Abstract
Acceptability of rapid HIV self-testing is high but potential users remain concerned about correct use, interpretation of test results, and linkage to care. This article describes user preferences for a smartphone app to mitigate these challenges and how these were integrated into the SMARTtest app to support self- and partner testing using the INSTI Multiplex®. Sixty men and transgender women who have sex with men self-tested for HIV and syphilis while guided by a prototype app that provided a video, pictorial step-by-step instructions, and sample test results presented textually (“positive,” “negative”). Subsequently, participants provided feedback on revisions and additional app content. Participants recommended offering different user modes (self, partner, both), and retaining the video, step-by-step instructions, and textual test results. They strongly favored the ability to save and send test results to sexual partners or providers. These features were integrated into the SMARTtest app to facilitate HIV/syphilis self- and partner testing, HIV/syphilis status awareness and disclosure, and linkage to care.
RESUMEN
La aceptabilidad de las auto-pruebas rápidas del VIH es alta pero sigue habiendo preocupaciones sobre el uso correcto, la interpretación de los resultados, y el enlace a la atención médica. Este artículo describe las preferencias de usuarios para una aplicación de teléfonos inteligentes para mitigar estos retos y como estas fueron integradas a la app SMARTtest para apoyar las auto-pruebas y pruebas con parejas para VIH y sífilis con el INSTI Multiplex®. Sesenta hombres y mujeres transgénero que tienen sexo con hombres se auto-realizaron una prueba de VIH y sífilis guiados por una app prototipo que proveyó instrucciones paso-a-paso en forma de video y foto, y resultados presentados en forma de texto (“positivo,” “negativo”). Después, los participantes fueron entrevistados a fondo para proveer retroalimentación sobre posibles revisiones y contenido adicional de la app. Los participantes sugirieron incorporar diferentes modos de usuarios (Yo, Mi pareja, Ambos), recomendaron retener el video, las instrucciones paso-a-paso, y los resultados en forma textual. Ellos favorecieron fuertemente la capacidad de guardar y enviar los resultados de las pruebas a parejas o a proveedores de salud. Todas estas funciones fueron integradas en la versión final de SMARTtest para facilitar el uso de pruebas de VIH/sífilis con parejas y para auto-pruebas, el conocimiento y divulgación de estados de VIH/sífilis, y el enlace a la atención médica.
INTRODUCTION
Increasing rates and frequency of HIV testing is critical to meeting End-the-Epidemic goals by alerting individuals to their HIV status and linking them to care. As such, facilitating access to HIV testing is essential. Since the approval of the OraQuick In Home HIV test by the FDA in 2012, the World Health Organization, U.S. Centers for Disease Control and Prevention, and the United Nations [1–3], have increased calls to implement and disseminate HIV self-testing, and its use is widening beyond the U.S. to Western Europe, Africa, and Asia.
Among men who have sex with men (MSM) and transgender women (TGW), HIV self-testing has high acceptability [4–10]. Prior studies have also shown that MSM are willing [11, 12] and able [13,14] to engage new and existing sexual partners in mutual HIV self-testing. However, potential users have expressed concerns that they may administer the test or read the results incorrectly [5,7], self-testing at home lacks the availability of expert support in case of an unfavorable test result [5–7], and that there may be uncertainty as to next steps or where to seek clinical treatment after receiving a positive result [4,6]. Potential users are also concerned that current HIV self-tests are designed to only detect HIV and do not detect other sexually transmitted infections (STIs) [6], especially given the sharp increase in incidence of STIs among at risk populations [14–18]. MSM users have expressed a clear preference for oral HIV self-tests [19,20]; however, that preference is eliminated if the fingerprick-based blood test also screens for other STIs [21]. Moreover, studies have found lower sensitivity performance for rapid oral HIV tests when compared to fingerprick-based blood tests [22, 23]. Given the high prevalence of HIV and syphilis among MSM, a dual HIV/syphilis rapid test could provide significant public health benefits by facilitating testing for HIV and syphilis and alerting those infected of their status so that they may seek care and reduce onward transmission to other sexual partners. As such, there is a need to develop approaches that are easily disseminated and might facilitate HIV/syphilis self- and partner-testing, even if it is a fingerprick-based blood test. Smartphone apps, which are being increasingly used in healthcare, including in HIV prevention and treatment [24–27], could fulfill such a role and help make potential users more comfortable with self- and partner-testing by providing clearer instructions, more easily readable results, and facilitating linkage to care.
This article describes the development process and resulting SMARTtest app to facilitate HIV and syphilis self- and partner-testing. The SMARTtest app has been designed as an adjunct to the INSTI Multiplex®, a blood-based rapid HIV and syphilis test developed by bioLytical Laboratories that provides results in one minute. The INSTI® HIV-1/HIV2 Antibody Test, an HIV-only test, is FDA-approved and available in the U.S., Canada, Europe, and Africa; there is also a self-test version available in Europe. Studies have shown high acceptability of the INSTI® HIV-1/HIV2 Antibody Test among providers [28] and self-testers [29]. The INSTI Multiplex® is available in Canada and Europe but is not yet FDA approved in the U.S. BioLytical plans to seek FDA approval of the INSTI Multiplex® for both a provider version, and subsequently a self-test version. Although not available as a self-test currently, user procedures to conduct the INSTI Multiplex® are identical to the INSTI® HIV self-test that is available in Europe, except that when reading results, there is an additional blue dot displayed for syphilis reactive results. In this article we present qualitative findings from MSM and TGW participants at high risk of infection about the components they would want in a smartphone app to support self- and partner-testing. Then, using screenshots of the app, we demonstrate how user feedback was incorporated into the design of the SMARTtest app to support correct use of the INSTI Multiplex, improve readability of results, offer options for serostatus disclosure or achieving results, and facilitating linkage to care.
METHODS
Recruitment
Sixty participants were recruited via geospatial sexual networking applications, online (Craigslist, etc.), and in-person (LGBT Center, etc.) for a study “to see whether people would screen their sexual partners using a new smartphone-based HIV/syphilis test. Recruitment was conducted in New York City between November 2016 and September 2017. To utilize the expertise of individuals with experience using a rapid HIV test for self-and partner-testing, twenty individuals were recruited following their participation in a different study in which they used the OraQuick In-Home HIV test to test themselves and potential sexual partners [30]. All participants were MSM or TGW, 18 years of age or older, HIV-uninfected, non-monogamous, reported at least three occasions of condomless anal intercourse over the past three months, and rarely or never using condoms during anal intercourse. Potential participants who were using PrEP, or who were unfamiliar with or did not own a smartphone were not excluded from the study. Ninety-nine participants were screened by telephone for eligibility, of which 75 screened eligible; of those, 60 came to our research offices to complete their study visit.
Procedures
This multi-phase study consisted of 1) in-depth qualitative interviews to inform app content; 2) 4-session focus groups to review and provide feedback on app development; 3) completion and pilot testing the app; and 4) revision of app based on pilot study feedback.
