Abstract
Although vaginal microbicides for HIV prevention are designed to be female-initiated, male partner influence has been identified as one of the most significant factors impacting women’s willingness and ability to use them. As a result, research teams have sought to increase male partner involvement by encouraging disclosure of product use to male partners, promoting male partner engagement in the study through attendance at the study clinic, and helping women to gamer male partner support for product use. This paper aims to assess the impact of these three elements of male partner involvement on women’s adherence to the dapivirine vaginal ring during MTN-020/ASPIRE, a phase III randomized placebo-controlled clinical trial involving 2,629 women in Malawi, South Africa, Uganda, and Zimbabwe. During the study, 64-80% of participants reported disclosure of ring use at each quarterly visit, and 13% reported that their partners had attended the study clinic at some point during the study. At study exit, 66% reported that their partner was supportive, 18% unsupportive, and 17% were unsure. After adjusting for age, site and time in study, women were more likely to have low ring adherence if they had an unsupportive male partner (aRR 1.29, 95% CI 1.03-1.62). Neither disclosure nor clinic attendance directly predicted ring adherence, but disclosure increased the probability of having a supportive partner (aRRR 24.17, 95% CI 16.38-35.66) or an unsupportive partner (aRRR 4.10, 95% CI 2.70-6.24), relative to an unknown level of partner support. Women were also more likely to have a supportive partner if their partner had attended the clinic (aRRR 3.77, 95% CI 1.36-10.42). This study suggests that although the vaginal ring is relatively discreet, lack of support from male partners remains a relevant barrier to use. Though both disclosure and clinic attendance may increase partner support, disclosure may also increase partner opposition. Interventions to reduce male partner opposition are needed to maximize the potential impact of the ring and other PrEP products for HIV prevention.
Keywords: Microbicides, HIV prevention, Women, Male involvement, Gender, Dapivirine vaginal ring
Introduction
Women, notably young women, continue to be disproportionally affected by HIV infection in comparison to their male counterparts both globally and in sub-Saharan Africa.(1) Microbicides research has aimed to mitigate this imbalance by identifying HIV prevention methods that women can initiate and use autonomously to protect themselves. Although many previous trials of female-initiated methods (including oral pre-exposure prophylaxis [PrEP] as well as vaginal microbicides and cervical barriers) have failed to demonstrate effectiveness (2–5), the dapivirine vaginal ring showed protective benefit in two phase III trials and in subsequent open-label extension studies, and licensure is currently being pursued.(6–11) As with oral PrEP, the level of HIV prevention efficacy with the ring was correlated with adherence.(12) Although adherence was higher in the open-label extension (OLE) studies than in the phase III trials, participants in the Microbicide Trials Network (MTN)-025/HOPE OLE still had adherence corresponding to low or no HIV protection at about 1/3 of visits. (11) Research has identified a number of multilevel factors that impact adherence among women(13–19) , and male partner influence has consistently been identified as one of the most important, despite vaginal microbicides being designed to be female-initiated.(20–24) As a result, research teams have implemented multiple strategies to increase male partner involvement, including encouraging and facilitating disclosure of study participation and product use to male partners, promoting male partner engagement in the studies, for example through attendance at the study clinics or at group events outside of scheduled visits, and utilizing community- and participant-focused strategies to help women garner their male partners’ support for product use.
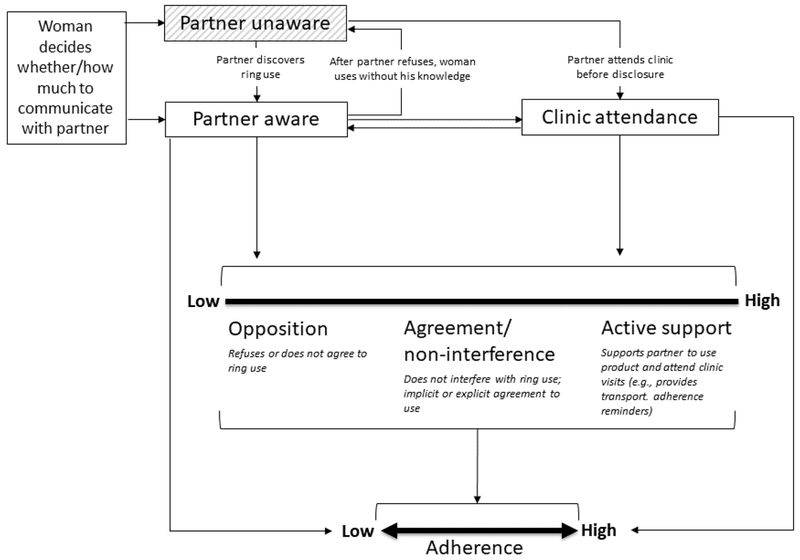
These efforts have pre-supposed that male partner involvement – in any form – would enhance women’s ability to consistently use an HIV prevention method. However, empirical evidence for this hypothesis is limited, and previous studies have focused predominantly on daily or coitally-dependent microbicide gels and oral PrEP tablets. The importance of male partner involvement may differ for a vaginal ring, which is inserted and left in place for a month or longer. The conceptual framework in Figure 1, adapted from Lanham et al (25), illustrates pathways by which such involvement may influence ring adherence. Male partner involvement usually begins with disclosure, though some women may bring their partner to the clinic to facilitate disclosure, so that clinic attendance happens simultaneously. Disclosure and clinic attendance may work independently or together to move partners along the continuum from opposition towards active support for ring use. Male partner support, or lack of opposition, may directly facilitate adherence, and disclosure or clinic attendance may also directly increase adherence without changing levels of support. For example, disclosure may reduce a woman’s anxiety around her partner feeling the ring during sex, and thus reduce the number of removals for sexual activity, even if her partner does not actively support ring use. During a clinic visit, a male partner may learn ways to better facilitate a woman’s ring adherence, even if he was already supportive before the visit.
