Abstract
With more than 1,800,000 cases and 110,000 deaths globally, COVID-19 is one of worst infectious disease outbreaks in history. This paper provides a critical review of the available evidence regarding the lessons learned from the Chinese experience with COVID-19 prevention and management. The steps that have led to a near disappearance of new cases in China included rapid sequencing of the virus to establish testing kits, which allowed tracking of infected persons in and out of Wuhan. In addition, aggressive quarantine measures included the complete isolation of Wuhan and then later Hubei Province and the rest of the country, as well as closure of all schools and nonessential businesses. Other measures included the rapid construction of two new hospitals and the establishment of “Fangcang” shelter hospitals. In the absence of a vaccine, the management of COVID-19 included antivirals, high-flow oxygen, mechanical ventilation, corticosteroids, hydroxychloroquine, tocilizumab, interferons, intravenous immunoglobulin, and convalescent plasma infusions. These measures appeared to provide only moderate success. Although some measures have been supported by weak descriptive data, their effectiveness is still unclear pending well controlled clinical trials. In the end, it was the enforcement of drastic quarantine measures that stopped SARS-CoV-2 from spreading. The earlier the implementation, the less likely resources will be depleted. The most critical factors in stopping a pandemic are early recognition of infected individuals, carriers, and contacts and early implementation of quarantine measures with an organised, proactive, and unified strategy at a national level. Delays result in significantly higher death tolls.
Résumé
Avec plus de 1 800 000 cas et 110 000 décès dans le monde, la COVID-19 est l'une des pires éclosions de maladies infectieuses de l'histoire. Ce document présente un examen critique des constats disponibles concernant les leçons tirées de l'expérience chinoise en matière de prévention et de gestion de la COVID-19. Les mesures qui ont conduit à la quasi-disparition des nouveaux cas en Chine comprenaient le séquençage rapide du virus pour établir des trousses de tests, ce qui a permis de suivre les personnes infectées à l'intérieur et à l'extérieur de Wuhan. En outre, des mesures agressives de quarantaine ont consisté à isoler complètement Wuhan, puis la province de Hubei et le reste du pays, ainsi qu'à fermer toutes les écoles et les entreprises non essentielles. D'autres mesures ont été prises, notamment la construction rapide de deux nouveaux hôpitaux et la création d'hôpitaux provisoires de type « Fangcang ». En l'absence de vaccin, la gestion de la COVID-19 a compris des antiviraux, de l'oxygène à haut débit, de la ventilation mécanique, des corticostéroïdes, de l'hydroxychloroquine, du tocilizumab, des interférons, des immunoglobulines en intraveineuses et des perfusions de plasma de patients convalescents. Ces mesures ne semblent avoir apporté qu'un succès modéré. Bien que certaines mesures aient été soutenues par des données descriptives modestes, leur efficacité n'est pas encore évidente, en attendant de meilleurs essais cliniques contrôlés. En fin de compte, c'est l'application de mesures de quarantaine drastiques qui a permis d'arrêter la propagation du SARS-CoV-2. Plus la mise en œuvre est précoce, moins il est probable que les limites des ressources soient atteintes. Les facteurs les plus critiques pour stopper une pandémie sont l’identification précoce des personnes infectées, des porteurs et des contacts et la mise en œuvre rapide de mesures de quarantaine grâce à une stratégie organisée, proactive et unifiée au niveau national. Tout retard entraîne une augmentation significative du nombre de décès.
Since mid-December 2019, there has been a worldwide outbreak of coronavirus disease (COVID)–19, caused by SARS-CoV-2 (formerly 2019-nCoV or HCoV-19) and first detected in Wuhan, China. The incubation period is 1-14 days (mean 5-6 days) in most cases, but can be as long as 24 days.1 The most commonly seen characteristics of COVID-19 are fever, cough, and abnormal chest computed tomography.2 , 3 At present, the Chinese chrysanthemum bat is thought to be the origin of SARS-CoV-2, based on sequence homology of 96% between SARS-CoV-2 and Bat-CoV-RaTG13.4 , 5 The pangolin has been proposed as an intermediate host, but this has not been confirmed.6 , 7 Human-to-human transmission of SARS-CoV-2 occurs mainly via respiratory droplets,1 direct contact,1 asymptomatic transmission,8 , 9 and intrafamilial transmission.3
SARS-CoV-2 can affect any demographic, including senior citizens, children, and pregnant women.3 , 10 According to the World Health Organisation (WHO), the Johns Hopkins Center for Systems Science and Engineering, and the State Council Information Office in Beijing, China, as of April 12, 2020, there have been more than 1,800,000 cases of COVID-19 worldwide, with 83,597 cases of COVID-19 confirmed in China, including 2,101 active cases (121 active severe cases), 1378 imported cases, 3351 deaths (mortality rate 3.99%), and 78,145 recovered cases (cure rate 93.5%). Today, normal life is slowly returning in most regions in an orderly and cautious manner, including in Wuhan, the epicenter of the epidemic in China.
To stop transmission of the virus and save lives, China adopted strategies and tactics that included a nationwide directive from the central government, governmental oversight, free testing (nucleic acid assay, gene sequencing, and IgM-IgG serology), free treatment, establishment of “Fangcang” hospitals, travel restrictions, new regulations, diagnostic algorithms, and the transfer of resources to the epicenter of the infection in Hubei Province. Very early, on January 23, 2020, when there were about 800 cases and 17 deaths, the entire city of Wuhan and subsequently Hubei Province were locked down and sealed off.
On March 2, the European Centers for Disease Control and Prevention (CDC) raised the SARS-CoV-2 risk level from moderate to high. On March 11, 2020, WHO declared COVID-19 a pandemic and recommended aggressive action by all countries in the world, warning that most countries were not prepared to handle the spread of SARS-CoV-2. As of April 12, 2020, SARS-CoV-2 has infected 1,770,469 people outside of China, causing 110,867 deaths (mortality rate 6.27%). It has been found in 6 continents, including 212 of 233 (91.0%) countries and international conveyances. The United States (560,402 cases), Spain (166,831 cases), Italy (156,363 cases), Germany (127,854 cases), and Iran (71,686 cases) are among the hardest hit, but cases in other countries are increasing rapidly.11
Managing the Spread of the Epidemic
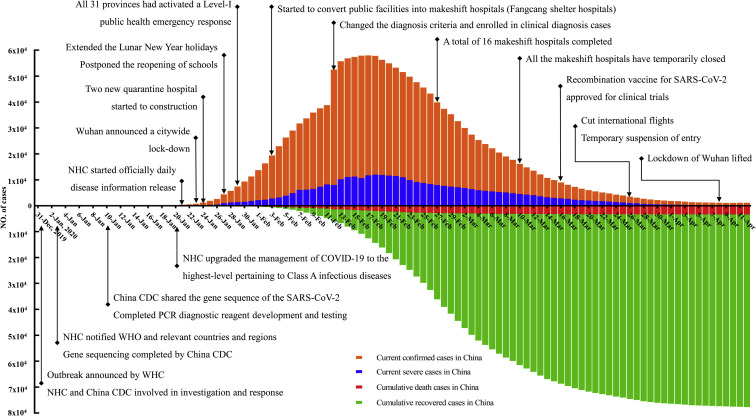
The most important strategy to combat a pandemic is to prevent it from even happening. This means that the spread of a virus needs to be countered as early as possible. The COVID-19 epidemic curves and major intervention measures that were implemented in China are shown in Figure 1 . Here is a description of the timeline.
Figure 1.
COVID-19 epidemiology curves and major intervention measures implemented in China. China CDC, Chinese Center for Disease Control and Prevention; NHC, National Health Commission of China; WHC, Wuhan Health Commission; WHO, World Health Organization. Data from the NHC, excluding Hong Kong and Macau Special Administrative Regions and Taiwan.
On December 31, 2019, the Wuhan Municipal Health Commission revealed a cluster of cases of pneumonia of unknown etiology. The Chinese CDC sent experts to Wuhan to support the investigation and control effort.12
On January 3, 2020, the first genomic sequence of the novel β-genus coronaviruses, now known as SARS-CoV-2, was determined by scientists of the National Institute of Viral Disease Control and Prevention.13 China informed the WHO.
On January 10, 2020, the whole genome sequence of the SARS-CoV-2 was shared with the WHO. Several rapid and sensitive detection tests were developed by the China CDC.
On January 16, 2020, the National Health Commission (NHC) issued the first of 7 versions of the protocol for the diagnosis and treatment of COVID-19.
On January 20, 2020, the NHC upgraded the management of the COVID-19 to the highest level pertaining to class A infectious diseases.
