Abstract
Rotator cuff (RC) tears are a common cause of upper extremity disability. Any tear size can result in subsequent muscle atrophy and fatty infiltration (FI)1. Preoperative muscle degeneration can predict repair and postoperative functional outcomes. Muscle residential fibro-adipogenic progenitors (FAPs) are found to be capable of differentiating into beige adipocytes that release factors to promote muscle growth. This study evaluated the regenerative potential of local cell transplantation of beige FAPs to mitigate muscle degeneration in a murine massive RC tear model. Beige FAPs were isolated from muscle in UCP1 reporter mice by flow cytometry as UCP1+/Sca1+/PDGFR+/CD31−/CD45−/integrin α7−. C57/BL6J mice undergoing supraspinatus (SS) tendon tear with suprascapular nerve transection (TT+DN) received either no additional treatment, PBS injection, or beige FAP injection 2 weeks after the initial injury. Forelimb gait analysis was used to assess shoulder function with DigiGait. Mice were sacrificed 6 weeks after cell transplantation. FI, fibrosis, fiber size, vascularity were analyzed and quantified via ImageJ. Our results showed that beige FAP transplantation significantly decreased fibrosis, FI, and atrophy, enhanced vascularization compared to saline injection and non-treatment groups. Beige FAP transplantation also significantly improved shoulder function as measured by gait analysis. This study suggests that beige-differentiated FAPs may serve as a treatment option for RC muscle atrophy and FI, thus improving shoulder function in patients with massive RC tendon tears.
Keywords: Rotator cuff tears, muscle atrophy, fatty infiltration, fibro-adipogenic progenitor, beige fat
INTRODUCTION
Muscle atrophy and fatty infiltration (FI) are commonly seen following rotator cuff (RC) tears. Although tears of all sizes can result in muscle degeneration, patients with anterior cable ruptures or large or massive tears are particularly susceptible1. Baseline muscle degeneration is associated with poor repair success and functional outcomes2–5. For patients in which repair is contraindicated, muscle atrophy and FI often continue to progress during nonoperative treatment, further impairing function6.
Fibro-adipogenic progenitors (FAPs) have been identified as the primary source of fibrosis and FI in skeletal muscle7; 8. We have recently shown that these same cells are the major source of adipocytes seen in RC FI9. Gorski has reported that FAPs can differentiate into brown/beige-like adipocytes (BAT) that express uncoupling protein 1 (UCP-1)10. In addition to thermogenesis and energy metabolism, BAT has been shown to have anabolic effects on bone and skeletal muscle11; 12. Meyer et al. has demonstrated that healthy RC muscle is, in fact, encapsulated by epimuscular adipose tissue that appears to adopt a beige phenotype when the RC tendon is torn13. Furthermore, Bryniarski et al has shown that transplantation of brown fat into supraspinatus muscle improved muscle regeneration after cardiotoxin injury, suggesting a positive role for BAT in RC muscle regeneration following injury14.
In this study, we tested the feasibility of treating RC muscle atrophy and FI by transplanting beige fat differentiated FAPs in a mouse massive RC tear model. We hypothesize that beige FAP transplantation can reduce RC muscle atrophy and FI and improve shoulder function after massive tendon tears.
METHODS
Cell Isolation/Culture
Beige FAPs were isolated from uncoupling-protein 1 (UCP-1) reporter mice acquired through a generous donation from Shingo Kajimura. Briefly, a luciferase-tdTomato cassette was inserted into the first exon of UCP-1. The following genetic construct was then inserted into the Y chromosome. Supraspinatus (SS), infraspinatus (IS), paraspinal (PS), tibialis anterior (TA) and gastrocnemius (GA) muscles from UCP-1 reporter mice were digested with 0.2% collagenase for 90 min followed by 0.4% dispase for 30 min. Muscles were chosen based on those exhibiting the highest density of FAPs .FAPs were isolated as Sca1+/PDGFRα+/CD31−/CD45−/integrin α7− as previously described7; 8. Beige FAPs were further isolated according to their expression of UCP1. Beige FAPs were then cultured in F10/20% FBS/10ng/mL bFGF. Upon reaching 75% confluency, cells were lifted with 0.25% trypsin-EDTA and then resuspended in PBS. All cells used in this study were primary cells and did not undergo any passages prior to transplantation.