After signing consent, participants completed a questionnaire using a computer assisted self-interview (CASI) that included assessments of demographic characteristics, sexual behavior, HIV and STI knowledge, testing experiences, risk perception, and pre-exposure prophylaxis (PrEP) use. They then self-tested for HIV and syphilis using either a custom-built smartphone accessory called the mChip dongle [31] (n = 40) or rapid HIV (INSTI) and Syphilis (Health Check) tests (n = 20) while being guided by a prototype smartphone app that provided video and step-by-step instructions on performing the tests and demonstrated results with written text (i.e., positive, negative) for HIV and syphilis. Afterwards, they underwent a qualitative in-depth interview (IDI) about their experience using the prototype app and subsequent self-testing. During the IDIs, participants were asked about what features, functions, and content they would prefer to have in a future version of the SMARTtest app. After self-testing for HIV and syphilis, one participant received a reactive HIV test result and did not complete the study visit, resulting in a total of 59 participants.
Once the IDIs were completed and preliminary analyses of app-related findings were conducted, the researchers employed a rapid user-centered design approach [32–36], a six- to eight-month process during which iterations of the app are presented to users to gather feedback to inform subsequent revisions in order to achieve an app with good functionality, simple features, and a usable interface [33]. During this period, four participants were invited back to take part in an ongoing focus group to assist in decision making about app content and features that would meet their needs when performing a self-test, in navigating the process with a sexual partner, and in accessing resources before, during, and after the testing process. Focus group participants were selected based on their active participation during the IDI and their expressed high likelihood of testing sexual partners, with consideration for age and ethnic/racial diversity. After preliminary qualitative findings from the individual in-depth interviews were shared with them, these participants reviewed initial app revisions and offered feedback on the revisions in a group setting. Subsequent app revisions were implemented following each focus group discussion over the course of six months; new changes were presented to focus group participants at the next session. Afterwards, ten participants were recruited for a one-month mini-pilot to test and provide feedback on the app. Pilot participants were selected using the same eligibility criteria as in the first phase of the study. Additionally, half were iPhone users, the other half were Android phone users. Participants were given six INSTI Multiplex test kits to take home (including all necessary materials for test use and a card on reading the test results) and had the app installed on their phones for their personal use. After one month, nine participants returned for a brief interview about their general impressions about the app and suggestions for further improvement; one participant could not be reached. This feedback was captured in debrief reports completed immediately following the brief interview. For this paper, feedback of the overall app and the different app components were summarized.
The Institutional Review Boards at the New York State Psychiatric Institute approved all study procedures.
Data Analysis
IDIs and focus groups were audio-recorded, transcribed, and reviewed for accuracy. Development of the codebook originated with the general topic areas of the IDI guide and was further refined through repeated reading of transcripts by a team of four researchers. Codes were defined with inclusion and exclusion criteria including examples. Subsequently, three staff members independently coded the interviews; 20% of the interviews were double-coded and discrepancies between coders were discussed until consensus was reached. For this manuscript, coding reports related to the app content (e.g., App recommendations, Referrals, Delivery of information, Instructions, Handling results, App screens, and saving, sharing and deleting results) were pulled and reviewed to identify modal responses and cases that contradicted the main trends as well as quotes to be included in-text. Quoted text has been edited for clarity and readability without compromising the integrity of its content.
RESULTS
Demographic Description of Participants
Participants (N=59) had a mean of 40.75 (SD=13.34) years of age and annual income of $37,916 (SD=$37,584). Almost all respondents (95%) identified as a man and nearly three-quarters (73%) as gay/homosexual (Table 1). Nineteen percent of participants reported their ethnicity as Latino/ Hispanic; 41% identified as African-American/Black (non-Latino). Fifty-six percent of the participants were college graduates, half of whom reported having a graduate degree. A large minority of participants (41%) reported full-time employment. Almost half of respondents (48%) reported a prior STI; 48% had used PrEP and 48% had used a rapid HIV self-test.
Table 1.
Sample description of SMARTtest participants (N=59)
| Characteristics | Mean (SD; Range) |
|---|---|
| Age | 40.75 (13.34; 20–73) |
| Annual income | $37,916 ($37,584; $0-$220,000) |
| N (%)1 | |
| Education | |
| Partial high school | 2 (3%) |
| High school graduate/GED | 10 (17%) |
| Partial college | 14 (24%) |
| College graduate | 17 (29%) |
| Graduate school degree | 16 (27%) |
| Currently a student | 7 (12%) |
| Race/Ethnicity | |
| Non-Hispanic/Latino | |
| African-American | 24 (41%) |
| White | 18 (31%) |
| Other/more than one | 6 (10%) |
| Hispanic/Latino | |
| African-American | 4 (7%) |
| White | 5 (8%) |
| Other/more than one | 2 (3%) |
| Gender identity | |
| Man | 56 (95%) |
| Woman | 1 (2%) |
| Transgender | 2 (3%) |
| Sexual identity | |
| Gay/homosexual | 43 (73%) |
| Bisexual | 13 (22%) |
| Straight/heterosexual | 1 (2%) |
| Other | 2 (3%) |
| Employment | |
| Full-time | 24 (41%) |
| Part-time | 21 (36%) |
| Not working | 14 (24%) |
| Lifetime history of STIs | |
| Any STI | 28 (48%) |
| Syphilis | 9 (15%) |
| Gonorrhea | 16 (27%) |
| Chlamydia | 14 (24%) |
| Hepatitis B | 1 (2%) |
| Hepatitis C | 0 (0%) |
| Ever used PrEP | 26 (48%) |
| Ever used in-home HIV test | 28 (48%) |
Ns may not sum to 59 due to missing data. Percents are of those with non-missing data.
SMARTtest App Components
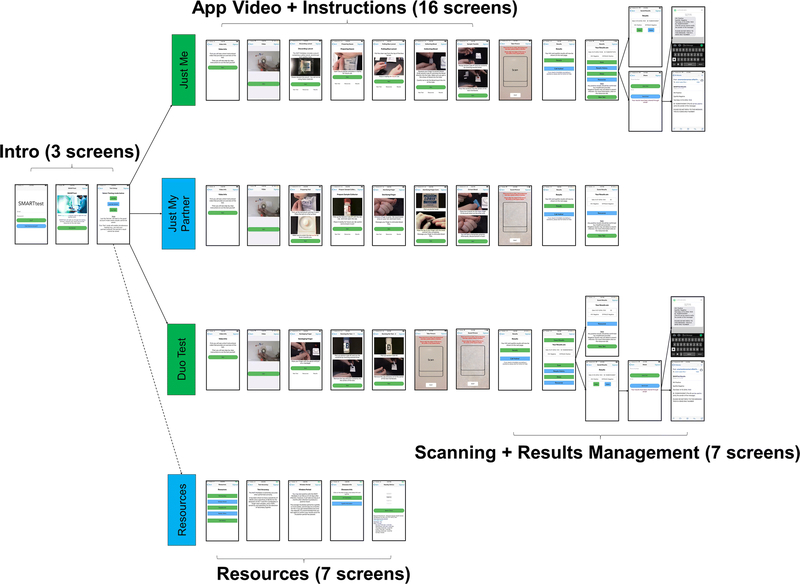
Existing SMARTtest app components were revised and new components developed based on feedback and recommendations from the IDIs, focus group discussions, and feedback from the mini-pilot. Table 2 presents examples of the qualitative findings that guided the development of the app. Below we summarize the feedback received from participants during the IDIs, how it was incorporated into the final version of the SMARTtest app, and feedback received during the mini-pilot (Table 3). Figure 1 presents an overview of the structure of the app (the total number of screens for each component is included). In subsequent figures, we provide more detailed screenshots of each app component: user mode, instructions, results management, saving and sending results, resources and linkage to care.
