Abstract
Using in vitro models, we previously reported that 4-methylumbelliferone (4-MU) blocked many of the pro-catabolic features of activated chondrocytes. 4-MU also blocked safranin O loss from human cartilage explants exposed to IL1β in vitro. However, the mechanism for this chondroprotective effect was independent of the action of 4-MU as a hyaluronan (HA) inhibitor. Interestingly, overexpression of HA synthase 2 (HAS2) also blocked the same pro-catabolic features of activated chondrocytes as 4-MU via a mechanism independent of extracellular HA accumulation. Data suggest that altering UDP-sugars may be behind these changes in chondrocyte metabolism. However, all of our previous experiments with 4-MU or HAS2 overexpression were performed in vitro. The purpose of this study was to confirm whether 4-MU was effective at limiting the effects of osteoarthritis (OA) on articular cartilage in vivo. The progression of OA was evaluated after destabilization of the medial meniscus (DMM) surgery on C57BL/6 mice in the presence or absence of 4-MU-containing chow. Mice fed 4-MU after DMM surgery exhibited a significant suppression of OA starting from an early stage in vivo. Mice fed 4-MU exhibited lower OARSI scores after DMM; reduced osteophyte formation and reduced MMP3 and MMP13 immunostaining. 4-MU also exerted pronounced chondroprotective effects on murine joint cartilage exposed to IL1β in vitro and, blocked IL1β-enhanced lactate production in cartilage explants. Therefore, 4-MU is effective at significantly reducing loss of proteoglycan and reducing MMP production both in vitro and in vivo as well as cartilage damage and osteophyte formation in vivo after DMM.
Keywords: osteoarthritis, DMM, matrix metalloproteinase, hyaluronan
INTRODUCTION
The glycosaminoglycan hyaluronan (HA) serves a primary role in retaining aggrecan proteoglycan in the extracellular matrix of cartilage; aggrecan that is necessary to maintain the hydrostatic, shock-absorbing properties of the tissue 1,2. Reversing deficits in HA, often associated with osteoarthritis (OA), has been a hypothetical strategy to preserve cartilage integrity and provide feed-back signaling from the extracellular matrix to chondrocytes for a return to steady-state metabolism. Thus, it was somewhat counterintuitive when we observed previously that a chemical inhibitor of HA biosynthesis, 4-methylumbelliferone (4-MU), blocked the synthesis and production of MMP13, ADAMTS4 and TSG6 in human OA chondrocytes and bovine chondrocytes 3. Of more relevance, the addition of 4-MU to intact bovine or human knee OA cartilage explants provided “chondroprotection” of the tissues, reducing the release of sulfated glycosaminoglycan (sGAG) into the medium and providing a near complete block in the loss of safranin O staining in the tissue. More intriguing was that 4-MU inhibition of MMP13 and other pro-catabolic markers did not require the inhibition of HA biosynthesis being just as effective in chondrocytes following knock-down of HAS2, conditions where there was little HA production. More recently, we examined the reverse situation, overexpression of HAS2 (HAS2-OE) 4. We observed that HAS2-OE also blocked the same pro-catabolic markers as 4-MU and promoted the retention of sGAG by chondrocytes. Surprisingly, the chondroprotective effects of HAS2-OE were not due to the enrichment of extracellular HA (outside-in signaling) but instead our data suggested induced changes in energy metabolism in the transduced chondrocytes.
Although intriguing, both the 4-MU and HAS2-OE studies were all performed using in vitro models including isolated human OA or bovine chondrocytes or cartilage explants treated with IL1β. These models are useful but likely do not fully represent OA in vivo 5-8. Questions arise as to whether the culture medium, serum, cytokines or normoxic conditions used in vitro truly reflect the environment of an OA joint in vivo. The destabilization of the medial meniscus (DMM) OA model in mice 9 has become the standard, effective approach to model OA in vivo 10,11. With relevance to our studies, the DMM model displays progressive changes in articular cartilage, with less contribution by inflamed synovial tissues. 4-MU has proven effective in mouse models of inflammation and in cases where inflammation is dependent on HA biosynthesis 12,13. Thus as a primary aim, we explored whether 4-MU is effective in limiting the progression of OA in mice following an induction by DMM.
The mechanism of action of 4-MU is complex. 4-MU is a low toxicity coumarin derivative that forms covalent glycoconjugates with D-glucuronic acid (GlcUA) via the transfer from UDP-GlcUA 14. The formation of these glycoconjugates restricts access of cytosolic UDP-GlcUA intermediates necessary for HA biosynthesis and serves as the primary mechanism for 4-MU blockage of HA production 15,16. However, 4-MU also blocks the transcription of HAS2 mRNA 3,17 providing an additional molecular dampener to HA biosynthesis. But in chondrocytes, 4-MU also blocks the transcription of a select set of genes activated after IL1β treatment in addition to HAS2 namely, MMP13, ADAMTS4 and TSG6 with modest enhancement of ACAN and COL2A 3. The mechanism for the selective transcriptional effects of 4-MU is as yet unknown.
