Highlights
-
•
In the context of the ongoing COVID-19 pandemic, management of exposure events is a concern.
-
•
There was a large COVID-19 exposure event at a long-term care hospital in Korea.
-
•
Post-exposure prophylaxis using hydroxychloroquine was provided to 211 individuals.
-
•
Disease development was successfully prevented without severe adverse events.
Keywords: Post-exposure prophylaxis, Hydroxychloroquine, SARS-CoV-2, COVID-19, Long-term care hospital
Abstract
In the context of the ongoing global outbreak of coronavirus disease 2019 (COVID-19), management of exposure events is a concern. Long-term care hospitals (LTCHs) are particularly vulnerable to cluster outbreaks because facilities for patient isolation and healthcare personnel to care for these patients in isolation are difficult to arrange in a large outbreak situation. Although several drugs have been proposed as treatment options, there are no data on the effectiveness and safety of post-exposure prophylaxis (PEP) for COVID-19. After a large COVID-19 exposure event in an LTCH in Korea, PEP using hydroxychloroquine (HCQ) was administered to 211 individuals, including 189 patients and 22 careworkers, whose baseline polymerase chain reaction (PCR) tests for COVID-19 were negative. PEP was completed in 184 (97.4%) patients and 21 (95.5%) careworkers without serious adverse events. At the end of 14 days of quarantine, all follow-up PCR tests were negative. Based on our experience, further clinical studies are recommended for COVID-19 PEP.
1. Introduction
The World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) a pandemic on March 11, 2020 [1]. South Korea has had a rapid surge in cases of COVID-19 since February 18, most of which were linked to the religious group Shincheonji [2]. To date, 81% of total Korean cases occurred as clusters [2]. Long-term care hospitals (LTCHs) are particularly vulnerable to cluster outbreaks because facilities for patient isolation and healthcare personnel to care for these patients in isolation are difficult to arrange in a large outbreak situation. Furthermore, patients in LTCHs are at high risk if they become infected. Although several drugs have been proposed as treatment options, there are no data on the effectiveness and safety of post-exposure prophylaxis (PEP) for COVID-19. On February 23, a hospital social worker at an LTCH in Busan was diagnosed with COVID-19 after attending a religious service of Shincheonji 7 days earlier, and inpatients and hospital staff were exposed to her. This paper describes the outbreak response strategy used in the LTCH, including PEP using hydroxychloroquine (HCQ).
2. Methods
2.1. Detection of the index patient, screening of the exposed, and quarantine measures
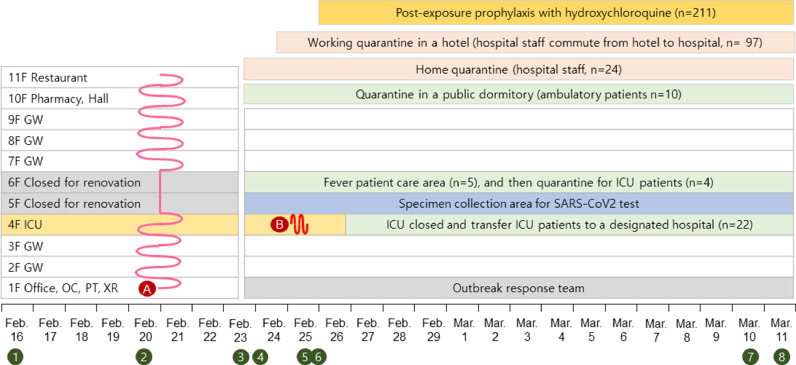
The index case was febrile and had myalgia 3 days before diagnosis, followed by malaise, headache and non-productive cough. She worked at the hospital while she was symptomatic for 2 days. Although she put on a facemask to walk in the corridors, it is uncertain whether she was wearing the mask in the patients’ rooms. All the 193 inpatients were considered to be exposed to her because she visited every floor of the hospital, including the intensive care unit (ICU), as observed on surveillance cameras (Fig. 1 , Supplement Table 1). Five febrile patients were isolated in single-bed rooms. Ten ambulatory patients were sent to a public dormitory for quarantine. Of the 123 hospital staff, 24 healthcare personnel (HCP) exposed to the index case were sent home to quarantine for 14 days. Other HCP who had to work were quarantined in a hotel and commuted to the hospital. Through screening of 313 individuals for SARS-CoV-2, a 63-year-old careworker (the second case) was found to be a COVID-19 case. She did not remember any contact with the index case. On February 25, the second case had a headache and was wearing a facemask while working in ICU from 6 am to noon. A total of 22 of 26 exposed ICU patients were transferred to a designated hospital and 4 were quarantined; 14 HCP exposed to the second case were quarantined separately.
