 50 μm for C and 100 μm for D and F (right panels).
Discussion
Chondrosarcoma is a rare disease and one that is difficult to study due to the relatively low incidence and broad spectrum of behavior. However, there is potential for improvement in our current approach and understanding of this disease. Large database studies from the Surveillance, Epidemology, and End Results (SEER) program through the National Cancer Institute have demonstrated that there has been no change in survival of this disease over the last 30 years.4 Recent clinical studies on the treatment of chondrosarcoma have focused on mutations in IDH, hedgehog pathway, src family tyrosine kinases, immunotherapy, angiogenesis, mTOR pathways, and histone deacetylase inhibitors.6–8 Unfortunately, prior studies are plagued by small patient numbers and often have a heterogenous patient group with different manifestations of chondrosarcoma, which is one of the challenges of studying a rare and variable disease. While we do acknowledge that we should examine the actions of Palovarotene and RARγ agonists on other chondrosarcoma cells lines and primary chondrosarcomas before making a definitive conclusion, our results suggest, however, that RARγ signaling may be a possible targeted pathway. Palovarotene, an orally bioavailable and selective RARγ agonist, has been identified as having therapeutic action against fibrodysplasia ossificans progressiva (FOP), heterotopic ossification, and osteochondroma, but has not been characterized previously for chondrosarcoma.16–19 Notably, Palovarotene is orally bioavailable, has been well tolerated in clinical trials, and is currently under investigation in phase 3 ( NCT03312634) and phase 2 ( NCT03442985) clinical trials for FOP and multiple hereditary exostosis, respectively. Our data indicates that RARγ is expressed in human chondrosarcomas, and its signaling is retained in a human chondrosarcoma cell line. In addition, we found that Palovarotene and other RARγ agonists result in decreased growth of a low-grade human chondrosarcoma cell line in a xenograft model. These findings encourage us to study the effects and actions of the RARγ agonists to better understand the utility of this drug for treatment of chondrosarcomas. Previous research on retinoic acid (RA) has demonstrated that oral administration of retinoic acid at 100 mg/kg for 7 days reduced the tumor size of rat chondrosarcoma cells that were subcutaneously transplanted in rats.14 This treatment reduced the metachromatic staining for proteoglycan, induced necrotic cells in the center part of the tumor mass, and increased collagen staining in Masson’s trichrome staining. These findings suggest that activation of retinoid signaling would be effective for chondrosarcomas. In this study, we also found similar tumor size regression and stimulation of collagen accumulation at much lower doses of the RARγ agonist. Retinoid treatment has been attempted for pathological conditions involving cartilage formation, but has not resulted in satisfactory outcomes. One of reasons for this is due to adverse side effects including dry skin, dry mucous membranes, headache, nausea and vomiting, rash, mouth sores, and vision changes.15, 26 Targeting retinoic signaling using selective agonists for one specific RAR receptor is likely more effective because RARγ agonists have shown good side effect profiles in preclinical and clinical studies.16, 20 The molecular mechanisms of RARγ agonists on inhibition of growth of HCS-2/8 cell tumors may include inhibition of synthesis of cartilaginous matrix proteins and suppression of cell proliferation. Gene expression analysis of the in vitro arm of this study revealed that RARγ agonists strongly inhibit expression of ACAN, COL2A1, COL9A1, and HAS2, which are cartilage extracellular matrix producing genes. A prior study by Hamada et al. reported that suppression of hyaluronan (HA) with 4-Methylumbelliferone (MU) resulted in attenuation of tumorgenicity of low-grade chondrosarcoma.27 Thus reduction in cartilage matrix may be an effective treatment approach for low grade chondrosarocoma as RARγ agonists may have the potential to effectively decrease matrix production and have been previously demonstrated to inhibit various types of cartilage matrix molecules. This may also increase the action of chemotherapy drugs on chondrosarcoma tumor cells since one of the proposed mechanisms of chemotherapy resistance is an abundant extracellular matrix that inhibits drug delivery.10 The actions of retinoid signaling on inhibition of matrix synthesis and cell proliferation are common with normal chondrocytes.28–30 In contrast, RARγ agonists did not stimulate MMP13 expression in HCS-2/8 cells while the retinoid signaling did increase MMP13 expression in normal growth plate chondrocytes.29 This difference may be explained by differences in the biology of normal and malignant cells. However, this interpretation should be limited to this specific cell line at this moment.
