Abstract
Despite advances in drug discovery, viral infections remain a major challenge for scientists across the globe. The recent pandemic of COVID-19 (coronavirus disease 2019), caused by a viral infection with SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), has created a disastrous situation all over the world. As no drugs are available to treat this life-threatening disease and the mortality rate due to COVID-19 is high, there is an utmost need to attempt to treat the infection using drug repurposing. Some countries are against the use of these drugs because of adverse effects associated with drug repurposing and lack of statistically significant clinical data, but they have been found to be effective in some countries to treat COVID-19 patients (off-label/investigational). This article emphasises possible drug candidates in the treatment of COVID-19. Most of these drugs were found to be effective in in vitro studies. There is a need to re-assess in vitro data and to carry out randomised clinical trials. Further investigations of these drugs are recommended on a priority basis.
Keywords: COVID-19, SARS-CoV-2, Drug repurposing, Coronavirus
Abbreviations: CoV, coronavirus; MERS-CoV, Middle East respiratory syndrome coronavirus; SARS-CoV, severe acute respiratory syndrome coronavirus
1. Introduction
COVID-19 (coronavirus disease 2019) is a respiratory tract infection caused by a novel coronavirus that was first identified in the city of Wuhan, Hubei Province, China, at the end of 2019. Genetically, the virus closely resembles the severe acute respiratory syndrome coronavirus (SARS-CoV) [1] and has been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It has spread across the globe to more than 190 counties within a short period, i.e. within 45–90 days of its initial recognition. The COVID-19 pandemic has created a devastating situation not only in developing countries but also in developed nations. To date, there is no specific treatment available to treat infection with SARS-CoV-2 and the disease COVID-19.
By the end of March 2020, approximately 750 000 people have been infected with SARS-CoV-2 globally and the situation is overwhelming in countries such as China, Italy, Spain and the USA. As no specific treatments or vaccine for COVID-19 are available, there is a need of drug repurposing, where approved drugs can be effectively used to treat novel diseases with minimal or no side effects. The benefits of drug repurposing are that the safety, optimal dosage and pharmacokinetics of drugs are well known.
In India, most of the drugs and antibiotics used to treat COVID-19 have been repurposed (off-label/investigational use) and have been found to be very effective in affected individuals. This might be one of the reasons for the low mortality rate in India (0.02 deaths per million persons) compared with Italy (178 deaths per million persons) [2].
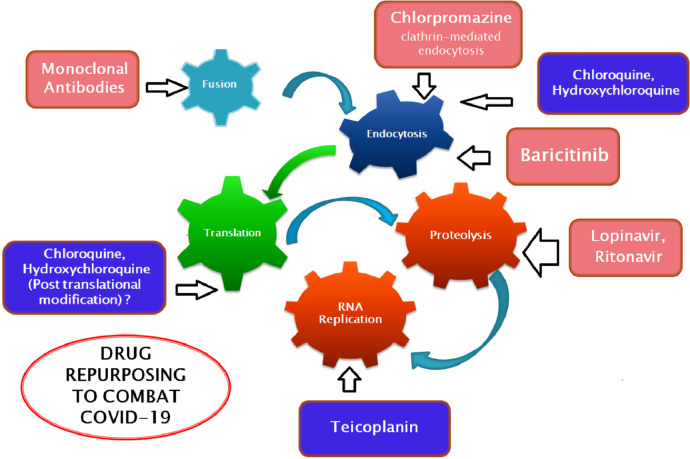
Chloroquine and its hydroxyl analogue hydroxychloroquine have been reported for their use as an antiviral agent in various studies. Apart from their antimalarial use, they have also shown in vitro activity against SARS-CoV-2 [3,4]. The pH increase induced by chloroquine and hydroxychloroquine within acidic organelles such as lysosomes, endosomes and Golgi vesicles is responsible for their antiviral activity [3,4]. In one mechanism of action, these drugs mainly inhibit virus entry into their host cell by a pH-dependent step. In another mechanism of action, chloroquine and hydroxychloroquine inhibit post-translational modification of the virus envelope glycoproteins inside the endoplasmic vesicles and trans-Golgi network [3,4]. The major stages of the coronavirus replication cycle and the probable sites of action of different drugs are shown in Fig. 1 .
Fig. 1.
Major stages of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) life cycle in host cells and probable site of action of different drugs.