As noted above, given that HAS2-OE also blocks the transcription of many of the same activated genes 4, some other non-canonical mechanism, in common to both 4-MU and HAS2-OE must be in play. Recent studies in human cancer cells demonstrate that experimental modulation of intracellular UDP-hexose pools exerts effects on cellular phenotype in large part by altering intracellular metabolism in addition to other concurrent effects on HA biosynthesis 18. HAS2-OE does give rise to changes in chondrocyte metabolism 4. If 4-MU and HAS2-OE are similar, 4-MU may provide a means to modulate the metabolomics of chondrocytes and thus contribute insight into how altering metabolism affects OA in the DMM model.
In this study, we examined knee joints from mice fed a diet of 5% 4-MU chow versus control chow and documented the effects on OA progression at various time points after DMM or sham surgery. 4-MU was effective at reducing features of OA in this in vivo model even at the earliest stage (2 weeks) when the most pronounced changes are observed primarily in the articular cartilage.
METHODS
Mice
All animal studies were approved by the Institutional Animal Care and Use Committee at East Carolina University. Male and female C57BL/6 mice (10-week old) and female CD-1 mice (10-week old) were purchased from Charles River, Wilmington, MA. All animals were housed in the same specific pathogen free facility with 1 male or 3 females per microisolator cage on a 12-hour light/dark cycle and provided food and water ad libitum.
Surgical induction of OA
Destabilization of medial meniscus (DMM) surgery was performed in the right knees of 12-week-old male and female C57BL/6 mice after 2-week acclimation as previously described 9. After oral administration of Meloxicam at 5 mg/kg by 1 hour prior to surgery, the mice were initially anesthetized with Isoflurane at a rate of 4-5% until reaching a surgical plane and then maintained at a 2-3% Isoflurane rate, with 0.8-1.0 liters/minute of O2. Under sterile conditions, a 3 mm longitudinal incision was made over the distal patella to proximal tibial plateau of the right knee and the joint capsule immediately medial to the patella tendon. The fat pad over the intercondylar area was dissected to expose the intercondylar region followed by transection of the medial meniscotibial ligament (MMTL). Then the joint capsule and the skin were closed with 8-0 nylon sutures. For sham surgery, performed on the right knee of an independent set of mice, the same procedure was performed except that the MMTL was not cut. All procedures were conducted by one surgeon (ST). Meloxicam treatment continued for 2 days following surgery.
Chow containing 4-MU
4-MU was pressed at 5% (w/w) into TestDiet® mouse chow with 2% chocolate flavor (4-MU chow; St. Louis, MO) and irradiated before shipment as previously described 19,20. This dose was determined to deliver approximately 150-250 mg/mouse/day using a previous study as reference 21. Control chows with comparable formulas were conventional Prolab® Isopro® RMH 3000 and TestDiet® chow with 2% chocolate flavor (no 4-MU).
Treatment Groups
Male C57BL/6 mice were randomly assigned into 4 groups at 12 animals per group 22,23, DMM with control chow, DMM with 4-MU chow, Sham with control chow and Sham with 4-MU chow. In all groups, the mice were provided with control or 4-MU chow ad libitum the next day after surgery until euthanasia at 2, 4 and 8 weeks after surgery (n = 12 per group unless otherwise stated). To confirm any sex differences, the same experimental format was performed using female C57BL6 mice wherein the mice were euthanized at 4 weeks (n = 10-12 per group). In an additional experiment, male C57BL/6 mice were randomly assigned into 2 groups, DMM with chocolate (C) control TestDiet® chow or, DMM with 4-MU TestDiet® chow, provided ad libitum from 1 week prior to surgery and for 2 weeks following surgery. The mice were euthanized at 2 weeks after surgery (n = 12 per group).
Histological and immunohistochemistrical evaluation of OA
For in vivo studies, at time of euthanasia, the right knee joints were prepared for histology and immunohistochemistry. Briefly, the knee joints were fixed in 4% paraformaldehyde for 18 h. The samples were incubated in Decalcifying Solution (22050130; Richard Allan Scientific, San Diego, CA) for one week before embedding in paraffin. In a coronal plane, 8 μm sections, that included the femoral condyles, menisci and tibial plateaus, were cut from anterior to posterior aspects at 100 μm intervals. These sections were stained with safranin O and fast green 3. Some adjacent sections were deparaffinized, rehydrated and antigen retrieval performed using 10 mM citrate buffer for 1 h at 60 °C. These sections were incubated with rabbit primary antibodies against MMP13 (1:130, ab39012, Abcam, Cambridge, MA) or MMP3 (1:100, NB100-91878, Novusbio, Centennial, CO) overnight at 4 °C, rinsed with PBS, and incubated with reagents included in a rabbit Vectastain ABC-HRP kit (Vector Laboratories, Burlingame, CA). All sections were visualized using a Nikon Eclipse E600 microscope; images were captured digitally using a Retiga 2000R digital camera and processed using NIS Elements BR 1.30 imaging software (Nikon, Melville, NY). The percentage of MMP13 or MMP3 positive chondrocytes within equivalent areas of the medial compartment, at 2 weeks after DMM surgery, was determined.