While we believe our study adds to the growing body of literature on the treatment of chondrosarcoma our study has several limitations. Human chondrosarcoma expresses RARγ but we should clarify whether there is correlation of the RARγ expression level to the tumor grade since this is clinically important to develop pharmacological treatments of this drug in humans. Furthermore, we did not assess systemic toxicity of palovarotene using standard blood tests, including levels of Palovarotene, as would be used in clinical trials. However, we did not find a decrease in body weight nor notice strong systemic adverse clinical effects in mice. Additionally, the same drug with similar treatment conditions did not show evidence of toxicity in laboratory testing.16 Second, we used a low grade chondrosarcoma cell line that is generally treated in humans with local resection and possible adjuvant treatments and at this time it is unknown if our findings are translatable to high grade chondrosarcoma cell lines. If RARγ agonist efficacy is limited to low grade chondrosarcoma, it may be of benefit in certain situations to reduce tumor volume, prevent more aggressive behavior, and potentially minimize surgical morbidity.
In conclusion, RARγ agonist treatment results in decreased growth of a low-grade human chondrosarcoma cell line in a mouse model both in vitro and in vivo. The in vivo arm of this study demonstrated a more pronounced effect when RARγ agonists were delivered locally with nanoparticles. Additionally, human chondrosarcoma specimens express RARγ regardless of tumor grade. Our findings support further study on the therapeutic potential of RARγ agonists in the setting of chondrosarcoma. Specifically, future research of this drug should include investigation on palovarotene as a possible radiation or chemotherapy sensitizing molecule in the setting of chondrosarcoma, effects of palovarotene on a higher grade chondrosarcoma cell line, and use in orthotopic and metastatic chondrosarcoma animal models.
Acknowledgment:
This study is partially supported by the NIH grants, R01AR073181 (MEI) and R01AR056837 (MI) and ICTR ATIP Grant Program at University of Maryland, Baltimore. The individual serving as co-investigator on the grants is a spouse.
References
- 1.Leddy LR, Holmes RE. 2014. Chondrosarcoma of Bone. Cancer Treat Res. 162:117–130. [DOI] [PubMed] [Google Scholar]
- 2.Murphey MD, Walker EA, Wilson AJ, et al. 2003. From the Archives of the Afip: Imaging of Primary Chondrosarcoma: Radiologic-Pathologic Correlation. Radiographics. 23(5):1245–1278. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Xu J, et al. 2010. Cancer Statistics, 2010. CA Cancer J Clin. 60(5):277–300. [DOI] [PubMed] [Google Scholar]
- 4.Giuffrida AY, Burgueno JE, Koniaris LG, et al. 2009. Chondrosarcoma in the United States (1973 to 2003): An Analysis of 2890 Cases from the Seer Database. J Bone Joint Surg Am. 91(5):1063–1072. [DOI] [PubMed] [Google Scholar]
- 5.Bovee JV, Hogendoorn PC, Wunder JS, et al. 2010. Cartilage Tumours and Bone Development: Molecular Pathology and Possible Therapeutic Targets. Nat Rev Cancer. 10(7):481–488. [DOI] [PubMed] [Google Scholar]
- 6.Nazeri E, Gouran Savadkoohi M, Majidzadeh AK, et al. 2018. Chondrosarcoma: An Overview of Clinical Behavior, Molecular Mechanisms Mediated Drug Resistance and Potential Therapeutic Targets. Crit Rev Oncol Hematol. 131:102–109. [DOI] [PubMed] [Google Scholar]
- 7.Mery B, Espenel S, Guy JB, et al. 2018. Biological Aspects of Chondrosarcoma: Leaps and Hurdles. Crit Rev Oncol Hematol. 126:32–36. [DOI] [PubMed] [Google Scholar]
- 8.Polychronidou G, Karavasilis V, Pollack SM, et al. 2017. Novel Therapeutic Approaches in Chondrosarcoma. Future Oncol. 13(7):637–648. [DOI] [PubMed] [Google Scholar]
- 9.Wyman JJ, Hornstein AM, Meitner PA, et al. 1999. Multidrug Resistance-1 and P-Glycoprotein in Human Chondrosarcoma Cell Lines: Expression Correlates with Decreased Intracellular Doxorubicin and in Vitro Chemoresistance. J Orthop Res. 17(6):935–940. [DOI] [PubMed] [Google Scholar]