On January 21, 2020, the NHC officially started to release daily disease information on the government website.
On January 23, 2020, the Chinese government began to limit movement of people in and out of Wuhan and announced that all public transportation, including city buses, subways, ferries, long-distance coaches, outbound channels at airports, and railway stations in Wuhan had been suspended or closed.
On January 24, 2020, the local government planned 2 new quarantine hospitals with 1000 and 1600 beds to be constructed within 10 days. By March 8, 2020, 42,600 health professionals from outside Hubei and 1,800 epidemiologic teams participated in battling COVID-19 in Hubei.14
On January 27, 2020, the Chinese government announced the extension of the Lunar New Year holidays, and postponement of reopening of schools and factories.
On January 29, 2020, all 31 provinces on the Chinese mainland had activated a level 1 public health emergency response, the highest in the country, to prevent further spread.
On February 3, 2020, public facilities such as conference venues and sports stadiums were converted into makeshift hospitals to isolate patients with mild to moderate COVID-19 from their families and communities, while providing medical care, disease monitoring, food, shelter, and social activity.15 As of March 10, 2020, more than 12,000 patients had recovered and all makeshift hospitals were temporarily closed.16
On February 7, 2020, the Chinese government announced that all medical expenses of confirmed patients would be covered by health insurance or financial compensation.
On February 8, 2020, the Chinese central and local governments began to take measures to ensure the orderly resumption of production by companies to provide material support for the control of the epidemic. These included offering free health check-ups and arranging chartered buses, trains, and airplanes to send migrant workers back to resume work in other regions.
Quarantine Strategies
Quarantine and surveillance are still the most effective means of controlling the spread of infectious diseases. Some of the strategies and tactics are described below.
-
1)
Limiting migration—Wuhan city announced a citywide lockdown on January 23, 2020. Subsequently, the Chinese government banned all domestic travel, extended the Lunar New Year holidays, and postponed reopening of schools and factories to reduce the nationwide migration of the population.
-
2)
Designated hospitals—In Wuhan, to consolidate patients, medical experts, resources, and treatment, there were 45 COVID-19–designated hospitals: 6 for critical patients and 39 for severe patients and patients > 65 years of age. All regional hospitals established standardized fever clinics to timely screen for fever and strengthen isolation management. Designated hospitals were increased as needed by conversion of public and nonpublic hospitals.
-
3)
Community isolation—Travelers from Wuhan and other epidemic areas were required to register at their destination along with their travel history with the use of smartphones and to self-quarantine for 2 weeks to prevent community transmission. Only 1 family member could leave the house to purchase daily living supplies every 2 days.
-
4)
Maintaining adequate supply of essential items and revamping supply chains—Express delivery companies conducted “noncontact delivery” in communities. Shelves in stores were kept stocked by criminalizing price gouging and preventing hoarding. Opening green channels and coordinating transportation of supplies helped to ensure the safe delivery of key supplies. In the early stages of the COVID-19 outbreak, there was a shortage of personal protection equipment (PPE), which was mitigated by the use of reserve supplies, acquiring donations, and increases of production (Supplemental Appendix S1; Supplemental Fig. S1).
-
5)
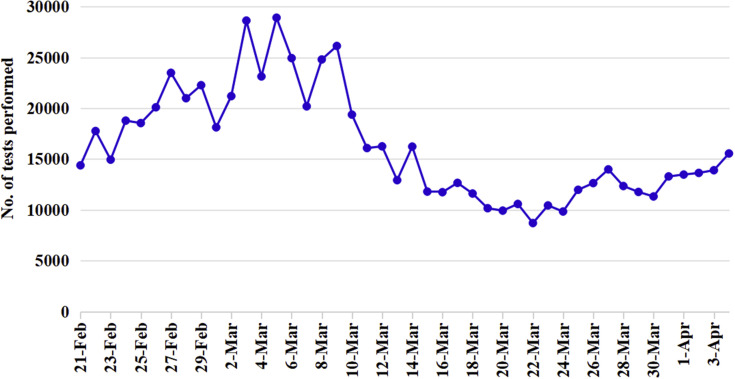
Testing—Early testing provided the basis for tracking and isolation in Wuhan to identify persons who could potentially spread the disease. The numbers of tests performed in Wuhan are shown in Figure 2 .
-
6)
Tracking—A major part of the success in controlling the spread of COVID-19 was rooted in the tracking strategy. All patients testing positive for COVID-19 were tracked and their contacts isolated. Big data and artificial intelligence (AI) were used to strengthen contact tracing and the management of priority populations.
-
7)
Temperature screening—Temperature screening checkpoints were established at supermarkets, residential area entrances, and transportation hubs. Everyone was screened for temperature when entering public areas. Temperature screening is a cheap, easily implemented, and rapid method of screening large numbers of the population for possible COVID-19. Fever is the most common symptom in patients with COVID-19. Persons who had traveled to Hubei or had close contact with COVID-19 patients in the preceding 14 days were required to undergo temperature checks.10
-
8)
Personal protection—All residents were required to wear medical surgical masks or N95 masks when accessing public places. The internet and media were leveraged to publicize the correct knowledge of protection and prevention of spread, such as using masks, daily disinfection, and washing hands correctly.
-
9)
Controlling cross-infection—Hospitals offered online consultation and medical services to reduce the frequency that patients visited hospitals. Online work and learning were used to avoid cross-infection.
Figure 2.
The daily number of nucleic acid tests performed in Wuhan. Data from the Wuhan Municipal Health Commission.
Managing Human and Equipment Resources
Physician scheduling was optimized.17 This scheduling is shown in Supplemental Table S1. Emergency staffing was arranged as needed within half an hour.
Nurses 1-3 jointly completed all of the nursing work for patients, including daily care, disinfecting the isolation areas, etc. Nurse 4 was the team leader, who was mainly responsible for counting supplementary materials, delegating resources, and completing nursing records.
Workflow was optimized and the correct use of protective equipment, cleaning, and disinfection measures was ensured.18
During the outbreak, many elective surgeries were not performed. In addition, surgery may accelerate and exacerbate disease progression of COVID-19.19
Treatment of COVID-19
Suspected and confirmed patients were quarantined and treated in designated hospitals. High-risk patients were hospitalized to proactively prevent complications and secondary infections, treat underlying diseases, and provide organ function support according to the patients’ conditions.10
Clinical classification
Physicians classified the disease severity of patients with SARS-CoV-2 infection according to the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia” issued by NHC (trial version 7)20 or “Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (2019-nCoV) Infection Is Suspected” from WHO.21 The severity definitions in the Chinese guidance were more widely used in clinical practice in China owing to its simplicity. According to the Chinese guidelines,20 , 89 the clinical classification of COVID-19 patients includes 4 levels of severity: mild, moderate, severe, and critical (Table 1 ). So far, there is no special COVID-19 clinical classification criteria for pregnant women, which may be due to the fact that clinical characteristics of COVID-19–infected pregnant women are generally similar to those of nonpregnant women with COVID-19 infection.22
Table 1.