Osteoarthritic cartilage changes in the medial tibial plateau were scored by 2 blinded observers (ST and YO) using the OARSI Scoring System developed by Pritzker et al 24. In brief, 5 safranin O/fast green stained serial sections around the anterior-midline region of each right knee joint were scored according to the formula: score (0-26) = grade (0 – 6.5 per 0.5) × stage (0 – 4). The score of each mouse was then calculated by summing the scores of 5 sections (0 – 130). The area of medial tibia osteophytes was measured using the 8-week after DMM surgery sections and presented as the average from 5 sections per knee.
Explant culture of mouse knee joints and bovine cartilage
Stock female, outbred CD-1 mice were used for tissue appropriation. For in vitro explant cultures, both left and right knee joints were harvested. Briefly, tibial-femoral joints were opened anteriorly but still articulated, placed into 12-well plates (one knee per well) and cultured for 2 days in 2.0 ml DMEM/F-12 medium (Mediatech, Menassas, VA) with 10% FBS (Hyclone, Pittsburgh, PA) followed by 10 days in serum-free medium without or with 3 ng/ml IL1β (R&D Systems, Minneapolis, MN) and 1.0 mM 4-MU. Full-thickness cores (6 mm) of bovine articular cartilage were isolated from metacarpophalangeal joints of 18–24-month-old adult steers as described previously 3,25. These cores were also cultured and treated as the murine joints. The conditioned medium of each explant culture was collected, clarified by centrifugation and stored at −80 ℃ until use. The explants were similarly processed for histology and immunehisto-chemistry in a similar fashion as described above.
Colorimetric assays
Equal volumes of diluted (1:5) conditioned media from explant cultures were analyzed for released sGAG by DMMB assay as described previously 3,26 and concentration determined by comparison to standard curve of bovine chondroitin sulfate (Sigma Chem, St. Louis, MO). For measurement of lactate, equal volume aliquots of diluted (1:5) conditioned media of bovine cartilage explant cultures were assayed for L-lactate according to the manufacturer’s instructions (Eton Bioscience, Research Triangle, NC) and concentration determined by comparison to a standard curve.
Western blotting
Equal volumes of 10x concentrated conditioned medium were loaded into 4-12% NuPAGE® Novex® Tris-acetate gradient mini gels (Thermo-Fisher, Waltham, MA). Following electrophoresis, proteins were transferred to a nitrocellulose membrane. The membranes were blocked in TBS containing 0.1% Tween 20 and 5% non-fat dry milk (TBS-T-NFDM) for 1 h at room temperature, then incubated overnight with primary antibody against MMP13 (1:6000, Abcam) 3 or rabbit anti-G1-ITEGE373 (1:600; also detects mouse G1-VTEGE373) 27 in TBS-T-NFDM at 4 °C. After rinsing and incubation with secondary antibody, immunoreactive bands were detected using chemiluminescence (Novex ECL, Thermo-Fisher) followed by exposure of X-ray films and visualization/digitalization using a GelDoc with ImageLab software (Biorad, Hercules, CA).
Statistical analysis.
All data in bar graphs are expressed as mean ± S.D and OARSI Scoring System data are shown in boxplots. A two-tailed unpaired Student’s t-test or a Mann-Whitney U test were used for direct comparison between 2 groups. A p-value of less than 0.05 was considered significant. One, two or three asterisks denotes a p-value < 0.05, < 0.01, < 0.001, respectively.
RESULTS
Effects of 4-MU on mouse knee explant culture
Before beginning in vivo studies, the chondroprotective effects of 4-MU were examined in an in vitro murine OA model. Excised, opened, but still articulated mouse knee joints as shown in Fig. 1B were treated with IL1β in the absence or presence of 4-MU and then processed for histology and immunohistochemistry. Interestingly, safranin O staining of sections of mouse knee explants (Fig 1A) revealed a substantial loss of safranin O in all layers of femoral and tibial cartilage following IL1β treatment as compared to untreated explants. Co-incubation with 4-MU and IL1β resulted in the marked retention of safranin O stained cartilage, providing morphology similar to that of untreated explants. Staining for MMP13 (Fig. 1C) and MMP3 (Fig. 1E) exhibited the highest protein expression in the medial compartment cartilage of IL1β-treated explants. The expression was substantially reduced in explants co-treated with IL1β and 4-MU, but not completely to the level observed in control joints. Quantification of MMP-positive cells is shown in Figures 1D and 1F. These data suggest that, like our previous observations in human and bovine cartilage explants 3, 4-MU is chondroprotective of murine joint cartilage degradation induced by IL1β at least in this in vitro explant OA model.