Animal Husbandry
All mice used in this study were housed in groups of 4 in cages located adjacent to one another. Mice were kept on a 12/12 hour light/dark cycle with free access to standard laboratory mouse diet and drinking water in their individual cages. Mice health status was monitored daily by a veterinary staff. Mice were allowed to engage in free exercise within their cages, both preoperatively and postoperatively.
Rotator Cuff Injury and Cell Transplantation
Three-month old female C57/BL6J mice underwent unilateral SS and IS tendon transection and suprascapular nerve transection (TT+DN), as previously described15. Sham surgery on the contralateral side was used as a control and was performed by incising the skin followed by immediate closure. Two weeks after the initial injury, mice (n=12; 4 per treatment group) received either no injection, 10µL of PBS or 10µL of 125,000 beige FAPs into both proximal and distal ends of the SS muscle (with a total of 250,000 cells per mouse). All injections were performed using a 28G insulin syringe. Mice were randomly allocated into each of the three groups. Experimental design is depicted in Figure 1. All experiments were approved by the Institutional Animal Care and Use Committee of our institution.
Figure 1.
Flow diagram of experimental design. 4 mice received no additional treatment, 4 mice 4 mice received PBS injections along with repair, and 4 mice received cell injections. TT+DN, tendon transection and denervation.
Sample size was determined based on a previous power analysis demonstrating that a sample size of n=4 would be sufficient to detect a significant difference in muscle weight loss in our rotator cuff injury model16. An additional power analysis with α = 0.05 and power of 0.80 was run for muscle weight loss and each histological parameter based on means and standard deviations from a pilot intramuscular supraspinatus injection study using these cells. This analysis provided further confidence that our sample size would be adequate to detect statistically significant differences between treatment and control group.
Gait Analysis
Gait analysis was conducted using a Digigait system (Mouse Specifics, Framingham, MA) to assess shoulder function every 2 weeks after the final surgery. Mice walked at 8 cm/s for 10 seconds on the DigiGait system with 10 degrees. Stride length, stride duration, and stance width were the parameters chosen to assess forelimb function and calculated as previously described17. Stride length was defined as the distance from the initial paw placement to the subsequent paw placement. Stride duration was defined as the time elapsed between initial paw placement and subsequent paw placement. Stance width was defined as the distance between the vertical axes that pass through the center of each forelimb paw.
Muscle Harvesting and Histology
All mice were sacrificed at 6 weeks after the injection/second surgery. Bilateral SS muscles were collected and weighed to assess muscle atrophy. Following wet weight measurement, muscle specimens were flash frozen by immersion in liquid nitrogen cooled isopentane and sectioned (10 µm) with a cryostat. For immunofluorescence staining, sections were fixed with 4% paraformaldehyde (PFA) for 10min, washed in PBST (1XPBS/0.1% TritonX-100), blocked in 5% BSA for 1h and then incubated with primary antibodies at 4⁰C overnight. Slides were then washed in PBS and incubated with secondary antibodies (1:200) for 1 hour at room temperature. Tissue sections were stained with DAPI and then mounted with Fluoromount G. For evaluation of fibrosis, staining was carried out using a Masson trichrome kit (American Mastertech, Lodi, CA) per manufacturer’s instructions. Fatty infiltration was determined by immunofluorescent staining for perilipin. Quantification was conducted using Image J. Collagen and fat fractional area was determined as area of fat or collagen divided by total sample section area. Vascularity was determined as area positive for CD31 staining divided by total section area.