COVID-19 clinical classification and treatment recommendations
| Classification | WHO interim guidance21 |
Chinese clinical guideline20,89 |
Treatment recommendations | ||
|---|---|---|---|---|---|
| Adult | Child | Adult | Child | ||
| Asymptomatic infection | NA | NA | NA | Laboratory-confirmed patients without any clinical symptoms or radiologic findings | Monitoring |
| Mild | Patients with nonspecific symptoms, such as fever, fatigue, cough, anorexia, malaise, muscle pain, sore throat, dyspnea, nasal congestion, or headache. Rarely, patients may also present with diarrhea, nausea, and vomiting∗ | Patients with mild clinical symptoms and no sign of pneumonia on imaging | General treatment, symptomatic treatment, antiviral treatment | ||
| Moderate | Adult with pneumonia but no signs of severe pneumonia and no need for supplemental oxygen | Child with nonsevere pneumonia who has cough or difficulty breathing plus fast breathing,† and no signs of severe pneumonia | Patients who have fever and respiratory symptoms with radiologic findings of pneumonia | Same as above, and supplemental oxygen therapy | |
| Severe | Adolescent or adult with fever or suspected respiratory infection, plus respiratory rate > 30 breaths/min, severe respiratory distress, or oxygen saturation ≤ 93% on room air | Child with cough or difficulty breathing, plus at least one of the following: 1) central cyanosis or oxygen saturation < 90%; 2) severe respiratory distress (eg, grunting, very severe chest retractions); or 3) signs of pneumonia with a general danger sign‡: inability to breastfeed or drink, lethargy or unconsciousness, or convulsions. Although the diagnosis is made on clinical grounds, chest imaging may identify or exclude pulmonary complications. | Adult who meets any of the following criteria: 1) respiratory distress (≥ 30 breaths/min); 2) oxygen saturation ≤ 93% at rest; 3) PaO2FiO2 ≤ 300 mm Hg (l mm Hg = 0.133 kPa)§ or chest imaging shows obvious lesion progression > 50% within 24-48 hours | Child who meets any of the following criteria: 1) tachypnea‖ independent from fever and crying; 2) oxygen saturation ≤ 92% according to finger pulse oximeter taken at rest; 3) laboured breathing,¶ cyanosis, and intermittent apnea; 4) lethargy and convulsion; 5) difficulty feeding and signs of dehydration; 6) HRCT shows infiltration in both lungs or multiple lobes, lesion progress in a short time, or pleural effusion | Same as above (for mild), and respiratory support (high-flow nasal oxygen and noninvasive ventilation or invasive mechanical ventilation) |
| Critical | |||||
| ARDS | Adults who meet the ARDS diagnostic criteria of the Berlin definition34 (oxygenation impairment identified according to the Berlin definition34) | Children who meet the ARDS diagnostic criteria of the Berlin definition34 | Patients who meet any of the following criteria: 1) respiratory failure and requiring mechanical ventilation; 2) shock; 3) with other organ failure that requires ICU care | Same as above (for mild), and respiratory support (invasive mechanical ventilation, extracorporeal membrane oxygenation) | |
| Sepsis | Adults with life-threatening organ dysfunction caused by a dysregulated host response to suspected or proven infection∗∗ | Children with suspected or proven infection and ≥ 2 age-based systemic inflammatory response syndrome criteria, of which one must be abnormal temperature or white blood cell count | |||
| Septic shock | Adults with persistent hypotension despite volume resuscitation, requiring vasopressors to maintain MAP (≥ 65 mm Hg) and serum lactate level > 2 mmol/L | Children who have any hypotension (SBP < 5th percentile or > 2 SD below normal for age) or 2 or 3 of the following: altered mental state; tachycardia or bradycardia (HR < 90 beats/min or > 160 beats/min in infants and HR < 70 beats/min or > 150 beats/min in children); prolonged capillary refill (> 2 s) or feeble pulse; tachypnea; mottled or cool skin or petechial or purpuric rash; increased lactate; oliguria; hyperthermia or hypothermia | |||
ARDS, acute respiratory distress syndrome; FiO2, fraction of inspired oxygen; HR, heart rate; HRCT, high-resolution computed tomography; ICU, intensive care unit; MAP, mean arterial pressure; NA, not available; PaO2, arterial partial pressure of oxygen; SBP, systolic blood pressure.
The elderly and immunosuppressed may present with atypical symptoms. Symptoms due to physiologic adaptations of pregnancy or adverse pregnancy events, such as dyspnea, fever, gastrointestinal symptoms,or fatigue, may overlap with COVID-19.
Fast breathing: age < 2 months ≥ 60 breaths/min; age 2-11 months: ≥ 50 breaths/min; age 1–5 years: ≥ 40 breaths/min.
Other signs of pneumonia may be present: chest retractions, fast breathing (in breaths/min†).
In high-altitude areas (> 1000 metres above sea level), PaO2/FiO2 should be corrected by the following formula: PaO2/FiO2 × [atmospheric pressure (mm Hg)/760].
Respiratory rate: age < 2 months: ≥ 60 breaths/min; age 2-12 months: ≥ 50 breaths/min; age 1–5 years: ≥ 40 breaths/min; age > 5 years: ≥ 30 breaths/min.
Moaning, nasal fluttering, and infrasternal, supraclavicular, and intercostal retractions.
Signs of organ dysfunction include: altered mental status, difficult or fast breathing, low oxygen saturation, reduced urine output, fast heart rate, weak pulse, cold extremities or low blood pressure, skin mottling, or laboratory evidence of coagulopathy, thrombocytopenia, acidosis, high lactate, or hyperbilirubinemia.
Nonpharmacologic Treatment—Supportive and Symptomatic Care
General treatment
Symptomatic and supportive treatment is essential and the main treatment for COVID-19.20 General supportive measures for SARS-CoV-2–infected patients include bed rest, adequate nutrition, water and electrolyte balance, and intensively monitoring vital signs (blood pressure, respiratory rate, heart rate, and oxygen saturation). Laboratory markers of disease progression and clinical outcomes, such as d-dimer, C-reactive protein, procalcitonin, neutrophil count, lymphocyte count, and inflammatory cytokines, were monitored.1 , 23, 24, 25
Symptomatic therapy
Fever is the most common symptom of SARS-CoV-2 infection.1 , 23, 24, 25 Continuous high fever may cause metabolic disorders and system dysfunction. Therefore, WHO guidance advocates the use of antipyretics and cooling measures.21 Multiple studies have shown that COVID-19 patients have underlying diseases, including hypertension and diabetes, leading to higher mortality.1 , 26 For these patients, blood pressure and blood sugar must be monitored, and if abnormal, it should be promptly treated. The onset of severe disease leading to liver, kidney, or cardiac injury should be anticipated and treated appropriately.
Respiratory support
Studies have shown that in severe cases, 66.7% of patients (124/186) received oxygen therapy, 44.7% (63/141) received high-flow nasal cannula (HFNC), 39.4% (108/274) received noninvasive mechanical ventilation, and 24.1% (66/274) required invasive mechanical ventilation. Only 6.2% (17/274) patients received extracorporeal membrane oxygenation (ECMO) (Table 2 ).1 , 23 , 24 , 27 , 28
Table 2.
Treatment, oxygen support, and death in severe cases with COVID-19
| Outcome | Guan et al.1 | Liu et al.28 | Yang et al.27 | Wang et al.24 | Huang et al.23 |
|---|---|---|---|---|---|
| ICU/severe cases, n∗ | 173 | 53 | 52 | 36 | 13 |
| Treatment, n (%) | |||||
| Antiviral treatment | 80 (46.2) | 52 (98.1) | 23 (44.2) | 34 (94.4) | 12 (92.3) |
| Antibiotic treatment | 139 (80.3) | 53 (100) | 49 (94.2) | NA | 13 (100) |
| Corticosteroid treatment | 77 (44.5) | 37 (69.8) | 30 (57.7) | 26 (72.2) | 6 (46.2) |
| Intravenous immunoglobulin | 58 (33.5) | 29 (54.7) | 28 (53.8) | NA | NA |
| CRRT | 9 (5.2) | NA | 9 (17.3) | 2 (5.6) | 3 (23.1) |
| Oxygen support, n (%) | |||||
| Oxygen therapy | 123 (71.1) | NA | NA | NA | 1 (7.7) |
| High-flow nasal cannula | NA | 26 (49.1) | 33 (63.5) | 4 (11.1) | NA |
| Noninvasive mechanical ventilation | 56 (32.4) | NA | 29 (55.8) | 15 (41.7) | 8 (61.5) |
| Invasive mechanical ventilation | 25 (14.5) | NA | 22 (42.3) | 17 (47.2) | 2 (15.4) |
| ECMO | 5 (2.9) | NA | 6 (11.5) | 4 (11.1) | 2 (15.4) |
| Death, n (%) | 14 (8.1) | 26 (49.1) | 32 (61.5) | 6 (16.7) | 5 (38.5) |
CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; NA, not available.
ICU/severe cases included severe and critical cases as defined in Table 1.
Supplemental oxygen
In a study of 1099 patients in China, 41.3% of confirmed COVID-19 patients (454/1099) and 35.7% of nonsevere patients (331/926) received oxygen therapy.1 WHO guidelines recommend that supplemental oxygen therapy be given to patients with severe acute respiratory infection (SARI), respiratory distress, hypoxemia, or shock.21 However, there are no restrictions on which patients can receive supplemental oxygen therapy in the Chinese guidelines,20 which may have resulted in the overuse of oxygen therapy. In general, patients with blood oxygen saturation (SPO2) ≥ 93% do not require supplemental oxygen, and hyperoxemia may induce further respiratory injury and even higher mortality.29 The indications for supplemental oxygen should be carefully applied in patients with COVID-19.21
High-flow nasal cannula oxygen therapy and noninvasive mechanical ventilation
HFNC and noninvasive positive-pressure ventilation (NIPPV) was used as step-up therapy in patients who failed to improve on supplemental oxygen.20 A Chinese expert consensus released in 2019 recommends that physicians can consider providing HFNC to mild or moderately ill patients (arterial oxygen partial pressure [PaO2]/fractional inspired oxygen [FiO2] 100-300 mm Hg). The clinical application of NIPPV on hypoxemic respiratory failure caused by severe pneumonia has long been controversial.30 , 31 WHO21 and Chinese20 guidelines both recommend using HFNC and NIPPV with extreme care and closely monitoring the condition of patients treated with HFNC or NIPPV for deterioration. Failure of a short trial (1 hour) of NIPPV may require proceeding to invasive mechanical ventilation.