Figure 1.
Articulated knee joints of female CD-1 mice (B: arrowhead) opened anteriorly, were cultured in serum-free media and treated without or with 3 ng/ml IL1β with co-treatment with 0 or 1.0 mM 4-MU for 10 days. Shown are the representative images of sections following safranin O / fast green staining (A), MMP13 staining (C) and MMP3 staining (E). Bars = 100 um. From each animal, equivalent areas of the tibial plateau and femoral condyle cartilage in the medial compartment of the knee from each condition were analyzed for the number of clearly immunopositive chondrocytes imaged in brightfield (digitally-enlarged examples are shown as insets in (C) and (E)). The percent of immunopositive chondrocytes was then calculated by comparison to the total number of chondrocytes within the same area as viewed in phase contrast (example images are shown for MMP13 stained images in (C)). This quantification of MMP13 (D) and MMP3 (F) positive chondrocytes (mean ± S.D., n = 3 mice per group) is shown. ** p < 0.01, *** p < 0.001 (Student’s t-test).
The release of sGAG (namely aggrecan) into the culture medium of these murine explant cultures was also quantified (Fig. 2A). sGAG accumulation inversely paralleled the loss of safranin O staining shown in Figure 1A. 4-MU blocked the background release of sGAG in control conditions as well as the IL1β-stimulated release of sGAG (Fig. 2A), confirming the histochemistry. The culture medium of these explants was also examined for the accumulation of degradation product of aggrecanase activity (G1-VTEGE domain of aggrecan) as well as released MMP13 protein (Fig. 2B). Both G1-VTEGE and MMP13 protein accumulation were enhanced in the medium of cultures treated with IL1β. Importantly, the release of both proteins was blocked by co-incubation of 4-MU with IL1β.
Figure 2.
(A) The ratio of sGAG content in the conditioned media of treated mouse knee joint explants (shown in Figure 1), compared to untreated controls was calculated after using a dimethyl-methylene blue (DMMB) assay. Data represent the mean ± S.D. of three independent experiments. * p < 0.05, ** p < 0.01. (B) Equivalent volume aliquots of conditioned media from treated mouse explant cultures (shown in Figure 1), were also processed for Western blotting to detect the aggrecan G1-VTEGE neoepitope and MMP13 protein. Shown is an example blot representative of three replicated, independent experiments. (C) The concentration of L-lactate in conditioned medium from bovine articular cartilage explants treated with IL1β and 4-MU as indicated. ** p < 0.01, *** p < 0.001 (Student’s t-test).
Using bovine cartilage explants under similar culture conditions as the murine explant model, accumulation of L-lactate in the medium were determined. IL1β treatment resulted in enhanced release of lactic acid into the medium as compared to controls (Fig. 2C). More importantly, the IL1β-enhanced lactate levels were significantly blocked by 4-MU. These results are not conclusive but reveal the wider scope of 4-MU action; inhibiting HA biosynthesis and cartilage degeneration as well as reversing metabolic imbalances associated with cytokine-activated chondrocytes 3.
Effects of 4-MU on OA development in mice following DMM surgery
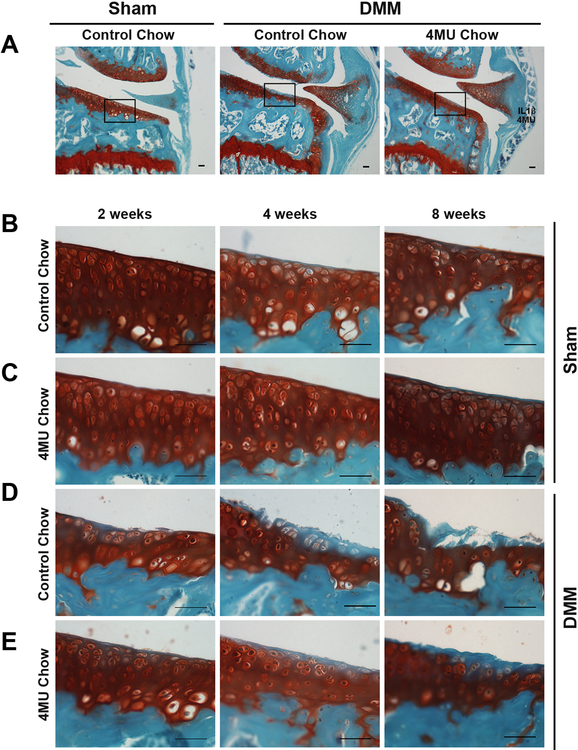
Mouse chow with 5% 4-MU (w/w) has been used successfully by investigators to deliver effective systemic doses that moderate a variety of functions 12,19-21. Figure 3A indicates the areas shown as representative images in Figures 3B-3E. Safranin O stained histological sections of sham-surgery mice, fed either control or 4-MU chow, exhibited no remarkable loss of stainable proteoglycan or morphological changes in articular cartilage at 2, 4 and 8 weeks after surgery (Fig. 3B and 3C). Although the sum of OARSI scores in all sham mice were comparatively low (between 0 and 12 points), slight increases were observed as time went on after sham surgery. However, no statistical differences were observed at 2, 4 and 8 weeks between control chow mice (sham) and 4-MU chow mice (sham) (Fig. 4A).
