Summary
Background
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19), which was declared a global pandemic by the World Health Organization on 11th March 2020. The treatment guidelines for COVID-19 vary between countries, yet there is no approved treatment to date.
Aim
To report any evidence of therapeutics used for the management of patients with COVID-19 in clinical practice since emergence of the virus.
Methods
A systematic review protocol was developed based on the PRISMA statement. Articles for review were selected from Embase, Medline and Google Scholar. Readily accessible peer-reviewed, full articles in English published from 1st December 2019 to 26th March 2020 were included. The search terms included combinations of: COVID, SARS-COV-2, glucocorticoids, convalescent plasma, antiviral and antibacterial. There were no restrictions on the types of study eligible for inclusion.
Results
Four hundred and forty-nine articles were identified in the literature search; of these, 41 studies were included in this review. These were clinical trials (N=3), case reports (N=7), case series (N=10), and retrospective (N=11) and prospective (N=10) observational studies. Thirty-six studies were conducted in China (88%). Corticosteroid treatment was reported most frequently (N=25), followed by lopinavir (N=21) and oseltamivir (N=16).
Conclusions
This is the first systematic review to date related to medication used to treat patients with COVID-19. Only 41 studies were eligible for inclusion, most of which were conducted in China. Corticosteroid treatment was reported most frequently in the literature.
Keywords: SARS-CoV-2, COVID-19, Hydroxychloroquine, Arbidol hydrochloride, Corticosteroids, Convalescent plasma therapy
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the causative agent of coronavirus disease 2019 (COVID-19), which was declared a global pandemic by the World Health Organization (WHO) on 11th March 2020. SARS-CoV-2 was discovered in December 2019 in Wuhan City, Hubei Province, China. The origin of the virus is unknown, but initially, newly diagnosed cases were linked to the Huanan Seafood Wholesale Market where people can buy wild animals, such as bats [1]. SARS-CoV-2, a novel enveloped RNA betacoronavirus, has phylogenetic similarity to severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus [2].
One of the characteristics of COVID-19 is that it is highly contagious; China and 164 other countries have been affected in less than 3 months. Despite China reaching 81,151 confirmed cases with 3242 deaths, the country has reported only one new domestic case since 18th March 2020. As of that date, the total worldwide confirmed cases was 193,475 with 7864 deaths (WHO). Although protective measures have been implemented in China (e.g. isolation from confirmed and suspected cases) to reduce spread of the virus, the need for effective treatment is imperative to stop the outbreak and reduce the morbidity and mortality of COVID-19 [1].
Since the onset of the outbreak, many agents that could have efficacy against COVID-19 have been proposed. Various antiviral agents were included in the latest guidelines from the National Health Commission, including interferon, lopinavir/ritonavir, chloroquine phosphate, ribavirin and arbidol [3]. Angiotensin receptor blockers, such as losartan, have also been suggested for the treatment of COVID-19 [4].
The treatment guidelines for COVID-19 vary between countries. The WHO guidelines are very general, recommending management of symptoms, and advise caution with paediatric patients, pregnant women and patients with underlying co-morbidities. There is no approved treatment for COVID-19; the recommendation is to provide supportive management according to each patient's need (e.g. antipyretics for fever, oxygen therapy for respiratory distress). Moreover, WHO recommendations indicate that severe cases should be given empiric antimicrobial therapy, with mechanical ventilation implemented depending on the patient's clinical condition. Some of the Asian guidelines (e.g. the Japanese guidelines) were not easy to interpret as they have not yet been translated into English. However, the treatment protocols across countries are similar, and include hydroxychloroquine, chloroquine phosphate, remedesivir and lopinavir/ritonavir [[5], [6], [7]]. Treatment guidelines between countries differ slightly, as shown in Table I [[8], [9], [10], [11]].
Table I.
Comparison between the treatment guidelines for coronavirus disease 2019 in Saudi Arabia, the USA, Europe and Egypt [[8], [9], [10], [11]]
| Saudi Arabia (Ministry of Health) | USA (Massachusetts General Hospital) | Europe (Ireland) | Egypt | |
|---|---|---|---|---|
| Mild-to-moderate | Hydroxychloroquine Chloroquine Chloroquine phosphate |
Clinical trial of remdesivir | Chloroquine (oral) Hydroxychloroquine (oral) Lopinavir/ritonavir (oral) Remdesivir (intravenous) |
Oseltamivir Hydroxychloroquine Chloroquine phosphate |
| Severe | Hydroxychloroquine Chloroquine Chloroquine phosphate Combination therapy (lopinavir/ritonavir) |
Hydroxychloroquine Chloroquine Lopinavir/ritonavir Darunavir/cobicistat |
Oseltamivir Hydroxychloroquine Chloroquine phosphate Lopinavir/ritonavir Serum ferritin, D-dimer |
|
| Critical | Combination therapy (lopinavir/ritonavir) Hydroxychloroquine Remdesivir |
With USA United States of America, interferon-β B1 (Betaseron) | Antibiotics Oseltamivir Hydroxychloroquine (or chloroquine phosphate) Azithromycin Hydrocortisone Therapeutic anticoagulants if D-Dimer Invasive |
In light of limited evidence in the literature regarding medication used to treat COVID-19, this review aims to retrospectively evaluate the therapeutic management received by patients with COVID-19 since emergence of the virus.
Methods
A systematic review protocol was developed based on PRISMA-P and the PRISMA statement. Articles for review were selected from Embase, Medline and Google Scholar. Readily accessible peer-reviewed, full articles in English, published from 1st December 2019 to 26th March 2020, were included. The search terms included combinations of: COVID-19, SARS-COV-2, glucocorticoids, chloroquine, convalescent plasma, antiviral, antibacterial, oseltamivir, hydroxychloroquine, chloroquine phosphate and monoclonal antibodies. There were no restrictions on the types of study eligible for inclusion; however, these were likely to be quantitative studies and randomized clinical trials. The focus of this review was therapeutics for the management of patients with COVID-19.
The primary outcomes were: (1) evidence of therapeutics used for the management of patients with COVID-19 in clinical practice, irrespective of patient characteristics, setting and outcome measures, in order to discuss the most commonly reported medicines; and (2) clinical outcomes of therapeutic treatment (i.e. recovery, mortality) in patients with COVID-19. The secondary outcome of this review was adverse events associated with treatment.
Duplicate articles were removed. Titles and abstracts were screened independently by two reviewers, followed by review of full articles where any doubt remained. Inclusions and exclusions were recorded following PRISMA guidelines, and detailed reasons for exclusion were recorded. Critical appraisal checklists appropriate to each study design were applied and checked by a second team member. Any bias or quality issues identified were considered prior to a quantitative meta-analysis and meta-narrative. Critical Appraisal Skills Programme checklist tools were used for quality assessment. A data extraction tool was designed to capture focus of interest, population, geographical location, methodology, specific mention of therapeutic treatment and adverse events, key findings and further research. Ethical approval was not required for this review of existing peer-reviewed literature.
Results
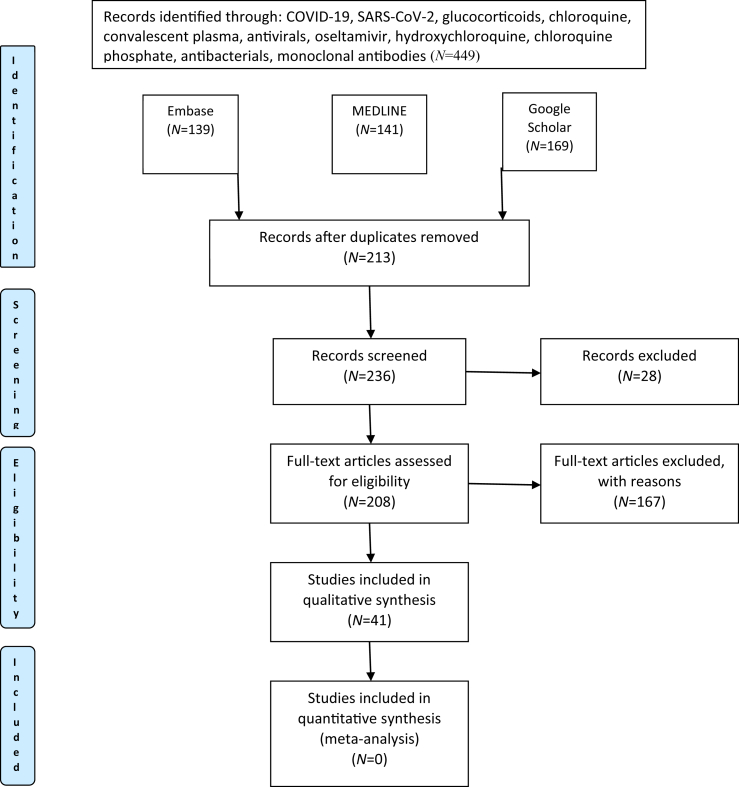
Four hundred and forty-nine articles were identified in the literature search. Inclusions and exclusions are reported following PRISMA guidelines in Figure 1, with reasons for exclusion recorded (Table II). In total, 213 duplicate studies were excluded. In addition, 18 studies were excluded due to language (Chinese N=9, Dutch N=2, Vietnamese N=1, Spanish N=1, Italian N=1, Russian N=1, Portuguese N=1, Iranian N=1, German N=1), and 10 studies were excluded for other reasons, including incomplete and irrelevant articles.