Invasive mechanical ventilation
According to Guan et al.,1 14.5% of severe patients (25/173) received invasive mechanical ventilation and 32.4% (56/173) received noninvasive mechanical ventilation. If standard oxygen therapy fails, Chinese guidelines recommend a trial of HFNC or noninvasive ventilation, but WHO guidelines recommend escalating to invasive mechanical ventilation.21 It is generally accepted that timely use of invasive mechanical ventilation is an important component of the treatment of severe respiratory failure and acute respiratory distress syndrome (ARDS).32 Based on a Chinese expert consensus,33 it is recommended that invasive mechanical ventilation be the first choice for moderate or severe ARDS patients (PaO2/FiO2 ≤ 150 mm Hg)34 or for patients after failure of HFNC and NIPPV. Lung-protective ventilation strategy, ie, low tidal volumes (6-8 mL/kg of predicted body weight) and low inspiratory pressures (platform pressure < 30 cm H2O) should be incorporated to prevent ventilator-related lung injury.32
Extracorporeal membrane oxygenation
ECMO is a form of extracorporeal life support for very ill patients that circulates blood flow through an artificial lung for gas exchange and then back into the bloodstream. It provides a period of pulmonary rest, artificially supports critical ill patents while their heart or lungs recover, and plays a role in the care of heart or lung transplant patients. ECMO has rarely been used in China owing to limited resources.1 , 23 Moreover, there are no published studies about the efficacy and safety of ECMO with severely ill COVID-19 patients. Previously, the experience of using ECMO in Middle East Respiratory Syndrome (MERS) and influenza has been controversial.35
Pharmacologic agents
We summarize the treatment of 327 pooled cases of severe cases with COVID-19 in Table 2.1 , 23 , 24 , 27 , 28 In severe cases, 87.3% of patients (254/291) received antibiotic treatment, 61.5% (201/327) received antiviral treatment, 53.8% (176/327) received corticosteroid treatment, and 41.3% (115/278) received intravenous immunoglobulin. Only 8.4% of patients (23/274) received continuous renal replacement therapy. The mortality rate of the 327 pooled severe cases was 25.4% (83/327) (Table 2).
Despite the use of various medications, the consensus of Chinese experts is that neuraminidase inhibitors (oseltamivir, peramivir, zanamivir, etc) and ganciclovir are not generally recommended. Routine prophylactic antibiotics, especially combined wide-spectrum antibiotics, are also not typically recommended.33 However, because of the widespread use of the medications, as indicated in the previous paragraph, ongoing trials are being conducted to clarify which medications may or may not be helpful in treating COVID-19.
Antiviral treatment
At present, there is no evidence to support the effectiveness of antiviral drugs for COVID-19, although these are commonly used in the treatment of COVID-19 in China. The guidelines of the NHC recommend interferon-α2b inhalation, lopinavir/ritonavir, ribavirin, chloroquine, and arbidol as antiviral therapy and does not recommend using more than 2 antiviral drugs at the same time (Table 3 ).10 , 36
Table 3.
Antiviral drugs for COVID-19 recommended in the guidelines of the NHC
| Medication instruction | Alpha-interferon | Lopinavir/ritonavir | Ribavirin | Chloroquine | Arbidol |
|---|---|---|---|---|---|
| Recommended dosage and course | 5 million units or equivalent dose per time for adults, adding 2 mL of sterile water, aerosol inhalation, twice per day. For children,∗ 100,000-200,000 IU/kg in mild cases, 200,000-400,000 IU/kg in severe cases, twice per day for 5-7 days. |
200 mg/50 mg per capsule, 2 capsules each time, twice per day for adults. The course of treatment should be ≤ 10 days. For children,∗ weight 7-15 kg, (12 mg/3 mg)/kg; weight 15-40 kg, (10 mg/2.5 mg)/kg; weight > 40 kg, same as adults. |
Combining with alpha-interferon or lopinavir/ritonavir, 500 mg for adults per time, intravenous injection 2-3 times per day. The course of treatment should be ≤ 10 days. For children,∗ 10 mg/kg per time (maximum 500 mg per time), intravenous injection 2-3 times per day. |
Only for adults aged 18-65 y. Weight > 50 kg, 500 mg per time, twice per day for 7 days. Weight < 50 kg, 500 mg per time, twice per day for 2 days and then 500 mg per time, once per day for 5 days. |
200 mg for adults, 3 times per day. The course of treatment should be ≤ 10 days. |
| Medication for special populations | |||||
| Children | Recommended | Recommended for children > 2 y old | Recommended | Not recommended | Unclear |
| Pregnant/lactating women | Prudent use | Recommended | Forbidden | Prudent use in lactating women | Unclear |
| Elderly | Recommended | Safety unclear; prudent use | Not recommended | Unclear | Unclear |
| Liver dysfunction patients | Prudent use | Prudent use | Prudent use | Prudent use | Unclear |
| Renal dysfunction patients | Prudent use | Recommended | CCR < 50 mL/min: not recommended | Prudent use | Prudent use in severe renal dysfunction |
| Contraindication | Patients with severe liver/renal dysfunction | Patients with severe liver dysfunction | Pregnant/lactating women | Pregnant women | Unclear |
| Approved indications | Broad-spectrum antiviral, immunomodulatory, and antitumor | HIV-1 infection | Respiratory syncytial virus pneumonia | Malaria | A and B influenza viruses |
| Adverse reactions | † | † | † | † | † |
| Off-label drug use | Yes | Yes | Yes | Yes | |
| Clinical trials‡ | 6 | 9 | 1 | 16 | 3 |
CCR, creatinine clearance rate; NHC, National Health Commission; HIV, human immunodeficiency virus.
The guidelines of the NHC did not recommend the dosage and course for children. The recommended dosage and course of the drugs for children in the table are from Chen et al.’s studies.36
Refer to product guidelines for specific information.
The number of clinical trials of COVID-19 in China as of April 3, 2020. Data from http://www.chictr.org.cn/.
Xu et al. reported that of 55 of 62 confirmed COVID-19 patients in Zhejiang province received antiviral treatment. Twenty-one patients (34%) received lopinavir/ritonavir and interferon-α2b inhalation, 17 (28%) received arbidol and lopinavir/ritonavir, and 8 (13%) received interferon-α2b by inhalation.37 Chen et al. reported that 75 of 99 confirmed patients in Wuhan received antiviral treatment, including oseltamivir, ganciclovir, and lopinavir/ritonavir.38 The duration of antiviral treatment was 3-14 days.38 However, the results of a clinical trial of lopinavir/ritonavir in China showed no clear benefit beyond standard care in hospitalized adult patients with severe COVID-19.39 Remdesivir may have the greatest potential for the successful treatment of SARS-CoV-2, but efficacy and safety in COVID-19 need further evaluation.40 , 41 Remdesivir has been studied in clinical trials to treat COVID-19 in China.
Favipiravir was approved for treatment of influenza on February 15, 2020, in China and is being studied in COVID-19 clinical trials. The preliminary results indicate that favipiravir has significantly more potent antiviral action and fewer adverse effects than lopinavir/ritonavir (P < 0.001).42 One of the clinical trials was conducted in Shenzhen, and the results showed that the median time to viral clearance was 4 days in the favipiravir treatment group compared with 11 days in the lopinavir/ritonavir treatment group (P < 0.001). In terms of chest imaging, the improvement rates of the favipiravir treatment group and the lopinavir/ritonavir treatment group were 91.4% and 62.2%, respectively. No significant adverse reactions were noted in the favipiravir treatment group, and there were significantly fewer adverse effects than in the lopinavir/ritonavir group.43
In early in vitro studies, chloroquine and hydroxychloroquine were found to inhibit SARS-CoV-2 infection efficiently,40 , 44 and several clinical trials have been quickly conducted in China to evaluate the efficacy and safety of chloroquine and hydroxychloroquine. Gao et al. summarized that compared with the control treatment, chloroquine phosphate was effective in inhibiting the exacerbation of pneumonia, improving lung imaging findings, promoting a virus-negative conversion, and shortening the disease course.45 The expert consensus on chloroquine phosphate recommends 500 mg twice daily for 10 days for patients diagnosed with COVID-19. Adverse effects of chloroquine and hydroxychloroquine must be carefully monitored.46
Zhou et al. proposed that hydroxychloroquine could serve as a better therapeutic agent than chloroquine, owing to reduced toxicity, fewer side-effects, lower cost, and relative safety in pregnancy.47 Yao et al. used physiologically based pharmacokinetics models and found that hydroxychloroquine was more potent than chloroquine at inhibiting SARS-CoV-2 in vitro. They recommended 400 mg hydroxychloroquine sulfate twice daily for 1 day, followed by 200 mg twice daily for 4 days to treat SARS-CoV-2 infection.48 The efficacy and safety data of chloroquine or hydroxychloroquine from high-quality clinical trials are urgently needed.