Antibodies
Primary antibodies and concentrations used for immunofluorescence in this study are as follows: rat anti-mouse CD31 (Biolegend cat#102501, 1:100), goat anti-mouse perilipin (Abcam ab61682,1:50), rabbit anti-laminin (Sigma L9393, 1:200). Secondary antibodies used in this study are: donkey anti-rabbit IgG Alexa Fluor® 488 (Abcam, ab150073, 1:200), donkey anti-rat IgG Alexa Fluor® 647 (Abcam, ab150155, 1:200), donkey anti-goat IgG Alexa Fluor® 647 (Abcam, ab150135).
Statistical Analysis
One way analysis of variance (ANOVA) with a post hoc Tukey test were used to analyze the difference between groups with regards to fibrosis, fatty infiltration, vascularity and gait parameters. Significance was defined as p<0.05.
RESULTS
Beige FAP transplantation mitigates muscle atrophy
Wet muscle weights loss was determined by the following equation: [(SSright – SSleft) ÷ SSleft] x 100%. Mice that were treated with beige FAPs transplantation exhibited significantly less weight loss compared to PBS and no treatment (−49.4 ± 8.3 vs. −65.3 ± 4.2 with PBS and 64.3 ± 5.7 with no treatment; mean ± standard deviation, P=0.03) (Figure 2).
Figure 2.
SS percent weight change. There was significantly less SS muscle weight loss in the mice receiving cell treatments compared to respective controls. Solid lines indicate p<0.05. Error bars indicate standard deviation.
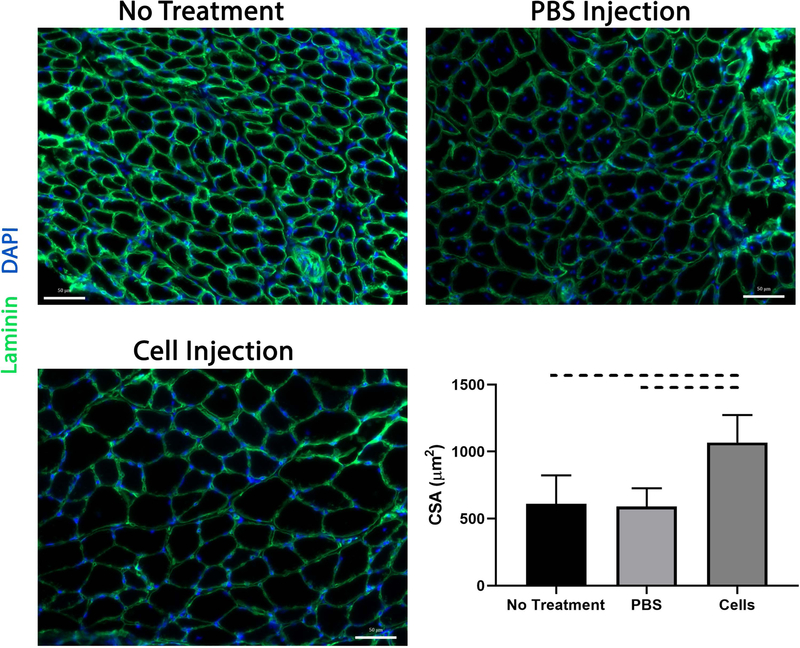
Beige FAP transplantation increases muscle fiber size
Consistent with muscle wet weight, SS muscle fiber CSA was significantly higher in mice receiving cell injections compared to control groups (1067 ± 205 µm2 vs 591 ± 136 µm2 in PBS and 610 ± 212 µm2 with no treatment, mean ± standard deviation, P=0.009) (Figure 3).
Figure 3.
SS fiber cross sectional area as determined via ImageJ. Fiber size was significantly larger in the cell treatment group compared to respective controls. Dashed lines indicate p<0.05.