- 10.Onishi AC, Hincker AM, Lee FY. 2011. Surmounting Chemotherapy and Radioresistance in Chondrosarcoma: Molecular Mechanisms and Therapeutic Targets. Sarcoma. 2011:381564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song K, Song J, Chen F, et al. 2019. Does Resection of the Primary Tumor Improve Survival in Patients with Metastatic Chondrosarcoma? Clin Orthop Relat Res. 477(3):573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weston AD, Hoffman LM, Underhill TM. 2003. Revisiting the Role of Retinoid Signaling in Skeletal Development. Birth Defects Res C Embryo Today. 69(2):156–173. [DOI] [PubMed] [Google Scholar]
- 13.Thein R, Lotan R. 1982. Sensitivity of Cultured Human Osteosarcoma and Chondrosarcoma Cells to Retinoic Acid. Cancer Res. 42(11):4771–4775. [PubMed] [Google Scholar]
- 14.Ettlin R, Galli B, Kistler A. 1982. Histological Changes During Regression Induced by Retinoic Acid in a Transplantable Rat Chondrosarcoma. Virchows Arch A Pathol Anat Histol. 396(1):1–8. [DOI] [PubMed] [Google Scholar]
- 15.David M, Hodak E, Lowe NJ. 1988. Adverse Effects of Retinoids. Med Toxicol Adverse Drug Exp. 3(4):273–288. [DOI] [PubMed] [Google Scholar]
- 16.Shimono K, Tung WE, Macolino C, et al. 2011. Potent Inhibition of Heterotopic Ossification by Nuclear Retinoic Acid Receptor-Gamma Agonists. Nat Med. 17(4):454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakkalakal SA, Uchibe K, Convente MR, et al. 2016. Palovarotene Inhibits Heterotopic Ossification and Maintains Limb Mobility and Growth in Mice with the Human Acvr1(R206h) Fibrodysplasia Ossificans Progressiva (Fop) Mutation. J Bone Miner Res. 31(9):1666–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheatley BM, Cilwa KE, Dey D, et al. 2018. Palovarotene Inhibits Connective Tissue Progenitor Cell Proliferation in a Rat Model of Combat-Related Heterotopic Ossification. J Orthop Res. 36(4):1135–1144. [DOI] [PubMed] [Google Scholar]
- 19.Inubushi T, Lemire I, Irie F, et al. 2018. Palovarotene Inhibits Osteochondroma Formation in a Mouse Model of Multiple Hereditary Exostoses. J Bone Miner Res. 33:658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wentworth KL, Masharani U, Hsiao EC. 2019. Therapeutic Advances for Blocking Heterotopic Ossification in Fibrodysplasia Ossificans Progressiva. Br J Clin Pharmacol. 85(6):1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takigawa M, Tajima K, Pan HO, et al. 1989. Establishment of a Clonal Human Chondrosarcoma Cell Line with Cartilage Phenotypes. Cancer Res. 49(14):3996–4002. [PubMed] [Google Scholar]
- 22.Tomayko MM, Reynolds CP. 1989. Determination of Subcutaneous Tumor Size in Athymic (Nude) Mice. Cancer Chemother Pharmacol. 24(3):148–154. [DOI] [PubMed] [Google Scholar]
- 23.Hind M, Stinchcombe S. 2009. Palovarotene, a Novel Retinoic Acid Receptor Gamma Agonist for the Treatment of Emphysema. Curr Opin Investig Drugs. 10(11):1243–1250. [PubMed] [Google Scholar]
- 24.Chorny M, Fishbein I, Danenberg HD, et al. Biodegradable Nanoparticles as Drug Delivery Systems for Parental Administration In: Yaszemski MJ, Trantolo DJ, Lewandroski K-U, Hasirci V, Altobelli DE, Wise DL, editors. Tissue Engineering and Novel Delivery Systems. New York: Marcel Dekker; 2003. p. 393–422. [Google Scholar]
- 25.Humbel R, Etringer S. 1974. A colorimetric method for the determination of sulfated glycosaminoglycans. Rev Roum Biochim. 11:21–24. [Google Scholar]
- 26.Lassus A 1980. Systemic Treatment of Psoriasis with an Oral Retinoic Acid Derivative (Ro 10–9359). Br J Dermatol. 102(2):195–202. [DOI] [PubMed] [Google Scholar]
- 27.Hamada S, Nishida Y, Zhuo L, et al. 2018. Suppression of Hyaluronan Synthesis Attenuates the Tumorigenicity of Low-Grade Chondrosarcoma. J Orthop Res. 36(6):1573–1580. [DOI] [PubMed] [Google Scholar]
- 28.Williams JA, Kane M, Okabe T, et al. 2010. Endogenous Retinoids in Mammalian Growth Plate Cartilage: Analysis and Roles in Matrix Homeostasis and Turnover. J Biol Chem. 285(47):36674–36681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwamoto M, Kitagaki J, Tamamura Y, et al. 2003. Runx2 Expression and Action in Chondrocytes Are Regulated by Retinoid Signaling and Parathyroid Hormone-Related Peptide (Pthrp). Osteoarthritis Cartilage. 11(1):6–15. [DOI] [PubMed] [Google Scholar]
- 30.Minegishi Y, Sakai Y, Yahara Y, et al. 2014. Cyp26b1 within the Growth Plate Regulates Bone Growth in Juvenile Mice. Biochem Biophys Res Commun. 454(1):12–18. [DOI] [PubMed] [Google Scholar]