Glucocorticoid therapy
Russell et al. recommended that corticosteroids should not be used in SARS-CoV-2–induced lung injury or shock.49 However, Shang et al. suggested using short courses (≤ 7 days) of corticosteroids at low to moderate dose (≤ 0.5-1 mg/kg/d methylprednisolone or equivalent) for critically ill patients with COVID-19.50 According to a Chinese experts’ consensus statement, the following criteria should be met before using corticosteroids in patients with COVID-19: 1) adults (≥ 18 years old), 2) laboratory-confirmed cases, 3) symptoms occurring within 10 days, 4) radiographic imaging consistent with COVID-19 pneumonia and progressing rapidly, and 5) SPO2 ≤ 93%, shortness of breath (respiratory rate ≥ 30/min), or oxygenation index ≤ 300 mm Hg at rest and with no oxygen therapy.51
A retrospective study found that low- to moderate-dose glucocorticoid therapy had no effect on the time to viral clearance in patients with COVID-19. Glucocorticoids are not recommended in mild cases, because there was no improvement in the rate of radiographic recovery.52 However, a single center in Wuhan shared that early low-dose and short-term corticosteroids (1-2 mg/kg/d for 5-7 days) was associated with a faster improvement of clinical symptoms and absorption of focal lung lesions in severe cases of COVID-19.53 Another study analyzed 15 critical cases and suggested that a low dose and short duration of corticosteroids (methylprednisolone < 1 mg/kg, < 7 days) may be beneficial for critically ill patients with COVID-19.54 Zhou et al. analyzed 10 patients with COVID-19 who received corticosteroids and found that short-term moderate-dose corticosteroids (160 mg/d) plus immunoglobulin (20 g/d) were effective for reversing the continued deterioration of COVID-19 patients who failed to respond to the low-dose therapy (40-80 mg/d corticosteroids and 10 g/d immunoglobulin).55 An open-label randomized controlled trial has been conducted to investigate the effectiveness of glucocorticoid therapy in patients with severe COVID-19.56
Convalescent plasma
Previous studies in MERS suggested that convalescent plasma may be effective.57 Mair-Jenkins et al. further suggested that convalescent plasma may reduce mortality in patients with SARI of viral etiology.58 The NHC (trial version 2) includes convalescent plasma as a potential treatment in severe cases, critical cases, and cases of rapidly worsening clinical status.59 Donors and recipients should be carefully selected, and serum-specific IgG antibodies for SARS-CoV-2 should be tested to guarantee the quality of convalescent plasma. Critical patients who received convalescent plasma showed significant improvement in clinical symptoms and laboratory findings.60 Multiple clinical trials have been registered in the Chinese Clinical Trial Registry to study the safety and efficacy of convalescent plasma treatment in COVID-19 patients.61
Intravenous immunoglobulin
In Guan et al.’s study1 of 173 severe COVID-19 cases, 58 patients (33.4%) received intravenous immunoglobulin treatment (IVIG). IVIG was considered as a potential therapy for immunologic injury in COVID-19 in clinical practice because of its antiinflammatory action in treating conditions such as hemophagocytic lymphohistiocytosis and cytokine storm. Similarly, two reviews recommended IVIG based on mechanisms of SARS-CoV-2–mediated inflammatory responses.62 , 63 However, further investigation and more clinical studies are needed.
Biologic modulators
The results of laboratory findings of COVID-19 patients in China showed an increase in plasma inflammatory cytokine levels, especially interleukin (IL) 6.23 , 38 Therefore, the anti-IL6 monoclonal antibody tocilizumab was considered as a potential drug for some COVID-19 cases with extensive lung lesions and elevated IL-6 levels.20 Three clinical trials have been initiated and registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn/index.aspx), but no results or data have been released at this time.
Other pharmaceutical measures
Traditional Chinese medicine treatment
The potentially beneficial role of traditional Chinese medicine is described in the guidelines of the NHC, and some Chinese patent medicines (CPMs) have been used to treat COVID-19.10 However, the experience with most of these treatments is anecdotal. Gu et al. reviewed 93 Chinese medicine prescriptions and 157 forms of constituent medicines for COVID-19 treatment and found that they may be effective in reducing fever, mucous production, cough, and asthma symptoms.64 Yan et al. discovered that 10 antiviral components from CPMs can directly bind to both host cell target angiotensin-converting enzyme (ACE) 2 receptor and viral target main protease, suggesting their potential for COVID-19 treatment.65
Other treatment measures
According to the guidelines of the NHC,10 vasoactive drugs may be required to supplement fluid resuscitation and to improve microcirculation. Continuous renal replacement therapy has been used in acute kidney injury.66 Apheresis methods, including plasma exchange, adsorption, perfusion, and filtration, were used in critical cases with severe inflammatory reactions.67
In addition to the treatment of the patients’ physical condition, psychologic support was provided to address the anxiety and fear that occurs in patients who are suffering from COVID-19 as well as in their loved ones and health workers. During the quarantine, mental health intervention provided online was especially recommended. According to Hu et al., the use of hypnotics can significantly improve clinical outcomes of COVID-19 patients. These improvements may be attributable to decreased oxygen consumption resulting from reduced periods of anxiety and improved immunity from experiencing high-quality sleep.68
Vaccine development
The development of a vaccine for SARS-CoV-2 has been accelerated as a priority project. On January 23, the Coalition for Epidemic Preparedness Innovations (CEPI) announced a $12.5 million grant to fund vaccine development by Queensland University in Australia, the National Institutes of Health in the United States, and the pharmaceutical companies Inovio and Moderna. On January 24, a virus strain of SARS-CoV-2 was successfully isolated from patients’ samples in China to provide the basis for vaccine development.69 On February 3, CEPI and GlaxoSmithKline announced collaboration to strengthen the global effort to develop a vaccine. Vaccine safety is a priority. Currently, strategies for SARS-CoV-2 vaccine development include recombinant proteins, DNA vaccines, mRNA vaccines, traditional live vaccines, and recombinant adenovirus vaccines. These are in various phases of development, ranging from nonhuman animal studies to clinical trials.3 , 70 , 71 On March 13, mRNA-1273 vaccine clinical trials began in the United States.70 On March 16, recombination vaccines for SARS-CoV-2 entered into phase I clinical trials.71 Although these are significant strides, the prospects for a commercially available vaccine are at least 6 months away, and probably much longer.
Respiratory nursing support
Studies have shown that prone positioning was associated with improved oxygenation and a decrease in CO2 retention, leading to reduced mortality, in patients with influenza, MERS, or ARDS.72 , 73 As a result, a Chinese expert consensus recommends that patients be prone for ≥ 12 hours a day when PaO2/FiO2 < 150 mm Hg.74 Before placing the patient in prone position, oral secretions should be suctioned regularly to help keep the artificial airway open. In addition, COVID-19 patients suffer from copious proteinaceous exudates in their lungs and airways.75 , 76 Aspiration of sputum can help to keep the airway intact and potentially improve sample collection and the accuracy of SARS-CoV-2 nucleic acid detection.77 The patient’s position should be assessed and changed regularly to avoid decubitus skin injury. Elevating the head of the bed 30-45 degrees,78 suctioning out oropharyngeal secretions, and monitoring the pressure of a rebreathing bag can help to prevent ventilator-associated pneumonia.