Table 2.
Participant Recommendations for SMARTtest App Components
| A. User Mode |
| • There should be an option if you’re testing yourself or someone else, and then once someone else is -- once the whole test is complete, I won’t see the results on my phone. It’ll be automatically texted or e-mailed to them and then once I’m done all their info should be wiped away. (PTID149) |
| • Definitely seems like there should be some kind of guest mode so that it’s clear this is not data for the primary user. And once that data gets sent along to the secondary user, that it is gone from your own phone so that you aren’t carrying someone else’s data around.(PTID115) |
| • If you want your result, then you need to download the app and buy the device. I can test you and we can see, but I can’t save it. (PTID126) |
| B. Instructions |
| • I thought it was fantastic. They were very simple. I like the video. I thought after the video you were just -- like that was it and you’re going to leave me in the forest and I was like, whoa, this is a lot for the first time. But the app instructions were great, because each step, you do the step and then you go on to the next one and it’s very straightforward. The images are clear. It’s easy. (PTID185) |
| • I got nervous during the test because I kept on bleeding, but I didn’t know if I could pause to put a gauze on my -- I got nervous that, oh my gosh, if I don’t put this in soon, I’m going to invalidate the result. So it might be nice if there were some instructions: OK, if you want to pause here and, you know, wipe your blood, or -- because I was, like, dripping and I was like, oh my God! (PTID109) |
| C. Results Display |
| • Maybe a little bit of a warning -- your results will be displayed in just a few moments. I guess, like, take a deep breath, or in the event that it’s positive, an 800-number will be displayed afterwards. Or -- (pause) yeah. I guess, maybe a recommendation. You know, like,“If this should come back positive, and this is your first time, it’s recommended to do the following: get a follow-up exam, contact your primary physician,” something to that effect. (PTID159) |
| • Well, when you say is there anything more, if it -- if the page comes up positive, I would always have right there, there is help. That you can get help. Because sometimes just leaving, and just looking at that and it’s just saying positive and just nothing else -- nothing to reinforce the fact that it's not a death sentence. (PTID150) |
| D. Results Management |
| D1. Saving Test Results |
| • I’ve been asked, ‘When’s the last date you’ve been tested?’ and sometimes I’m like, ‘You know, I know I got tested in December, I think it was around—’ and so to be able to pull up an app and go, ‘Yeah, I got tested December 4th, and that was the result.’ (PTID126) |
| • I think that would be great just to be like,“Look, here is the history of every month I’ve tested myself. Here’s the whole history of—they’re all negative, right?”… It’s keeping data. It’s like, I keep track of how many steps I take every day. (PTID115) |
| •The results should be sent straight to e—mail or text. We don’t see it…I should just know that the test is complete. All the info should be wiped out of my history, and then keep it moving.(PTID149) |
| • You don’t want to take a chance on letting anybody see what you don’t want them to see. So, that [delete] should definitely be an option, whether it’s used or not. (PTID124) |
| • Disadvantages of saving it is that, once it’s on your phone, anyone can break into your phone. Like, these phones are easily hacked, so you just don’t know who’s going to hack into your phone, or who’s going to see those results, or who can be nosy, and you leave your phone on the bed, and you’re with a partner, or you’re going to be sexual with this person, and this person decides to go through your phone. (PTID192) |
| D2. Sharing Results with Sexual Partners |
| • It’s almost like an ID card. You need to know my status? Here’s proof, this is my status. (PTID153) |
| • Some gay men are like“I need lab results from you before we even go on a date because I’m not going to be with somebody who’s HIV-positive, so I need to know that you’re completely STD-free”. So being able to take the test at home and being able to then send them to people through some kind of authenticated system, that would be advantageous. (PTID109) |
| • You can throw it in the cloud someplace and be able to grab it afterwards. … Just so no one can get into it. Just so I’d be the only one. It’d have to be some type of passcode or something like that, that I can go in and retrieve it afterwards… no name, just have a number on it (#174) |
| • I love that notion of putting it on the [hookup] apps because they’re already advancing with like, statuses and what do those statuses mean.” (PTID141) |
| • If it could link to hook-up apps, like directly, like get your status out there and maybe—instead of a person’s claimed status, it could be like a verified status. (PTID123) |
| • So kind of like on social media where you have a share button and often with different options of what to share through, same thing. Something that will just get a nice little picture of me, my ID, my status, so that I can use as proof that say I’m negative and be able to text it, email it, WhatsApp it, Facebook, Messenger it, whatever. (PTID132) |
| • You should never have the results on a dating app. I mean, there’s already dating apps for HIV-positive men. Like, that’s some really personal stuff, not just personal, but, like, I don’t know that it should be as simplified or as conversational as that. (PTID204) |
| • Now I have his negative result on my phone, and I take that to the next person.“Hey, I’m negative.” You know, there’s no way … that doesn’t mean shit. That could be anybody’s. It could be your friend sent it to you, so I think there should be some sort of identifying. (PTID122) |
| • How do I know those are your results? How do I know? But you’ll never know unless you’re actually standing there with someone when they take the test. … So there’s no way for you to know. So I don’t think that’s an issue that we could do anything about. (PTID126) |
| • That seems very fundamental. Like I said, I should be able to send a text message to someone that was like,“Hey, yo! You should go get tested!” Right? (PTID115) |
| D3. Sharing results with physicians |
| • Well, if it tested positive, I would definitely send it to my doctor. And then I’ll delete them from my phone. (PTID185) |
| • If it’s all negative, then you would have a share button,“share on Grindr or GROWlr,” or, if it’s positive,“share with your physician,” doctor, or whoever. While setting up the app with your username, your email, everything, maybe there's a suggestion that you connect your physician to the app, or a hospital or whatever. (PTID122) |
| • I actually suggested incorporating it with apps like MyChart or Helo. They’re communication apps that you can use for your test results and to communicate with a doctor in a secure environment and check your appointments and lab results and stuff like that. So you could use that to transmit it to the doctor directly. (PTID151) |
| • I think it depends on the results. I think if they’re negative, then it’s kind of nice to have to share with people (partners, doctor), just like a confirmation. If they’re positive, then that’s a little harder to swallow, but probably want to share it with a medical provider, your doctor. (PTID165) |
| E. Information |
| E1. About the test |
| • I would want to know what the window is between HIV infection and when it can be detected on the blood test… if there’s a guy who is more sexually active, and he’s had a lot of unprotected sex in the past 90 days, it might make me feel a little bit more reluctant because maybe he’s picked up something that the test can’t pick up on yet. (PTID109) |
| • What the window period actually is and means. Because if you test this date, it really should say well this only covers up until three months ago. (PTID124) |
| E2. About HIV and syphilis |
| • What to look for: Signs, symptoms, and how to protect oneself as far as risky behavior. The things that you don’t want to do, unknowingly. Same thing with HIV. Just basic stuff. Nothing too -- you know, pre- and post-prophylactic stuff like that. Seroconversion, just basic stuff. (PTID125) |
| • On the app I think there should be links. You don’t want to put too much stuff on apps because then people are like“Yeah, yeah, yeah. Too much.” But you want to put links to the CDC site. This is HIV and this is what the test means. To the New York City Department of Health site about syphilis because they would probably say, you can go to these clinics. (PTID126) |