Fig. 1.
Timeline of exposure period, quarantine and post-exposure prophylaxis.
Ⓐ Pink line indicates the route of patient A (the index case) while symptomatic, Ⓑ Red line indicates the route of patient B (the second case) while symptomatic, ① Patient A, attended a religious service in Daegu, ② Patient A, symptom onset, ③ Patient A, diagnosed with COVID-19, ④ Hospital closed and contacts quarantined, COVID-19 tests for all patients and hospital staff, ⑤ Patient B, diagnosed with COVID-19, ICU patients transferred to the other hospital, ⑥ Patients and careworkers, initiated post-exposure prophylaxis with hydroxychloroquine, ⑦ Patients and hospital staff, follow-up COVID-19 tests, ⑧ Discontinuation of isolation, quarantine and post-exposure prophylaxis with hydroxychloroquine. F, floor; GW, general ward; ICU, intensive care unit; OC, outpatient clinic; PT, physical therapy room; XR, X-ray room
2.1. PEP
As the exposed patients had to remain in multi-bed rooms receiving the care of similarly exposed careworkers, and some of them might be in incubation periods, this study addressed the repetitive exposure episodes to newly developing patients. PEP with HCQ for patients and careworkers was started on February 26 (Supplement Fig. 1). Physicians and pharmacists were educated about potential adverse events. HCQ was administrated orally at a dose of 400 mg daily until the completion of 14 days of quarantine. A checklist for common adverse events was distributed (Supplement Fig. 2). The study was approved and informed consent was waived by the Institutional Review Board of Pusan National University Hospital (H-2003-014-089).
3. Results
3.1. Demographic characteristics and COVID-19 exposure
The baseline demographic characteristics of 193 patients, 30 careworkers and 93 other hospital staff are shown in Supplement Table 1. There was a significant age difference between the three groups. The mean age was significantly higher in patients (82 years) and careworkers (65.8 years) compared with other hospital staff (52.3 years). The proportion of females was higher in hospital staff (86.2%) compared with patients (70.5%). Most patients (99.5%) had one or more comorbidities, with dementia (47.7%) the most common. Of the 26 patients admitted to ICU, 15 (57.7%) required supplemental oxygen and 13 (50%) also required frequent suctioning.
Of the 314 individuals, 9 hospital staff who had worked in the same office or had lunch with the index case were classified as high-risk exposure. Forty-three patients who had participated in a social program with the index case, 26 ICU patients who had contact with the second case, and 15 hospital staff who reported contact with the index case were classified as intermediate-risk exposure. Nine nurses and 5 careworkers who had contact with the second case in ICU were classified as low-risk exposure. A total of 206 individuals (65.6%) were not classified because their exposure could not be specified.
3.2. Acceptance and safety of PEP with HCQ
Among 193 patients and 121 hospital staff, PEP with HCQ was offered to 193 patients and 29 careworkers. Of the 193 patients, 4 (2.1%) were excluded because of death for other reasons (2) or refusal (2); therefore, 189 patients (97.9%) started PEP. Six (20.7%) of 29 careworkers quit their work and one (3.5%) disagreed with PEP, so HCQ was given to 22 careworkers (75.9%) (Supplement Fig. 1). PEP was initiated within a median of 58 h (range 48-106 h) and a median 18 h (range 6-66 h) after detection of the index case and the second case, respectively. Overall, PEP was completed in 184 (97.4%) patients and 21 (95.5%) careworkers. Median duration of PEP was 10 days (range 2-15 days). A total of 32 individuals (15.6%) reported one or more symptoms during the course of PEP (Supplement Table 2). The most common symptoms were diarrhea or loose stool (9%), skin rash (4.3%), gastrointestinal upset (0.95%) and bradycardia (0.95%). PEP was discontinued in 5 patients (2.7%) due to gastrointestinal upset (2), bradycardia (2) and need for fasting (1) (Supplement Fig. 1). PEP was also arbitrarily discontinued in a careworker.
