Dear Editor,
In the journal, a comprehensive review of COVID-19 was published to summarize the nature of SARS-CoV-2 and the timing of its clinical characteristics.1 Since the emerging infectious disease emerged in Wuhan, China, it has spread rapidly around the world.2 According to the latest epidemiological statistics, by March 20, 2020, the number of confirmed cases worldwide has exceeded 240,000, with a fatality rate of 4.1%. The course of COVID-19 illness can progress rapidly, causing acute respiratory distress syndrome, septic shock, metabolic acidosis and blood coagulation dysfunction.1 , 3 – 5 Due to a previous lack of in-depth research on the characteristics of the disease, the mortality of severe illness is high.4 It is very important to analyse the clinical characteristics of COVID-19 in international regions and identify risk factors to reduce the incidence of severe and critical illness in the early stage. In this letter, we present discrepancies of patients with different disease severities and risk factors for severe COVID-19 by comparing and analysing epidemiological and clinical data of 167 confirmed patients in Anhui, China.
In the present study, the rate of severely ill patients was as high as 17.9%. Comparisons of demographics and clinical characteristics between 30 severe and 137 non-severe patients are shown in Table 1 . The mean age was 49.03 years in severe patients and 40.83 years in non-severe patients, with a significant difference (p = 0.007. There were 95 males (56.89%) and 72 females (43.11%); however, there was no significant differences in sex between the two groups. Among 167 patients, 146 had fever (87.43%), 132 had cough (79.04%) and 61 had shortness of breath (36.53%). The prevalence of shortness of breath was 73.33% in severe patients, which was significantly greater than the 28.47% prevalence in non-severe patients (p < 0.001). There were 44 patients (26.35%) with comorbidities, of which 9 had multiple comorbidities (5.39%). Among patients with diabetes, severe cases were significantly more common than in non-severe patients (p < 0.001). Compared to non-severe patients, fingertip oxygen saturation decreased significantly in severe patients (p < 0.001), which predisposed patients with chronic obstructive pulmonary disease to acute exacerbation. Since SARS-CoV-2 affected multiple organs by binding angiotensin converting enzyme 2 (ACE2) receptor6 and many severe COVID-19 patients had comorbidities, multidisciplinary team (MDT) consultation played an important role in reducing the mortality of severe infection. There were significant differences in the use of mechanical ventilation, glucocorticoids and immunoglobulin between severe and non-severe patients (all p < 0.05).
Table 1.
Comparison of demographics and clinical characteristics between severe and non-severe patients with COVID-19.
| Variables | All patients (n = 167) |
Disease severity |
||
|---|---|---|---|---|
| Non-severe (n = 137) |
Severe (n = 30) |
P-value | ||
| Age, years | 42.31(15.29) | 40.83(15.47) | 49.03(12.60) | 0.007 |
| Mal sex, n (%) | 95(56.89%) | 75(54.74%) | 20(66.67%) | 0.232 |
| BMI, kg/m2 | 24.52(3.41) | 24.22(22.19,26.12) | 24.55(3.19) | 0.830 |
| Exposure history, n (%) | ||||
| History of cluster onset | 91(54.49%) | NA | NA | NA |
| Exposure to Wuhan | 78(46.71%) | 60(43.80%) | 18(60.00%) | 0.107 |
| Non-exposure to Wuhan | 12(7.19%) | 12(8.76%) | 0(0.00%) | 0.196 |
| Signs and symptoms at admission, n (%) | ||||