Figure 3.
(A) Representative images of safranin O and fast green stained sections of the medial compartment of male C57BL/6 knee joints at 4 weeks after sham or DMM surgery. The middle area in the medial tibial plateau of mice (rectangle boxes in A) was selected to show representative images of osteoarthritic changes at 2, 4 and 8 weeks after sham surgery with control chow (B) or 4-MU chow (C) or DMM surgery with control chow (D) or 4-MU chow (E). Bars = 50 um.
Figure 4.
Histological evaluation of knee osteoarthritis of male C57BL/6 mice using the sum of OARSI scores24 for five serial sections at the medial tibial plateau of each knee. Mice treated with control or 4-MU chow were evaluated at 2, 4 and 8 weeks after (A) sham surgery; sample numbers (control/4-MU) were 12/12; 12/16; 9/11, respectively or (B) DMM surgery; sample numbers (control/4-MU) were 12/12; 13/16; 12/14, respectively. (C) Chocolate control chow or 4-MU chow was provided to mice (sample numbers 12/12) from 1 week before DMM surgery and for 2 weeks following surgery. The evaluation was conducted at 2 weeks after DMM surgery. Data are shown with the Tukey boxplot with overlay of individual scores as filled circles. Statistical outliers are depicted as open circles.
Significantly higher OARSI scores were observed in the articular cartilage of DMM mice compared to sham mice as early as 2 weeks after surgery (Fig. 4B). These results confirmed that the DMM surgery used in this study provided for progressive longitudinal changes in OA that could be evaluated and quantified histologically. Representative images of each condition highlight the main histological features (Fig. 3D-3E). Focal discontinuity of the cartilage superficial zone and staining loss of safranin O in the mid zone was observed at 2 weeks in DMM mice fed control chow. Continued cartilage matrix loss occurring mainly in the superficial zone was accompanied by much wider and deeper loss of safranin O staining, readily observed at 4 weeks after surgery. Furthermore, OA changes were most prominent at 8 weeks post-surgery, with observations of severe loss of matrix well into the deep zone (Fig. 3D). Reflecting these changes, the sum of OARSI scores of DMM mice consuming control chow increased continuously from 2 to 4 to 8 weeks (Fig. 4B). DMM mice consuming 4-MU chow displayed reduced changes in cartilage degeneration and OARSI scores at each time period evaluated. Discontinuity in the superficial zone was not severe at 2 weeks and cartilage matrix was preserved without severe loss of safranin O at 4 weeks in the 4-MU chow DMM mice. Also different from control chow DMM mice, destructive damages (clefts and delamination) were not observed at 8 weeks in the 4-MU chow DMM mice (Fig. 3E). Although a continuous increase of the sum of OARSI scores was observed during the time course in DMM mice consuming the 4-MU chow, there were significant decreases in these summed scores as compared to DMM mice on control chow. A few DMM mice on 4-MU chow were distinct outliers (open circles). However, none of them exceeded the maximum score of DMM mice fed control chow (Fig. 4B). The significant difference at 2 weeks was p = 0.038; at both 4 and 8 weeks p = 0.006. Analysis of 4 week data on female mice was similar to males with the summed scores for DMM mice on 4-MU chow (median score 33.0) significantly lower than DMM mice on control chow (median score 47.8) with p = 0.007.
Osteophytes at medial tibia were not detected in sham mice at 8 weeks after surgery, but DMM mice with control chow showed development of osteophytes. The size of osteophytes observed in DMM mice with 4-MU chow (Fig. 5A) was significantly reduced. The percentage of MMP13 (Fig. 5B) and MMP3 (Fig. 5C) positive chondrocytes was significantly lower in 4-MU fed mice than control mice at 2 weeks after DMM surgery.
Figure 5.
(A) Representative images of osteophyte morphology of male C57BL/6 mouse medial tibia (encircled areas outlined by black lines) 8 weeks after surgery. These areas were quantified to determine changes in osteophyte size (area in μm2, n = 12 mice per group). Bars = 100 um. *** P < 0.001 (Student’s t-test). Representative images of MMP13 (B) and MMP3 (C) staining of the medial tibia of DMM mice at 2 weeks after surgery and fed control or 4-MU chow. The percentage of positive chondrocytes was determined (n = 6 mice per group) as described in Fig. 1. Bars = 50um. * p < 0.05 (Student’s t-test).