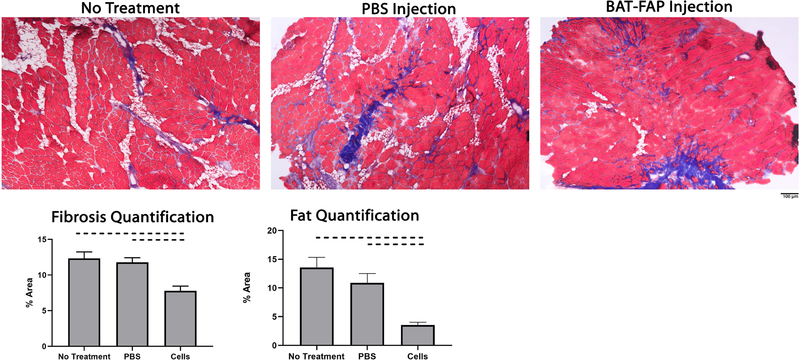
Beige FAP transplantation reduces muscle fibrosis
Mice treated with beige FAPs exhibited significantly less fibrosis compared to PBS injection and no treatment groups (7.8% ± 0.7% vs. 11.8% ± 0.7% in PBS and 12.3% ± 0.9% with no treatment; mean ± standard deviation, P=0.006) (Figure 4).
Figure 4.
Trichrome staining (x100) of the supraspinatus (SS) showed significantly more fibrosis and fatty infiltration in repair and PBS groups compared to group receiving cell injection. Area fraction of collagen quantified as area occupied by collagen divided by total area of the image. Area fraction of fat was quantified as area of perilipin staining as a percentage of the total area. Dashed lines indicate p<0.05.
Beige FAP transplantation reduces muscle fatty infiltration
Mice treated with beige FAPs exhibited significantly less fatty infiltration in the SS compared to their respective control groups (5.2% ± 1.4% vs 15.4% ± 2.8% in PBS vs 14.5% ± 0.3% with no treatment; mean ± standard deviation, P=0.001) (Figure 4). Transplanted cells are seen to lie in regions adjacent to intramuscular fat and maintain their beige phenotype as seen by persistent UCP-1 expression 6 weeks after transplantation (Figure 5).
Figure 5.
Close adjunctive position of transplanted cells with intramuscular fat and blood vessels. (A) Transplanted cells lie in regions adjacent to fat and maintain its beige phenotype as seen by persistent UCP-1 expression (B) Transplanted cells cluster around blood vessels.
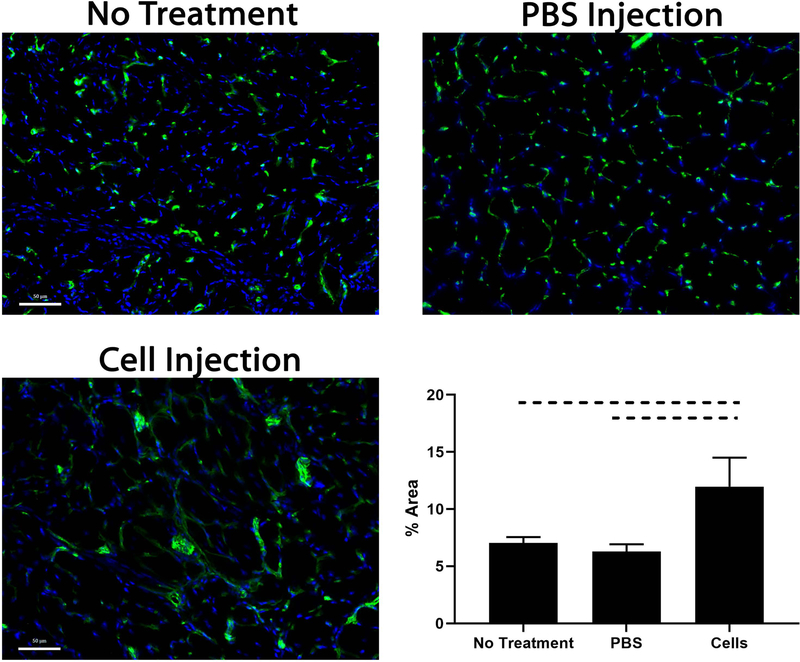
Beige FAP transplantation increases muscle vascularity
There was significantly more vascularization in the cell treatment group compared to their respective controls (11.9% ± 2.5% vs 6.3% ± 0.7% in PBS and 7.0% ± 0.5% with no treatment; mean ± standard deviation p=0.0005) (Figure 6). Transplanted cells also appeared to cluster around blood vessels, suggesting a possible pro-angiogenic role of beige FAPs (Figure 5).