Figure 1: Conceptual framework of male partner involvement and ring adherence1.
1 adapted from Lanham et al (25)
This paper aims to assess the impact of male partner involvement, including disclosure, engagement, and support, on women’s adherence to the vaginal ring during the MTN-020/ASPIRE trial. Insofar as male partners influence women’s adherence to the ring, and adherence influences efficacy, this analysis offers valuable insight into how male partner involvement might strengthen or diminish the public health impact of this promising HIV prevention method for women.
Methods
Study design and participants
ASPIRE was a randomized, double-blind, placebo-controlled phase III trial to assess the safety and effectiveness of the dapivirine vaginal ring for HIV prevention (ClinicalTrials.gov number NCT01617096). The study design, methods and outcomes of the trial have been described elsewhere. (6) Briefly, 2,629 women across 15 sites in Malawi, South Africa, Uganda, and Zimbabwe were enrolled between August 2012 and June 2014. Women were randomized in a 1:1 ratio to receive either a silicone elastomer vaginal matrix ring containing 25 mg of dapivirine or a placebo vaginal ring. Follow-up occurred monthly for up to 2.6 years (median: 1.6 years, interquartile range [IQR]: 1.1-2.3 years). The study demonstrated 27% efficacy overall and greater than 50% efficacy with higher levels of product use.(6, 12) Approval for this study was received from research ethics committees and drug regulatory authorities specific to each participating site. All participants provided written informed consent in English or their local language.
Procedures to Engage Male Partners
Male partner involvement was encouraged throughout the study: male partners could come to the study clinic at any time for information, individual or couples counselling, STI testing and/or treatment, or HIV counselling and testing, depending on site resources. Disclosure of study participation or ring use to male partners was not a requirement for study participation but was encouraged. Adherence support for women included monthly participant-centered counselling sessions with trained counsellors. These sessions were designed to help women explore challenges and facilitators of ring use, identify her specific needs to improve or maintain adherence, explore new strategies or continued use of established strategies to address identified needs, and set a goal to try or continue one or more strategies to support ring use. The counseling manual was not prescriptive on how to address disclosure: if a participant identified a goal to disclose to a partner or any other person, the counselor would work with her to achieve that goal. Building partner support was encouraged whenever possible, but it was acknowledged that this is not always feasible. If a participant preferred to use the ring covertly, counseling would focus on other adherence goals identified by the participant, such as getting better at re-inserting the ring on her own or keeping her clinic appointments. Each site designed and implemented unique participant engagement activities to support adherence, including activities to engage men in the community and male partners such as community education programs, male-only workshops, couples workshops, and social events like movie days or soccer games over weekends. The number of events and level of male partner engagement varied widely across sites.
Data Collection
Data on male partner involvement were collected through interviewer-administered questionnaires and through audio computer-assisted self-interview (ACASI). To encourage honest reporting and minimize response bias, these interviews preceded adherence counselling and were conducted by staff not involved in that procedure.
Disclosure.
For the purposes of this study, disclosure of ring use to male partners was defined by the participant’s report of whether her primary sex partner knew that she had been asked to use a ring as a part of the study. Answer options were “Yes”, “No”, and “Not sure”. Disclosure was assessed via interviewer-administered questionnaire at enrolment, quarterly follow up visits, and the last study visit (study exit).
Engagement.
Male partner engagement was measured by visits to the study clinic. At enrolment, quarterly follow-up visits, and study exit, participants were asked via an interviewer-administered questionnaire whether her primary sex partner had visited the study clinic in the past month and, if so, whether he attended with the participant, received counselling or other services, and/or came to the clinic for another reason.
Support.
Participants were asked two measures of male partner support or opposition via ACASI at study exit: first, if the ring was acceptable to their primary partner, with response options of “Yes”, “No”, and “Don’t know”, and second, if her primary partner had ever asked her to stop wearing the ring (“Yes” or “No” only).
Adherence.
Adherence was measured by levels of residual dapivirine in used rings. After the first year of the trial, all used rings were tested for dapivirine with the use of acetone extraction and high-pressure liquid chromatography (PAREXEL). Women were defined as having low adherence if the returned ring contained more than 22 mg of dapivirine (i.e., with ≤3 mg released from the original 25 mg ring). This cut-off approximately corresponds to no or low adherence versus medium-to-high adherence.(12)
Statistical Analysis
Descriptive statistics were calculated for each measure of male partner involvement. Women were categorized as having “always”, “sometimes”, or “never” disclosed ring use to male partners based on the proportion of follow-up visits at which they reported disclosure. Disclosure at the enrolment visit is reported separately because women had not yet used the ring or committed to using it at the time of that visit, and subsequent acceptability of and comfort with ring use may have influenced the decision to disclose at follow up visits. We calculated the frequency of male partner attendance at the study clinic at or before study enrolment, and at any point during the study (i.e. at any enrolment or follow-up visit). For male partner support at study exit, a summary variable was created to combine the two available measures. A participant had a “supportive” partner if the ring was acceptable to her primary partner and he never asked her to stop wearing it. Her partner was “not supportive” if the ring was not acceptable to her primary partner or if her primary partner had ever asked her to stop wearing the ring. The level of partner support was “unknown” if the participant did not know if the ring was acceptable to her partner and he had never asked her to stop wearing the ring. We used multinomial logistic regression to model the impact of disclosure and clinic attendance on male partner support, comparing supportive and unsupportive partners to “unknown” partner support and including an interaction term between disclosure and clinic attendance to estimate the impact of the two exposures both separately and together. Because male partner support status was only assessed at study exit, this was a cross-sectional analysis. Disclosure was modeled as the current disclosure status at the exit visit, and clinic attendance categorized according to whether the participant reported that her partner had ever attended the clinic during the study.