Figure 1.
PRISMA flow diagram reporting search results. COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
Table II.
Excluded papers and reasons for exclusion
| No. | Authors | Title | COVID-19 Yes/no |
Reason for exclusion |
|---|---|---|---|---|
| 1 | Chughtai et al., 2020 | Policies on the use of respiratory protection for hospital health workers to protect from coronavirus disease (COVID-19) | Yes | No details on therapeutics/commentary |
| 2 | Gurwitz, 2020 | Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics | Yes | Commentary |
| 3 | Wang et al., 2020 | Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro | Yes | Commentary |
| 4 | Colson et al., 2020 | Chloroquine and hydroxychloroquine as available weapons to fight COVID-19 | Yes | Commentary |
| 5 | Liu et al., 2020 | Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy | Yes | No details on therapeutics/commentary |
| 6 | Baron et al., 2020 | Teicoplanin: an alternative drug for the treatment of coronavirus COVID-19? | Yes | Commentary |
| 7 | Mitja and Clotet, 2020 | Use of antiviral drugs to reduce COVID-19 transmission | Yes | Commentary |
| 8 | Colson et al., 2020 | Chloroquine for the 2019 novel coronavirus SARS-CoV-2 | Yes | Commentary |
| 9 | Morse et al., 2020 | Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV | Yes | Commentary |
| 10 | Thevarajan et al., 2020 | Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19 | Yes | Commentary |
| 11 | Elfiky, 2020 | Anti-HCV, nucleotide inhibitors, repurposing against COVID-19 | Yes | Commentary |
| 12 | Ung, 2020 | Community pharmacist in public health emergencies: quick to action against the coronavirus 2019-nCoV outbreak | Yes | Commentary |
| 13 | Gupta, 2020 | Clinical considerations for patients with diabetes in times of COVID-19 epidemic | Yes | Commentary |
| 14 | Dong et al., 2020 | Discovering drugs to treat coronavirus disease 2019 (COVID-19) | Yes | Commentary |
| 15 | Zhang et al., 2020 | Liver injury in COVID-19: management and challenges | Yes | Commentary |
| 16 | Cunningham et al., 2020 | Treatment of COVID-19: old tricks for new challenges | Yes | Commentary |
| 17 | Ko et al., 2020 | Arguments in favour of remdesivir for treating SARS-CoV-2 infections | Yes | Commentary |
| 18 | Arabi et al., 2020 | COVID-19: a novel coronavirus and a novel challenge for critical care | Yes | Commentary |
| 19 | Wang and Shi, 2020 | Managing neonates with respiratory failure due to SARS-CoV-2 | Yes | Commentary |
| 20 | Stebbing et al., 2020 | COVID-19: combining antiviral and anti-inflammatory treatments | Yes | Commentary |
| 21 | Touret and Lamballerie, 2020 | Of chloroquine and COVID-19 | Yes | Commentary |
| 22 | Porcheddu et al., 2020 | Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China | Yes | No therapeutic data/commentary |
| 23 | Zhang et al., 2020 | Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics | Yes | Commentary |
| 24 | Baden and Rubin, 2020 | COVID-19 – the search for effective therapy | Yes | Commentary |
| 25 | Baud et al., 2020 | COVID-19 in pregnant women | Yes | No therapeutic data/commentary |
| 26 | Ortega et al., 2020 | Unrevealing sequence and structural features of novel coronavirus using in silico approaches: the main protease as molecular target | Yes | No therapeutic data |
| 27 | Ma et al., 2020 | 2019 novel coronavirus disease in hemodialysis (HD) patients: report from one HD center in Wuhan, China | Yes | No therapeutic data |
| 28 | Columbus et al., 2020 | 2019 novel coronavirus: an emerging global threat | Yes | Commentary |
| 29 | Barry et al., 2020 | COVID-19 in the shadows of MERS-CoV in the Kingdom of Saudi Arabia | Yes | Commentary |
| 30 | Wang et al., 2020 | A precision medicine approach to managing 2019 novel coronavirus pneumonia | Yes | No therapeutic data/commentary |
| 31 | Singhal, 2020 | A Review of coronavirus disease-2019 (COVID-19) | Yes | Review article |
| 32 | Li et al., 2020 | A simple laboratory parameter facilitates early identification of COVID-19 patients | Yes | Retrospective case-negative control study |
| 33 | Guo et al., 2020 | A survey for COVID-19 among HIV/AIDS patients in two districts of Wuhan, China | Yes | No therapeutic data |
| 34 | Gao et al., 2020 | Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies | Yes | Commentary |
| 35 | Deng et al., 2020 | Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study | Yes | Retrospective control study |
| 36 | Murthy et al., 2020 | Care for critically ill patients with COVID-19 | Yes | Commentary |
| 37 | Deng and Peng, 2020 | Characteristics of and public health responses to the coronavirus disease 2019 outbreak in China | Yes | Review |
| 38 | Wang et al., 2020 | Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China | Yes | No therapeutic data |
| 39 | Xiong et al., 2020 | Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes | Yes | No therapeutic data |
| 40 | Chen et al., 2020 | Clinical and immunologic features in severe and moderate forms of coronavirus disease 2019 | Yes | No therapeutic data |
| 41 | Chen et al., 2020 | Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records | Yes | No therapeutic data |
| 42 | Hong et al., 2020 | Clinical characteristics of novel coronavirus disease 2019 (COVID-19) in newborns, infants and children | Yes | Perspectives/no therapeutic data |
| 43 | Ye et al., 2020 | Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation | Yes | No therapeutic data |
| 44 | Anderson et al., 2020 | Clinical management of suspected or confirmed COVID-19 disease | Yes | Review |
| 45 | Zhang et al., 2020 | Clinical trials for the treatment of coronavirus disease 2019 (COVID-19): a rapid response to urgent need | Yes | Commentary |
| 46 | Chen et al., 2020 | Convalescent plasma as a potential therapy for COVID-19 | Yes | Commentary |
| 47 | Yang et al., 2020 | Corona virus disease 2019: a growing threat to children? | Yes | Commentary/no therapeutic data |
| 48 | Kooraki et al., 2020 | Coronavirus (COVID-19) outbreak: what the department of radiology should know | Yes | Commentary/no therapeutic data |
| 49 | Rasmussen et al., 2020 | Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know | Yes | Commentary/no therapeutic data |
| 50 | Liu et al., 2020 | Coronavirus disease 2019 (COVID-19) during pregnancy: a case series | Yes | No therapeutic data |
| 51 | Mclntosh et al., 2020 | Coronavirus disease 2019 (COVID-19) | Yes | Review |
| 52 | He and Li, 2020 | Coronavirus disease 2019 (COVID-19): what we know? | Yes | Review |
| 53 | Xiong et al., 2020 | Coronaviruses and the cardiovascular system: acute and long-term implications | Yes | Commentary |
| 54 | Gong et al., 2020 | Correlation analysis between disease severity and inflammation-related parameters in patients with COVID-19 pneumonia | Yes | No therapeutic data |
| 55 | Dong et al., 2020 | Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China | Yes | No therapeutic data |
| 56 | Shereen et al., 2020 | COVID-19 infection: origin, transmission, and characteristics of human coronaviruses | Yes | Review |
| 57 | Rio and Malani, 2020 | COVID-19 – new insights on a rapidly changing epidemic | Yes | Review |
| 58 | Yi et al., 2020 | COVID-19: what has been learned and to be learned about the novel coronavirus disease | Yes | Review |
| 59 | Rezaeetalab et al., 2020 | COVID-19: a new virus as a potential rapidly spreading in the worldwide | Yes | Review |
| 60 | Shaker et al., 2020 | COVID-19: pandemic contingency planning for the allergy and immunology clinic | Yes | No therapeutic data |
| 61 | Aslam and Mehra, 2020 | COVID-19: yet another coronavirus challenge in transplantation | Yes | Commentary |
| 62 | Padmanabhan, 2020 | Potential dual therapeutic approach against SARS-CoV-2/COVID-19 with nitazoxanide and hydroxychloroquine | Yes | Commentary |
| 63 | Hick et al., 2020 | Duty to plan: health care, crisis standards of care, and novel coronavirus SARS-CoV-2 | Yes | Discussion |
| 64 | Yang et al., 2020 | Epidemiological and clinical features of COVID-19 patients with and without pneumonia in Beijing, China | Yes | No therapeutic data |
| 65 | Khan, 2020 | Epidemiology of corona virus in the world and its effects on the China economy | Yes | Review |
| 66 | Hoehl et al., 2020 | Evidence of SARS-CoV-2 infection in returning travelers from Wuhan, China | Yes | Commentary |
| 67 | Yang et al., 2020 | Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome | Yes | Review |
| 68 | Cascella et al., 2020 | Features, evaluation and treatment of coronavirus (COVID-19) | Yes | Review |
| 69 | Erol, 2020 | High-dose intravenous vitamin C treatment for COVID-19 (a mechanistic approach) | Yes | Review |
| 70 | Liu et al., 2020 | Highly ACE2 expression in pancreas may cause pancreas damage after SARS-CoV-2 infection | Yes | Commentary |
| 71 | Zhang et al., 2020 | Immune phenotyping based on neutrophil-to-lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID-19 | Yes | No therapeutic data |
| 72 | Mao et al., 2020 | Implications of COVID-19 for patients with pre-existing digestive diseases | Yes | Commentary |
| 73 | Ferguson et al., 2020 | Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand | Yes | No therapeutic data |
| 74 | Qiu et al., 2020 | Intensive care during the coronavirus epidemic | Yes | Commentary |
| 75 | Poon et al., 2020 | ISUOG interim guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals | Yes | Review |
| 76 | Khan et al., 2020 | The emergence of a novel coronavirus (SARS-CoV-2), their biology and therapeutic options | Yes | Discussion |
| 77 | Sun et al., 2020 | Lower mortality of COVID-19 by early recognition and intervention: experience from Jiangsu Province | Yes | Commentary |
| 78 | Guzzi et al., 2020 | Master regulator analysis of the SARS-CoV-2/human interactome | Yes | No therapeutic data |
| 79 | Memish et al., 2020 | Middle East respiratory syndrome | No | Review |
| 80 | Nicastri, 2020 | Recommendations for COVID-19 clinical management | Yes | Commentary |
| 81 | Li et al., 2020 | Network bioinformatics analysis provides insight into drug repurposing for COVID-2019 | Yes | No therapeutic data |
| 82 | Xiong et al., 2020 | Novel and potent inhibitors targeting DHODH, a rate-limiting enzymein de novo pyrimidine biosynthesis, are broad-spectrum antiviral against RNA viruses including newly emerged coronavirus SARS-CoV-2 | Yes | No therapeutic data |
| 83 | Rezabakhsh et al., 2020 | Novel coronavirus (COVID-19): a new emerging pandemic threat | Yes | Survey/no therapeutic data |
| 84 | Ai et al., 2020 | Optimizing diagnostic strategy for novel coronavirus pneumonia, a multi-center study in Eastern China | Yes | No therapeutic data |