Figure 6.
SS from mice receiving cell injections exhibited significantly more vascularization compared to respective controls. Dashed lines indicate p<0.05. (Green: CD31)
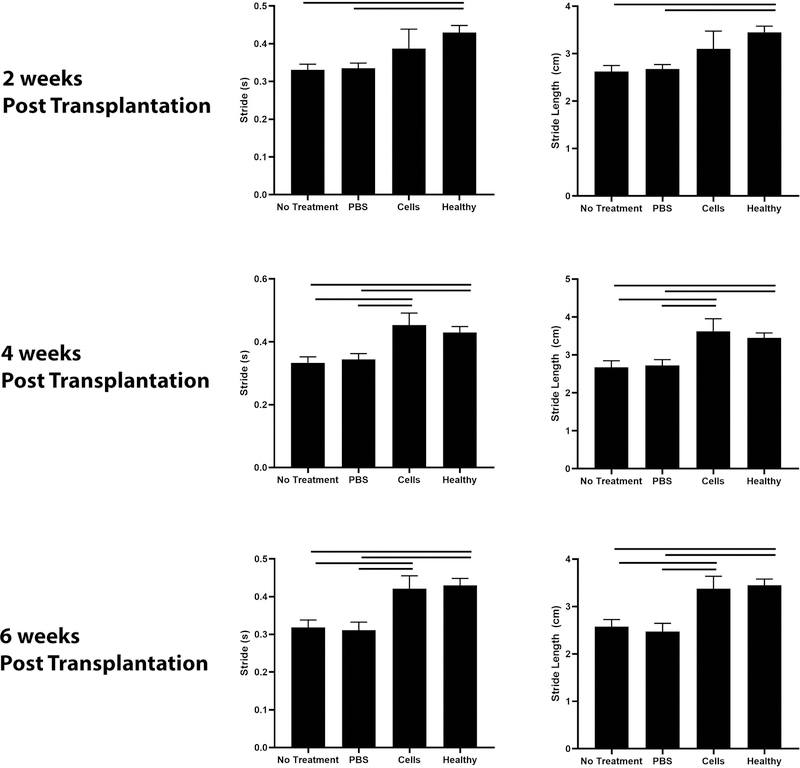
Beige FAP transplantation improves shoulder function
Mice treated with beige FAPs exhibited improved stride time and stride length 4 and 6 weeks post transplantation compared to mice receiving saline injections or no treatment. Mice in all groups improved in these three parameters with time (Figure 7).
Figure 7.
Gait analysis of injured forelimb. Mice in the cell treatment arm exhibited significantly enhanced stride and stride length. Dashed lines indicate p<0.05.
Discussion
RC tears are a common cause of upper extremity pain and disability. Irreversible muscle degeneration has major clinical implications for patients with RC tears including repair failure and increased postoperative disability2; 4; 5. Fibrosis and fatty infiltration are chronic manifestations of muscle degeneration following RC tears for which there is currently no treatment. The ability of BAT to facilitate muscle growth has previously been reported. Bryniarski et al has shown that intramuscular grafting of brown fat results in increased fiber size and improved RC contractility in a cardiotoxin injury model14. However, a cardiotoxin injury does not represent any injury that might be encountered in a clinical setting, thus limiting the clinical relevance of these results. In addition, auto-transplantation of brown fat to RC muscle has limited translational potency to patients due to limited BAT depots in adults. Beige adipocytes offer an intriguing alternative due to their existence within white fat depots in adult humans18. Beige adipocytes typically express low basal levels of UCP-1 but upon stimulation, can express high levels of UCP-1 and exhibit a thermogenic potential similar to that of BAT19. The efficacy of brown or beige adipocytes to mitigate muscle degeneration and promote muscle growth in a RC tear model has not been reported in the literature. Our study seeks to establish a therapeutic model of cell transplantation for treating RC degeneration by delivering beige FAPs two weeks after the massive tendon tears.