In the adherence analyses, ring disclosure and clinic attendance were modelled as time-varying exposures. The disclosure status reported at the end of the quarter was back-filled to apply to the first two monthly visits of the quarter as well as the visit at which it was measured. Clinic attendance was categorized as “yes” if the participant reported that her partner had attended the clinic at any visit up to, and including, the current visit, and “no” if she did not report any clinic attendance by that visit. The association between ring disclosure, clinic attendance, and ring adherence at each visit was analyzed using bivariate and multivariable (adjusted) generalized estimating equation (GEE) Poisson regression models with robust standard errors and an exchangeable correlation matrix. The GEE structure accounted for repeated measures for each participant. Because male partner support was only assessed once, we conducted a separate analysis of the association between support and adherence at the study exit visit only, using bivariate and multivariable (adjusted) Poisson models with robust standard errors and the same categories described above (“supportive”, “not supportive” or “unknown”).
All analyses excluded visits that occurred during permanent or temporary study-imposed product holds (6% of all visits), and visits at which a participant did not report having a primary sex partner (3% of all visits), as adherence was unlikely to be influenced by male partner involvement in these periods, and it may have been difficult for participants to interpret questions about disclosure or support. Because residual dapivirine levels are only a valid adherence measure for participants in the arm that received the dapivirine ring, all analyses of adherence excluded participants in the placebo arm.
The multivariable models adjusted a priori for age, study site, and time in study. We also evaluated the following variables as potential confounders, but their inclusion did not result in meaningful (>10%) changes to the risk ratios, and they were not retained in the final models: baseline measures of marital status, mobile phone ownership, partner circumcision status, engagement in transactional sex, participant’s belief that her partner has other partners, and worries about partner not liking or approving of the ring; and time-varying measures of knowledge of partner HIV status, participant having additional partners, and clinic attendance and ring disclosure (for the model of partner support and adherence).
Missing data:
Participant retention was high and there was little to no missing data for most measures. An important exception is that data on male partner support was missing for 360 participants (13.8%). The primary predictor of missing data on this variable was study site: data were missing for 86% of participants at one site and 42% of participants at a second site due to errors in data transmission. When these sites were excluded, data were missing for <7% of participants, ranging from 3%-11% across the remaining 13 sites. The majority of participants with missing data from these sites (82%) were lost to follow-up or refused further contact, and therefore did not complete the exit ACASI survey. Exclusion of the two sites with high levels of missing data did not change the findings on male partner support and ring adherence.
Results
Participant Characteristics
Baseline characteristics are displayed in Table 1. The majority of participants were older than 21 years (80.4%) and were from South African sites (54.2%). Nearly all (99.5%) had a primary partner at enrollment, though most were unmarried (59.1%). Most women reported the HIV status of her partner as being negative (55.2%) or unknown (43.5%), with 1.3% reporting an HIV positive partner. Just over half of participants had not completed secondary school (54.4%) and did not earn their own income (54.9%) (Table 1).
Table 1:
Demographic Characteristics at Baseline
| N | % | ||
|---|---|---|---|
| Total | 2629 | 100.0 | |
| Age | 18-21 | 514 | 19.6 |
| 22-26 | 842 | 32.0 | |
| 27+ | 1273 | 48.4 | |
| Median (IQR) | 26 | 22-31 | |
| Country | Malawi | 272 | 10.3 |
| South Africa | 1426 | 54.2 | |
| Uganda | 253 | 9.6 | |
| Zimbabwe | 678 | 25.8 | |
| Has primary partner* | |||
| Yes | 2616 | 99.5 | |
| No | 12 | 0.05 | |
| Currently married* | |||
| Yes | 1074 | 40.9 | |
| No | 1553 | 59.1 | |
| Partner HIV status** | |||
| HIV negative | 1444 | 55.2 | |
| HIV positive | 35 | 1.3 | |
| Unknown | 1137 | 43.5 | |
| Highest level of education | |||
| No schooling | 23 | 0.9 | |
| Primary school, not complete | 239 | 9.1 | |
| Primary school, complete | 142 | 5.4 | |
| Secondary school, not complete | 1026 | 39.0 | |
| Secondary school, complete | 1044 | 39.7 | |
| Attended college or university | 155 | 5.9 | |
| How she earns income | |||
| Formal employment | 266 | 10.1 | |
| Self-employment | 551 | 21.0 | |
| Other | 369 | 14.0 | |
| No income | 1443 | 54.9 | |
Missing data on primary partnership for 1 participant and on marital status for 2 participants.
Denominator is women who reported a current partner (n=2616)
Male partner involvement in ASPIRE: Disclosure and clinic attendance
Almost two-thirds (64.2%) of study participants reported disclosure at study enrollment, meaning that their primary partner knew that they had been asked to use the ring. During follow-up, 65.1% reported disclosure at every visit, 21.6% reported disclosure at some (but not all) visits, and 13.4% never reported disclosure (Table 2). The largest increase in disclosure was during the first quarter of study participation, from 64% to 74%. Thereafter, 76-80% of women reported disclosure at each visit after enrolment, and the proportion of women who disclosed increased by about 1% for each quarter after study enrolment (p<0.001, data not shown).