| Fingertip oxygen saturation (%) | 98.00(96.00,99.00) | 98.00(97.00,99.00) | 94.00(91.00,97.25) | 0.000 |
| Cough | 132(79.04%) | 106(77.37%) | 26(86.67%) | 0.257 |
| Fever | 146(87.43%) | 118(86.13%) | 28(93.33%) | 0.439 |
| Shortness of breath | 61(36.53%) | 39(28.47%) | 22(73.33%) | 0.000 |
| Sore throat | 25(14.97%) | 21(15.33%) | 4(13.33%) | 1.000 |
| Diarrhea | 56(33.53%) | 50(36.50%) | 6(20.00%) | 0.083 |
| Nausea and vomiting | 17(10.18%) | 16(11.68%) | 1(3.33%) | 0.300 |
| Multiple symptoms | 147(88.02%) | 119(86.86%) | 28(93.33%) | 0.497 |
| Comorbidity, n (%) | ||||
| Any | 44(26.35%) | 30(21.90%) | 14(46.67%) | 0.005 |
| Cardiovascular diseases | 24(14.37%) | 17(12.41%) | 7(23.33%) | 0.209 |
| Diabetes | 11(6.59%) | 4(2.92%) | 7(23.33%) | 0.000 |
| Digestive diseases | 9(5.39%) | 6(4.38%) | 3(10.00%) | 0.430 |
| Respiratory diseases | 4(2.40%) | 2(1.46%) | 2 (6.67%) | 0.148 |
| Central nervous system diseases | 2(1.20%) | 2(1.46%) | 0(0.00%) | 1.000 |
| Hematological diseases | 1(0.60%) | 0(0.00%) | 1(3.33%) | 0.180 |
| Immune diseases | 2(1.20%) | 2(1.46%) | 0(0.00%) | 1.000 |
| Treatment, n (%) | ||||
| Oxygen therapy | 133(79.64%) | 104(75.91%) | 29(96.67%) | 0.011 |
| Mechanical ventilation | 22(13.17%) | 2(1.46%) | 20(66.67%) | 0.000 |
| Invasive | 4(2.40%) | 0(0.00%) | 4(13.33%) | 0.001 |
| Non-invasive | 18(10.78%) | 2(1.46%) | 16(53.33%) | 0.000 |
| Antiviral treatment | 166(99.40%) | 137(100.00%) | 29(96.67%) | 0.180 |
| Glucocorticoids | 42(25.15%) | 20(14.60%) | 22(73.33%) | 0.000 |
| Immunoglobulin | 27(16.17%) | 9(6.57%) | 18(60.00%) | 0.000 |
| Length of stay in hospital | 15.00(12.00,20.00) | 15.00(12.00,20.00) | 17.06(4.98) | 0.260 |
Notes: Data are presented as number (%) or means (standard deviation) or median (interquartile range).
P values indicate differences between severe and non-severe patients.
Abbreviation: COVID-19, Coronavirus disease-19; BMI, Body Mass Index; NA, not applicable.
Table 2 presents comparisons of laboratory parameters between severe and non-severe patients. Lymphocyte, CD4 and CD8 cell counts were decreased significantly in severe patients compared to non-severe patients (p = 0.004, 0.021 and 0.002), suggesting that T lymphocytes were seriously destroyed. The increased level of c-reactive protein (CRP) in severe patients was significantly higher than that in non-severe patients (p = 0.001). Interleukin-6 (IL-6) levels increased in 122 patients (73.05%); the increase was more significant in severe patients than in non-severe (p = 0.001). By clearing or blocking inflammatory factors,7 artificial liver therapy and tocilizumab, a monoclonal antibody of IL-6 receptor, may prevent serious injuries in the lungs in severe patients. The lactate dehydrogenase (LDH) concentration was higher and the albumin concentration was lower in severe patients, with significant differences (p = 0.002 and p<0.001). In severe patients, the fibrinogen concentration was significantly higher (p = 0.008) than in non-severe patients, suggesting that severe patients were more likely to experience myocardial infarction or sudden death. Between the two groups, there was a significant difference in the neutrophil to lymphocyte ratio (NLR), a predictor for severe infection8 (p = 0.033). Other laboratory parameters that changed in COVID-19 patients were not significantly different between the two groups (all p > 0.05).
Table 2.
Comparison of laboratory parameters between severe and non-severe patients with COVID-19.