| 85 | Qiu et al., 2020 | Outcome reporting from protocols of clinical trials of coronavirus disease 2019 (COVID-19): a review | Yes | No therapeutic data |
| 86 | Bajema et al., 2020 | Persons evaluated for 2019 novel coronavirus – United States, January 2020 | Yes | Commentary |
| 87 | Shanmugaraj et al., 2020 | Perspectives on monoclonal antibody therapy as potential therapeutic intervention for coronavirus disease-19 (COVID-19) | Yes | Review |
| 88 | Zhou and Zhao, 2020 | Perspectives on therapeutic neutralizing antibodies against the novel coronavirus SARS-CoV-2 | Yes | Review |
| 89 | Hoffmann et al., 2020 | SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor | Yes | No therapeutic data |
| 90 | Zhang and Liu, 2020 | Potential interventions for novel coronavirus in China: a systematic review | Yes | Review |
| 91 | Vasylyeva, 2020 | Pregnancy and COVID-19: a brief review | Yes | Review |
| 92 | Alamri et al., 2020 | Pharmacoinformatics and molecular dynamic simulation studies reveal potential inhibitors of SARS-CoV-2 main protease 3CLpro | Yes | No therapeutic data |
| 93 | Fisher and Heymann, 2020 | Q&A: The novel coronavirus outbreak causing COVID-19 | Yes | Commentary |
| 94 | Goh et al., 2020 | Rapid progression to acute respiratory distress syndrome: review of current understanding of critical illness from COVID-19 infection | Yes | No therapeutic data |
| 95 | Chen et al., 2020 | Restoration of leukomonocyte counts is associated with viral clearance in COVID-19 hospitalized patients | Yes | No therapeutic data |
| 96 | Bouadma et al., 2020 | Severe SARS-CoV-2 infections: practical considerations and management strategy for intensivists | Yes | Review |
| 97 | Zhu et al., 2020 | Systematic review of the registered clinical trials of coronavirus disease2019 (COVID-19) | Yes | Review |
| 98 | Yang et al., 2020 | The deadly coronaviruses: the 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China | Yes | Review |
| 99 | Li et al., 2020 | The neuroinvasive potential of SARS CoV2 may play a role in the respiratory failure of COVID 19 patients | Yes | Review |
| 100 | Naicker et al., 2020 | The novel coronavirus 2019 epidemic and kidneys | Yes | Review |
| 101 | Fang et al., 2020 | Transmission dynamics of the COVID 19 outbreak and effectiveness of government interventions: a data driven analysis | Yes | No therapeutic data |
| 102 | Sun et al., 2020 | Understanding of COVID 19 based on current evidence | Yes | Review |
| 103 | Wang et al., 2020 | Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures | Yes | Review |
| 104 | Maoujoud et al., 2020 | What nephrologist should know about COVID-19 outbreak? | Yes | Commentary |
| 105 | Cortegiani et al., 2020 | A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19 | Yes | Review |
| 106 | Ryu et al., 2020 | An interim review of the epidemiological characteristics of 2019 novel coronavirus | Yes | Review |
| 107 | Yang and Shen, 2020 | Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19 | Yes | Review |
| 108 | Fan et al., 2020 | Bat coronaviruses in China | Yes | Review |
| 109 | Russell et al., 2020 | Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury | Yes | Commentary |
| 110 | Liang et al., 2020 | Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells | Yes | No therapeutic data/commentary |
| 111 | Wu et al., 2020 | Co-infection with SARS-CoV-2 and influenza A virus in patient with pneumonia, China | Yes | Commentary |
| 112 | Martinez et al., 2020 | Compounds with therapeutic potential against novel respiratory 2019 coronavirus | Yes | Commentary |
| 113 | Tang et al., 2020 | Coronavirus disease 2019 (COVID-19) pneumonia in a hemodialysis patient | Yes | No therapeutic data |
| 114 | Chang et al., 2020 | Coronavirus disease 2019: coronaviruses and blood safety | Yes | Review |
| 115 | Walker, 2020 | COVID-19, Australia: Epidemiology Report 2 | Yes | Commentary |
| 116 | Lu, 2020 | Drug treatment options for the 2019-new coronavirus (2019-nCoV) | Yes | Commentary |
| 117 | Hellewell et al., 2020 | Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts | Yes | No therapeutic data |
| 118 | Prompetchara et al., 2020 | Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic | Yes | Review |
| 119 | Ashour et al., 2020 | Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks | Yes | Review |
| 120 | Zhou et al., 2020 | Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2 | Yes | No therapeutic data |
| 121 | Devaux et al., 2020 | New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? | Yes | Review |
| 122 | Cauchi and Locht, 2020 | Non-specific effects of live attenuated pertussis vaccine against heterologous infectious and inflammatory diseases | Yes | Review |
| 123 | Chang et al., 2020 | Potential therapeutic agents for COVID-19 based on the analysis of protease and RNA polymerase docking | Yes | No therapeutic data |
| 124 | Pang et al., 2020 | Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review | Yes | Review |
| 125 | Chen et al., 2020 | Recurrence of positive SARS-CoV-2 RNA in COVID-19: a case report | Yes | Commentary |
| 126 | Liu et al., 2020 | Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases | Yes | Review |
| 127 | Gralinski and Menachery, 2020 | Return of the coronavirus: 2019-nCoV | Yes | Commentary |
| 128 | Cao et al., 2020 | SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics | Yes | Commentary |
| 129 | Walls et al., 2020 | Structure, function and antigenicity of the SARS-CoV-2 spike glycoprotein | Yes | Commentary |
| 130 | Xu et al., 2020 | Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS- | Yes | Review |
| 131 | Garrett, 2020 | The art of medicine COVID-19: the medium is the message | Yes | Commentary |
| 132 | Habibzadeh and Stoneman, 2020 | The novel coronavirus: a bird's eye view | Yes | Review |
| 133 | Wu et al., 2020 | The SARS-CoV-2 outbreak: what we know | Yes | Review |
| 134 | Nezhad et al., 2020 | Therapeutic approaches for COVID-19 based on the dynamics of interferon-mediated immune responses | Yes | No therapeutic data |
| 135 | Lu, 2020 | Timely development of vaccines against SARS-CoV-2 | Yes | Commentary |
| 136 | Kim et al., 2020 | Viral load kinetics of SARS-CoV-2 infection in first two patients in Korea | Yes | Commentary |
| 137 | Sekhar, 2020 | Virtual screening based prediction of potential drugs for COVID-19 | Yes | No therapeutic data |
| 138 | Park et al., 2020 | Virus isolation from the first patient with SARS-CoV-2 in Korea | Yes | Commentary |
| 139 | Lake, 2020 | What we know so far: COVID-19 current clinical knowledge and research | Yes | Review |
| 140 | Ralph et al., 2020 | 2019-nCoV (Wuhan virus), a novel coronavirus: human-to-human transmission, travel-related cases, and vaccine readiness | Yes | Review |
| 141 | Jin, 2020 | A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) | Yes | Review |
| 142 | Liu et al., 2020 | Association of cardiovascular manifestations with in-hospital outcomes in patients with COVID-19: a hospital staff data | Yes | No therapeutic data |
| 143 | Lai et al., 2020 | Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths | Yes | Review |
| 144 | Bordi et al., 2020 | Differential diagnosis of illness in patients under investigation for the novel coronavirus (SARS-CoV-2), Italy, February 2020 | Yes | Commentary |
| 145 | Li, 2020 | Diagnosis and clinical management of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: an operational recommendation of Peking Union Medical College Hospital (V2.0) | Yes | Review |
| 146 | Song and Karako, 2020 | COVID-19: real-time dissemination of scientific information to fight a public health emergency of international concern | Yes | Commentary |
| 147 | Vankadari and Wilce, 2020 | Emerging WuHan (COVID-19) coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26 | Yes | Review |
| 148 | Hsih et al., 2020 | Featuring COVID-19 cases via screening symptomatic patients with epidemiologic link during flu season in a medical center of central Taiwan | Yes | No therapeutic data |
| 149 | Stoecklin et al., 2020 | First cases of coronavirus disease 2019 (COVID-19) in France: surveillance, investigations and control measures, January 2020 | Yes | No therapeutic data |
| 150 | Chan et al., 2020 | Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan | Yes | No therapeutic data |
| 151 | Boulos and Geraghty, 2020 | Geographical tracking and mapping of coronavirus disease COVID-19/severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) epidemic and associated events around the world: how 21st century GIS technologies are supporting the global fight against outbreaks and epidemics | Yes | No therapeutic data |
| 152 | Zeng et al., 2020 | Mortality of COVID-19 is associated with cellular immune function compared to immune function in Chinese Han population | Yes | No therapeutic data |
| 153 | Ahmed et al., 2020 | Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies | Yes | No therapeutic data |
| 154 | Lai et al., 2020 | Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges | Yes | Review |
| 155 | Alhazzani et al., 2020 | Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) | Yes | No therapeutic data |
| 156 | Guo et al., 2020 | The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status | Yes | Review |
| 157 | Yang et al., 2020 | Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective | Yes | Review |
| 158 | Liu et al., 2020 | Therapeutic effects of dipyridamole on COVID-19 patients with coagulation dysfunction | Yes | No therapeutic data |
| 159 | World Health Organization, 2020 | Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected | Yes | Guidelines |
| 160 | Li et al., 2020 | Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis | Yes | No therapeutic data |
| 161 | Mao et al., 2020 | Clinical and pathological characteristics of 2019 novel coronavirus disease (COVID-19): a systematic reviews | Yes | Review |
| 162 | Cui et al., 2020 | Clinical features and sexual transmission potential of SARS-CoV-2 infected female patients: a descriptive study in Wuhan, China | Yes | No therapeutic data |
| 163 | Saw Swee Hock School of Public Health, 2020 | COVID-19 science report: therapeutics | Yes | Report |
| 164 | Yao, 2020 | In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) | Yes | Commentary |
| 165 | Pongpirul et al., 2020 | Journey of a Thai taxi driver and novel coronavirus | Yes | No therapeutic data |
| 166 | Liu et al., 2020 | A locally transmitted case of SARS-CoV-2 infection in Taiwan | Yes | No therapeutic data |
| 167 | Velavan and Meyer, 2020 | The COVID-19 epidemic | Yes | Commentary |
Consensus on final inclusion of studies (N=41) (negotiated without the need for a third reviewer) is presented in Table III.