The molecular mechanisms by which beige adipocytes support muscle growth are not fully understood. Chen et al has identified a novel pathway of beige adipogenesis that does not rely on sympathetic nervous system activation. Beige adipocytes generated through this pathway develop from WAT depots and exhibit a myogenic signature20. In addition, multiple studies have suggested that BAT may possess non-thermogenic roles that may be important in both fat metabolism and muscle function. Meyer et al has demonstrated that beige fat depots surrounding the rotator cuff can promote myogenesis in a paracrine fashion13. Braga et al has shown that BAT express high levels of follistatin, a secreted glycoprotein that has high affinity binding for activins and myostatin and functions as an inhibitor of myostatin activity11; 21; 22. Previous work has suggested that follistatin is also a soluble mediator of functional interactions between FAP and myogenic satellite cells during muscle regeneration23. It is likely that beige FAPs stimulate RC muscle regeneration through a similar paracrine manner. It is also possible that beige FAPs originate from a distinct beige adipose population that is intrinsically more myogenic. Future work is needed to define beige FAP origin and beige FAP-derived promyogenic factors that mitigate RC muscle atrophy.
Fatty infiltration of RC muscles has major clinical implications as it correlates with shoulder function and is often irreversible in the setting of repair3; 5; 6. As such, methods to reduce or diminish adipogenesis following RC tears are critical. Browning of white adipose tissue has emerged as a recent topic of interest due to its potential as a treatment for obesity. Established methods of white adipocyte browning that have been described in the literature include exercise, cold stimulation and pharmaceuticals such as beta adrenergic agonists and PPAR agonists24–26. However, prolonged cold exposure is difficult to implement clinically and treatment with pharmaceuticals such as beta adrenergic agonists often carry unintended side effects and require repeated dosing. Intramuscular cell transplantation offers the potential for sustained local growth factor and cytokine release that can induce white adipocyte browning and thus mitigate fatty degeneration. Fisher et al and Lee et al have demonstrated that brown adipocytes can promote white fat browning via BAT secretion of growth factors including fibroblast growth factor-21 (FGF21) and vascular endothelial growth factor (VEGF)27; 28. Our study suggests that transplantation of beige FAPs significantly reduces fatty infiltration in a massive RC tear model. Transplanted beige FAPs may diminish fatty degeneration by releasing trophic factors that promote browning of intramuscular white fat.
Vascularization is critical to regenerating skeletal muscle. It has been reported that endothelial cells (EC) promotes muscle progenitors (MPC) migration, proliferation and terminal differentiation29. Delayed angiogenesis results in impaired muscle regeneration in vivo30. Previous studies have shown that vascularity is decreased in the SS compared to the contralateral uninjured side following RC tears31; 32. In this study, we demonstrate that transplantation of beige FAPs results in improved SS vascularity compared to PBS and no treatment. Improvements in vascularity correlate with enhanced fiber CSA and reduced atrophy, suggesting a critical role for angiogenesis in promoting muscle growth following RC tears. The mechanism by which beige FAP promotes intramuscular angiogenesis has yet to be determined and warrants further investigation. BAT is known to release regulatory factors that promote angiogenesis. VEGF is the main angiogenic factor secreted by BAT. VEGF promotes endothelial cell activation, proliferation, and migration and has been shown to be critical for BAT survival and differentiation33. It is possible that VEGF secretion by transplanted beige FAPs is secondarily promoting angiogenesis within the muscle.
Forced treadmill running is a commonly used method for evaluating shoulder function in shoulder injury models34; 35. Gait analysis of the injured forelimb correlate with histological parameters of RC injury35; 36. In this study, mice receiving beige FAPs exhibited improved stride and stride length at 4 and 6 weeks after injection, suggesting that improvements in muscle quality may predict positive functional outcomes. While gait analysis offers the ability to assess range of motion and ability to bear weight, it does not measure muscle strength and force production. As such, future work will focus on assessing the contractility of isolated RC muscles following cell transplantation.