| • I would keep it to basic facts about HIV and syphilis, but focus on referrals and next steps rather than trying to be a major resource within the app. I wouldn’t want someone to think that the app had all the answers, so the fewer answers it has the less risk there is of that and the more likely for someone to go and speak with a medical professional if they get a positive result. (PTID137) |
| • Just the information on syphilis itself and how you get it, how long it stays in your system, stuff like that. I think most people is more in tune with HIV and how you get it and how it lays there, so definitely the information about syphilis is important. (PTID174) |
| E.3 Linkage to Care |
| • Probably a link, or like a numbers to call. I guess just to give people a safe place to go and speak to the doctor. Probably places that -- with low cost or no cost at all. Probably a page there for people whose -- in the LGBT community, because I know this is not only for just people who are gay, but straight people as well can use this, and I guess like -- because sometimes, some people don’t like going to regular clinics. (PTID103) |
| • I would say mainly department of health they are the ones that do the free services. So knowing any free clinics and/or CBOs that provide STI screenings and/or treatment. (PTID135) |
| • Since it’s smart phone-enabled it’s definitely going to have GPS, so it can recognize the clinics that are sort of near you. Then it can show you the clinics that are nearby, the hours of operation” and it can probably be linking all the websites of each clinic. (PTID160) |
| • Besides nearest doctors and testing locations, being basic bare bones would be much better. Resources are always good, but sometimes, people have their own resources. (PTID165) |
| • I’m a gay man living in Chelsea, New York. I have a gay doctor. My friends are all gay. Many of my gay friends are doctors. I have no trouble seeking good medical care. (PTID147) |
| • I would say, primary doctors, because a clinic is a clinic. So, you can just go by the clinic. But maybe a list of resources with doctors who specifically have knowledge of how to deal with someone who’s HIV positive. I will go to a doctor that only knows about hormones, you know? I’m not going to go to a doctor that doesn’t know nothing about hormones. So, a doctor like that, like, ah, OK, these are doctors that are special -- a specialist in HIV care. (PTID192) |
Table 3.
Phase 1B App Feedback and Recommendations
| General Impressions of the App |
| • Going through the testing process with the help of the app was user-friendly and cool since I have this thing no one else has. (PTID 132) |
| • The app was an interesting thing to use; anticipatedpartners to react negatively to using the app with the test but was surprised by how nicely they reacted although they were nervous about the finger prick. My partners went from ‘happy ’ to ‘very happy’ to ‘excited’” [about the testing process]. (PTID 165) |
| • Everything worked as it should have. Didn’t have any challenges using the app. Having the test and the app allowed to determine if sex was safer. (PTID 1021) |
| • The app was great and very easy to use. (PTID 1022) |
| User Mode |
| • What we did was do it together in unison. We went through the video, we both did the swipe and lets both do the blood drop. (PTID 1006) |
| • I thought the “duo test” option worked wellfor doing simultaneous tests. (PTID 165) |
| Instructions |
| • I had a root canal that morning and wasn ’t thinking clearly and I wanted to review it. It opened very easy. It was very easy to follow and understand. Actually, I was going to watch it and then do everything, but I realized it goes at a slow enough pace that I could get everything opened up and prepared as it was talking so I thought the video was pretty good. (PTID 1002) |
| • Would have the instructions speak the instructions over. Should include more details in the video to help with the finger-prickprocess. It got cumbersome to get the drop into the vial. (PTID 1021) |
| • The video guided me through (the test) very easily. [The test] seemed simple when I was here (study office) but realized there were a lot of steps. If I didn ’t have the app, I would not have been able to do the test. (PTID 1022) |
| Results Management |
| • I do not think it ’s necessary to scan the results. I don’t think it ’s a heavy lift to askpeople to look at dots and then to interpret dots, and as I was processing the false positive, “well was it my camera? Was it gunk?” so I’d rather just look at it. (PTID 1006) |
| • I was skeptical about the scanning but was glad it worked well and the results were accurate. You should add a flash feature to the scanning component to help eliminate shadows cast by the user’s phone. (PTID 165) |
| • The app should have a disclaimer after scanning about the potential for receiving false positive results. (PTID 1004) |
| • Results sharing should continue to be at the discretion of the user, since some people may not be willing to share results. (PTID 132) |
| Resources and Information |
| • I tried to read the results in the piece of paper that came with the tests and I found they were really hard to follow but then when I opened up the resources tab—and I expected the resources to be a list of doctors or whatever. I was surprised to see the result reading (interpretation) portion was in the resources. It just seemed to be an oddplace to put it (PTID 1002) |
| • I appreciated having a results interpretation page on the app. It was easy to interpret the resultthat way and it was nice to reassure my partner that his results were in fact negative. (PTID 1006) |
| • Thought the information in the resources tab was comprehensive. (PTID 1004) |
| • App should include more information about what happens if you test positive. (PTID 1003) |
| • If someone tests positive, then the app should automatically display linkage-to-care information after the test results are shown. (PTID 1003) |
| • Partners looked at the resources tab and took pictures of the list of clinics. (PTID 165) |
Figure 1.
SMARTtest App Overall Structure
User Mode
Study participants recognized that testing encounters might differ depending on the individual(s) being tested (i.e., self, partner, both), which had implications across content areas of the SMARTtest app in relation to duplication of processes and privacy. For example, participants expressed discomfort with users being able to save partners’ test results on their phone. As such, participants wanted different app modes depending on who was being tested to streamline the testing process. As such, after signing in to the SMARTtest app, users can select who will undergo HIV/syphilis testing on each occasion: Just Me, Just my Partner, Duo Test. All approaches contain the same sequence of content (instructions, results scanning, results page, results management, and linkage to care), but with important differences. Whereas the “Just Me” user can save or share results with others, these options are not available in the “Just my Partner” mode. The “Duo Test” mode is designed so both the user and a partner can watch the instructions together on a single smartphone and, at the appropriate time, use that same phone to scan and receive test results individually for each test. This reduces the time it takes for both partners to test simultaneously, by eliminating the need to run through all the procedures again for the second test. As with the “Just my Partner” mode, partner results cannot be saved or forwarded to others.
Instructions
Having seen both video and step-by-step instructions in the prototype app, participants strongly favored retaining both types of instructions in the revised app. Many felt the video provided a comprehensive overview of the process, although it was too fast to pace a self-test alongside the video. In comparison, the step-by-step instructions allowed the user to proceed at their own pace. Participants recommended adding additional details regarding the testing process, such as when to bandage their finger, to the instructions.
Observations of participants self-testing during the study visit highlighted key issues to address in the instructions, including 1) incorporating clearer instructions to first watch the video completely and begin self-testing only during the step-by-step instructions; and 2) preparing the finger to obtain a blood sample, including massaging it beforehand, placement of the lancet, and appropriate time to bandage the finger to prevent bloodying of test materials. Participants also recommended increasing the font size for clarity, and an audio narration of the step-by-step instructions. Lastly, it was recommended that the app allow instructions to be skipped or repeated based on the user’s familiarity with the test.