Cardiac complications
Cardiac abnormalities were found to be present in a high proportion of patients who died of COVID-19 and may be more associated with severe disease. Based on current studies, COVID-19 patients with cardiovascular diseases are more likely to develop into critical cases and have higher mortality.1 , 23 , 24 , 38 , 79 Clinicians should be alert to the manifestations of heart injury by closely monitoring patients’ vital signs (blood pressure and heart rate), laboratory tests including creatine kinase, lactate dehydrogenase, high-sensitivity cardiac Troponin I, creatine kinase MB, B-type natriuretic peptide (BNP) and N-terminal proBNP, and electrocardiography. Antiviral drugs, such as ribavirin and lopinavir/ritonavir, have been widely used in cardiac patients, although there no well-designed trials to support that. The hypothesis that COVID-19 directly causes myocardial injury may support the usage of antiviral drugs, but the side-effects of these drugs on the cardiovascular system, such as sudden cardiac death and bradycardia, should be carefully considered.80
Owing to the fact that cardiac insufficiency can lead to a coagulation disorder and that severe COVID-19 patients were reported to have increased level of d-dimer, low-molecular-weight heparin was recommended to treat COVID-19 patients in the early phase of disease.62 As with SARS-CoV, ACE2 has been identified as the receptor for SARS-CoV-2 to enter cells.81 The use of ACE inhibitors (ACEIs) may not be of any benefit, because they does not bind to the ACE2 receptor. This also means that discontinuing ACEIs in patients with COVID-19 is not necessary. Regarding angiotensin receptor blockers (ARBs), there is evidence that ARBs could lead to increased expression of ACE2, thus worsening disease, but this remains controversial. There is no current recommendation to discontinue ARBs during treatment of COVID-19 in China. Monteil et al. found that SARS-CoV-2 infection in engineered human blood vessel organoids and human kidney organoids could be inhibited by human recombinant soluble ACE2.82 Further investigations are required to shed light on the role of ACE2 in cardiac manifestations of COVID-19.
Regarding acute myocardial infarction in COVID-19 patients, Peking Union Medical College Hospital and Zhongnan Hospital of Wuhan University provided the following experience.83 , 84 For acute ST-segment-elevation myocardial infarction (STEMI) in patients with confirmed COVID-19, strict isolation should start immediately and thrombolytic contraindications should be evaluated. Patients with thrombolytic contraindications should be transferred to the local infectious disease specialist hospitals immediately for further treatment. Patients without thrombolytic contraindications should first be started on intravenous thrombolysis and then transferred to the local infectious disease specialist hospitals for further treatment.83 Considering the increased risk of exposure due to the lack of negative-pressure catheterization chambers and shortages of PPE, and the greater difficulty of finely manipulating the guidewires under level 3 protection, fibrinolysis is preferred when both percutaneous coronary intervention (PCI) and fibrinolysis are available. Once PCI is required, all medical workers should be under level 3 protection and thorough environmental disinfection must be given after each PCI.84
Effectiveness and Importance of Public Health Interventions
The WHO-China joint mission report reported that China’s vigorous public health measures to prevent the COVID-19 are the most “ambitious, agile, and aggressive disease containment effort in history.”85 The drastic measures taken are listed in Table 4 . If not for a national strategy that incorporated all of these measures simultaneously, it was estimated there would have been 744,000 COVID-19 cases outside Wuhan on day 50 of the epidemic, compared with the actual number of fewer than 20,000.86
Table 4.
Specific lockdown measures in Wuhan later extended to the entire country
| Measure | Content |
|---|---|
| Specific limitations |
|
| Enforcement measures |
|
| Required measures |
|
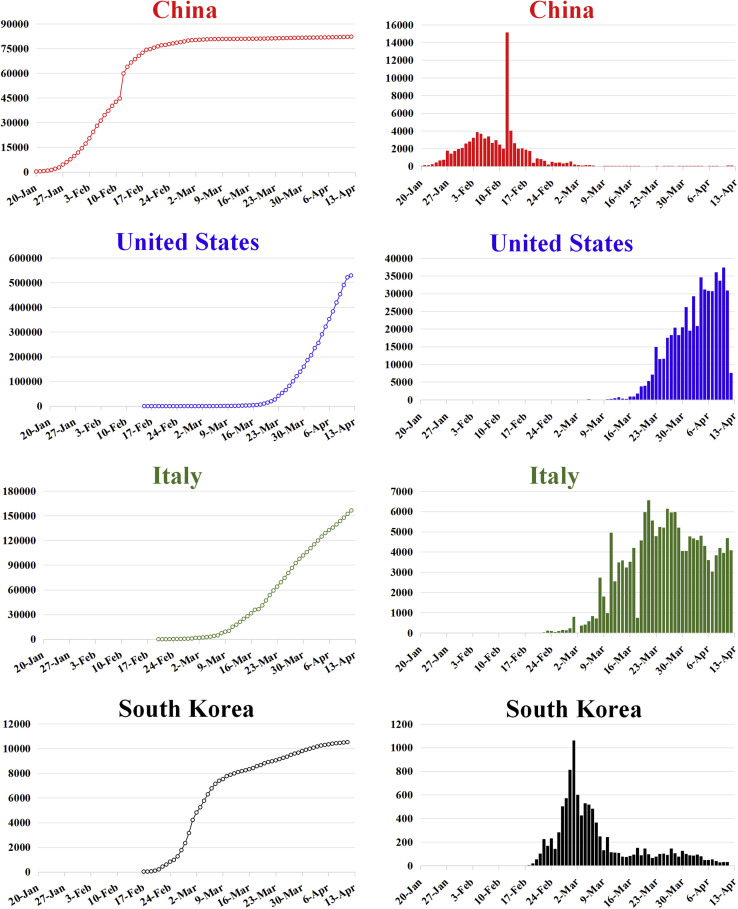
As of April 13, 2020, COVID-19 has spread to 6 continents and 212 countries and regions. We compare the epidemiologic characteristics among China, Italy, South Korea, and the United States in Figure 3 . The data from China show that the daily number of infected patients has been less than 50 for the past week. However, there are a steady daily number of imported cases. Similarly, in South Korea, the strategy that worked also included early testing, tracking, and isolation. Unfortunately, lessons learned in China have not been universally adopted, and the numbers of cases in many countries are rapidly increasing.
Figure 3.
Epidemic curves of the confirmed cases of COVID-19 in various countries. Left, timeline of breakouts of SARS-CoV-2. Right, daily numbers of increasing cases. Data for China were obtained from the National Health Commission of China, which excluded Hong Kong and Macau Special Administrative Regions and Taiwan. Data for the remaining countries were obtained from the World Health Organisation.
The lessons learned in China and South Korea are that in these times of crises, the earlier the intervention from a societal perspective the better. China had implemented an electronic monitoring system for infectious disease outbreaks after SARS in 2003. The question is how early one can reasonably expect to determine that something is out of the ordinary. People die of respiratory failure due to many causes, including influenza, every day. When should there be suspicion that something like a new virus is involved? The lesson here is that one should always have a high index of suspicion, but this must be balanced against causing mass hysteria and panic.
Once it is recognized that there is a new, potentially lethal, virus, the virus needs to be isolated, the genome sequenced, and testing kits validated and released for rapid distribution. Infected individuals and their contacts need to be tracked and isolated. The earlier this is done, the less impact there will be on the personal and professional lives of people and the economy, and the fewer resources will be needed. When more people are infected, the drastic measures including shutting down cities and restricting travel will need to be more widespread, health care resources will be exhausted, and the economy will take a bigger hit.
It is perhaps significant to evaluate the number and distribution of cases in China as a basis for aggressive testing, tracking, and quarantining. In the early days of the epidemic, the genomic sequence of the virus was determined and tests kits that would deliver results within hours were developed. It should be emphasized that the lockdown in Wuhan was a complete lockdown, other than bringing in necessary medical personnel, ventilators, and PPE, as well as everyday goods and supplies. The difference between this type of lockdown and the shelter in place recommendations that have been put forward in many Western countries is that the lockdown in Wuhan was strictly enforced. Laws in Wuhan limited grocery shopping to 1 person per household once every 2 days, with identity checks when leaving and returning. This type of enforced lockdown was later extended to the rest of the country, so that even in cities of more than 7 million and only 200 cases, the population experienced the same restrictions (Table 4). The entire country stayed on lockdown for more than 2 months. When lockdown measures were eased over the past few weeks, temperature checks were still being performed and one had to show electronic medical data on one’s electronic device. The lesson here is that if effective quarantine is what needs to occur, these measures must be supported by personnel to enforce them, and they cannot be voluntary or simply recommended.
Downgrading After the Epidemic or Pandemic Has Been Brought Under Control
According to relevant laws and regulations in China, when the number of confirmed cases has a steady decline and the risk of the spread of the epidemic is effectively controlled, each province should reduce the emergency response levels accordingly. In fact, local governments adjust emergency response levels according to the local epidemic situation. Provincial governments use counties as a unit to divide different regions into high-risk, medium-risk, and low-risk areas and implement different prevention and control measures in the different risk areas.87
Low-risk districts have no confirmed cases or no new confirmed cases for 14 consecutive days. Medium-risk districts have new confirmed cases within the previous 14 days but the cumulative number does not exceed 50 confirmed cases, or there are cumulatively more than 50 confirmed cases but no clustered epidemics within the previous 14 days. High-risk districts have a cumulative number of more than 50 confirmed cases, with clustered epidemics within the previous 14 days.88 Even so, there is always a risk of a second wave if restrictions are relaxed too soon and too drastically.