Previous studies by other investigators suggested that a 1-week loading period may be required for mice on 4-MU chow to obtain maximal systemic levels of 4-MU 21. To explore whether this contributed to the lower significant differences observed at 2 weeks as compared to those at 4 and 8 weeks, a 1-week pre-loading regimen was undertaken. Thus, mice were provided 4-MU chow or control chow containing chocolate flavor for 1 week prior to surgery and, for 2 weeks following DMM surgery. DMM mice fed chocolate-flavored control chow (Fig. 4C) showed similar OARSI scores as DMM mice fed control chow (Fig. 4B) by 2 weeks after DMM surgery. There was no significant difference between mice fed chocolate-flavored and unflavored control chows (p = 0.67). This result suggests that the chocolate flavoring, or other minor differences in the recipes of TestDiet control mouse chows, had no effects on OA progression after DMM surgery. More importantly, DMM mice fed 4-MU chow 1-week pre-surgery displayed lower summed OARSI scores and protection from cartilage damage as compared to DMM mice on chocolate-flavored control chow mice, 2 weeks after surgery (Fig. 4C). The significant difference was in the same range (p < 0.05) in the comparison of 2 weeks post-surgery as shown in Fig. 4B. There was no significant difference between mice fed 4-MU chow without and with pre-loading (p = 0.58). Taken together, 4-MU contributes to the suppression of OA development from an early stage.
DISCUSSION
The primary result of this study was that inclusion of 4-MU in the diet of mice limits OA damage to cartilage following DMM (Figs. 3, 4). This suppression was seen even at 2 weeks, the earliest time when OA changes are observable 11 and a time when MMPs are just becoming relevant (Fig. 5B). In later stages after DMM (8 weeks), osteophyte formation is clearly recognizable (Fig. 5A). Osteophyte size was also significantly reduced in DMM mice fed a 4-MU diet.
Many studies have demonstrated the in vivo usefulness of 4-MU in mouse models but primarily as an inhibitor of HA production 28,29 and especially for models of inflammation dependent on HA production 12,13,20,21,30. For example, Yoshioka et al. 12 observed that the application of 4-MU blocked the onset and severity of rheumatoid arthritis (RA) in a collagen-induced arthritis model (CIA). The primary effect of 4-MU was reduced synovitis related to a 4-MU-mediated reduction in HA production. However, they also observed protection of articular cartilage from damage associated with the CIA model including reduced loss of safranin O staining and reduced MMP13 immunostaining. Our hypothesis is that chondroprotection in this CIA model includes direct effects of 4-MU on chondrocytes in addition to reduction of inflammation of the synovium. Our goal was to examine the in vivo effects of 4-MU in an OA model more related to degenerative changes primarily in cartilage and less on the contribution of inflammation. Further supporting this effort we choose to examine the earliest time points after DMM (namely 2, 4 and 8 weeks), and not include 12 weeks wherein synovial inflammation might become a factor. Additionally, the rescue of safranin O staining and reduction in MMP deposition by 4-MU in the DMM OA model in vivo (Figures 3 and 5) closely matched the rescue of IL1β-treated mouse cartilage in vitro model (Fig. 1)—the latter with no possibility of a contribution by inflammation.
Our previous study documented that, while 4-MU does function as an effective HA biosynthesis inhibitor, it also exhibits another inhibitory potential completely independent of effects on HA production 3. 4-MU blocked the transcriptional expression of a select group of pro-catabolic genes related to OA including MMP13 and ADAMTS4 even in the absence of ongoing HA production 3. Moreover, co-treatment of human or bovine cartilage explants (with no synovium) with 4-MU and IL1β, provides a near total block of safranin O staining loss. While an interesting model, direct exposure of cartilage surfaces to IL1β under serum-free and normoxic conditions in vitro may not represent authentic OA 5-8. DMM provides a more gradual and consistent model for OA 11 and, while all tissues of the joint are affected, initial changes occur primary in the medial compartment articular cartilage and subchondral bone.
A pharmacokinetics study of 5% 4-MU/chow documented that 4-MU is readily metabolized in mice with a large proportion of 4-MU present in the circulation as glycoconjugates, lowering the effective dose of systemic free 4-MU 21. Interestingly, oral 4-MU was more effective at disease modification than intraperitoneal injection of 4-MU. Thus, one limitation of our study is that the effective 4-MU dose available to the joint tissues of DMM mice was likely lower than the concentrations used in our previous in vitro studies 3. Nonetheless, although less dramatic than the chondroprotection observed in mouse joint explants exposed to IL1β and 4-MU in vitro (Fig. 2), the effects of 4-MU in vivo, covering a period of months of natural, progressive degeneration, is the more relevant observation.