Few researchers are against the use of antibacterial agents and antibiotics to treat viral infections, but drugs such as teicoplanin can inhibit the growth of viruses in human cells [5]. Staphylococci infections can be treated with teicoplanin and it was also shown to be efficacious in the first stage of the Middle East respiratory syndrome coronavirus (MERS-CoV) viral cycle. Teicoplanin mainly inhibits the low-pH cleavage of the spike (S) protein by cathepsin L in the late endosomes, hence preventing viral RNA release and replication of virus [5,6].
Other glycopeptide antibiotics such as oritavancin, dalbavancin and telavancin also have the potential to inhibit the entry of SARS-CoV, Ebola virus and MERS-CoV transcription and replication-competent virus-like particles (trVLPs) [5,6].
The major mechanism by which MERS-CoV [7,8] and SARS-CoV [9] enter host cells is clathrin-mediated endocytosis. A drug that acts by inhibiting clathrin-mediated endocytosis is chlorpromazine, which can inhibit the entry of these coronaviruses into host cells [10]. Chlorpromazine is a widely used antipsychotic agent that is safely used to treat schizophrenia. It may be efficacious to treat COVID-19 patients provided that adequate clinical trials are conducted.
The macrolide antibiotic bafilomycin A1 may be a promising candidate to treat COVID-19. It is an endo/lysosomal V-ATPase inhibitor that mainly interrupts the function of angiotensin-converting enzyme 2 (ACE2), the receptor for SARS-CoV and SARS-CoV-2, by which it may stop the viral cycle at the early entry stage [9].
Remdesivir, an antiviral agent initially developed for Ebola virus infection, revealed more effective results against SARS-CoV-2 in vitro [11]. It is an adenosine analogue that incorporates into nascent viral RNA chains and results in premature termination. Further investigations of remdesivir are anticipated in human COVID-19 patients on an urgent basis.
Nitazoxanide is used to treat parasitic infections and has also been found to be effective in treating a wide range of viruses, including human coronaviruses, in vitro at very low concentrations. It selectively blocks haemagglutinin intracellular trafficking and insertion of this protein into the host plasma membrane, a key step for correct assembly and exit of the virus from the host cell [12].
Padmanabhan reported a combination therapy approach using hydroxychloroquine and nitazoxanide [12]. A synergistic effect can be produced by using both drugs, as hydroxychloroquine inhibits viral entry and fusion whilst nitazoxanide upregulates the innate immune response to prevent ongoing viral replication.
Tocilizumab, an immunosuppressive agent originally used to treat rheumatoid arthritis, was also found to be effective in vivo in COVID-19 patients in China [13]. Tocilizumab effectively reduces the clinical symptoms of viral infection, however the number of patients tested in the study was very small [13].
Another antibiotic found to be effective in viral infections is the macrolide azithromycin. The drug effectively inhibits the growth of Zika virus and Ebola virus in vitro [14,15]. The synergistic effects of the combination of azithromycin and hydroxychloroquine were also reported in the treatment of COVID-19 patients [16]. The report highlights the efficacy of this combination in clearing viral nasopharyngeal carriage within a short time in COVID-19 patients compared with patients receiving only hydroxychloroquine. Hydroxychloroquine increases the pH within acidic organelles and also inhibits entry of the virus [3,4], producing an antiviral effect (as explained earlier), whilst the exact antiviral action of azithromycin is not known. It may have immunomodulatory properties that could be beneficial in the treatment of pulmonary viral infections. The molecule may decrease the inflammatory responses and the production of excessive cytokines (‘cytokine storm’) accompanying viral infections. The immunomodulatory mechanism may be due to decreased chemotaxis of neutrophils to the lungs by inhibiting cytokines as well as the formation of reactive oxygen species [16,17].
In India, hydroxychloroquine plus the antiviral combination lopinavir/ritonavir have been used to treat COVID-19 patients. Lopinavir/ritonavir affects the viral protease 3CLpro responsible for proteolysis in the coronavirus replication cycle [18].
The Japanese antiviral drug favipiravir used to treat influenza, developed by FUJIFILM Toyama Chemical Co., Ltd., showed considerable success in clinical trials of more than 340 patients [19] where it was found to be safe and effective in the treatment of COVID-19 patients. Further investigations are recommended in this context.
Ascorbic acid (vitamin C) possesses antioxidant properties. It does not have a direct lethal effect on viruses, but it has been reported that viral respiratory infections in humans are affected by vitamin C levels [20]. When viral infection occurs, the subsequent cytokine surge is activated and neutrophils accumulate in the lungs, destroying alveolar capillaries. Early clinical studies have shown that vitamin C has the potential to inhibit these processes [21]. Combining ascorbic acid with other drugs will definitely be helpful for affected COVID-19 individuals.