Different prevention and control measures were implemented in different risk areas. In low-risk areas the main focus was to prevent import of outside cases with the economy restored back to normal. In medium-risk areas, both preventing internal and external spread was implemented, and screening of body temperature and disinfection of public facilities was continued. The entertainment industry remained closed, but other business activities resumed. In high-risk areas, strict quarantine and control measures were continued, with continued closure of nonessential businesses.
Conclusion
The experience in China is unique in that it was the first country to implement drastic measures to combat COVID-19. The strategy in China was early testing, diligent tracking, and strict isolation of patients and contacts, first in Wuhan and then nationwide. Even with this, there were more than 80,000 cases in China and more than 3000 deaths. All countries, even China, can learn from this. Although the drastic measures that China implemented appear for now to have stopped the viral spread, earlier implementation could have reduced the number of cases and deaths even more.
Viral epidemics need to be countered with a unified national strategy that can be implemented quickly on a very large scale. The ability to mobilize and to stay nimble in adapting to these challenges is paramount. Public buy-in is crucial for all citizens to abide by the rules and recommendations. Rules must be mandatory and cannot be simply recommended and voluntary. Designated officers must be deployed to enforce these rules. The negative short-term public and economic consequences that may result from shutting down services, restricting travel, and aggressive quarantining must be accepted or the virus will keep spreading. Collaboration and sharing among countries are imperative to establish more effective policies to control the spread of future epidemics and minimize mortality and morbidity.
Funding Sources
This study was supported in part by the Beijing Municipal Natural Science Foundation General Program, China (7192197), CAMS Innovation Fund for Medical Sciences, China (2016-I2M-1-003), the National Natural Science Foundation of China (31671371 and 81700490), the Central Public-Interest Scientific Institution Basal Research Fund, China (2016ZX310195, 2017PT31026, and 2018PT31016), and the Epidemic Research Project of Guangzhou Regenerative Medicine and Health Guangdong Laboratory, China (2020GZR110406001).
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 927 for disclosure information.
To access the supplementary material accompanying this article, visit the online version of the Canadian Journal of Cardiology at www.onlinecjc.ca and at https://doi.org/10.1016/j.cjca.2020.04.010.
Contributor Information
Taijiao Jiang, Email: taijiao@ibms.pumc.edu.cn.
Jinlyu Sun, Email: sunjinlv@pumch.cn.
Guogang Xu, Email: guogang_xu@qq.com.
Christopher Chang, Email: chrchang@mhs.net.
Supplementary Material
References
- 1.Guan W.-J., Ni Z.-Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: A single arm meta-analysis. [e-pub before print]. J Med Virol 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed]
- 3.China Centers for Disease Control China-WHO new coronavirus pneumonia (COVID-19) joint inspection report. http://www.nhc.gov.cn/jkj/s3578/202002/87fd92510d094e4b9bad597608f5cc2c.shtml Available at: Accessed February 29, 2020.
- 4.Zhou P., Yang X.-L., Wang X.-G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu A., Peng Y., Huang B. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Zai J, Zhao Q, et al. Evolutionary history, potential intermediate animal host, and cross-species analyses of SARS-CoV-2 [e-pub before print]. J Med Virol 10.1002/jmv.25731. [DOI] [PMC free article] [PubMed]
- 7.Liu P., Jiang J.-Z., Hua Y. Are pangolins the intermediate host of the 2019 novel coronavirus (2019-nCoV)? PLoS Pathog. 2020;16:e1008421. doi: 10.1371/journal.ppat.1008421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F.-S., Zhang C. What to do next to control the 2019-nCoV epidemic? Lancet. 2020;395:391–393. doi: 10.1016/S0140-6736(20)30300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Health Commission of the People's Republic of China . China National Health Commission; Beijing: 2020. [New coronavirus pneumonia prevention and control program (seventh trial edition)]http://www.nhc.gov.cn/xcs/zhengcwj/202002/3b09b894ac9b4204a79db5b8912d4440.shtml2020 [in Chinese]. Available at: Accessed March 4, 2020. [Google Scholar]
- 11.World Health Organisation. Coronavirus disease (COVID-19) outbreak. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed April 12, 2020.
- 12.Novel Coronavirus Pneumonia Emergency Response Epidemiology Team The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua liu xing bing xue za zhi. 2020;41:145–151. [Google Scholar]
- 13.Wenjie T., Xiang Z., Xuejun M. A novel coronavirus genome identified in a cluster of pneumonia cases—Wuhan, China 2019-2020. China CDC Wkly. 2020;2:61–62. [PMC free article] [PubMed] [Google Scholar]
- 14.The Joint Prevention and Control Mechanism of the State Council Caring frontline healthworks in epidemic prevention and control. http://www.nhc.gov.cn/xwzb/webcontroller.do?titleSeq=11257&gecstype=12020 Available at: Accessed March 08, 2020.
- 15.Chen S., Zhang Z., Yang J. Fangcang shelter hospitals: a novel concept for responding to public health emergencies. Lancet. 2020;395:1305–1314. doi: 10.1016/S0140-6736(20)30744-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xinhuanet Ma Xiaowei: The Fangcang hospital did “zero infection, zero death and zero return.” February 28, 2020. http://www.xinhuanet.com/politics/2020-02/28/c_1125641312.htm Available at: Accessed February 28, 2020.
- 17.Wu X.Y.Y., Ke S. Practical effect of continuous seamless scheduling and level management model in isolated wards during COVID-19. Chin Gen Pract Nurs. 2013;11:3041–3042. [Google Scholar]
- 18.Hubei Province Pediatric Medical Quality Control Center Practice for management of pediatric ward and prevention of infection during the epidemic period of COVID-19. Chin J Appl Clin Pediatr. 2020;35:105–111. [in Chinese] [Google Scholar]
- 19.Lei S., Jiang F., Su W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organisation; 2020. National Health Commission of the People's Republic of China. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7) Available at: http://www.nhc.gov.cn/xcs/zhengcwj/202002/3b09b894ac9b4204a79db5b8912d4440.shtml2020. Accessed February 5, 2020. [Google Scholar]
- 21.World Health Organisation. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. 2020. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed March 13, 2020.
- 22.Chen H., Guo J., Wang C. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J-J, Dong X, Cao Y-Y, et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China [e-pub before print]. Allergy, doi: 10.1111/all.14238. [DOI] [PubMed]
- 26.Zhou F., Yu T., Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y., Sun W., Li J. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019 [preprint] medRxiv. 2020 02.17.20024166. [Google Scholar]
- 29.Mach W.J., Thimmesch A.R., Pierce J.T., Pierce J.D. Consequences of hyperoxia and the toxicity of oxygen in the lung. Nurs Res Pract. 2011;2011:260482. doi: 10.1155/2011/260482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He H., Sun B., Liang L. A multicenter RCT of noninvasive ventilation in pneumonia-induced early mild acute respiratory distress syndrome. Crit Care. 2019;23:300. doi: 10.1186/s13054-019-2575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrillo A., Gonzalez-Diaz G., Ferrer M. Non-invasive ventilation in community-acquired pneumonia and severe acute respiratory failure. Intensive Care Med. 2012;38:458–466. doi: 10.1007/s00134-012-2475-6. [DOI] [PubMed] [Google Scholar]
- 32.Del Sorbo L., Goligher E.C., McAuley D.F. Mechanical ventilation in adults with acute respiratory distress syndrome. Summary of the experimental evidence for the clinical practice guideline. Ann Am Thorac Soc. 2017;14:S261–S270. doi: 10.1513/AnnalsATS.201704-345OT. [DOI] [PubMed] [Google Scholar]
- 33.Chinese Research Hospital Association of Critical Care Medicine, Youth Committee of Chinese Research Hospital Association Of Critical Care Medicine. [Chinese experts' consensus on diagnosis and treatment of severe and critical coronavirus disease 2019 (Revised Edition)]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020;32:269‐74 [in Chinese]. [DOI] [PubMed]
- 34.Force A.D.T., Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 35.Arabi Y.M., Fowler R., Hayden F.G. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46:315–328. doi: 10.1007/s00134-020-05943-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Z., Shu Q., Wang Diagnosis and treatment recommendation for pediatric coronavirus disease-19 (the second edition) J Zhejiang Univ (Med Sci) 2020;49:139–146. doi: 10.3785/j.issn.1008-9292.2020.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu X.-W., Wu X.-X., Jiang X.-G. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao B., Wang Y., Wen D. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M., Cao R., Zhang L. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 42.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 43.China National Health Commission Press conference of the Joint Prevention and Control Mechanism of the State Council. March 17, 2020. http://www.nhc.gov.cn/xcs/s3574/202003/01426fc0590249ecac89a2874214e523.shtml2020 Available at: Accessed March 17, 2020.