Table 2:
Descriptive statistics for male partner disclosure, engagement, and support
| Ring Disclosure to Primary Partner | N | Percent |
|---|---|---|
| Enrollment (N=2616*) | ||
| Disclosed | 1680 | 64.2% |
| Not disclosed | 936 | 35.8% |
| Follow-up (N=2588Ψ) | ||
| Disclosed at all visits | 1,684 | 65.1% |
| Disclosed at some visits | 558 | 21.6% |
| Disclosed at no visits | 346 | 13.4% |
| Primary Partner Clinic Attendance | N | Percent |
| Enrollment (N=2615*) | ||
| No | 2561 | 97.9% |
| Yes | 54 | 2.1% |
| At any point in study (N=2626^) | ||
| No | 2,288 | 87.1% |
| Yes | 338 | 12.9% |
| Number of visits throughout study: | ||
| 1 | 254 | 75.2% |
| 2 | 57 | 16.9% |
| 3 | 20 | 5.9% |
| 4 | 6 | 1.8% |
| 7 | 1 | 0.3% |
| Timing of clinic attendance relative to disclosure (N=403 visits†) | ||
| Participant reported disclosure at current visit and the previous visit | 359 | 88.6% |
| Participant reported disclosure at current visit, no disclosure at the previous visit | 21 | 5.2% |
| No disclosure at current visit | 23 | 5.7% |
| Partner Support of Ring Use (N=2269¥) | N | Percent |
| Ring acceptable | ||
| Yes | 1610 | 71.0% |
| No | 250 | 11.0% |
| Don’t know | 409 | 18.0% |
| Ever asked to stop wearing ring | ||
| Yes | 239 | 10.5% |
| No | 2029 | 89.4% |
| Composite | ||
| Supportive: Ring acceptable and never asked to stop wearing | 1,495 | 65.9% |
| Not supportive: Ring not acceptable or asked to stop wearing | 396 | 17.5% |
| Unknown: Does not know if ring is acceptable, never asked to stop wearing | 378 | 16.7% |
Excludes 12 women with no primary partner at enrollment and 1-2 women with missing data.
Excludes 29 women with no follow-up behavioral assessments and 12 women who did not report a primary sex partner during follow-up
Excludes 3 women with no follow-up data
405 male partner clinic visits were reported after study enrollment. Table excludes 2 participants who had currently disclosed but were missing data on disclosure at the previous visit
Data not collected for 360 participants
Male partner clinic attendance was reported by 54 participants (2.1%) at study enrolment, of whom 49 (90.7%) had disclosed ring use. By the end of the study, 388 participants (12.9%) of participants reported that their male partner had attended the clinic at least once (Table 2). Most visited either once (75.2%) or twice (16.9%), and the reported maximum number of visits by any one partner was seven (Table 2). Of the 405 clinic visits that occurred after enrollment, the vast majority (359; 88.6%) took place after the participant had disclosed ring use. There were few visits at which the participant’s male partner was not aware of ring use (23; 5.7% ), or when disclosure and the clinic visit had both taken place since the last study visit (21; 5.2%). Accompanying the study participant or seeking services related to HIV counselling and testing and STI treatment at the research site were the main reasons for male partner visits.
Male partner support for ring use:
At study exit, most participants reported that their partners accepted the ring (71.0%) and never asked them to stop wearing it (89.4%, Table 2). For the composite support variable, nearly two-thirds of the participants (65.9%) were classified as having supportive partners at study exit because their partners both found the ring acceptable and did not ask the participant to remove the ring. Almost 18 percent (17.5%) of participants had partners who were not supportive, including 10.5% who reported that their partner asked them to stop wearing the ring during study participation. The level of partner support was unknown for 16.7% of women, of whom 66.2% had not disclosed ring use to their primary partner at the visit at which support was assessed (data not shown).
Male partner support was significantly associated with current disclosure status (i.e. at study exit) and with any history of male partner clinic attendance during the study (Table 3). Among women who had disclosed, 78.4% reported a supportive partner, compared to only 22.5% among women who had not disclosed. Among women whose partner had attended the clinic, 81.2% reported a supportive partner, compared to 64.2% among those whose partners had not attended. As expected, women who reported disclosure, compared to non-disclosure, had a significantly higher probability of having a supportive partner, compared to an unknown level of partner support in both bivariate and multivariate analyses (adjusted relative risk ratio [aRRR] for disclosure, 24.17, 95% CI 16.38-35.66, p<0.001). They also had a significantly higher probability of having an unsupportive partner, compared to an unknown level of partner support (aRRR 4.10, 95% CI 2.70-6.24, p<0.001), though the effect size was not as strong. Women who reported male partner clinic attendance had a significantly higher probability of having a supportive partner (aRRR 3.77, 95% CI 1.36-10.42, p=0.01), but not of having an unsupportive partner (aRRR 1.45, 95% CI 0.42-5.07, p=0.56), compared to an unknown level of partner support. There was no evidence of interaction between disclosure and clinic attendance on male partner support (global p for interaction term =0.33).
Table 3:
Association of male partner support with disclosure and clinic attendance (N=1942*)
| Unadjusted Relative risk ratio | 95% CI | p | Adjusted for age, site, and time in study Relative risk ratio | 95% CI | p | |||
|---|---|---|---|---|---|---|---|---|
| Outcome: Supportive male partner | ||||||||
| Exposures: | ||||||||
| Disclosure of ring use to primary partner | ||||||||
| Yes | 1,534 | 1,202 (78.4) | 24.92 | 17.87-34.75 | <0.001 | 24.17 | 16.38-35.66 | <0.001 |
| No/not sure | 408 | 92 (22.6) | Ref | Ref | ||||
| Primary partner attended clinic to date | ||||||||
| Yes | 276 | 224 (81.2) | 3.05 | 1.10-8.47 | 0.03 | 3.77 | 1.36-10.42 | 0.01 |
| No | 1,666 | 1,070 (64.2) | Ref | Ref | ||||
| Interaction: Disclosure x clinic attendance | 0.61 | 0.18-2.04 | 0.42 | 0.44 | 0.13-1.50 | 0.19 | ||
| Outcome: Unsupportive male partner | ||||||||
| Exposures: | ||||||||
| Disclosure of ring use to primary partner | ||||||||
| Yes | 1,534 | 227 (14.8) | 3.82 | 2.72-5.37 | <0.001 | 4.10 | 2.70-6.24 | <0.001 |
| No/not sure | 408 | 112 (27.5) | Ref | Ref | ||||
| Primary partner attended clinic to date | ||||||||
| Yes | 276 | 34 (12.3) | 1.04 | 0.30-3.64 | 0.95 | 1.45 | 0.42-5.07 | 0.56 |
| No | 1,666 | 305 (18.3) | Ref | Ref | ||||
| Interaction: Disclosure x clinic attendance | 1.25 | 0.29-5.32 | 0.76 | 0.95 | 0.22-6.24 | 0.94 | ||
| Outcome: Partner support unknown | Ref | Ref | ||||||
Study exit visits only. Excludes visits with missing data on partner support, product hold, or no primary partner reported.