Baricitinib is another drug used in the treatment of rheumatoid arthritis. It can be also used to treat novel coronavirus [22], possibly by targeting the process of endocytosis. The receptor used by SARS-CoV-2 to infect lung cells is ACE2, which are prone to viral infection. The AP2-associated protein kinase 1 (AAK1) is one of the known regulators of endocytosis, and disruption of AAK1 might interrupt the passage of virus into cells and also the intracellular assembly of virus particles [23].
2. Recommended drugs (off-label) for treatment of COVID-19
Recommended drugs for the treatment (off-label/investigational use) of COVID-19 are shown in Table 1 .
Table 1.
Recommended drugs (off-label) for treatment of COVID-19 (coronavirus disease 2019) [18]
| Drug | Dosage |
|---|---|
| Hydroxychloroquine | 400 mg b.i.d. × two doses, then 200 mg b.i.d. for 5 days |
| Remdesivir | 200 mg i.v. loading dose, then 100 mg i.v. for up to 10 days |
| Oseltamivir | 150 mg b.i.d. for 5 days |
| Lopinavir | 400 mg b.i.d. for 10 days |
| Ritonavir | 100 mg b.i.d. for 10 days |
| Ribavirin | 2 g loading dose, then 600 mg t.i.d. |
b.i.d., twice daily; i.v. intravenous; t.i.d., three times daily.
3. Current status of investigational drugs to treat COVID-19
Table 2 summarises the current status of investigational drugs/vaccines to treat COVID-19 across the globe.
Table 2.
List of investigational drugs/vaccines to treat COVID-19 (coronavirus disease 2019)
| Name | Organisation | Study phase | Current status | Reference |
|---|---|---|---|---|
| Remdesivir | Gilead Sciences | III | Initiated two phase III clinical studies. Randomised studies will enrol ∼1000 patients: (i) 400 patients with severe clinical manifestations of COVID-19; and (ii) 600 patients with moderate clinical manifestations of COVID-19 | [24] |
| Kevzara® (sarilumab) | Regeneron Pharmaceuticals and Sanofi | II/III | Assessing safety and efficacy in 400 hospitalised adult COVID-19 patients using the IL-6 receptor antagonist Kevzara® (sarilumab) | [25] |
| Lopinavir/ritonavir combination | AbbVie | I/II (?) | Clinical trial for use of the anti-HIV combination medication lopinavir/ritonavir to treat COVID-19 has been initiated | [26] |
| Antibody | Regeneron Pharmaceuticals Inc. | Preclinical/clinical (?) | Initiated development of a novel multi-antibody cocktail for COVID-19 | [27] |
| Antibody (TJM2) | I-Mab Biopharma | Phase I completed | Completed phase I study; safety, tolerability and immunogenicity studies showed favourable results | [28] |
| Antibody | Medicago | Preclinical | Successfully formulated virus-like particle (VLP) | [29] |
| OYA1 (investigational) | OyaGen, Inc. | Preclinical | Safety and efficacy studies are ongoing | [30] |
IL-6, interleukin-6; HIV, human immunodeficiency virus.
4. Concluding remarks
In conclusion, based on previous studies, use of these drugs in the treatment of viral diseases should be thoroughly investigated to save the lives of affected individuals, despite some adverse effects. All of the above-listed drugs may be potential candidates to treat COVID-19 patients, and sufficient in vivo data regarding their interactions and efficacy are urgently required. Preliminary in vitro data are required to be reassessed and clinical studies are recommended at the earliest opportunity.
Acknowledgments
Acknowledgments
The author is highly indebted to Prin. Dr Y.T. Pawar and Prin. Dr R.S. Bhambar for their valuable guidance in pursuing this research.
Funding
None.
Competing Interests
None declared.
Ethical Approval
Not required.
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 coronavirus pandemic. Confirmed cases and deaths by country, territory, or conveyance. https://www.worldometers.info/coronavirus/#countries[accessed 15 April 2020].
