Highlights
-
•
The pandemic of coronavirus disease 2019 (COVID-19) has emerged as a major health crisis, with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) having infected over a million people around the world within a few months of its identification as a human pathogen.
-
•
Initially, SARS-CoV-2 infects cells in the respiratory system and causes inflammation and cell death.
-
•
Subsequently, the virus spreads out and damages other vital organs and tissues, triggering a complicated spectrum of pathophysiological changes and symptoms, including cardiovascular complications.
-
•
Acting as the receptor for SARS-CoV entering mammalian cells, angiotensin converting enzyme-2 (ACE2) plays a pivotal role in the regulation of cardiovascular cell function.
-
•
Diverse clinical manifestations and laboratory abnormalities occur in patients with cardiovascular injury in COVID-19, characterizing the development of this complication, as well as providing clues to diagnosis and treatment.
-
•
This review provides a summary of the rapidly appearing laboratory and clinical evidence for the pathophysiology and therapeutic approaches to COVID-19 pulmonary and cardiovascular complications.
Keywords: Coronavirus, Lung, Heart, Blood vessels, Infection, Injury
Abstract
The pandemic of coronavirus disease 2019 (COVID-19) has emerged as a major health crisis, with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) having infected over a million people around the world within a few months of its identification as a human pathogen. Initially, SARS-CoV-2 infects cells in the respiratory system and causes inflammation and cell death. Subsequently, the virus spreads out and damages other vital organs and tissues, triggering a complicated spectrum of pathophysiological changes and symptoms, including cardiovascular complications. Acting as the receptor for SARS-CoV entering mammalian cells, angiotensin converting enzyme-2 (ACE2) plays a pivotal role in the regulation of cardiovascular cell function. Diverse clinical manifestations and laboratory abnormalities occur in patients with cardiovascular injury in COVID-19, characterizing the development of this complication, as well as providing clues to diagnosis and treatment. This review provides a summary of the rapidly appearing laboratory and clinical evidence for the pathophysiology and therapeutic approaches to COVID-19 pulmonary and cardiovascular complications.
Graphical abstract
- α-HBDH
α-hydroxybutyrate dehydrogenase
- ARDS
acute respiratory distress syndrome
- CCL2
C-C motif chemokine ligand 2
- COVID-19
Coronavirus disease 2019
- EAT
epicardial adipose tissue
- MERS-CoV
middle east respiratory syndrome coronavirus
- RAS
renin–angiotensin system
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SARS-CoV
severe acute respiratory syndrome coronavirus
- TACE
Tumor Necrosis Factor-α-converting enzyme
- TMPRSS2
type II transmembrane serine proteases
1. Introduction
Since December 2019, an acute severe viral infection involving primarily the respiratory system has emerged with rapid transmission around the world to over a million people within a few months. Named coronavirus disease 2019 (COVID-19) by the World Health Organization [1], the disease pandemic has resulted in a major health crisis. The pathogen of COVID-19 has been attributed to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel beta coronavirus closely related to severe acute respiratory syndrome coronavirus (SARS-CoV) [2]. COVID-19 has resulted in many infections and death throughout the world [3]. Unlike those seen in influenza, the morbidity and transmission modality of COVID-19 appear more severe and uncontrollable [4]. The primary pulmonary injury and subsequent cardiovascular complications constitute the key pathophysiology of this deadly disease. This review updates and summarizes the pathophysiological features, possible underlying mechanisms, and clinical characteristics of pulmonary and cardiovascular injury of COVID-19.