Association between male partner involvement and ring adherence.
Data on residual dapivirine in used rings was available for 20,699/27,904 (74.2%) of follow-up visits included in our analysis, of which 7,123 (34.4%) had residual dapivirine concentrations >22mg, meeting our definition of low adherence. When modeled jointly, there were no significant associations between ring adherence and ring disclosure or male partner clinic attendance in bivariate analysis or after adjusting for age, site and time in study. When compared to the majority of visits at which women had disclosed ring use but had never had a partner attend the clinic, the risk of low ring adherence was slightly higher at visits when women had not disclosed ring use to their primary partner (adjusted risk ratio [aRR] 1.09, 95% CI 0.99-1.19, p=0.07) and slightly lower at visits when their partners had come to the study clinic at any point prior (aRR 0.88, 95% CI 0.77-1.00, p=0.06), (Table 4). There was no evidence of effect modification between male partner clinic attendance and disclosure status (p=0.5).
Table 4:
Associations between male partner disclosure, engagement, and support with low ring adherence, measured by residual ring concentrations >22mg*
| Model # | Exposure | # visits | n (%) with low adherence | Unadjusted Risk ratio | 95% CI | p | Adjusted for age, site, and time in study Risk ratio | 95% CI | p |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Primary partner knows about ring use | ||||||||
| Yes | 16,117 | 5,493 (34.1%) | Ref | Ref | |||||
| No/not sure | 4,582 | 1,630 (35.6%) | 1.07 | 0.98 - 1.17 | 0.13 | 1.09 | 0.99 - 1.19 | 0.07 | |
| Primary partner attended clinic to date | |||||||||
| No | 18,471 | 6,372 (34.5%) | Ref | Ref | |||||
| Yes | 2,228 | 751 (33.7%) | 0.90 | 0.79-1.02 | 0.09 | 0.88 | 0.77-1.00 | 0.06 | |
| Interaction: Disclosure x clinic attendance | 1.12 | 0.82-1.55 | 0.47 | 1.12 | 0.81-1.55 | 0.51 | |||
| 2 | Primary partner support^ | ||||||||
| Supportive | 651 | 210 (32.3) | Ref | Ref | |||||
| Not supportive | 149 | 63 (42.3) | 1.31 | 1.05-1.63 | 0.02 | 1.29 | 1.03-1.62 | 0.03 | |
| Unknown | 160 | 44 (27.5) | 0.85 | 0.65-1.12 | 0.26 | 0.87 | 0.66-1.15 | 0.32 | |
Both models exclude visits with product hold or no primary partner reported.
Study exit visits only.
Reporting an unsupportive male partner at the study exit visit was significantly associated with low ring adherence at that visit in both bivariate and adjusted analyses (aRR 1.29, 95% CI 1.03-1.62, p=0.03), while the risk of low adherence was similar among women who reported supportive partners and women whose partner support was classified as “unknown” (aRR 0.87, 95% CI 0.66-1.15, p=0.32). These associations did not change meaningfully when the model controlled for disclosure and clinic attendance (aRR 1.25, 95% CI 0.99-1.57, p=0.06), or when we restricted the analysis to women who had disclosed ring use (aRR 1.23, 95% CI 0.94-1.60, p=0.13), though in both cases the p-values increased to >0.05.
Discussion
This study confirms an association between male partner support and women’s adherence to the dapivirine vaginal ring. Women with an unsupportive male partner were 30% more likely to have low adherence than women who had supportive partners. Disclosure of ring use and clinic attendance were both associated with a higher probability of having a supportive partner, but were not directly associated with ring adherence.
Although most women reported that their partners supported their ring use at the end of the study, a substantial number faced unsupportive attitudes or requests to not wear the ring or did not know how their partner felt. Even among women who had disclosed ring use, those who did not know whether their partner was supportive had adherence levels similar to or higher than women with supportive partners, which may suggest that active support provides little benefit over passive acceptance. This hypothesis also helps to explain why disclosure did not predict ring adherence in this study. Partners who are unaware of ring use cannot actively oppose it, and while disclosure may lead to partner support, it may also result in partner opposition that could, in turn, inhibit ring use. These results are consistent with other studies among oral PrEP and vaginal gel users finding that tacit acceptance (also labelled neutrality(26) or non-interference(25)) is sufficient for most women, and with findings that experience of social harm from male partners is associated with lower ring adherence.(27) Here we add that opposition from male partners remains a relevant barrier to adherence with the vaginal ring, even when it does not lead to a reportable social harm during study participation.