- 44.Liu J., Cao R., Xu M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72–73. doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 46.Multicenter Collaboration Group of Department of Science And Technology of Guangdong Province and Health Commission of Guangdong Province for Chloroquine in the Treatment of Novel Coronavirus Pneumonia. [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:185–188. doi: 10.3760/cma.j.issn.1001-0939.2020.03.009. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 47.Zhou D, Dai SM, Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression [e-pub before print]. J Antimicrob Chemother, 10.1093/jac/dkaa114. [DOI] [PMC free article] [PubMed]
- 48.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [e-pub before print]. Clin Infect Dis, doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed]
- 49.Russell C.D., Millar J.E., Baillie J.K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J.P., Hu Y., Du R.H. [Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:183–184. doi: 10.3760/cma.j.issn.1001-0939.2020.03.008. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 52.Ni Qin D.C., Li Y., Zhao H.H. Retrospective study of side effects of low-dose glucocorticoids in rheumatoid arthritis. Chin J Clin Infect Dis. 2020:43. [Google Scholar]
- 53.Wang Y., Jiang W., He Q. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China [preprint] medRxiv. 2020 03.06.20032342. [Google Scholar]
- 54.Zhou W., Liu Y., Tian D. al. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther. 2020;5:18. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Z., Xie S., Zhang J. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy [preprint] Preprints. 2020:2020030065. [Google Scholar]
- 56.Zhou YH, Qin YY, Lu YQ, et al. Effectiveness of glucocorticoid therapy in patients with severe novel coronavirus pneumonia: protocol of a randomized controlled trial [e-pub before print]. Chin Med J 10.1097/CM9.0000000000000791. [DOI]
- 57.Brown C, Carson G, Chand M, Dunning J, Zambon M. Treatment of MERS-CoV: information for clinicians clinical decision-making support for treatment of MERS-CoV patients. London: 2014, Public Health England.
- 58.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.China National Health Commission. The clinical guideline of convalescent plasma treatment (trial version 2). 2020. Available at: http://www.chinadaily.com.cn/a/202002/16/WS5e48cbf6a310128217277d72.html. Accessed February 16, 2020.
- 60.Zhang Y, Wang X. 32 recovered patients donate plasma to others with coronavirus infection. China Daily 2020 Feb 16. Available at: https://www.chinadaily.com.cn/a/202002/16/WS5e48cbf6a310128217277d72.html. Accessed February 16, 2020.
- 61.Xiang Y. Clinical trial registration information analysis of COVID-19 in China. Chin J Clin Pharmacol Ther. 2020;25:135–140. [Google Scholar]
- 62.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection—a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2–mediated inflammatory responses: from mechanisms to potential therapeutic tools [e-pub before print]. Virol Sin, 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed]
- 64.Gu M., Shi N., Li X. Analysis of property and efficacy of traditional Chinese medicine in staging prevention and treatment of corona virus disease 2019. Zhongguo Zhong Yao Za Zhi. 2020;45:1253–1258. doi: 10.19540/j.cnki.cjcmm.20200225.501. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 65.Yan Y-M, Shen X, Cao Y-K, Zhang J-J, Wang Y, Cheng Y-X. Discovery of anti–2019-nCoV agents from 38 Chinese patent drugs toward respiratory diseases via docking screening. Preprints 2020:2020020254.
- 66.Expert Team of Chinese Medical Association Nephrology Branch Expert consensus on diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infection with acute kidney injury. Chin J Nephrol. 2020:242–246. [Google Scholar]
- 67.Li L. Translation: Expert consensus on the application of artificial liver blood purification system in the treatment of severe and critical COVID-19: National Clinical Research Center for Infectious Diseases. State Key Laboratory for Diagnosis and Treatment of Infectious Diseases. 2020:10. [Google Scholar]
- 68.Hu L., Chen S., Fu Y. Risk factors associated with clinical outcomes in 323 COVID-19 patients in Wuhan, China [e-pub before print] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention [e-pub before print]. JAMA, doi: 10.1001/jama.2020.2648. [DOI] [PubMed]
- 70.First vaccine clinical trials begin in the United States. March 17, 2020. https://www.nature.com/articles/d41586-020-00154-w Available at: Accessed March 17, 2020. [DOI] [PubMed]
- 71.China has successfully developed recombination vaccines of SARS-CoV-2. Available at: https://news.qudong.com/2020/0317/653095.shtml. Accessed March 17, 2020.
- 72.Guerin C., Reignier J., Richard J.C. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 73.Xu Y., Deng X., Han Y. A multicenter retrospective review of prone position ventilation (PPV) in treatment of severe human H7N9 avian flu. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nursing Department of Tongji Hospital Affiliated to Tongji Medical College Hust; Nursing Department of Peking Union Medical College Hospital; Intensive Care Professional Committee of the Chinese Nursing Association; Writing Committee Members; Wang H, Zeng T, Wu X, Sun H. Holistic care for patients with severe coronavirus disease 2019: an expert consensus. Int J of Nurs Sci 2020;7:128-34. [DOI] [PMC free article] [PubMed]
- 75.Tang S., Hu W., Niu L. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15:700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:P420-2. [DOI] [PMC free article] [PubMed]
- 77.Yang P., Shao F., Wang G. Two cases of increased positive rate of SARS-CoV-2 nucleic acid test by aerosol inhalation to induce sputum excretion. Chin J Tuberc Respir Dis. 2020:E018. [Google Scholar]
- 78.Infectious Disease Group, Respiratory Disease Branch, Chinese Medical Association Guidelines for the diagnosis and treatment of hospital-acquired pneumonia and ventilator-associated pneumonia in adult in China (2018 edition) Chin J Tuberc Respir Dis. 2018;4:255–280. [Google Scholar]
- 79.Chinese Society of Cardiology of Chinese Medical Association; Editorial Board of Chinese Journal of Cardiovascular Disease [Expert consensus on principal of clinical management of patients with severe emergent cardiovascular diseases during the epidemic period of COVID-19] Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:189–194. doi: 10.3760/cma.j.cn112148-20200210-00066. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 80.Deng P., Zhong D., Yu K. Pharmacokinetics, metabolism, and excretion of the antiviral drug arbidol in humans. Antimicrob Agents Chemother. 2013;57:1743–1755. doi: 10.1128/AAC.02282-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wrapp D., Wang N., Corbett K.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Monteil V., Prado P., Hagelkrüys A. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181 doi: 10.1016/j.cell.2020.04.004. 905‐913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jing Z.-C., Zhu H.-D., Yan X.-W. Recommendations from the Peking Union Medical College Hospital for the management of acute myocardial infarction during the COVID-19 outbreak. Eur Heart J. 2020;41:1791–1794. doi: 10.1093/eurheartj/ehaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang L., Fan Y., Lu Z. Experiences and lesson strategies for cardiology from the COVID-19 outbreak in Wuhan, China, by “on the scene” cardiologists. Eur Heart J. 2020;41:1788–1790. doi: 10.1093/eurheartj/ehaa266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.COVID-19: too little, too late? [editorial]. Lancet 2020;395(10266):755. [DOI] [PMC free article] [PubMed]
- 86.Tian H., Liu Y., Li Y. The impact of transmission control measures during the first 50 days of the COVID-19 epidemic in China [preprint] medRxiv. 2020 doi: 10.1126/science.abb6105. 01.30.20019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.China National Health Commission Covid-19 prevention and control scheme. http://www.nhc.gov.cn/xcs/zhengcwj/202003/4856d5b0458141fa9f376853224d41d7.shtml Available at: Accessed March 7, 2020.
- 88.Comprehensive Office of the Leading Group for the Prevention and Control of New Coronavirus Pneumonia in Chongqing, Chongqing Municipal People’s Government. [Chongqing COVID-19 epidemic area classification and classification prevention and control implementation scheme] http://www.cq.gov.cn/zwgk/fdzdgknr/lzyj/qtgw/202002/t20200219_5273455.html Available at: Accessed February 19, 2020.
- 89.Yi Jiang Y., Lu X., Jin R. Diagnosis, treatment and prevention of 2019 novel coronavirus infection in children: experts' consensus statement (Second Edition). [J] Chinese Journal of Applied Clinical Pediatrics. 2020;35:143–150. doi: 10.1007/s12519-020-00343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.