Table III.
Data extracted from included papers
| Author/title/DOI | Sample size | Mean age (years) | Gender | Type of study | Therapeutic treatment | Type: N (%) | Outcomes (recovery/mortality) | Adverse events | Quality assessment (applicable/inapplicable) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cao et al. A trial of lopinavir–ritonavir in adults hospitalized with severe COVID-19. N Engl J Med 2020. https://doi.org/10.1056/NEJMoa2001282 | 199 | 58 | 120 M 79 F |
Randomized clinical trial | Lopinavir and ritonavir | Lopinavir and ritonavir: 50%Standard care: 50% | In hospitalized adult patients with severe COVID-19, no benefit was observed with lopinavir–ritonavir treatment beyond standard careNineteen deaths among patients who received the intervention | 14% of patients who received lopinavir–ritonavir developed gastrointestinal adverse events, including anorexia, nausea, abdominal discomfort or diarrhoea, as well as two serious adverse events (both acute gastritis)Two recipients had self-limited skin eruptions | The study addressed a focused issue Randomization with intention-to-treat analysis The population who entered the study were accounted for properly in the conclusion Not blinded The two groups who entered the study were similar and treated equally The primary outcome was specified clearly |
| 2 | Cao et al. Clinical features and short-term outcomes of 18 patients with coronavirus disease 2019 in intensive care unit. Intensive Care Med 2020.https://doi.org/10.1007/s00134-020-05987-7 | 41 | 49 | 30 M 11 F |
Prospective | Antibiotics and oseltamivir (orally 75 mg twice daily)Corticosteroid was given as a combined regimen if severe community-acquired pneumonia was diagnosed by physicians at the designated hospital | All patients received empirical antibiotic treatmentAntiviral (oseltamivir): 38 (93%)Systemic corticosteroid: 9 (22%) | Antiviral: 12 ICU admissions (92%) Antibiotic: 13 ICU admissions (100%) Corticosteroid: six ICU admissions (46%) |
Not reported | Adverse events not reported Treatment given not specified Types of antibiotics given not mentioned |
| 3 | Chen et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv 2020. https://doi.org/10.1101/2020.03.17.200 | 236 | 56 (25–86) | Favipiravir group:59 M 57 FArbidol group:51 M 69 F |
Randomized controlled trial | Favipiravir Arbidol |
Antiviral: 116 Antiviral: 120 |
71 patients recovered | Abnormal liver function tests, raised serum uric acid, psychiatric symptom reactions and digestive tract reactions | No effective antiviral drug was reported, and the drugs mentioned were based on the sixth edition of the guidelines of Chinese diagnosis and treatment plan of COVID-19 patients |
| 4 | Chen et al. Thalidomide combined with low-dose glucocorticoid in the treatment of COVID-19 pneumonia 2020. Preprints 2020; 2020020395. https://www.preprints.org/manuscript/202002.0395/v1 | 1 | 45 | F | Case report | Thalidomide and low-dose glucocorticoid. The patient was first treated with oral ofloxacin and oseltamivir, but her condition deteriorated. The patient was subsequently treated with lopinavir/ritonavir | Thalidomide inhibits the cytokine surge and regulates immune functions. In addition, it can be used to calm patients down in order to reduce oxygen consumption and relieve digestive symptoms | Not reported | Randomized controlled trials are needed | |
| 5 | Chen et al. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering 2020. https://doi.org/10.1016/j.eng.2020.02.006 | 61 | 62 | Not mentioned | Open labelled clinical trial | Oseltamivir or peramivir (according to standard therapy) and antibiotics were given based on positive blood test results | Not mentioned | 17.6% of patients in the experimental group and 54.5% of patients in the control group died | Not reported | With only 17 patients using mesenchymal stem cells, it cannot be guaranteed that every step was perfect during the phase with a single clinical trialSome patients refused to attend and some did not complete follow-up. Thus, there is still concern about the long-term safety of mesenchymal stem cell transplantation for the treatment of H7N9-induced ARDS, despite the lack of side-effects observed in this clinical trial This study was undertaken on patients with H7N9 not COVID-19 |
| 6 | Chen et al. Retrospective analysis of clinical features in 101 death cases with COVID-19. medRxiv 2020. https://doi.org/10.1101/2020.03.09.20033068 | 101 | 65.46 | 64 M 37 F |
Single centre and observational study (retrospective) | Antiviral drugs, including oseltamivir, ribavirin, lopinavir, ritonavir, ganciclovir and interferon Glucocorticoids, IV immunoglobulins and thymosin preparations Antibiotic treatment, including cephalosporins, quinolones, carbapenems, linezolid and tigecycline |
Antiviral: 61 (60.4%) Glucocorticoid: 59 (58.42%)IV immunoglobulin: 63.37% Thymosin: 44.55%Antibiotic: 101 (100%)Restricted antibiotic: 63 (62.38%)Antifungal: 23 (22.78%) |
101 patients died | Not reported | Only the critical death patients are included No comparison was made between the improvement groups |
| 7 | Chen et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect 2020. https://doi.org/10.1016/j.jinf.2020.03.004 | 249 | 51 | 126 M 123 F |
Retrospective, single-centre study | Antiviral drugs (e.g. lopinavir/ritonavir, arbidol) were used in a small proportion of patients Corticosteroids were not used unless considered necessary by an expert panel (e.g. ARDS) | Not mentioned | Two patients died (0.8%) 22 patients were admitted to ICU (8.8%) Eight patients developed ARDS (3.2%) 215 patients were discharged (86.3%) |
Not reported | A small proportion the patients were still hospitalized at the time of manuscript submission. Therefore, clinical outcomes in these patients were not available and continued observations are needed SARS-CoV-2 was not tested daily for all patients. Hence, the actual time to viral clearance should be shorter than the estimated value |
| 8 | Chen et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13. | 99 | 55.5 | 67 M 32 F |
Retrospective, single-centre descriptive study | Antibiotic: cephalo-sporins, quinolones, carbapenems, tigecycline against meticillin-resistant Staphylococcus aureus, linezolid Antifungal Antiviral: oseltamivir, ganciclovir and lopinavir/ritonavir Glucocorticoid: methylprednisolone sodium succinate, methylprednisolone and dexamethasone- Immunoglobulin |
Antibiotic: 70 (71%) Antifungal: 15 (15%) Antiviral: 75 (76%), including oseltamivir (75 mg every 12 h, orally), ganciclovir (0.25 g every 12 h, intravenously), and lopinavir/ritonavir (500 mg twice daily, orally). The duration of antiviral treatment was 3–14 days Glucocorticoid: 19 (19%) IV immunoglobulin: 27 (27%) |
11 (11%) patients died | Not reported or NA | Suspected but undiagnosed cases were ruled out in the analyses More detailed patient information, particularly regarding clinical outcomes, was unavailable at the time of analysis |
| 9 | Chen et al. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. medRxiv 2020. https://doi.org/10.1101/2020.03.03.20030353 | 291 | 46 | 145 M 146 F |
Double-centre observational study | Antiviral including lopinavir and ritonavir Recombinant human interferon-α2b Recombinant cytokine gene-derived protein Arbidol hydrochloride Chinese medicine | Antiviral: 285 (97.9%)Lopinavir/ritonavir: 75.9% Recombinant human interferon-α2b: 45.4% Recombinant cytokine gene-derived protein: 18.9% Arbidol hydrochloride: 17.2% Chinese medicine: 281 (96.6%) |
Two (0.7%) patients died | Not reported | Due to limitations of the retrospective study, laboratory examinations were performed according to the clinical care needs of the patients; as such, some laboratory examinations were not completed Given the short observation period, nearly half of the patients were still receiving treatment in hospital at the end of the follow-up period, and it was not possible to determine mortality and prognosis of the whole case series |
| 10 | Cui et al. A 55-day-old female infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J Infect Dis 2020. https://doi.org/10.1093/infdis/jiaa113 | 1 | 55 days | F | Case report | Inhaled interferon-α1b (15 μg, bid); amoxicillin potassium clavulanate (30 mg/kg, Q8H, ivgtt) | NA | NA | NA | Case report for infant patient Adverse events and outcomes not reported |
| 11 | Du et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. SSRN 2020. https://ssrn.com/abstract=3546088 | 191 | 56 | 119 M 72 F |
Retrospective, multi-centre cohort study | Antibiotic Antiviral (lopinavir and ritonavir) Corticosteroid IV immunoglobulin |
Antibiotic: 181 (95%) Antiviral (lopinavir and ritonavir): 41 (21%) Corticosteroid: 57 (30%) IV immunoglobulin: 46 (24%) |