One potential limitation of this study: the pharmacokinetics study documented that even with chocolate additive, mice do not eat the 4-MU chow well in the first week, lose weight in the first week followed by progressive regain of weight parallel to control animals 21. We observed similar initial weight loss. Even with progressive recovery, the weight of these animals was lower than control animals at every time point. In another study, mice leaner than controls showed the same DMM outcomes 22. The OARSI scores shown in Fig. 4 (4 week time point) of each individual mouse was normalized to its individual weight; the DMM mice fed 4-MU chow still exhibited a statistically-significant lower OARSI score (as a group p=0.040) as compared to control chow DMM mice. Interestingly, female DMM mice with 4-MU chow lost less weight yet exhibited similar reduced OARSI scores as male mice; p-value = 0.009 as a group even after correcting individual female mice for weight loss. Most DMM mouse model studies utilize primarily male mice as females (129S6/SvEv mice) were previously determined to be less susceptible to OA following DMM 31. We included female C57BL/6 mice at 4 weeks post-DMM for completeness of our study and found overall DMM OARSI scores were only slightly lower than in males.
Another possible limitation was that only the percentage of MMP13 or MMP3 positive cells per unit area were calculated for quantification of immunostaining shown in Figures 1C, 1E, 5B and 5C. It was more difficult to accurately quantify the MMP13 or MMP3 present in the cartilage extracellular matrix of pro-catabolically active cartilage. Thus, counting MMP-positive cells was a more stringent analysis but likely provided an under-estimate of the potential of 4-MU to block the accumulation of MMPs.
The mechanism for the transcriptional inhibition effects of 4-MU is currently unknown. What is known is that 4-MU blocks HA production by diminishing UDP-hexose pools (UDP-GlcUA) 14 and in some cells, disturbing UDP-hexoses affects energy metabolism which in turn, affects cell phenotype 18. Coincidentally, HAS2-OE also puts stress on intracellular UDP-hexose pools, inhibits the pro-catabolic phenotype of chondrocytes and reduces the rate of IL1β-enhanced acidification of chondrocytes culture media (ECAR) 4. In this study, 4-MU suppressed IL1β-enhanced accumulation of lactic acid (Fig. 2C). In a separate recent study, we have demonstrated that pro-catabolically activated chondrocytes exhibit an enhanced dependence of glycolysis for ATP production and, both 4-MU and HAS2-OE reverse this dependency, rescue their use of oxidative phosphorylation and rescue their deficits in p-AMPK 32. Moreover, these changes can be mimicked, even in cartilage explants, by glycolysis blocking compounds that have no direct on HA biosynthesis including 2-deoxyglucose or dichloroacetate 32. Thus, it appears that the blocking effects of 4-MU on the glycolytic pathway, driving a switch in energy metabolism, is the primary mechanism for reversing the pro-catabolic phenotype and providing chondroprotection.
ACKNOWLEDGEMENTS.
The authors thanks Ms. Joani Zary Oswald for histological / immunohistological technical assistance with this project as well as assistance from the veterinary/animal care staff of the Department of Comparative Medicine at East Carolina University. The authors thank Dr. Amanda Fosang (Melbourne University Department of Pediatrics and Murdoch Children’s Research Institute, Arthritis Research Group, Royal Children’s Hospital, Parkville, Australia) for graciously providing the anti-G1-ITEGE antibody. This work was supported in part by NIH grant R21-AR072682 (WK).
Grants: NIH R21-AR072682 (WK)
REFERENCES
- 1.Roughley PJ, Mort JS. 2014. The role of aggrecan in normal and osteoarthritic cartilage. J Exp Orthop 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudson W, Ishizuka S, Terabe K, et al. 2019. The pericellular hyaluronan of articular chondrocytes. Matrix Biol 78-79:32–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishizuka S, Askew EB, Ishizuka N, et al. 2016. 4-Methylumbelliferone Diminishes Catabolically Activated Articular Chondrocytes and Cartilage Explants via a Mechanism Independent of Hyaluronan Inhibition. J Biol Chem 291:12087–12104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishizuka S, Tsuchiya S, Ohashi Y, Terabe K, Askew EA, Ishizuka N, Knudson CB, Knudson W. 2019. Hyaluronan synthase 2 (HAS2) overexpression diminishes the procatabolic activity of chondrocytes by a mechanism independent of extracellular hyaluronan. J Biol Chem 294:13562–13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldring MB. 2000. The role of the chondrocyte in osteoarthritis. Arthritis Rheum 43:1916–1926. [DOI] [PubMed] [Google Scholar]
- 6.Liu-Bryan R, Terkeltaub R. 2015. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol 11:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandy JD, Chan DD, Trevino RL, et al. 2015. Human genome-wide expression analysis reorients the study of inflammatory mediators and biomechanics in osteoarthritis. Osteoarthr Cartil 23:1939–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunn SL, Soul J, Anand S, et al. 2016. Gene expression changes in damaged osteoarthritic cartilage identify a signature of non-chondrogenic and mechanical responses. Osteoarthr Cartil 24:1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glasson SS, Blanchet TJ, Morris EA. 2007. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr Cartil 15:1061–1069. [DOI] [PubMed] [Google Scholar]