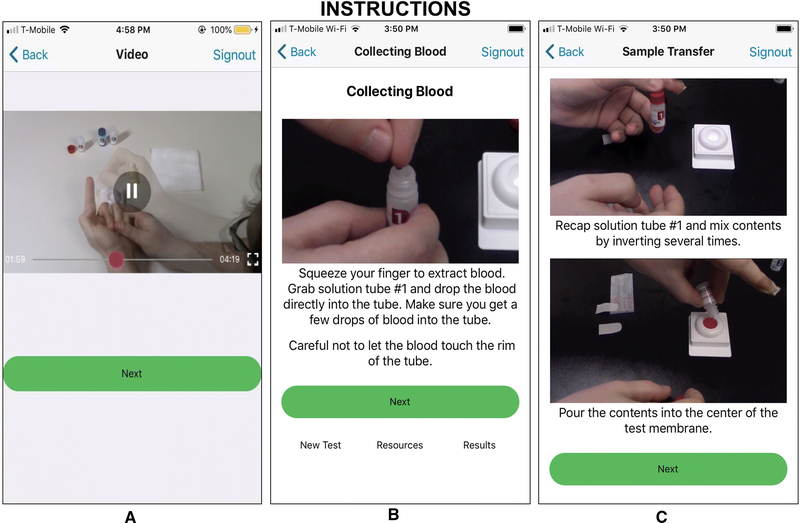
Figure 2 displays the Instructions component of the SMARTtest app, retaining the video and step-by-step instructions and incorporating key details regarding finger-prick preparation (e.g., massaging the finger, pushing the lancet firmly against the tip of the finger, etc.) and bandaging of the finger.
Figure 2.
Test Use Instructions Screenshots
Test Results Display
Having seen the test results in the prototype app that accompanied use of the INSTI and Syphilis Health Check, which displayed HIV and syphilis tests results as “positive” or “negative,” all participants preferred textual test results to those of existing rapid tests that required interpretation (e.g. deciphering lines or dots). However, some participants recommended that an app screen alert the user that the next page will show test results. About half the participants also recommended including information on the results page about what the results mean, next steps, and support.
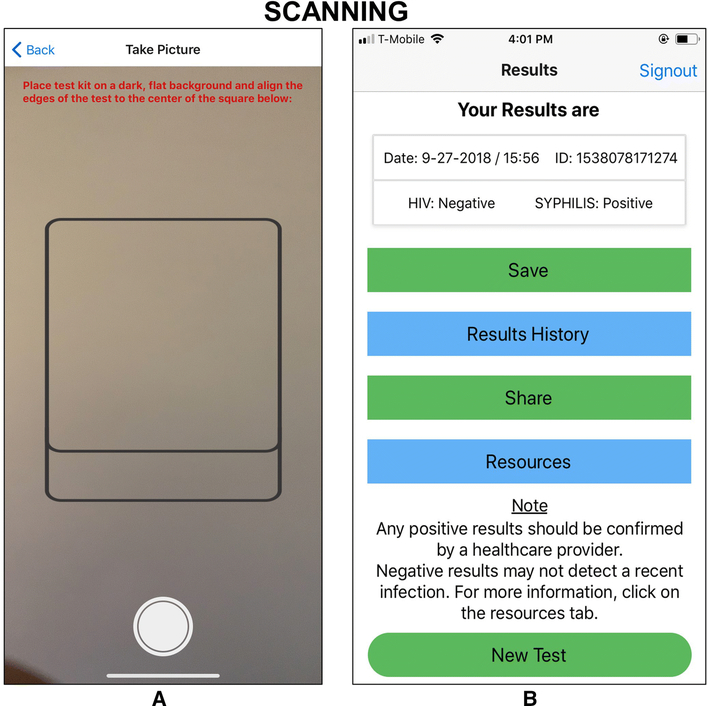
Figure 3 shows the SMARTtest app screens for scanning the INSTI Multiplex results and the textual presentation of test results. Once the INSTI test results emerge, the user places their smartphone over the test, ensuring that the image of the test completely fills in the outline on the scanning screen. Then, the user clicks on the SNAP button for the image to be captured. The image is then sent to the cloud-based server for image processing and the test results are sent back to the phone to provide the user with their results in words (“positive” or “negative”) instead of the blue dots on the INSTI Multiplex test. This feature removes subjective user interpretation of the test kit to determine if a particular test is positive, negative, or invalid for HIV and/or syphilis. Prior to seeing their test results, the user sees a screen notifying them that the next screen will show their results. As seen in Figure 3, the results screen shows the date of the test and separate results of their HIV and syphilis test. This screen includes a reminder that positive results need to be confirmed and that negative results may not be detecting a recent infection, with a referral to the Resources tab for more information (also provided to Guest users). Lastly, it also provides tabs for the user to save or send results, access their testing history, and access information and referral resources.
Figure 3.
Results Scanning Screenshots
Results management
We assessed participant attitudes toward saving and sharing test results, first in CASI surveys and then in IDIs.
Saving Test Results
The majority of participants wanted to save test results within the app or phone, primarily to show partners or track their own testing histories. Some imagined the convenience of a ubiquitous smartphone to simply, quickly, and directly show someone else their results, or to maintain an accessible history of their HIV and syphilis tests on their devices. Some participants, however, expressed reticence to save test results to their phone due to privacy concerns. As such, we incorporated a secure and private data management server to store and manage test results instead of allowing results to be saved locally on the phone. Most participants also felt uncomfortable with the idea of saving a partner’s test result on their phone, although a few favored being able to send a partner’s own test results to that partner.
Sharing Results
Sharing results with sexual partners
A majority of participants indicated they would send their own results directly to sexual partners while a minority reported interest in posting their results to sexual networking sites and apps; a few also spoke of wanting to post results to social networking apps. Their enthusiasm seemed aligned with trends in online communication, but also hinged on the desire to keep profiles up-to-date with some kind of proof of their own or other people’s most recent test results. However, there was also strong reticence against sharing any results online. These participants referenced concerns about privacy and the authenticity of online information. Some shared suspicions that test result information might be forged and wished for a safeguard to ensure the latest and most accurate test result of a particular person.
Sharing results with physicians
A majority of participants intended to share results with their physicians. Some considered automating the process of forwarding results straight to their doctor. Further, the method of communication also seemed important. Although some participants thought that sending positive results by email was acceptable, others suggested greater security by integrating results into HIPAA-secure apps, like MyChart or Helo, which communicate directly with primary care providers.
Thus, in summary, respondents voiced considerable interest in communicating test results with sexual partners and health care providers. However, it was also clear that participants wanted options regarding saving, sharing, and deleting results in order to make individual choices based on the test results, the potential recipient of the results, and their privacy concerns. Figure 4 shows how these options were incorporated into the Results Management section of the SMARTtest app.