181 (95%) patients received antibiotics: 53 (98%) died, 128 (93%) survived (P=0.15) 41 (21%) patients received antivirals: 12 (22%) died, 29 (21%) survived (P=0.87) 57 (30%) patients received corticosteroid: 26 (48%) died, 31 (23%) survived (P=0.0005) 46 (24%) patients received IV immunoglobulin: 36 (67%) died, 10 (7%) survived (P<0.0001) 54 patients died in hospital |
Not reported | Lack of effective antivirals, inadequate adherence to standard supportive therapy, and high-dose corticosteroid use may also have contributed to the poor clinical outcomes in some patients |
| 12 | Gautret et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents 2020:105949. https://doi.org/10.1016/j.ijantimicag.2020.105949 | Treated: 20 Control: 16 Total: 36 |
45.1 | 15 M 21 other |
Open label non- randomized clinical trial | Hydroxychloroquine and azithromycin | Hydroxychloroquine sulfate 200 mg, three times per day for 10 days | On day 6 post inclusion, 100% of patients treated with a combination of hydroxychloroquine and azithromycin were virologicaly cured, compared with 57.1% of patients treated with hydroxychloroquine alone and 12.5% of patients in the control group | One patient stopped treatment on day 3 post inclusion due to nausea | Clinical follow-up and occurrence of side-effects were not discussed |
| 13 | Guan et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020. https://doi.org/10.1056/NEJMoa2002032 | 1099 | 47.9 | 41.1% F | Retrospective observational study | IV antibiotic Oseltamivir Antifungal Systemic glucocorticoid |
Antibiotic: 637 (58%) Oseltamivir: 393 (35.8%) Antifungal: 31 (2.8%) Glucocorticoid: 204 (18.6%) |
5.0% of patients were admitted to the ICU, 2.3% underwent invasive mechanical ventilation and 1.4% died among the 173 patients with severe disease | Not reported | Drug dose, frequency and duration were not included |
| 14 | Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020. https://doi.org/10.1056/NEJMoa2001191 | 1 | 35 | M | Case report | Antipyretic consisting of guaifenesin | 650 mg 600 mg |
Discharged with no symptoms | Not reported | This was only a single case study and does not represent the whole population As this was a case report, it is not certain that the positive impact on the patient's health was due to the medication taken Randomized controlled trials are needed |
| 15 | Huang et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. | 41 | 49 | 30 M (73%) 11 F (27%) |
Prospective collection and analysed data for patients with pneumonia | Antiviral: 38 (93%) Antibiotic: 41 (100%) Corticosteroid: 9 (22%) |
Not mentioned | One patient was admitted to ICU Six patients died |
Not reported | As the causative pathogen has just been identified, kinetics of viral load and antibody titres were not available at the time of the study |
| 16 | Huang et al. Early and critical care in severe patients with COVID-19 in Jiangsu Province, China: a descriptive study. 2020. https://doi.org/10.21203/rs.3.rs-17397/v1 | 60 | 57 | 58.3% M 42.8% other |
Multi-centre retrospective cohort study was conducted to extract and analyse epidemiological, clinical and laboratory data and treatment of 60 severe cases | Antiviral: 60 (100%) Abidor: 50 (83.3) Lopinavir/ritonavir: 41 (68.3) Interferon: 12 (20.0) Ribavirin: 7 (11.7) Oseltamivir: 2 (3.3) Fluoroquinolone: (61.7%) |
34 patients (56.7%) received IV glucocorticoids at doses ranging from 40 to 80 mg/day 28 patients (46.7%) received immunoglobulin (IgG enriched) injections for 5–9 days of immunoregulation |
50 patients improved significantly Two patients were discharged Eight patients remained in a serious condition |
Four patients who developed secondary infections received glucocorticoids | Most drug doses, frequencies and durations were not included The effect of glucocorticoids was not significant |
| 17 | Huang et al. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. medRxiv 2020. https://doi.org/10.1101/2020.02.27.20029009 | 36 | 69.22 | 25 M (69.44%) 11 F (30.56%) |
Retrospective, single-centre study | Antibiotic: 36 (100%) Antiviral: 35 (97.22%) Glucocorticoid: 25 (69.44%) |
Not mentioned | All patients died | All patients died | Drug dose, frequency and duration were not included |
| 18 | Jian-ya G. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. medRxiv 2020. https://doi.org/10.1101/2020.02.20.20025536 | 51 | 45 | 32 M (62.7%) 19 F (37.3%) |
Retrospective, single-centre case series | Oseltamivir (oral): 7 (13.7%) Interferon (oral): 51 (100%) Kaletra (oral): 51 (100%) Thymopentin (IM): 48 (94.1%) Traditional Chinese medicine decoction (oral): 28 (54.9%) Reduling (IV): 30 (58.8%) Xuebijing (IV): 2 (3.9%) |
Not mentioned | One patient died with shock complications | Six patients had an obvious decline in appetite . |
Drug dose, frequency and duration were not included |
| 19 | Liang et al. Clinical characteristics of 457 cases with coronavirus disease 2019. Available at SSRN. 2020. https://doi.org/10.2139/ssrn.3543581 | 457 | Varies | 267 M (58%) 9 pregnant women (2%) |
Systematic review | Antiviral: 352 (77%) Antibacterial: 258 (56%) Glucocorticoid: 130 (28%) |
Not mentioned | 195 patients improved and were discharged | 35 patients died | Drug dose, frequency and duration were not included |
| 20 | Liao et al. Epidemiological and clinical characteristics of COVID-19 in adolescents and young adults. medRxiv 2020. https://doi.org/10.1101/2020.03.10.20032136 | 46 | Not mentioned because they were two groups | 17 M (53.1) 15 F (46.9) |
Retrospective case series data | Antiviral: 46 (100.0%) Antifungal: 5 (10.9%) Glucocorticoid |
Not mentioned | 78.3% of patients were discharged | Three patients developed acute kidney injury during treatment | At the end of this study, nearly 20% of the patients were still hospitalized |
| 21 | Lim et al. Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J Korean Med Sci 2020; 35. https://doi.org/10.3346/jkms.2020.35.e79 | 1 | 54 | M | Case report | Lopinavir/ritonavir | 200 mg 50 mg (two tablets bid) |
Reduced viral load and improved clinical symptoms | The patient also complained of psychiatric symptoms such as depression, insomnia and suicidal thoughts after isolation | This was a single case and does not represent the whole population Randomized controlled trials are needed |
| 22 | Liu et al. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis 2020. https://doi.org/10.1016/j.ijid.2020.03.013 | 10 | 42 | 6 F 4 other |
Retrospective observational single-centre study | Lopinavir, Interferon-α2b atomization inhalation |
400 mg every twelve hours |
Oeosinophil counts presented potential as predictor of the development of COVID-19 Seven patients were discharged Three patients stopped lopinavir: two deteriorated and one was hospitalized for longer than other patients who continued taking lopinavir |
Digestive adverse effect and hypokalaemia | Small sample size |
| 23 | Liu et al. Epidemiological, clinical characteristics and outcome of medical staff infected with COVID-19 in Wuhan, China: a retrospective case series analysis. medRxiv 2020. https://doi.org/10.1101/2020.03.09.20033118 | 64 | 35 (29–43) | 23 M 41 F |
Single centre- retrospective-observational study | Immunoglobulin Thymosin Corticosteroid | Antibody: 23 Hormone: 33 Steroid hormone: 7 | 34 patients were discharged 30 patients were still hospitalized |
Not reported | Preliminary insight into epidemiological features and clinical outcomes Single centre |
| 24 | Liu et al. Detection of COVID-19 in children in early January 2020 in Wuhan, China. N Engl J Med 2020. https://doi.org/10.1056/NEJMc2003717 | Six | 3 (1–7) | 2 M 4 F |
Retrospective case series analysis | Ribavirin Oseltamivir Glucocorticoid IV immunoglobulin |
Antiviral: 2 Antiviral: 6 Steroid hormone: 4 Antibody: 1 |
Six patients recovered | Not reported | Small sample size |
| 25 | Liu et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. medRxiv 2020. https://doi.org/10.1101/2020.02.17.20024166 | 109 | 55 | 59 M 50 F |
Retrospective case series analysis | Glucocorticoid IV immunoglobulin | Steroid hormone: 43 Antibody: 32 Antibiotic: 105 Antiviral: 105 |
31 patients died | Not reported | This study did not mention the names of the therapeutic treatment used among patients with ARDS |
| 26 | Lo et al. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci 2020; 16:1698–707. https://doi.org/10.7150/ijbs.45357 | 10 | 54 (27–64) | 3 M 1 teenager 6 other |
Retrospective case series analysis | Lopinavir Ritonavir |
Antiviral: 10 | Five patients were discharged Five patients were still hospitalized |
Not reported | Small sample size, so difficult to draw a definite conclusion Single centre Half of the enrolled patients were still hospitalized at the time of submission of this paper. Therefore, there may have been bias regarding the prognosis of the patients |
| 27 | Mo et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis 2020. https://doi.org/10.1093/cid/ciaa270 | 155 | 54 (42–66) | 86 M 69 other |
Single-centre, retrospective case series analysis | Arbidol Lopinavir and ritonavir Interferon inhalation Immune enhancer |