There are several other limitations in this study. The model used to induce RC tears involves a suprascapular nerve transection. Although suprascapular neuropathy has been reported following RC tears, complete transection of the nerve is different from chronic nerve compression injury37. In addition, the robust murine healing response often results in scar tissue bridging the gap between the transected tendon and the humerus, a phenomenon not observed in human RC tears. This spontaneous healing may be responsible for the functional improvements observed in the setting of complete tendon transection. We have previously shown that this scar bridge is biomechanically weaker than surgically repaired tendon and results in considerable functional impairment when compared to RC tendon repair35. Future work will evaluate the efficacy of beige FAPs in promoting muscle regeneration in the setting of RC repair. Finally, beige FAPs used in this study were isolated from whole mouse muscle. While the results of this study are encouraging, a human ortholog of these cells will need to be identified to determine if the same results can be achieved with human cells.
In summary, we show that intramuscular transplantation of beige FAPs in the setting of massive rotator cuff tears reduces muscle atrophy and improves shoulder function in a murine massive RC tear model. Patients with Goutallier class 2–4 FI in RC muscles are currently considered relative contraindications to repair due to high re-tear rates38. The results of this study suggest that beige FAPs may offer a bridge to repair for patients with advanced RC pathology. Future work will evaluate the efficacy of BAT-FAPs in promoting muscle regeneration in the setting of RC repair.
Acknowledgements
This material is based upon work supported by and NIH/NIAMS Research Grant (1R01AR072669-01A1, PI: Feeley) and U.S. Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Merit Review Grant (1I01BX002680, PI: Kim). All authors have no professional or financial disclosures to report.
References:
- 1.Namdari S, Donegan RP, Dahiya N, et al. 2014. Characteristics of small to medium-sized rotator cuff tears with and without disruption of the anterior supraspinatus tendon. Journal of shoulder and elbow surgery 23:20–27. [DOI] [PubMed] [Google Scholar]
- 2.Gladstone JN, Bishop JY, Lo IK, et al. 2007. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. The American journal of sports medicine 35:719–728. [DOI] [PubMed] [Google Scholar]
- 3.Lansdown DA, Lee S, Sam C, et al. 2017. A Prospective, Quantitative Evaluation of Fatty Infiltration Before and After Rotator Cuff Repair. Orthopaedic journal of sports medicine 5:2325967117718537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shen PH, Lien SB, Shen HC, et al. 2008. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. Journal of shoulder and elbow surgery 17:1S–7S. [DOI] [PubMed] [Google Scholar]
- 5.Valencia AP, Lai JK, Iyer SR, et al. 2018. Fatty Infiltration Is a Prognostic Marker of Muscle Function After Rotator Cuff Tear. The American journal of sports medicine 46:2161–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melis B, DeFranco MJ, Chuinard C, et al. 2010. Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clinical orthopaedics and related research 468:1498–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uezumi A, Fukada S, Yamamoto N, et al. 2010. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature cell biology 12:143–152. [DOI] [PubMed] [Google Scholar]
- 8.Uezumi A, Ito T, Morikawa D, et al. 2011. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. Journal of cell science 124:3654–3664. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Ning AY, Chang NC, et al. 2016. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles, ligaments and tendons journal 6:6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorski T, Mathes S, Krutzfeldt J. 2018. Uncoupling protein 1 expression in adipocytes derived from skeletal muscle fibro/adipogenic progenitors is under genetic and hormonal control. Journal of cachexia, sarcopenia and muscle 9:384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong X, Yao T, Zhou P, et al. 2018. Brown Adipose Tissue Controls Skeletal Muscle Function via the Secretion of Myostatin. Cell metabolism 28:631–643 e633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahman S, Lu Y, Czernik PJ, et al. 2013. Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology 154:2687–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer GA, Gibbons MC, Sato E, et al. 2015. Epimuscular Fat in the Human Rotator Cuff Is a Novel Beige Depot. Stem cells translational medicine 4:764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryniarski AR, Meyer GA. 2019. Brown fat promotes muscle growth during regeneration. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. [DOI] [PMC free article] [PubMed]