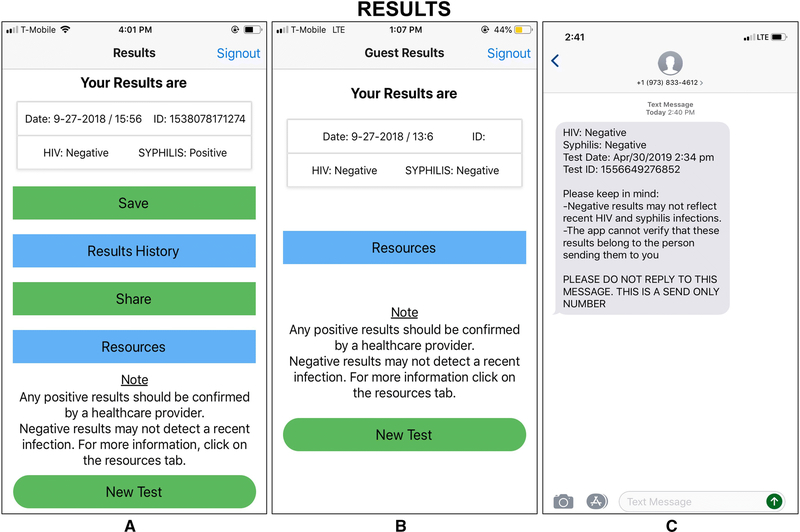
Figure 4.
Results Management Screenshots
As seen in the figure, the SMARTtest app allows saving, sharing, and deleting of self-test results. Nonetheless, partner-test results are only available and displayed during the testing process and are unable to be saved or shared to provide greater privacy to partners. Buttons on the lower half of the test results screen allow the self-test user to save or share the results. Clicking on the “Save” results button adds those results to the list of test results, which can be accessed through the “Results History” button on the same screen. Clicking on the “Share” button allows the anonymized result (or any result selected from the saved list) to be sent via email or text message from the server where results are housed to the email address or phone number entered by the user. Recommendations by few participants, such as the ability to post to social media or integrate with existing electronic medical records, were omitted in this version of the app.
Resources
Participants favored succinct information about HIV and Syphilis that focused on symptoms, modes of transmission, and how to prevent infection. Information about syphilis was considered critical because they perceived it to be less disseminated than information about HIV. A few participants also mentioned including information about the INSTI Multiplex, such as test reliability and window period. In general, participants favored information that could be accessed by the user, but was not mandatorily imposed on the user as part of the testing experience.
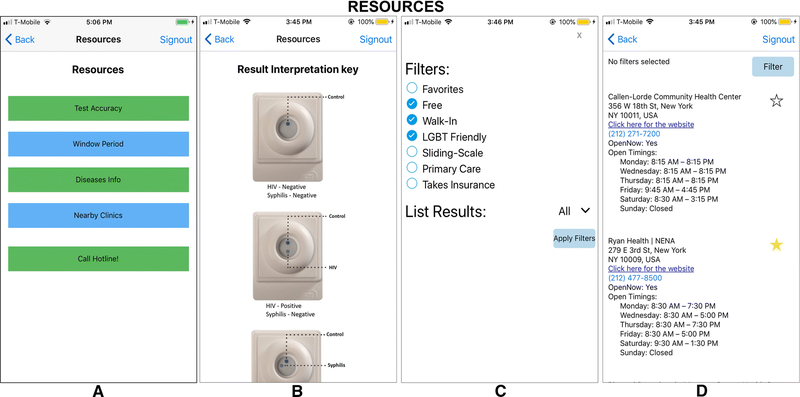
Figure 5 shows the Informational Resources page on the SMARTtest App, which is accessed through a tab at the bottom of the screen and available beginning on the third screen of the app. It can also be accessed through a tab on the page displaying test results. Tabs within the screen provide information on test accuracy and the window period. Links within the “HIV information” and “Syphilis Information” tabs lead the user to the corresponding websites at the Centers for Disease Control and Prevention where they can access updated information provided to the public. This app section also allows the user to contact an AIDS Hotline directly and find nearby clinics that can offer confirmatory testing and follow-up care (described below).
Figure 5.
Resources Screenshots
Linkage to Care
Almost every participant commented on the need for the SMARTtest app to facilitate linkage to care. Recommendations included providing users with next steps following positive test results and a way to identify and contact nearby organizations that offered confirmatory testing and follow-up care or to community-based organizations that could facilitate necessary referrals. The approach for providing this linkage varied significantly across participants. Some favored just a list of places they could go that included addresses, phone numbers, and costs/insurance information while others wanted geospatial mapping of nearby clinics. Discussion about benefits and drawbacks of the different approaches (i.e., ease of use of GPS, inability of GPS to specifically identify LGBT-friendly clinics, privacy issues regarding GPS capturing location data, etc.) during the Focus Groups expressed a preference for knowing which referral sites were specifically LGBT-friendly, versus a listing of all available resources.
As Figure 5 demonstrates, the app allows users to filter clinic searches by location (ZIP code or nearby) and by clinic attributes (i.e., Free, Walk-In), as well as select and save a “favorite” clinic to allow them to go directly to that saved resource. Search results opens a page with listings including address, contact information, website link, and hours of operation. Clicking on the listed phone number automatically places the call for the user.
Participant Feedback from Mini-Pilot
Table 3 presents feedback obtained from the nine participants in the one-month mini-pilot of the SMARTtest app. These individuals reported conducting nine self-tests and eleven partner tests. Overall, participants found the app useful and engaging. They also reported that the video and step-by-step instructions were comprehensive and easy to follow. Although many participants felt comfortable enough from their self-testing experience at the study visit to perform the INSTI without reviewing the app instructions, almost all viewed the video before using the test. All nine participants scanned their results with the app, but the accuracy of the scanned test interpretations varied. Some participants obtained invalid or incorrect results but were able to visually interpret their results correctly with the use of the “results interpretation” key that accompanied the INSTI or the one included in the app. Finally, participants re-iterated that saving and sharing results should remain at the discretion of the user as it was often easier to read the results visually (although this would not allow the participant to save or send results). Lastly, participants appeared content with the resources page, while some suggested more guidance in case of a positive result, some may not have received the existing guidance if they did not scan the results as the app provides such guidance in the event of a positive result.
Feedback from the mini-pilot resulted in changes to the app. Most importantly was a change to the scanning processing software to create greater contrast between the INSTI background membrane and test results so that the photo image could more accurately read the results. Small changes were also made to the instructions to address challenges in blood collection. These changes were incorporated into the version of the app presented in this paper. Further changes may come based on feedback from participants in the current pilot study (N=50) who will use the current version of the app for self- and partner testing over a period of three months.
DISCUSSION
Findings from this study show that SMARTtest, a user-informed smartphone app to facilitate the use of the INSTI Multiplex for self- and partner-testing has the potential to address ongoing concerns about the use of HIV self-tests, such as correct use, correct reading of results, and linkage to care. Overcoming these concerns is critical to increasing rates of HIV among users and their sexual partners, which is a key component of attaining the goal of Ending the Epidemic. Questions and issues raised by participants during IDIs highlighted that, to be useful, the app had to provide more than instructions on how to use the test and instead had to comprehensively facilitate all aspects of the testing process, especially guidance in case of reactive results. Users also favored components for testing partners that would minimize duplication of steps (i.e., the Duo mode) and provide partners with privacy of their test result (i.e., not allowing those to be saved). These features may facilitate partner testing, given its acceptability among MSM as well as heterosexual couples [13, 39,40]. However, it was also clear that participants did not want to overburden the app with information that could be obtained through other sources or that were not directly related to the testing experience (i.e, general sexual health, PrEP, PEP).