Our observed lack of association between disclosure and ring adherence contrasts with findings from recent oral PrEP studies among young women in sub-Saharan Africa, in which disclosure has been one of the strongest determinants of adherence.(16, 28) Although our study population was older, our findings suggest that the ring may be a preferable option for women who wish to use an HIV prevention product without disclosure. Despite this potential advantage, the proportion of women that reported disclosure to their partners was similar to other microbicide and PrEP studies such as CAPRISA 004 (67%) (13) , MDP301 (84%)(29), and VOICE (87%, personal communication, E.T. Montgomery). The ring is arguably more discreet than these other methods, suggesting that disclosure seems to be less dependent on the dosing modality and more a product of social context. This is consistent with qualitative studies in which men, women and community stakeholders have endorsed the idea that women should disclose microbicide use to male partners to avoid suspicions, prevent relationship problems and to demonstrate respect. However, similar to microbicide gels, women have also reported fearing that the ring would be felt by her partner during vaginal intercourse or digital foreplay, and that the male partner’s discovery of undisclosed use could result in violence, anger, or relationship discord.(30–32) Therefore, disclosure patterns may differ with a completely undetectable intervention delivery method such as an injection.
Only a small proportion of male partners ever attended the study clinic during ASPIRE, despite concerted efforts by study staff. Similar challenges have been noted in previous HIV prevention studies,(23, 33) and male engagement in the HIV prevention and treatment cascade is a widely cited challenge to achieving the 90/90/90 targets.(34–36) Although effective strategies to encourage male engagement in the health care system are needed to improve men’s health outcomes, we did not find evidence that male partners’ clinic attendance enhanced ring adherence, although it contributed to gaining their support.
Our findings suggest that future interventions to shift male partner attitudes should be targeted at reducing opposition to ring use rather than promoting disclosure or male engagement for their own sake. In qualitative studies, male partner opposition to the ring has related to actual or feared changes to the feeling of sex and concerns about short and long-term side effects (e.g. infertility, cancer)(30, 31, 37, 38). Strategies that offer male partners the opportunity to interact directly with clinical staff may address men’s concerns and simultaneously provide a forum to promote the ring in a way that emphasizes the positive impact it could have on health and sex. That said, social scientists have suggested that male resistance to female-initiated methods may be more indicative of broader, structural-level concerns about shifts in gender power, enhanced female independence and access to knowledge than in specific features of product use. (32) Given this perspective, and the low rates of male partner clinic attendance in ASPIRE, it may be more effective to implement community-based interventions that address gender norms and other sources of male resistance than labor- and resource-intensive strategies to get men into clinics for direct one on one interventions. Alternatively, or in complement, counseling interventions for women to help them to gain male partner support after disclosure could be effective, especially given the social pressures to disclose, described above. We observed that most disclosure happened within the first three months of the study, suggesting that such interventions would be most effective at the early stages of product initiation. However, the process of disclosing and building support is often continuous, rather than a one-time decision. From qualitative studies, we learn that some women who disclosed and received unsupportive reactions pretended to discontinue ring use, returning to a state of non-disclosure (31), while others changed partners during the study, and may have waited until the partnership was more established before choosing to disclose.(30) Interventions to help women gain their partner’s support should be offered throughout product use to accommodate changes in women’s lives, in combination with efforts to address other factors known to contribute to poor adherence, such as initial concerns about using an unfamiliar product (19), discomfort with using the ring during menses (18), negative peer influence, and perceived side effects (30).
Several limitations of this study should be noted. Because this is a secondary data analysis, the measures of male involvement were not originally designed to answer the questions we pose here and were not administered at the ideal time points or with the ideal phrasing. Our disclosure variable was based on the participant’s report of whether her partner was aware that she had been asked to use the ring as part of the study, and we cannot distinguish whether she told him about the ring or whether he found out in another way, whether he was aware that the ring was intended for HIV prevention, or whether she told him that she would actually be using the ring (rather than having been asked to use it and declined). Our measure of support was likewise imperfect. Because it was only measured at the end of the study, we don’t know how much women’s perceptions of male partner support changed throughout the study timeline, nor the reasons for any changes (e.g. partnership change, increasing acceptance, or unintentional disclosure). We were unable to account for partner change or a change in a partner’s level of support in this analysis, or for the existence of multiple concurrent partnerships with differing levels of disclosure or support. Further, women’s perceptions of her partner’s support for ring use may be based on her own fears or subjective interpretations as opposed to actual experiences. Indeed, the concepts of disclosure, engagement, and support are not universally defined nor understood, and operationalization has not been systematic in previous studies, highlighting the difficulties in quantifying “male involvement”.(39) Finally, this study of male engagement was conducted in the context of the ASPIRE trial, and women’s experiences could be different outside of a trial setting. Study-related male engagement activities are likely to have facilitated disclosure to partners and increased male partner support, and frequent clinic visits for the trial follow-up may have made it more difficult for women to use the ring covertly. Rates of partner opposition may be higher under non-study conditions, and rates of disclosure lower. Alternatively, the provision of the ring outside of research context may normalize it and increase rates of partner support, as described during an open-label implementation trial of the tenofovir gel in South Africa. (40) Overall rates of ring adherence are also likely to be different in non-study conditions. In open label extension studies of oral PREP and the vaginal ring, adherence rates were higher than in clinical trials (10, 11, 41, 42), likely because participants knew that they were using an effective HIV prevention product instead of an experimental product or placebo. However, if the ring becomes available in non-research settings, adherence rates may be lower if women receive less frequent or comprehensive adherence counseling and support.(28, 43) Future studies should evaluate the relationship between male involvement and ring use in programmatic settings.
Conclusions
We assessed the association between ring adherence and three aspects of male partner involvement: partner clinic attendance, disclosure to partners, and partner support for ring use. The perception of a non-supportive male partner was the greatest barrier to ring adherence, while disclosure and clinic attendance were each associated with a higher likelihood of support. The results suggest that the ring is a method that can be used successfully by women without partner disclosure, highlighting its potential as a woman-initiated HIV prevention method. Despite this positive news, women with unsupportive partners had inconsistent ring use at significantly more visits, and previous studies strongly suggest that, for many women, partner opposition is detrimental to product adherence. Future roll-out should include efforts to identify women with unsupportive partners; offer them strategies to win support through disclosure, education, and counseling; and assist them to use the ring safely and consistently despite partner opposition. Given the challenges of bringing men into clinics, supplemental community-based activities may reach a wider audience and help to normalize ring use. These findings also have implications for other current and future PrEP products such as oral PrEP, injections, and implants: regardless of the level of discretion they provide, many women will desire or seek partner support, or will fear the consequences of covert use, while others will use the products without disclosing to partners. By providing support for women in their choice to use HIV prevention products independently or to engage their partners to reduce opposition, we can make these products fit more easily into the lives of more women, increasing their potential to reduce HIV risk.