| Variables | All patients (n = 167) |
Disease severity |
||
|---|---|---|---|---|
| Non-severe (n = 137) |
Severe (n = 30) |
P-value | ||
| Blood routine | ||||
| Leucocytes (× 10⁹ /L) | 4.99(3.85,6.25) | 5.00(3.88,6.24) | 4.62(3.49,6.83) | 0.552 |
| normal | 132(79.04%) | |||
| Neutrophils (× 10⁹/L) | 3.39(2.32,4.46) | 3.43(2.39,4.40) | 3.93(2.23) | 0.812 |
| Increased | 14(8.38%) | 10(7.30%) | 4(13.33%) | 0.474 |
| Lymphocytes (× 10⁹/L) | 1.13(0.77,1.41) | 1.17(0.86,1.42) | 0.77(0.65,1.32) | 0.006 |
| Decreased | 77(46.11%) | 56(40.88%) | 21(70.00%) | 0.004 |
| Blood biochemistry | ||||
| Albumin(/µL) | 41.20(38.90,43.90) | 41.60(39.75,44.35) | 38.89(3.74) | 0.000 |
| Decreased | 57(34.13%) | 38(27.74%) | 19(63.33%) | 0.000 |
| ALT (U/L) | 23.00 (15.00,37.00) | 23.00(13.50,37.00) | 24.00(16.75,37.00) | 0.551 |
| AST (U/L) | 26.00(20.00,33.00) | 25.00(19.00,33.00) | 28.00(23.00,30.25) | 0.108 |
| LDH (U/L) | 233.00(201.00,281.00) | 232.00(197.50,266.50) | 291.20(84.83) | 0.002 |
| Increased | 66(39.52%) | 48(35.04%) | 18(60.00%) | 0.011 |
| Glucose (mmol/l) | 5.95(5.42,6.78) | 5.90(5.37,6.73) | 6.23(5.54,7.71) | 0.141 |
| Creatine kinase (U/L) | 65.00(44.00,88.00) | 65.00(45.50,88.00) | 65.00(43.75,91.00) | 0.796 |
| CK-MB (U/L) | 8.00(5.00,12.00) | 8.00(4.99,12.00) | 9.50(5.75,13.00) | 0.329 |
| Blood urea nitrogen (mmol/L) | 3.80(3.00,4.70) | 3.70(3.05,4.70) | 4.10(2.98,5.30) | 0.191 |
| Creatinine clearance(ml/min) | 64.91(16.52) | 64.01(16.43) | 74.50(53.50,80.00) | 0.135 |
| Decreased | 134(80.24%) | 112(81.75%) | 22(73.33%) | 0.294 |
| Coagulation function | ||||
| D-dimer (mg/L) | 0.28(0.20,0.53) | 0.26(0.19,0.51) | 0.35(0.23,0.58) | 0.106 |
| Fibrinogen (g/L) | 3.29(2.56,4.13) | 3.17(2.55,3.84) | 4.04(1.46) | 0.008 |
| Increased | 47(28.14%) | 31(22.63%) | 16(53.33%) | 0.001 |
| Other related biomarkers | ||||
| Procalcitonin (ng/mL) | 0.03(0.02,0.06) | 0.03(0.02,0.05) | 0.05(0.02,0.10) | 0.101 |
| Increased | 47(28.14%) | 33(24.09%) | 14(46.67%) | 0.013 |
| CRP (mg/L) | 12.40(2.70,30.60) | 9.00(2.35,24.45) | 30.35(8.75,74.05) | 0.001 |
| Increased | 107(64.07%) | 83(60.58%) | 24(80.00%) | 0.045 |
| IL-6(pg/ml) | 17.50(5.30,36.10) | 15.40(5.05,28.90) | 36.20(16.25,59.90) | 0.001 |
| Increased | 122(73.05%) | 96(70.07%) | 26(86.67%) | 0.064 |
| NLR | 3.12(1.93,4.83) | 3.05(1.86,4.43) | 4.77(3.44) | 0.033 |
| CD4 cell count(/µL) | 465.63(263.82) | 490.40(232.64) | 282.00(183.00,574.75) | 0.002 |
| Decreased | 74(44.31%) | 55(40.15%) | 19(63.33%) | 0.021 |
| CD8 cell count(/µL) | 291.00(190.00,425.00) | 316.00(234.50,452.25) | 191.00(135.75,326.50) | 0.002 |
| Decreased | 41(24.55%) | 27(19.71%) | 14(46.67%) | 0.002 |
| CD4/CD8 | 1.51(1.08,1.91) | 1.51(1.11,1.91) | 1.51(0.71) | 0.904 |
Notes: Data are presented as number (%) or means (standard deviation) or median (interquartile range).
P values indicate differences between severe and non-severe patients.
Abbreviation: COVID-19, Coronavirus disease-19; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; LDH, Lactic dehydrogenase; CRP, C-reactive protein; NLR, Neutrophil-to-Lymphocyte Ratio; CK-MB, creatine kinase isoenzyme-MB.