3.3. Follow-up PCR for COVID-19
Postmortem polymerase chain reaction (PCR) tests with upper respiratory tract specimens were performed for 2 patients who died during the quarantine period and the results were negative. The two mortality cases, a 96-year-old male and an 84-year-old female with Alzheimer disease, had been receiving end-of-life care. For the remaining 191 patients and 121 hospital staff who had initially tested negative for COVID-19, follow-up PCR tests were conducted one or two days prior to discontinuing the 14-day quarantine and all were negative. After confirming the results, PEP with HCQ was stopped and quarantine was discontinued sequentially by March 11.
4. Discussion
Many patients would be expected to become infected with COVID-19 in the setting of cluster outbreaks associated with LTCHs. In this study, there were no additional confirmed cases among exposed patients and caregivers; however, it is not clear whether PEP was effective because there was no control group. Both chloroquine and HCQ had antiviral activity against SARS-CoV-2 in vitro [3], [4], [5], [6]. Furthermore, clinical data from China and France showed chloroquine was superior to control treatment, leading to the recommendation that chloroquine could be administered to patients with mild to severe COVID-19 pneumonia [7], [8], [9], [10]. Although there is currently no approved PEP for individuals exposed to COVID-19, PEP with HCQ was initiated for the patients and careworkers in this study because these were elderly patients who are at risk from adverse outcomes and HCQ is a safe drug widely used for rheumatoid arthritis treatment. Several different regimens of HCQ are recommended to treat COVID-19 patients: 800 mg qd, 600 mg qd, 200 mg tid, and 400 mg qd, with or without loading doses. No data are available on regimens for PEP. A dose of 400 mg qd, with no initial loading dose, was chosen for the current study because the patients in the LTCH had low body weights and a lower dose was required to minimize adverse events. In this study, HCQ was associated with mild adverse events. One patient had skin rash requiring steroids but did not discontinue PEP. Five patients discontinued PEP because of gastrointestinal upset, bradycardia, and for fasting.
Although there was no adequate control group and the study was conducted at a single center, this is the first study to use PEP with HCQ as an outbreak response strategy against COVID-19 in an LTCH. A total of 92 hospital staff, including physicians and nurses, showed negative PCR results after 14 days of quarantine even though they did not receive PEP. From these results, we could not conclude that PEP is effective for prevention of COVID-19 in close contacts. However, there were differences in the level of risk exposure: patients and careworkers might have had close contact with the index case (high-risk exposure) and most hospital staff were at low-risk exposure. Nonetheless, this study showed that all 189 patients and 22 careworkers who received PEP did not develop COVID-19.
5. Conclusion
In this study, PEP with HCQ was implemented safely under proper monitoring and no additional patients were diagnosed with COVID-19. Randomized clinical studies are needed to evaluate whether PEP is an effective option for an outbreak response strategy against COVID-19 in LTCHs.
Acknowledgments
Contributors
LSH and SH were involved in data collection and study organization. LSH and SH wrote the first draft of the manuscript. KRP designed the study and did the final revision of the manuscript. All authors revised the first and last draft of the manuscript.
Acknowledgement
We would like to express our sincerest condolences to the patients and families who suffered from the COVID-19 outbreak. We also greatly appreciate the healthcare personnel and staff members who worked together to overcome the COVID-19 outbreak.
Declarations
Funding: No funding.
Competing Interests: There are no competing financial interests.
Ethical Approval: The study was approved and informed consent was waived by the Institutional Review Board of Pusan National University Hospital (H-2003-014-089).
Editor: Jean-Marc Rolain
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2020.105988.
Appendix. Supplementary materials
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korean Society of Infectious Diseases KSoPID, Korean Society of Epidemiology, Korean Society for Antimicrobial Therapy, Korean Society for Healthcare-associated Infection Control and Prevention, and Korea Centers for Disease Control and Prevention Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35:e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6:67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquinone for the treatment of severe acute respiratory syndrome coronarvirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 Mar 9 doi: 10.1093/cid/ciaa237. ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colson P, Rolain JM, Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 9.Multicenter collaboration group of Department of S, Technology of Guangdong P, Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus p [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E019. doi: 10.3760/cma.j.issn.1001-0939.2020.0019. [DOI] [PubMed] [Google Scholar]
- 10.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.