We were struck by the enthusiasm for saving and sharing results. The ability to save results to show or send to sexual partners would allow users to avoid having to repeatedly test with each new partner, which, depending on their sexual practices, might be as frequent as multiple times per week in the absence of such a record [13]. Participants appreciated that, rather than relying on self-report, the SMARTtest app could send partners an “official” statement of their HIV and syphilis status that was derived from a scanned test result and sent by the app’s dedicated and secure system. Yet, they also recognized that it would be possible for an individual to purposefully misrepresent their HIV and syphilis status by entering the self-test mode in the app to scan and save another person’s non-reactive test results to later use as their own. Users who recently test HIV or syphilis positive could also show partners a prior test result showing non-reactive results. Lastly, a user could also inadvertently send a false negative result, whether due to test error or because the test was conducted during the window period before antibodies can be detected. While these same situations can occur with clinic testing if an individual wants to misrepresent their HIV status, the system generated message might offer the recipient of a test report a false sense of security. However, alerting recipients of these caveats in the message they receive from the system will heighten their awareness of these limitations and allow them to make a more accurate assessment of the validity of the results. Prior study findings have shown that even after receiving non-reactive HIV rapid self-test results, individuals remain concerned about the window period and some opt to use condoms for subsequent anal intercourse [14]. Potentially, this screen can also include the links provided in the Resources tab to allow these recipients to easily find relevant information on the CDC website. While at this time it is not possible to overcome purposeful deceit in results or the window period for the test, participants in the study recognized these limitations and a significant majority found app-based sharing of results was acceptable, suggesting it could offer novel pathways to promote disclosure of HIV and syphilis status to sexual partners.
Surprisingly, there was relatively limited concerns about privacy expressed by the participants, especially with non-reactive results. While some participants expressed concerns about phone hacking, most were comfortable with non-reactive results being saved on their phones, though all supported password protection as a secured gateway into the app. The few participants who wanted to be able to post results on social media sites, such as Facebook, spoke of wanting to share good news that they had gone for an HIV test and received non-reactive results, highlighting the potential for such posts to de-stigmatize a proactive approach to HIV testing and inspire others to do the same. Predictably, there were greater privacy concerns about reactive results, and most participants spoke of deleting those results immediately.
The SMARTtest app has been designed to facilitate the use of self- and partner-testing, accurate reading of test results, disclosure of HIV and syphilis status, and linkage to care when needed, which have been identified as concerns by potential users of HIV self-tests. By supporting users through the testing process, we hope to facilitate frequent HIV and syphilis self-testing in accordance with CDC recommendations [29]. Furthermore, by actively incorporating partner-testing into the app functions, the app may encourage users to regularly test partners, which has been shown to decrease sexual risk behavior when partners test HIV positive [13], and at times, even when the results are non-reactive, as individuals assess remaining risk due to window period limitations of the test [14] and their overall risk awareness increases [38]. Key to the success of the SMARTtest app in facilitating self- and partner-testing will be its utility in the context where these tests may be conducted, where factors such as dim lighting, use in imperfect settings (i.e., cars, etc), recent or concomitant substance use, and sexual excitement may affect the use of the app as well as the INSTI Multiplex for self- and partner testing. To this end, the SMARTtest app and the INSTI Multiplex are now being piloted in a study with 50 participants who are provided access to the app and given 10 INSTI Multiplex kits to use for self- and partner-testing over the course of three months who will then return for a quantitative survey and an IDI about their experiences using the app and the INSTI Multiplex in this context.
Findings from this study have provided valuable insights that informed the design of the SMARTtest app. However, it is important to note limitations to this study. First, the app has been designed based on the needs of these particular participants, who are not monogamous, regularly engage in condomless anal intercourse in the absence of PrEP use and may decide to frequently use HIV and syphilis self- and partner-testing as a risk reduction approach. Other user groups may have recommended other components or content. Second, these are self-reported data on potential use, not actual use, and intention to use a product does not always result in actual adoption.
While potential users might vary in the specific components of the SMARTtest app they use regularly, we believe that the app will be useful for a broad range of MSM and TGW who wish to use the INSTI Multiplex to self- or partner-test. Furthermore, knowledge gained from this study about the app components desired by potential users of HIV self-tests can inform the adaptation of the app for use with other tests, such as the OraQuick® In Home HIV Test or other STI self-tests, where changes may be made to portions of the content as well as to the algorithm developed for scanning of results, yet other components such as the test results management or linkage-to-care are retained as is.
ACKNOWLEDGMENTS
This research was supported by grants from the U.S. National Institutes of Health: R01-HD088156 (PI: I. Balán) and P30-MH43520 (PI: R. Remien). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.World Health Organization. (2016). WHO recommends HIV self-testing. Retrieved December 10, 2017 from http://www.who.int/hiv/pub/vct/who-recommends-hiv-self-testing/en/
- 2.Centers for Disease Control. (2017). HIV/AIDS Special Studies and Diagnostics Team. Retrieved December 10, 2017 from https://www.cdc.gov/hiv/dhap/bcsb/ssdt/index.html
- 3.UNAIDS. (2017). WHO, UNAIDS statement on HIV testing services: New opportunities and ongoing challenges. Retrieved December 10, 2017 from http://www.unaids.org/sites/default/files/media_asset/2017_WHO-UNAIDS_statement_HIV-testing-services_en.pdf
- 4.Pai NP, Sharma J, Shivkumar S, Pillay S, Vadnais C, Joseph L, …Peeling RW (2013). Supervised and unsupervised self-testing for HIV in high-and low-risk populations: a systematic review. PLoS Med, 10(4), e1001414 PMCID: PMC3614510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause J, Subklew-Sehume F, Kenyon C, & Colebunders R (2013). Acceptability of HIV self-testing: a systematic literature review. BMC public health, 13(1), 735 PMCID: PMC3750621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilardi JE, Walker S, Read T, Prestage G, Chen MY, Guy R, Bradshaw C, & Fairley CK (2013). Gay and bisexual men’s views on rapid self-testing for HIV. AIDS and Behavior, 17(6), 2093–2099. [DOI] [PubMed] [Google Scholar]
- 7.Figueroa C, Johnson C, Verster A, & Baggaley R (2015). Attitudes and Acceptability on HIV Self-testing Among Key Populations: A Literature Review. AIDS and behavior. Epub ahead of publication. DOI 10.1007/s10461-015-1097-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher M, Wayal S, Smith H, Llewellyn C, Alexander S, Ison C,…Brighton Home Sampling Kit Project Steering Group. (2015). Home sampling for sexually transmitted infections and HIV in men who have sex with men: A prospective observational study. PLoS One. DOI: 10.1371/journal.pone.0120810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belza MJ, Rosales-Statkus ME, Hoyos J, Segura P, Ferreras E, Sánchez R,…the Madrid Rapid HIV Testing Group. (2012). Supervised blood-based self-sample collection and rapid test performance: a valuable alternative to the use of saliva by HIV testing programmes with no medical or nursing staff. Sexually transmitted infections, Epub prior to publication. doi: 10.1136/sextrans-2011-050131. [DOI] [PubMed] [Google Scholar]