Antiviral: 31 Antiviral: 27 Antiviral: 30 Immune enhancer: 14 |
22 patients died | Not reported | Selection bias may have occurred, and a large-scale nationwide study is needed |
| 28 | Wang et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020. https://doi.org/10.1001/jama.2020.1585 | 138 | 56 (42–68) | 75 M 63 F |
Retrospective, single-centre case series | Oseltamivir Moxifloxcain Ceftriaxone Azithromycin Glucocorticoid |
Antiviral: 124 Antibacterial: 89 Antibacterial: 34 Antibacterial: 25 Glucocorticoid: 62 |
47 patients were discharged Six patients died 85 patients were still hospitalized |
Not reported | Most patients were still hospitalized at the time of manuscript submission. Therefore, there may have been bias regarding the prognosis of the patients. |
| 29 | Wang et al. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020. https://doi.org/10.1093/cid/ciaa272 | 69 | 42 (35–62) | 32 M 37 F |
Retrospective case series | - | Antiviral: 66 Antibiotic: 66 Antifungal: 8 Corticosteroid: 10 Arbidol: 36 |
44 patients were still hospitalized 18 patients were discharged Five patients died |
Not reported | Drug dose, frequency and duration were not included |
| 30 | Wu et al. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19). medRxiv 2020. https://doi.org/10.1101/2020.02.26.20028589 | 188 | 52 | 119 M 69 other |
Retrospective cohort study | - | Antibiotic: 185 Antiviral: 158 Corticosteroid: 59 |
43 patients died 145 patients were discharged 12 patients were still hospitalized |
Not reported | Drug dose, frequency and duration were not included |
| 31 | Wu F et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579:265–9. | 1 | 41 | M | Epidemiological investigations | Antiviral Antibiotic Glucocorticoid Oxygen |
Oseltamivir Cefoselis Not mentioned Mechanical ventilation |
Recovered | Not reported | Applicable |
| 32 | Xu et al. Clinical characteristics of SARS-CoV-2 pneumonia compared to controls in Chinese Han population. medRxiv 2020. https://doi.org/10.1101/2020.03.08.20031658 | Patients: 69 Controls: 14,117 |
57 | 50.7% M 49.3% F |
Retrospective, multi-centre case series | Antiviral Antibiotic Oxygen |
Oseltamivir: 38 (55.1%) Moxifloxacin, ceftriaxone, azithromycin, tigecycline or linezolid: 31 (44.9%) Mechanical ventilation: 2 Invasive ventilator: 2 |
Three patients were discharged One patient recovered One patient died |
Six patients with a significant increase in IL6 were also treated with methylprednisolone | Applicable |
| 33 | Xu et al. Clinical findings in critical ill patients infected with SARS-CoV-2 in Guangdong Province, China: a multi-center, retrospective, observational study. medRxiv 2020. https://doi.org/10.1101/2020.03.03.20030668 | 45 | 56.7 | 29 M (64.4%) 16 F (35.6%) |
Multi-centre, retrospective, observational study | Antiviral: 45 (100) patients Antibacterial: 45 (100) Antifungal: 19 (42.2) Convalescent plasma: 6 (13.3) Glucocorticoid: 21 (46.7) Immunoglobulin: 28 (62.2) Albumin: 35 (77.8) |
Osehamivir ribavirin Not mentioned Not mentioned Not mentioned Not mentioned Not mentioned Not mentioned |
23 (51.1%) patients were discharged from the ICU 11 (24.2%) patients were discharged One (2.2%) patient died |
37 (82.2%) patients developed ARDS and 13 (28.9%) patients developed septic shock 20 (44.4%) patients required intubation and nine (20%) patients required extracorporeal membrane oxygenation |
At the time of study submission, half of the patients had not been discharged from the ICU; as such, it was difficult to estimate ICU stay, ventilation-free days, case fatality rate and the predictors of fatality Drug dose, frequency and duration were not included |
| 34 | Xu et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020; 368:m606. | 62 | 41 | 35 M (56%) 27 F (44%) |
Retrospective study | Antiviral: 55 (89%) Antibiotic Corticosteroid and gamma globulin |
Interferon-α inhalation: 8 (13%) Lopinavir/ritonavir: 4 (6%) Arbidol + interferon-α inhalation: 1 (2%) Lopinavir/ritonavir + interferon-α inhalation: 21 (34%) Arbidol + lopinavir/ritonavir: 17 (28%) Arbidol + lopinavir/ritonavir + interferon-α inhalation: 4 (6%) 28 (45%) 16 (26%) |
No deaths | Not reported | At the time of study submission, most patients had not been discharged, so it was difficult to estimate the case fatality rate or the predictors of fatality |
| 35 | Xu et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020. https://doi.org/10.1016/S2213-2600(20)30076-X | 1 | 50 | M | Postmortem biopsies | Antiviral Antibiotic Corticosteroid |
Interferon-α2b atomization Lopinavir + ritonavir Moxifloxacin Methylprednisolone |
Died due to cardiac arrest | Chest x-ray showed progressive infiltrate and diffuse gridding shadow in both lungs. Hypoxaemia and shortness of breath worsened and patient had sudden cardiac arrest | This was a single case study and does not represent the whole population The patient refused ventilator support in the ICU repeatedly because he suffered from claustrophobia; therefore, he received high-flow nasal cannula There is a need for randomized controlled trials |
| 36 | Yang et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020. https://doi.org/10.1016/S2213-2600(20)30079-5 | 52 | 59.7 | 35 M (67%) 17 F (33%) |
Single-centre retrospective, observational study | Vasoconstrictor Antiviral: 23 (44%) Antibacterial Glucocorticoid Immunoglobulin |
18 (35%) Oseltamivir: 18 (35%) Ganciclovir: 14 (27%) Lopinavir: 7 (13.5%). 49 (94%) 30 (58%) 28 (54%) |
32 (61.5%) patients died | Not reported | Due to the exploratory nature of the study, which was not driven by formal hypotheses, the sample size calculation was waived The researchers acknowledged that some specific information from the ICU was missing, such as mechanical ventilation settings Drug dose, frequency and duration were not included |
| 37 | Young et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020. https://doi.org/10.1001/jama.2020.3204 | 18 | 47 | 9 M (50%) 9 F (50%) |
Descriptive case series | Antiretroviral Antiviral Antibiotic |
Lopinavir/ritonavir Oseltamivir Not reported |
No deaths | Not reported | Small sample size Drug dose, frequency and duration were not included |
| 38 | Zhang et al. Clinical characteristics of 82 death cases with COVID-19. medRxiv 2020. https://doi.org/10.1101/2020.02.26.20028191 | 82 | 72.5 | 65.9% M | Death cases | Antiviral Antibiotic Corticosteroid |
82 (100%) 82 (100%) 29 (35.3%) |
Not reported | The study was performed in one setting. No information was given about the hospital's capabilities in terms of personnel or equipment because the mortality rate from this centre was a little higher than other centres Traditional Chinese medicine was given Drug dose, frequency and duration were not included |
|
| 39 | Zhang et al. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. medRxiv 2020. https://doi.org/10.1101/2020.03.02.20030452 | 221 | 55 | 108 M (48.9%) 113 F (51.1%) |
Retrospective case study | Antiviral: 196 (88.7%) Antibiotic Corticosteroid: 115 (52.0%) |
Oseltamivir Arbidol hydrochloride Interferon-α atomization inhalation Lopinavir/ritonavir Moxifloxacin hydrochloride Piperacillin sodium tazobactam sodium Cefoperazone sulbactam Glucocorticoid: 64 (49.6%) |
12 (5.4%) patients died | Not reported | The dose and duration of IV glucocorticoid treatment showed no difference in symptomatic relief and death Drug dose, frequency and duration were not included |
| 40 | Zhang et al. The potential role of IL-6 in monitoring coronavirus disease 2019. https://doi.org/10.1101/2020.03.01.20029769 | 80 | 53 | 46 F (57.5%) 34 M (42.5%) |
Data collection (clinical data from patients with COVID-19 diagnosed by laboratory test in study institution) Observation of clinical manifestation |
Antibiotic: 73 (91.25%) Oseltamivir: 20 (25%) Ribavirin, ganciclovir or peramivir: 47 (58.75%) Arbidol: 49 (61.25%) Antifungal: 10 (12.5%) IV immunoglobin: 36 (45%) Corticosteroid: 29 (36·25%) |
Not mentioned | IL-6 may be used as a biomarker for disease monitoring in severe cases of COVID-19 | Not reported | Drug dose, frequency and duration were not included IL-6 and the pathogenesis of COVID-19 remains elusive |
| 41 | Zhou et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020. https://doi.org/10.1016/S0140-6736(20)30566-3 | 191 | 56 | 119 M (62%) 72 F (38%) |
Retrospective cohort study | Antibiotic: 181 (95%) Antiviral: 41 (21%) Corticosteroid: 57 (30%) IV immunoglobin: 46 (24%) |
Lopinavir/ritonavir | 137 patients were discharged 54 patients died |
191 patients | There was no observation of a shortening of the duration of viral shedding after lopinavir/ritonavir treatment Drug dose, frequency and duration were not included |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; ICU, intensive care unit; ARDS, acute respiratory distress syndrome; IL-6, interleukin-6; NA, not applicable; IV, intravenous; IM, intramuscular.