Acknowledgements
We are grateful to the study participants for their participation and dedication. We thank the study team members at the research sites, at the MTN-020/ASPIRE Protocol Management team and at the MTN Leadership Operations Center for their contributions to data collection.
Funding: The MTN-020/ASPIRE study was designed and implemented by the Microbicide Trials Network (MTN). The MTN is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the U.S. National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The vaginal rings used in this study were developed and supplied by the International Partnership for Microbicides (IPM).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.UNAIDS. UNAIDS Reports on the Global AIDS Epidemic 2010 2010. [Available from: http://www.unaids.org/globalreport/default.htm.
- 2.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. New England Journal of Medicine. 2015;372(6):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, et al. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. The Lancet. 2010;376(9749):1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padian NS, van der Straten A, Ramjee G, Chipato T, de Bruyn G, Blanchard K, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. The Lancet. 2007;370(9583):251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med. 2016;375(22):2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, et al. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. N Engl J Med. 2016;375(22):2133–43. [DOI] [PubMed] [Google Scholar]
- 8.Nel A, Van Neikerk N, Van Baelen B, Roseberg Z. HIV Incidence and Adherence in DREAM: An Open-Label Trial of Dapivirine Vaginal Ring. Conference on Retroviruses and Opportunistic Infections (CROI); March 4-7, 2018; Boston, MA, USA. [Google Scholar]
- 9.Baeten JM, Palanee-Phillips T, Mgodi N, Mayo A, Nel A, Roseberg Z, et al. High Uptake and Reduced HIV-1 Incidence in an Open-Label Trial of the Dapivirine Ring. Conference on Retroviruses and Opportunistic Infections (CROI); March 4-7, 2018; Boston, MA, USA. [Google Scholar]
- 10.Nel A, Malherbe M, Mans W, Van Baelen B, Van Niekerk N, Louw C, et al. Safety, Adherence and HIV-1 Seroconversion in DREAM – An Open-label Dapivirine Vaginal Ring Trial. 9th SA AIDS Conference; June 11-14, 2019; Durban, South Africa [Google Scholar]
- 11.Baeten J, Palanee-Phillips T, Mgodi N, Ramjee G, Gati B, Mhlanga F, et al. High uptake and sustained impact on HIV-1 incidence: final results of an open-label extension trial of the dapivirine ring. 10th IAS Conference on HIV Science; July 21-24, 2019; Mexico City, Mexico. [Google Scholar]
- 12.Brown E, Palanee-Philips T, Marzinke M, Hendrix C, Dezutti C, Soto-Torres L, et al. Residual dapivirine ring levels indicate higher adherence to vaginal ring is associated with HIV-1 protection. 21st International AIDS Conference (AIDS 2016); July 11-22, 2016; Durban, South Africa. [Google Scholar]
- 13.Mngadi KT, Maarschalk S, Grobler AC, Mansoor LE, Frohlich JA, Madlala B, et al. Disclosure of microbicide gel use to sexual partners: influence on adherence in the CAPRISA 004 trial. AIDS and Behavior. 2014; 18(5):849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C qualitative study in Johannesburg, South Africa. PLoS ONE. 2014;9(2):e89118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Straten A, Montgomery ET, Musara P, Etima J, Naidoo S, Laborde N, et al. Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS. 2015;29(16):2161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Celum C, Gill K, Morton J, Stein G, Myers L, Thomas K, et al. Incentives conditioned on tenofovir levels to increase adherence among young women on PrEP in Cape Town. 10th IAS Conference on HIV Science; July 21-24, 2019; Mexico City, Mexico. [Google Scholar]
- 17.Chitukuta M, Duby Z, Katz A, Nakyanzi T, Reddy K, Palanee-Phillips T, et al. Negative rumours about a vaginal ring for HIV-1 prevention in sub-Saharan Africa. Cult Health Sex. 2019;Epub ahead of print: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duby Z, Katz AWK, Browne EN, Mutero P, Etima J, Zimba CC, et al. Hygiene, blood flow, and vaginal overload: why women removed an HIV prevention vaginal ring during menstruation in Malawi, South Africa, Uganda and Zimbabwe. AIDS Behav. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Straten A, Browne EN, Shapley-Quinn MK, Brown ER, Reddy K, Scheckter R, et al. First impressions matter: how initial worries influence adherence to the dapivirine vaginal ring. J Acquir Immune Defic Syndr. 2019;81(3):304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montgomery E, Napierala S, Zvivamwe P, Mtetwa S, Hammond N, Chipato T, et al. Male involvement in a diaphragm and microbicide safety study in Zimbabwe Microbicides 2006; 23-26 April, 2006; Cape Town, South Africa. [Google Scholar]
- 21.Montgomery CM. The role of partnership dynamics in determining the acceptability of condoms and microbicides. AIDS Care. 2008;20(6):733–40. [DOI] [PubMed] [Google Scholar]
- 22.Sahin-Hodoglugil NN, van der Straten A, Cheng H, Montgomery ET, Kacanek D, Mtetwa S, et al. Degrees of disclosure: a study of women’s covert use of the diaphragm in an HIV prevention trial in sub-Saharan Africa. Soc Sci Med. 2009;69(10): 1547–55. [DOI] [PubMed] [Google Scholar]