- 10.Lippman SA, Moran ME, Ventura A, Castillo LS, Buchbinder S, Treves-Kagan S, & Sevelius J Home HIV testing among transgender women in San Francisco: A pilot feasability and acceptability study. (2015, July). Poster presented at the 8th International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention Vancouver, Canada. [Google Scholar]
- 11.Carballo-Dieguez A, Frasca T, Dolezal C, & Balán I (2012). Will gay and bisexually active men at high risk of infection use over-the-counter rapid HIV tests to screen sexual partners? Journal of Sex Research, 49(4), 379–387. PMCID: PMC3600862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma A, Chavez PR, MacGowan RJ, McNaghten AD, Mustanski B, Gravens L, … & Sullivan PS (2017). Willingness to distribute free rapid home HIV test kits and to test with social or sexual network associates among men who have sex with men in the United States. AIDS care, 29(12), 1499–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carballo-Dieguez A, Frasca T, Balán I, Ibitoye M, & Dolezal C (2012). Use of a rapid HIV home test prevents HIV exposure in a high risk sample of men who have sex with men. AIDS & Behavior, 16(7), 1753–1760. PMCID: PMC3458207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balán IC, Carballo-Diéguez A, Frasca T, Dolezal C, & Ibitoye M (2014). The impact of rapid HIV home test use with sexual partners on subsequent sexual behavior among men who have sex with men. AIDS and Behavior, 18(2), 254–262. PMCID: PMC3815512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.New York City Department of Health and Mental Hygiene. (2013, updated 2015). New York City HIV/AIDS Surveillance Slide Sets. Retrieved from: http://www.nyc.gov/html/doh/html/data/epi-surveillance.shtml
- 16.Centers for Disease Control and Prevention. (2015, April 15). HIV among Transgender people. Retrieved from: http://www.cdc.gov/hiv/group/gender/transgender/index.html
- 17.Center for Disease Control and Prevention. Primary and Secondary Syphilis-united State ‒ 2005–2013.(2014). MMWR Morbidity and Mortality Weekly Report, 63(18), 402–406. [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. (2014, Dec 17). STDs in Men Who Have Sex with Men. Retrieved from: http://www.cdc.gov/std/stats13/msm.htm
- 19.Figueroa C, Johnson C, Verster A, & Baggaley R (2015). Attitudes and acceptability on HIV self-testing among key populations: a literature review. AIDS and behavior, 19(11), 1949–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause J, Subklew-Sehume F, Kenyon C, & Colebunders R (2013). Acceptability of HIV self-testing: a systematic literature review. BMC public health, 13(1), 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balán I, Frasca T, Ibitoye M, Dolezal C, & Carballo-Diéguez A (2017). Fingerprick versus oral swab: acceptability of blood-based testing increases if other STIs can be detected. AIDS and Behavior, 21(2), 501–504. PMCID: PMC5250595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Figueroa C, Johnson C, Ford N, Sands A, Dalal S, Meurant R, … & Baggaley R (2018). Reliability of HIV rapid diagnostic tests for self-testing compared with testing by health-care workers: a systematic review and meta-analysis. The lancet HIV, 5(6), e277–e290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaspard M, Le Moal G, Saberan-Roncato M, Plainchamp D, Langlois A, Camps P, … Prazuck T (2014). Finger-stick whole blood HIV-1/−2 home-use tests are more sensitive than oral fluid-based in-home HIV tests. PloS one, 9(6), e101148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Payne HE, Lister C, West JH, & Bernhardt JM (2015). Behavioral functionality of mobile apps in health interventions: a systematic review of the literature. JMIR mHealth and uHealth, 3(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donker T, Petrie K, Proudfoot J, Clarke J, Birch MR, & Christensen H (2013). Smartphones for smarter delivery of mental health programs: a systematic review. Journal of medical Internet research, 15(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang ETY, Williams H, Hocking JS, & Lim MS (2016). Safe sex messages within dating and entertainment smartphone apps: a review. JMIR mHealth and uHealth, 4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swendeman D, Ramanathan N, Baetscher L, Medich M, Scheffler A, Comulada WS, & Estrin D (2015). Smartphone self-monitoring to support self-management among people living with HIV: Perceived benefits and theory of change from a mixed-methods, randomized pilot study. Journal of acquired immune deficiency syndromes, 69(1), S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amyai N, Darling KEA, D’acremont V, Castro E, Ebert S, Diserens MM, … & Cavassini M (2018). A prospective multicentre study of healthcare provider preference in rapid HIV testing kits: Determine versus INSTI. International journal of STD & AIDS, 29(1), 51–56. [DOI] [PubMed] [Google Scholar]
- 29.Bwana P, & Mwau M (2018). Performance and usability evaluation of the INSTI HIV self-test in Kenya for qualitative detection of antibodies to HIV. PloS one, 13(9), e0202491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carballo-Dieguez A, Giguere R, Balan IC, Dolezal C, Brown W, Lopez-Rios J,… Febo I (2019). Few Aggressive or Violent Incidents are Associated with the Use of HIV Self-Tests to Screen Sexual Partners among Key Populations. JAIDS. In Press. [DOI] [PMC free article] [PubMed]
- 31.Laksanasopin T, Guo TW, Nayak S, Sridhara AA, Xie S, Olowookere OO,…Sia SK (2015). A smartphone dongle for diagnosis of infectious diseases at the point of care. Science translational medicine, 7(273), 273re1–273re1. [DOI] [PubMed] [Google Scholar]
- 32.Lank E, Withee K, Schile L, & Parker T (2006). User centered rapid application development In Rapid Integration of Software Engineering Techniques (pp. 34–49). Springer; Berlin Heidelberg. [Google Scholar]
- 33.Kumar S, & Sharma T (2014). User Centric Rapid Application Development. International Journal of Research, 1(10), 959–964. [Google Scholar]
- 34.Martin J (1992) Rapid Application Development. Prentice-Hall, Englewood Cliffs. [Google Scholar]
- 35.Årsand E, & Demiris G (2008). User-centered methods for designing patient-centric self-help tools. Informatics for Health and Social Care, 33(3), 158–169. [DOI] [PubMed] [Google Scholar]
- 36.Dabbs ADV, Myers BA, Mc Curry KR, Dunbar-Jacob J, Hawkins RP, Begey A, & Dew MA (2009). User-centered design and interactive health technologies for patients. Computers, informatics, nursing: CIN, 27(3), 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiNenno EA, Prejean J, Irwin K, Delaney KP, Bowles K, Martin T, …Lansky A (2017). Recommendations for HIV Screening of Gay, Bisexual, and Other Men Who Have Sex with Men — United States. MMWR Morbidity and Mortality Weekly Report, 66, 830–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frasca T, Balan I, Ibitoye M, Valladares J, Dolezal C, & Carballo-Diéguez A (2014). Attitude and behavior changes among gay and bisexual men after use of rapid home HIV tests to screen sexual partners. AIDS and Behavior, 18(5), 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Masters SH, Agot K, Obonyo B, Mavedzenge SN, Maman S, & Thirumurthy H (2016). Promoting partner testing and couples testing through secondary distribution of HIV self-tests: A randomized clinical trial. PLoS Medicine, 13(11), e1002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maman S, Murray KR, Mavedzenge SN, Oluoch L, Sijenje F, Agot K, & Thirumurthy H (2017). A qualitative study of secondary distribution of HIV self-test kits by female sex workers in Kenya. PloS One, 12(3), e0174629. [DOI] [PMC free article] [PubMed] [Google Scholar]