- 3.Rolain J.M., Colson P., Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int J Antimicrob Agents. 2007;30:297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colson P., Rolain J.M., Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colson P., Raoult D. Fighting viruses with antibiotics: an overlooked path. Int J Antimicrob Agents. 2016;48:349–352. doi: 10.1016/j.ijantimicag.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C., et al. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of Ebola virus, Middle East respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J Biol Chem. 2016;291:9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkard C., Verheije M.H., Wicht O., van Kasteren S.I., van Kuppeveld F.J., Haagmans B.L., et al. Coronavirus cell entry occurs through the endo-/lysosomal pathway in a proteolysis-dependent manner. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004502. Erratum in: PLoS Pathog 2015;11:e1004709. doi: 10.1371/journal.ppat.1004709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkard C., Verheije M.H., Haagmans B.L., van Kuppeveld F.J., Rottier P.J., Bosch B.J., et al. ATP1A1-mediated Src signaling inhibits coronavirus entry into host cells. J Virol. 2015;89:4434–4448. doi: 10.1128/JVI.03274-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., et al. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M., et al. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J Virol. 2007;81:8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padmanabhan S.Potential dual therapeutic approach against SARS-CoV-2/COVID-19 with nitazoxanide and hydroxychloroquine. doi: 10.13140/RG.2.2.28124.74882.
- 13.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. ChinaXiv. 2020 doi: 10.1073/pnas.2005615117. 202003.00026, http://chinaxiv.org/abs/202003.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madrid P.B., Panchal R.G., Warren T.K., Shurtleff A.C., Endsley A.N., Green C.E., et al. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect Dis. 2015;1:317–326. doi: 10.1021/acsinfecdis.5b00030. [DOI] [PubMed] [Google Scholar]
- 15.Bosseboeuf E., Aubry M., Nhan T., de Pina J.J., Rolain J.M., Raoult D., et al. Azithromycin inhibits the replication of Zika virus. J Antivir Antiretrovir. 2018;10:6–11. doi: 10.4172/1948-5964.1000173. [DOI] [Google Scholar]
- 16.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Kanoh S., Rubin B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010;23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph T., Ashkan M., editors. International pulmonologist's consensus on COVID 19. https://scts.org/wp-content/uploads/2020/03/Pulmonologist-Consensus-COVID-19-March-2020.pdf[accessed 15 April 2020].
- 19.Japanese flu drug ‘clearly effective’ in treating coronavirus, says China. The Guardian 2020 Mar 18. https://www.theguardian.com/world/2020/mar/18/japanese-flu-drug-clearly-effective-in-treating-coronavirus-says-china[accessed 15 April 2020].
- 20.Hemilä H., Douglas R.M. Vitamin C and acute respiratory infections. Int J Tuberc Lung Dis. 1999;3:756–761. [PubMed] [Google Scholar]
- 21.ClinicalTrial.gov. Vitamin C infusion for the treatment of severe 2019-nCoV infected pneumonia. ClinicalTrials.gov Identifier: NCT04264533. https://clinicaltrials.gov/ct2/show/NCT04264533[accessed 15 April 2020].
- 22.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilead Sciences. Gilead Sciences initiates two phase 3 studies of investigational antiviral remdesivir for the treatment of COVID-19. https://www.gilead.com/news-and-press/press-room/press-releases/2020/2/gilead-sciences-initiates-two-phase-3-studies-of-investigational-antiviral-remdesivir-for-the-treatment-of-covid-19[accessed 15 April 2020].
- 25.Genetic Engineering & Biotechnology News. Regeneron, Sanofi launch clinical trial of Kevzara as coronavirus treatment. https://www.genengnews.com/news/regeneron-sanofi-launch-clinical-trial-of-kevzara-as-coronavirus-treatment/[accessed 15 April 2020].
- 26.AbbVie. AbbVie partnering with global authorities to determine efficacy of HIV drug in treating COVID-19. https://news.abbvie.com/news/press-releases/abbvie-partnering-with-global-authorities-to-determine-efficacy-hiv-drug-in-treating-covid-19.htm[accessed 15 April 2020].
- 27.Regeneron Pharmaceuticals Inc. Regeneron announces important advances in novel COVID-19 antibody program. https://www.prnewswire.com/news-releases/regeneron-announces-important-advances-in-novel-covid-19-antibody-program-301025247.html[accessed 15 April 2020].
- 28.Contract Pharma. I-Mab explores TJM2 in treating severe COVID-19 disease. https://www.contractpharma.com/contents/view_breaking-news/2020-03-13/i-mab-explores-tjm2-in-treating-severe-covid-19-disease/[accessed 15 April 2020].
- 29.The Pharma Letter. Medicago claims to have a viable vaccine candidate for COVID-19. https://www.thepharmaletter.com/article/medicago-claims-to-have-a-viable-vaccine-candidate-for-covid-19/[accessed 15 April 2020].
- 30.P&T Community. OyaGen, Inc. announces a compound in development with broad antiviral activity against coronaviruses, including SARS-CoV-2. https://www.ptcommunity.com/wire/oyagen-inc-announces-compound-development-broad-antiviral-activity-against-coronaviruses[accessed 15 April 2020].