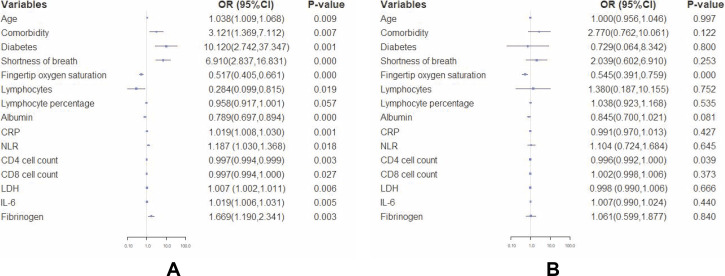
Fig. 1 A shows the risk factors for severe patients based on the results of mono-factor logistic regression analysis. The analysis demonstrated that increased age, existence of comorbidities, NLR, increased levels of CRP, LDH and IL-6, decreased fingertip oxygen saturation, decreased lymphocytes, and CD4 and CD8 cell counts were risk factors for severe infections (all p<0.05). The results of multi-factor logistic regression analysis then proved that fingertip oxygen saturation (OR=0.545, 95%CI: 0.391–0.759, p<0.001) and decreased CD4 cell count (OR=0.996, 95%CI: 0.992–1.000, p = 0.039) were independent risk factors, as shown in Fig. 1B.
Fig. 1.
Forest plots of hazard ratios (HRs) for severe COVID-19 illness.
A. Mono-factor logistic regression analysis was used to analyse risk factors for severe illness using R software; B. Multi-factor logistic regression analysis was used to analyse independent risk factors for severe illness using R software.
Notes: P<0.05 indicates a risk factor in Fig. 1A and an independent risk factor in Fig. 1B for severe patients.
There are still no specific therapies for COVID-191; nevertheless, assessing risk factors and symptomatic treatment in the early stage of the disease can improve the prognosis. Of 167 patients, 99.40% received initial antiviral therapy using lopinavir and ritonavir (400 mg/100 mg, bid) for no more than 10 days and/or atomized inhalation of interferon-α except for a 2-year-old patient, who was given interferon-α only, and 25.15% of patients received 40 mg methylprednisolone for 3 to 5 days. A total of 16.17% of patients were supported with immunoglobulins, and 13.18% of patients were managed with suitable oxygen therapy. All severe patients received MDT consultation, and three critical patients were treated with artificial liver therapy every other day, three times consecutively. Finally, all patients in our study, both severe or non-severe, improved and were discharged without death.
The clinical characteristics of 167 COVID-19 patients are summarized in Tables 1 and 2. The similarities and differences between severe and non-severe patients in this letter suggested that elderly patients with multiple comorbidities, hypoxia, decreased CD4 and CD8 cell counts and increased levels of CRP and IL-6 are all closely associated with disease severity and prognosis, which should be assessed seriously during diagnosis and treatment. A rapid decline in T lymphocytes and significant increases in the levels of inflammatory factors, including CRP and IL-6, can be clinical warnings of severe infection. MDT consultation and artificial liver therapy are very effective methods for severe patients with COVID-19. With these integrated prevention and treatment strategies, there will be a good prognosis for COVID-19.
Declaration of Competing Interest
The authors reported no conflicts of interest in this work.
Acknowledgments
Acknowledgments
We thank the medical workers fighting against COVID-19 for their hard work and sincere responses to our information requests.
Funding
This work was funded by the Key Technology Research and Development Program of Anhui Province (No: 1804h08020237).
References
- 1.Han Q., Lin Q., Jin S., You L. Coronavirus 2019-nCoV: a brief perspective from the front line. J Infect. 2020;80(4):373–377. doi: 10.1016/j.jinf.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogoch I.I., Watts A., Thomas-Bachli A., Huber C., Kraemer M.U.G., Khan K. Potential for global spread of a novel coronavirus from China. J Travel Med. 2020;27(2) doi: 10.1093/jtm/taaa011. taaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diagnosis and Treatment of Pneumonia Caused by 2019-nCoV (version 5) 2020 [Available from:http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml.
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, et al. Aberrant pathogenic GM-CSF T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus, bioRxiv. 2020.02.12.945576; doi: 10.1101/2020.02.12.945576. [DOI]
- 8.Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C. Neutrophil-to-Lymphocyte ratio predicts severe illness patients with 2019 novel coronavirus in the early stage. medRxiv. 2020;2020 doi: 10.1186/s12967-020-02374-0. 02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]