- 15.Liu X, Laron D, Natsuhara K, et al. 2012. A mouse model of massive rotator cuff tears. The Journal of bone and joint surgery American volume 94:e41. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Joshi SK, Samagh SP, et al. 2012. Evaluation of Akt/mTOR activity in muscle atrophy after rotator cuff tears in a rat model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 30:1440–1446. [DOI] [PubMed] [Google Scholar]
- 17.Hampton TG, Stasko MR, Kale A, et al. 2004. Gait dynamics in trisomic mice: quantitative neurological traits of Down syndrome. Physiology & behavior 82:381–389. [DOI] [PubMed] [Google Scholar]
- 18.Yang W, Seale P. 2018. Stepping Up Human Beige Fat Cell Production. Cell reports 25:2935–2936. [DOI] [PubMed] [Google Scholar]
- 19.Harms M, Seale P. 2013. Brown and beige fat: development, function and therapeutic potential. Nature medicine 19:1252–1263. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Ikeda K, Yoneshiro T, et al. 2019. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 565:180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, Lee YS, Zimmers TA, et al. 2010. Regulation of muscle mass by follistatin and activins. Molecular endocrinology 24:1998–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braga M, Reddy ST, Vergnes L, et al. 2014. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. Journal of lipid research 55:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mozzetta C, Consalvi S, Saccone V, et al. 2013. Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice. EMBO molecular medicine 5:626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Cohen P, Spiegelman BM. 2013. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes & development 27:234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aldiss P, Betts J, Sale C, et al. 2018. Exercise-induced ‘browning’ of adipose tissues. Metabolism: clinical and experimental 81:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohno H, Shinoda K, Spiegelman BM, et al. 2012. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell metabolism 15:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee P, Linderman JD, Smith S, et al. 2014. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell metabolism 19:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fisher FM, Kleiner S, Douris N, et al. 2012. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & development 26:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Latroche C, Weiss-Gayet M, Muller L, et al. 2017. Coupling between Myogenesis and Angiogenesis during Skeletal Muscle Regeneration Is Stimulated by Restorative Macrophages. Stem cell reports 9:2018–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochoa O, Sun D, Reyes-Reyna SM, et al. 2007. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. American journal of physiology Regulatory, integrative and comparative physiology 293:R651–661. [DOI] [PubMed] [Google Scholar]
- 31.Gigliotti D, Xu MC, Davidson MJ, et al. 2017. Fibrosis, low vascularity, and fewer slow fibers after rotator-cuff injury. Muscle & nerve 55:715–726. [DOI] [PubMed] [Google Scholar]
- 32.Lee C, Liu M, Bertoy L, et al. 2019. Effect of amibegron on blood vessel and nerve density in a mouse rotator cuff injury model. . Orthopaedic Research Society; Austin, Tx. [Google Scholar]
- 33.Bagchi M, Kim LA, Boucher J, et al. 2013. Vascular endothelial growth factor is important for brown adipose tissue development and maintenance. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 27:3257–3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lakes EH, Allen KD. 2016. Gait analysis methods for rodent models of arthritic disorders: reviews and recommendations. Osteoarthritis and cartilage 24:1837–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Z, Liu X, Davies MR, et al. 2018. A Mouse Model of Delayed Rotator Cuff Repair Results in Persistent Muscle Atrophy and Fatty Infiltration. The American journal of sports medicine 46:2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter JE, Thomopoulos S, Flanagan CL, et al. 1998. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. Journal of shoulder and elbow surgery 7:599–605. [DOI] [PubMed] [Google Scholar]
- 37.Kong BY, Kim SH, Kim DH, et al. 2016. Suprascapular neuropathy in massive rotator cuff tears with severe fatty degeneration in the infraspinatus muscle. The bone & joint journal 98-B:1505–1509. [DOI] [PubMed] [Google Scholar]
- 38.Somerson JS, Hsu JE, Gorbaty JD, et al. 2016. Classifications in Brief: Goutallier Classification of Fatty Infiltration of the Rotator Cuff Musculature. Clinical orthopaedics and related research 474:1328–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]