Forty-one studies were included in this review. These were clinical trials (N=3), case reports (N=7), case series (N=10), and retrospective (N=11) and prospective (N=10) observational studies. Thirty-six studies were conducted in China, and one in each of Korea, the USA, France, Singapore and Macau.
Patient characteristics
In total, 8806 patients were reported in the 41 studies included in this review. The mean age of patients was 50.8 years based on 39 studies; age was not specified in two studies.
Reported therapeutics
Corticosteroids, an anti-inflammatory medication, were reported most commonly in this systematic review (N=25), using different names and product characteristics (corticosteroid N=21, methylprednisolone N=3, dexamethasone N=1). Use of lopinavir, an antiviral HIV medication (N=21) – in combination with ritonavir (N=18) or alone (N=3) – oseltamivir (N=16) and arbidol hydrochloride (N=8) was also reported.
In terms of antibacterial agents, moxifloxacin (N=4) and tigecycline were reported most frequently.
Convalescent plasma therapy was reported in one multi-centre retrospective observational study of six patients.
Treatment outcome
The outcome measures recorded were patient discharge and recovery, ongoing hospitalization and mortality (Table III).
Discussion
To the authors' knowledge, this is the first systematic review related to medication used to treat patients with COVID-19. Only 41 eligible research articles were identified and included in this review [2,5,[12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49]]. Of these, three studies were clinical trials; the rest were case reports, case series, or prospective or retrospective observational studies. Systemic corticosteroids with different names and formulations were reported most frequently, followed by the antivirals lopinavir, oseltamivir and arbidol hydrochloride. Convalescent plasma therapy was reported in one multi-centre retrospective observational study and was administered to six patients.
Although quality assessment was applied to the research articles included in this review, there was insufficient evidence to conduct a meta-analysis, and it was not possible to conduct subgroup analysis (adults and children; different formulations, dosages and durations).
Most studies included in this review were of low quality, with incomplete or inconsistent information on study design and outcome. As such, it was difficult to analyse the medication in terms of efficacy and safety.
Despite these limitations, this is the first systematic review on medication used to treat patients with COVID-19, and provides up-to-date insight on the current therapeutic guidelines for management of these patients. Most of the medications reported in this review are available in the USA, Saudi Arabia, Europe and Egypt (Table I).
Corticosteroids were the most commonly reported medication in this review; however, they are not recommended in any guidelines. In the absence of conclusive scientific evidence, WHO and the US Centers for Disease Control and Prevention (CDC) have recommended that corticosteroids should not be used routinely in patients with COVID-19 for treatment of viral pneumonia or acute respiratory distress syndrome (ARDS) unless indicated for other conditions, such as asthma or chronic obstructive pulmonary disease exacerbation, or septic shock [5,50,51]. Careful use of low-to-moderate doses of corticosteroids as a short course is advised. Hyperglycaemia, hypernatraemia and hypokalaemia are the most common adverse effects associated with the use of corticosteroids and should be monitored routinely [5,51].
Lopinavir/ritonavir (Kaletra) was the second most commonly reported medication in this review. A randomized clinical trial reported that this HIV treatment had negative outcomes for patients with COVID-19 (Table II) [30,[52], [53], [54]]. No benefit of lopinavir/ritonavir treatment compared with standard care was observed in this study, and 19 patients who received the intervention died. However, some study limitations were observed, including lack of blinding. RCT NCT04252885 and the SOLIDARITY trial are ongoing to determine the efficacy of lopinavir/ritonavir in patients with COVID-19 [52].
Oseltamivir (Tamiflu), used to treat influenza A and influenza B, was the third most commonly reported medication in this review. Oseltamivir has been recommended by WHO for people at high risk of infection for prevention of pandemic influenza. A retrospective observational study reported the use of oseltamivir in 1099 patients with COVID-19; however, the study was not able to provide any solid data on the effectiveness of oseltamivir in the prevention or treatment of COVID-19. Study limitations included incomplete documentation of patient data and recall bias [55,56].
Arbidol hydrochloride was the fourth most commonly reported medication in this review. It is a broad-spectrum inhibitor of influenza A and B virus, parainfluenza virus and other viruses, including hepatitis C virus. Arbidol hydrochloride is used in Russia and China, but has not yet been approved for use in other countries [52]. However, no conclusive evidence of its efficacy in patients with COVID-19 was reported. In this review, it was reported together with favipiravir, which was approved for the treatment of novel influenza on 15th February 2020 in China [52].
Chloroquine phosphate and hydroxychloroquine were reported in this review and showed favourable outcomes in the recovery of patients with COVID-19 [6,7,[57], [58], [59], [60]]. These two medications are likely to share the same mechanism of action. Chloroquine, an antimalarial, has shown positive outcomes in patients with COVID-19. Furthermore, hydroxychloroquine has shown significant effectiveness against intracellular pathogens such as Coxiella burnetii, the agent of Q fever [22]. This French open label, non- randomized clinical trial was promising and the first clinical trial of these medications in patients with COVID-19. The effect of hydroxychloroquine was significant because it showed a reduction in the viral load compared with the control group [22]. Moreover, the effect of hydroxychloroquine was significantly more potent when given in conjunction with azithromycin. However, clinical follow-up and the occurrence of adverse effects were not discussed in the study, and further work is needed to reduce the morbidity and mortality of COVID-19 [[57], [58], [59]]. Although chloroquine and hydroxychloroquine have shown promising activity against SARSCoV-2, there is a risk of arrhythmia associated with their administration. Therefore, caution is required for use at higher cumulative dosages. It is recommended that their use in cases of suspected/confirmed COVID-19 should be restricted to hospitalized patients. On 30th March 2020, the US Food and Drug Administration (FDA) issued an emergency use authorization for chloroquine and hydroxychloroquine to treat patients hospitalized with COVID-19 [60].
Convalescent plasma therapy was reported in a multi-centre cohort research trial of 45 critically ill patients with COVID-19 admitted to an intensive care unit in Wuhan. The findings showed that convalescent plasma therapy was administered to six patients and no transfusion reactions occurred; however, the study did not provide adequate information about the efficacy of convalescent plasma therapy due to the limited sample size and lack of a randomized control group [61,62].
Convalescent plasma therapy could be a promising treatment method for patients with COVID-19. A recent case series from China showed that five critically ill patients with laboratory-confirmed COVID-19 (who had ARDS) improved. After receiving plasma transfusion, their body temperature normalized within 3 days (in four of the five patients), their viral loads became undetectable within 12 days, and three of the five patients were discharged from hospital and were in a stable condition at 37 days post transfusion [63]. On 24th March 2020, the US FDA approved convalescent plasma therapy for investigational use under the traditional Investigational New Drug Applications regulatory pathway, and for eligible patients who have confirmed COVID-19 and severe or immediately life-threatening conditions such as respiratory failure, septic shock, and/or multiple organ dysfunction or failure [64,65]. Notably there are potential risks and ethical issues associated with convalescent plasma therapy, including increased risk of a thrombotic event (from 0.04% to 14.9%), lack of high-quality research in this particular area, and the selection of donors with high neutralizing antibody titres [65].
In conclusion, this is the first systematic review of medication used to treat patients with COVID-19. Only 41 research articles were eligible for inclusion in this review, mainly conducted in China, of which only three were clinical trials.
The use of corticosteroids to treat patients with COVID-19 was reported most frequently in this review, despite safety alerts issued by WHO and CDC, followed by lopinavir, oseltamivir and arbidol hydrochloride.
Although further research is warranted as the amount of the evidence increases, this review presents the current picture of treatment modalities used for COVID-19. Efficacy and safety profiles of treatments for COVID-19 will need to be characterized in future studies.
Conflict of interest statement
None declared.
Funding sources
None.