- 23.Montgomery ET, van der Straten A, Chidanyika A, Chipato T, Jaffar S, Padian N. The importance of male partner involvement for women’s acceptability and adherence to female-initiated HIV prevention methods in Zimbabwe. AIDS Behav. 2010;15(5):959–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montgomery E, Stadler J, Hartmann M, Magee C, Magazi B, Mathebula F, et al. , editors. Male partner roles and influence on women’s use of HIV pre-exposure prophylaxis in Johannesburg AIDS Impact; Sept 29-Oct 3, 2013; Barcelona, Spain. [Google Scholar]
- 25.Lanham M, Wilcher R, Montgomery ET, Pool R, Schuler S, Lenzi R, et al. Engaging male partners in women’s microbicide use: evidence from clinical trials and implications for future research and microbicide introduction. J Int AIDS Soc. 2014;17(3 Suppl 2): 19159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Succop SM, MacQueen KM, van Loggerenberg F, Majola N, Karim QA, Karim SS. Trial participation disclosure and gel use behavior in the CAPRISA 004 tenofovir gel trial. AIDS Care. 2014;26(12): 1521–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palanee-Phillips T, Roberts ST, Reddy K, Govender V, Naidoo L, Siva S, et al. Impact of Partner-Related Social Harms on Women’s Adherence to the Dapivirine Vaginal Ring During a Phase III Trial. J Acquir Immune Defic Syndr. 2018;79(5):580–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celum C, Mgodi N, Bekker LG, Hosek S, Donnel D, Anderson P, et al. PrEP use in young African women in HPTN 082: Effect of drug level feedback. 10th IAS Conference on HIV Science; July 21-24, 2019; Mexico City, Mexico. [Google Scholar]
- 29.Gafos M, Pool R, Mzimela MA, Ndlovu HB, McCormack S, Elford J, et al. Communication About Microbicide Use Between Couples in KwaZulu-Natal, South Africa. AIDS Behav. 2015;19(5):832–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery ET, van der Straten A, Chitukuta M, Reddy K, Woeber K, Atujuna M, et al. Acceptability and use of a dapivirine vaginal ring in a phase III trial. AIDS. 2017;31(8): 1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Laborde ND, Pleasants E, Reddy K, Atujuna M, Nakyanzi T, Chitukuta M, et al. Impact of the Dapivirine Vaginal Ring on Sexual Experiences and Intimate Partnerships of Women in an HIV Prevention Clinical Trial: Managing Ring Detection and Hot Sex. AIDS Behav. 2018;22(2):437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stadler J, Delany-Moretlwe S, Palanee T, Rees H. Hidden harms: women’s narratives of intimate partner violence in a microbicide trial, South Africa. Soc Sci Med. 2014;110:49–55. [DOI] [PubMed] [Google Scholar]
- 33.Montgomery ET, Upkong M, White R, van der Straten A, editors. Involving male partners in trials of female-initiated HIV prevention methods in Africa: a review of strategies and evidence. International Conference on HIV Pathogenesis, Treatment, and Prevention; July 19-22, 2009; Cape Town, South Africa2009. [Google Scholar]
- 34.Kojima N, Klausner JD. Strategies to Increase Human Immunodeficiency Virus Testing Among Men to Reach UNAIDS 90-90-90 Targets. Clin Infect Dis. 2018;67(9):1468–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huerga H, Van Cutsem G, Ben Farhat J, Puren A, Bouhenia M, Wiesner L, et al. Progress towards the UNAIDS 90-90-90 goals by age and gender in a rural area of KwaZulu-Natal, South Africa: a household-based community cross-sectional survey. BMC Public Health. 2018;18(1):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joint United Nations Programme on HIV/AIDS (UNAIDS). Reaching out to men and boys: Addressing a blind spot in the response to HIV. Geneva, Switzerland: UNAIDS; 2017. [Google Scholar]
- 37.Montgomery ET, Chidanyika A, Chipato T, van der Straten A. Sharing the trousers: gender roles and relationships in an HIV-prevention trial in Zimbabwe. Cult Health Sex. 2012;14(7):795–810. [DOI] [PubMed] [Google Scholar]
- 38.van der Straten A, Montgomery ET, Cheng H, Wegner L, Masenga G, von Mollendorf C, et al. High acceptability of a vaginal ring intended as a microbicide delivery method for HIV prevention in African women. AIDS Behav. 2012;16(7): 1775–86. [DOI] [PubMed] [Google Scholar]
- 39.Montgomery E, van der Straten A, Torjesen K. “Male Involvement” in Women and Children’s HIV Prevention: Challenges in Definition and Interpretation. JAIDS 2011;57(5):e114. [DOI] [PubMed] [Google Scholar]
- 40.MacQueen KM, Dlamini S, Perry B, Okumu E, Sortijas S, Singh C, et al. Social Context of Adherence in an Open-Label 1 % Tenofovir Gel Trial: Gender Dynamics and Disclosure in KwaZulu-Natal, South Africa. AIDS Behav. 2016;20(11):2682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baeten JM, Heffron R, Kidoguchi L, Mugo NR, Katabira E, Bukusi EA, et al. Integrated Delivery of Antiretroviral Treatment and Pre-exposure Prophylaxis to HIV-1-Serodiscordant Couples: A Prospective Implementation Study in Kenya and Uganda. PLoS Med. 2016;13(8):e1002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gill K, Dietrich J, Gray G, Pidwell T, Kayamba F, Bennie T, et al. Pluspills: an open label, safety and feasibility study of oral pre-exposure prophylaxis (PrEP) in 15-19 year old adolescents in two sites in South Africa. 9th IAS Conference on HIV Science; July 23-26, 2017; Paris, France. [Google Scholar]