- 10.Culley KL, Dragomir CL, Chang J, et al. 2015. Mouse models of osteoarthritis: surgical model of posttraumatic osteoarthritis induced by destabilization of the medial meniscus. Methods Mol Biol 1226:143–173. [DOI] [PubMed] [Google Scholar]
- 11.Ratneswaran A, Beier F. 2017. An approach towards accountability: suggestions for increased reproducibility in surgical destabilization of medial meniscus (DMM) models. Osteoarthr Cartil 25:1747–1750. [DOI] [PubMed] [Google Scholar]
- 12.Yoshioka Y, Kozawa E, Urakawa H, et al. 2013. Suppression of hyaluronan synthesis alleviates inflammatory responses in murine arthritis and in human rheumatoid synovial fibroblasts. Arthritis Rheum 65:1160–1170. [DOI] [PubMed] [Google Scholar]
- 13.Nagy N, Kuipers HF, Frymoyer AR, et al. 2015. 4-methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front Immunol 6:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakizaki I, Kojima K, Takagaki K, et al. 2004. A novel mechanism for the inhibition of hyaluronan biosynthesis by 4-methylumbelliferone. J Biol Chem 279:33281–33289. [DOI] [PubMed] [Google Scholar]
- 15.Vigetti D, Ori M, Viola M, et al. 2006. Molecular cloning and characterization of UDP-glucose dehydrogenase from the amphibian Xenopus laevis and its involvement in hyaluronan synthesis. J Biol Chem 281:8254–8263. [DOI] [PubMed] [Google Scholar]
- 16.Hascall VC, Wang A, Tammi M, et al. 2014. The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biol 35:14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kultti A, Pasonen-Seppanen S, Jauhiainen M, et al. 2009. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res 315:1914–1923. [DOI] [PubMed] [Google Scholar]
- 18.Chanmee T, Ontong P, Izumikawa T, et al. 2016. Hyaluronan Production Regulates Metabolic and Cancer Stem-like Properties of Breast Cancer Cells via Hexosamine Biosynthetic Pathway-coupled HIF-1 Signaling. J Biol Chem 291:24105–24120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagy N, de la Zerda A, Kaber G, et al. 2018. Hyaluronan content governs tissue stiffness in pancreatic islet inflammation. J Biol Chem 293:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagy N, Freudenberger T, Melchior-Becker A, et al. 2010. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: novel insights into the role of hyaluronan synthesis. Circulation 122:2313–2322. [DOI] [PubMed] [Google Scholar]
- 21.Kuipers HF, Nagy N, Ruppert SM, et al. 2016. The pharmacokinetics and dosing of oral 4-methylumbelliferone for inhibition of hyaluronan synthesis in mice. Clin Exp Immunol 185:372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ulici V, Kelley KL, Azcarate-Peril MA, et al. 2018. Osteoarthritis induced by destabilization of the medial meniscus is reduced in germ-free mice. Osteoarthr Cartil 26:1098–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowe MA, Harper LR, McNulty MA, et al. 2017. Reduced Osteoarthritis Severity in Aged Mice With Deletion of Macrophage Migration Inhibitory Factor. Arthritis Rheumatol 69:352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pritzker KP, Gay S, Jimenez SA, et al. 2006. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil 14:13–29. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi N, Knudson CB, Thankamony S, et al. 2010. Induction of CD44 cleavage in articular chondrocytes. Arthritis Rheum 62:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farndale RW, Sayers CA, Barrett AJ. 1982. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res 9:247–248. [DOI] [PubMed] [Google Scholar]
- 27.Ariyoshi W, Knudson CB, Luo N, et al. 2010. Internalization of aggrecan G1 domain neoepitope ITEGE in chondrocytes requires CD44. J Biol Chem 285:36216–36224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urakawa H, Nishida Y, Wasa J, et al. 2012. Inhibition of hyaluronan synthesis in breast cancer cells by 4-methylumbelliferone suppresses tumorigenicity in vitro and metastatic lesions of bone in vivo. Int J Cancer 130:454–466. [DOI] [PubMed] [Google Scholar]
- 29.Ikuta K, Ota T, Zhuo L, et al. 2017. Antitumor effects of 4-methylumbelliferone, a hyaluronan synthesis inhibitor, on malignant peripheral nerve sheath tumor. Int J Cancer 140:469–479. [DOI] [PubMed] [Google Scholar]
- 30.Mueller AM, Yoon BH, Sadiq SA. 2014. Inhibition of hyaluronan synthesis protects against central nervous system (CNS) autoimmunity and increases CXCL12 expression in the inflamed CNS. J Biol Chem 289:22888–22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma HL, Blanchet TJ, Peluso D, et al. 2007. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthr Cartil 15:695–700. [DOI] [PubMed] [Google Scholar]
- 32.Terabe K, Ohashi Y, Tsuchiya S, Ishizuka S, Knudson CB, and Knudson W Chondroprotective effects of 4-methylumbelliferone and hyaluronan synthase-2 overexpression involve changes in chondrocyte energy metabolism. J Biol Chem, in press; doi: 10.1074/jbc.RA119.009556. [DOI] [PMC free article] [PubMed] [Google Scholar]