References
- 1.Yang W., Cao Q., Qin L., Wang X., Cheng Z., Pan A. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;S0163–4453(20):388–393. doi: 10.1016/j.jinf.2020.02.016. [published online ahead of print, 2020 Feb 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J. Clinical characteristics of coronavirusdisease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 4.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020:1–4. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C., Qi F., Shi K., Li Y., Li J., Chen Y. Thalidomide combined with low-dose glucocorticoid in the treatment of COVID-19 pneumonia. Preprints. 2020:2020020395. https://www.preprints.org/manuscript/202002.0395/v1 [Google Scholar]
- 6.Colson P., Rolain J.M., Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J Antimicrob Agents. 2020:105923. doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colson P., Rolain J.-M., Lagier J.-C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55(4):1–3. doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Massachusetts General Hospital . MGH; Boston: 2020. COVID-19 treatment guidance.https://www.massgeneral.org/news/coronavirus/treatment-guidance Available at: [last accessed 17 march 2020] [Google Scholar]
- 9.Egypt Ministry of Health and Population . Egypt Ministry of Health and Population; Cairo: 2020. Diagnosis and treatment protocol for COVID 19.https://madamasr.com/en/2020/03/19/feature/politics/your-guide-to-coronavirus-in-egypt/ Available at: [last accessed March 2020] [Google Scholar]
- 10.Saudi Arabia Ministry of Health . Saudi Arabia Ministry of Health; Riyadh: 2020. Coronavirus disease 19 (COVID-19) guidelines.https://www.moh.gov.sa/en/HealthAwareness/EducationalContent/PublicHealth/Pages/corona.aspx Available at: [last accessed March 2020] [Google Scholar]
- 11.Health Protection Surveillance Centre . HPSC; Dublin: 2020. Treatment guidelines for COVID-19 in Ireland.https://www.hpsc.ie/a-z/respiratory/coronavirus/novelcoronavirus/casesinireland/epidemiologyofcovid-19inireland/ Available at: [last accessed March 2020] [Google Scholar]
- 12.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavireritonavir inadults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. https://www.nejm.org/doi/full/10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao J., Hu X., Cheng W., Yu L., Tu W., Liu Q. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med. 2020;(46):851–853. doi: 10.1007/s00134-020-05987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C., Zhang Y., Huang J., Yin P., Cheng Z., Wu J. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.03.17.20037432. [DOI] [Google Scholar]
- 15.Chen J., Hu C., Chen L., Tang L., Zhu Y., Xu X. Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering. 2020 doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J., Fan H., Zhang L., Hunag B., Zhu M., Zhou Y. Retrospective analysis of clinical features in 101 death cases with COVID-19. medRxiv. 2020 doi: 10.1101/2020.03.09.20033068. [DOI] [Google Scholar]
- 17.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80-(5):e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen X., Zheng F., Qing Y., Ding S., Yang D., Lei C. Epidemiological and clinical features of 291 cases with coronavirus disease 2019 in areas adjacent to Hubei, China: a double-center observational study. medRxiv. 2020 doi: 10.1101/2020.03.03.20030353. [DOI] [Google Scholar]
- 20.Cui Y., Tian M., Huang D., Wang X., Huang Y., Fan L. A 55-day-old female infant infected with COVID 19: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020;221(11):1775–1781. doi: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gautret P., Lagier J., Parola P., Hoang V., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label nonrandomized clinical trial. Int J Antimicrob Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Holshue M.L., DeBolt C., Lindquist S., Lofy K., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang M., Yang Y., Shang F., Zheng Y., Zhao W., Luo L. Early and critical care in severe patients with COVID-19 in Jiangsu Province, China: a descriptive study. Res Square. 2020 doi: 10.21203/rs.3.rs-17397/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang Y., Zhou H., Yang R., Xu Y., Feng X., Gong P. Clinical characteristics of 36 non-survivors with COVID-19 in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.02.27.20029009. [DOI] [Google Scholar]
- 27.Jian-ya G. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing, China. medRxiv. 2020 doi: 10.1101/2020.02.20.20025536. [DOI] [Google Scholar]
- 28.Liang B., Zhao Y., Zhang X., Lu J., Gu N. 2020. Clinical characteristics of 457 cases with coronavirus disease 2019. Available at: SSRN. [DOI] [Google Scholar]
- 29.Liao J., Fan S., Chen J., Wu J., Xu S., Guo Y. Epidemiological and clinical characteristics of COVID-19 in adolescents and young adults. medRxiv. 2020 doi: 10.1101/2020.03.10.20032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim J., Jeon S., Shin H., Kim M., Seong Y., Lee W. Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35(6) doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu F., Xu A., Zhang Y., Xuan W., Yan T., Pan K. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis. 2020;95:183–191. doi: 10.1016/j.ijid.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J., Ouyang L., Guo P., Wu H., Fu P., Chen Y. Epidemiological, clinical characteristics and outcome of medical staff infected with COVID-19 in Wuhan, China: a retrospective case series analysis. medRxiv. 2020 doi: 10.1101/2020.03.09.20033118. [DOI] [Google Scholar]
- 33.Liu W., Zhang Q., Chen J., Xiang R., Song H., Shu S. Detection of COVID-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382:1370–1371. doi: 10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y., Sun W., Li J., Chen L., Wang Y., Zhang L. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. medRxiv. 2020 doi: 10.1101/2020.02.17.20024166. [DOI] [Google Scholar]
- 35.Lo I.L., Lio C.F., Cheong H.H., Lei C.I., Cheong T., Zhong X. Evaluation of SARS-CoV-2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID-19 in Macau. Int J Biol Sci. 2020;16:1698–1707. doi: 10.7150/ijbs.45357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mo P., Xing Y., Xiao Y., Deno L., Zhao Q., Wang H. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu C., Hu X., Song J., Du C., Xu J., Yang D. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19) medRxiv. 2020 doi: 10.1101/2020.02.26.20028589. [DOI] [Google Scholar]
- 40.Wu F., Zhao S., Yu B., Chen Y., Wang W., Song Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. https://www.nature.com/articles/s41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Y., Li Y., Zeng Q., Lu Z., Li Y., Wu W. Clinical characteristics of SARS-CoV-2 pneumonia compared to controls in Chinese Han population. medRxiv. 2020 doi: 10.1101/2020.03.08.20031658. [DOI] [Google Scholar]
- 42.Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-CoV-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang B., Zhou X., Qiu Y., Feng F., Feng J., Jia Y. Clinical characteristics of 82 death cases with COVID-19. medRxiv. 2020 doi: 10.1101/2020.02.26.20028191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J. Clinical features and outcomes of 221patients with COVID-19 in Wuhan, China. medRxiv. 2020 doi: 10.1101/2020.03.02.20030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang J.C., Zhang X., Wu G., Yi J. The potential role of IL-6 in monitoring coronavirus disease 2019. medRxiv. 2020 doi: 10.1101/2020.03.01.20029769. [DOI] [Google Scholar]
- 49.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors formortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-CoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lamontagne F., Rochwerg B., Lytvyn L., Guyatt H.G., Moller M.H., Annane D. Corticosteroid therapy for sepsis: a clinical practice guideline. BMJ. 2018;362:k3284. doi: 10.1136/bmj.k3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J., Ling Y., Xi X., Liu P., Li F., Li T. Efficacies of lopinavir/ritonavir and abidol in the treatment of novel coronavirus pneumonia. Chin J Infect Dis. 2020;E008 https://clinicaltrials.gov/ct2/show/NCT04252885 [Google Scholar]
- 53.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., etal Atrial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. https://www.nejm.org/doi/10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welliver R., Monto A.S., Carewicz O., Schatteman E., Hassman M., Hedrick J. Effectiveness of oseltamivir in preventing influenza in household contacts: a randomized controlled trial. JAMA. 2001;285(6):748–754. doi: 10.1001/jama.285.6.748. [DOI] [PubMed] [Google Scholar]
- 56.World Health Organization . Interim guidance. 2020 Mar 13. WHO; Geneva: 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected.https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Available at: [last accessed April 2020] [Google Scholar]
- 57.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woodyatt A., Hollingsworth J., Westcott B., Renton A., Wagner M., Hayes M. March 30 coronavirus news. CNN 30 March 2020. https://edition.cnn.com/world/live-news/coronavirus-outbreak-03-30-20-intl-hnk/h_7a11b7b7563b288686587c03a6e28e88 Available at: [last accessed April 2020]
- 61.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.-M., Lim W.S. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Arabi Y., Balkhy H., Hajeer A.H., Bouchama A., Hayden F.G., Al-Omari A. Feasibility, safety, clinical, and laboratory effects of convalescent plasma therapy for patients with Middle East respiratory syndrome coronavirus infection: a study protocol. Springer Plus. 2015;4:709. doi: 10.1186/s40064-015-1490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hung I.F., To K.K., Lee C.-K., Lee K.-L., Chan K., Yan W.-W. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Wilde A.H., Jochmans D., Posthuma C.C., Zevenhoven-Dobbe J.C., van Nieuwkoop S., Bestebroer T.M. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother. 2014;58:4875–4884. doi: 10.1128/AAC.03011-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.US Food and Drug Administration . FDA; White Oak, MD: 2020. Recommendations for investigational COVID-19 convalescent plasma.https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/investigational-covid-19-convalescent-plasma-emergency-inds Available at: [last accessed April 2020] [Google Scholar]