2. Pathogen(s) of COVID-19
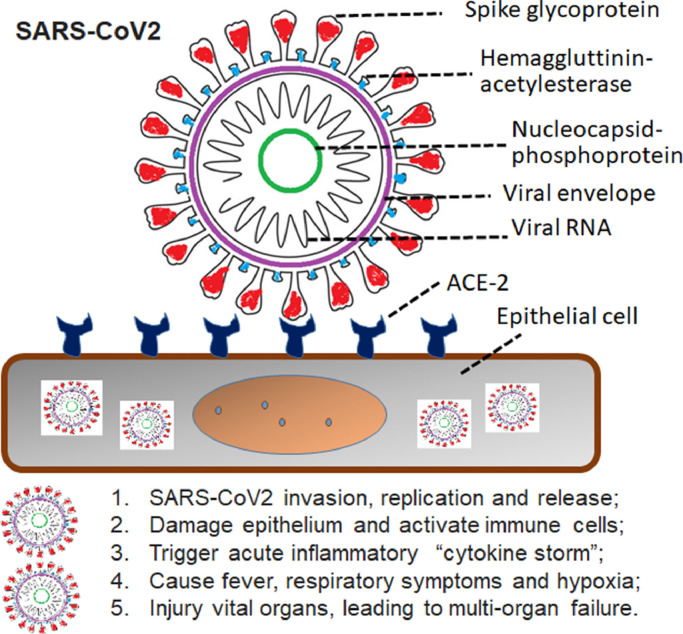
The highly contagious virus, SARS-CoV-2, has been identified as the primary pathogen responsible for the development of COVID-19. It belongs to the Coronaviridae family [5]. Structurally and functionally similar to most members of the Betacoranavirus Subgroup B, SARS-CoV-2 (Fig. 1 ) has thought to be descended from a bat gene pool as the seventh member of coronavirus family known to infect humans, and comprises a positive-sense single-stranded RNA with 50–200 nm in size [6]. Among the other 6 coronaviruses capable of causing illnesses, only SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV) reportedly cause severe disease and fatalities [7]. Infection by the other 4 coronaviruses remains asymptomatic or mildly symptomatic in normal people. According to the full-length genome sequencing, SARS-CoV-2 is 79.5% homologous with SARS-CoV. Like SARS-CoV, SARSomatic or mildly symptomatic in normal peells by receptor-mediated endocytosis in association with angiotensin converting enzyme II (ACE2) [8]. An epidemiological study enrolling 44,672 confirmed cases in China has indicated that the overall case-fatality rate of SARS-CoV-2 was about 2.3% [9], whereas it was 9.6% (774/8096) in the SARS-CoV epidemic [10] and 34.4% (858/2494) in the MERS-CoV outbreak [11]. Mortality in Italy, Spain, and France may be higher and closer to that of SARS-CoV. This may be due to strain variation, yet to be determined. However, in consideration of rapidly increasing numbers of confirmed cases and evidence of human-to-human transmission [12,13], the SARS-CoV-2 infectivity seems to be stronger than SARS-CoV and MERS-CoV. Ultrastructural examination of SARS-CoV-2 by cryo-electron microscopy has demonstrated that the binding affinity of SARS-CoV-2 to ACE2 appears approximately 10- to 20-fold higher than SARS-CoV, structurally explaining why SARS-CoV-2 has a high contagiousness [14].
Fig. 1.
Schematic representation of the COVID-19 pathogenic virus, SARS-CoV2, invasion and triggering organ injury, and symptoms. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin converting enzyme II.
In spite of the fact that SARS-CoV-2 has infected more than a million individuals it is largely unknown how and when the virus has been evolving and interacts with other microorganisms (Table 1 ) in the lung and other vital organs, such as heart and brain. Shen et al. [15] have recently reported a genomic diversity of SARS-Cov-2 in patients with COVID-19. They observed, by meta-transcriptomal sequencing for the bronchoalveolar lavage fluid samples from of COVID-19, community-acquired pneumonia, and healthy individuals. They observed a limited polymorphism and diversity in the intrahost setting, and a substantial proportion of bacteria in several COVID-19 patients, similar to other patients with noncoronaviral pneumonia. As a common complication of viral infection, especially for respiratory viruses, secondary bacterial infection often results in a significant increase in morbidity or even mortality. Indeed, in the retrospective observational study of 85 fatal cases of COVID-19, Du et al. [16] reported that in addition to SARS-Cov-2 infection, simultaneously or secondarily, other pathogens may participate in the COVID-19 development and complications, contributing to the severity and mortality of COVID-19. Thus, co-infection of other pathogens certainly complicates the pathogenesis and management of COVID-19.
Table 1.
Co-pathogens of COVID-19*
| Virus | % cases |
|---|---|
| Sars-CoV2 | 100% |
| Respiratory syncytial virus | 33.3% |
| Influenza A virus | 9.1% |
| Influenza B virus | 5.3% |
| Other microorganisms | |
| Mycoplasma | 26.5% |
| Chlamydia | 34.1% |
| Fungus | 33.3% |
*Reference [16]
3. Diagnosis of COVID-19
As recommended by WHO, most countries, including the United States, have adapted similar diagnostic procedures for COVID-19 and harvest certain clinical and epidemiologic information for diagnosis (Table 2 ). In the United States, criteria have been developed for persons under investigation (PUI) for COVID-19. People with confirmed COVID-19 usually develop fever and/or symptoms of acute respiratory illness (e.g., cough, difficulty breathing). For the PUI individuals, health care providers should immediately put him or her under infection control and prevention measures. Initially, testing for all other sources of respiratory infection is implemented while assessing epidemiologic factors to assist their diagnosis, including the information as to whether the person has had close contact with a patient with laboratory-confirmed COVID-19 within 14 days of symptom onset or a history of travel from affected geographic areas or epicenters within 14 days.
Table 2.
Criteria implemented for assessment and diagnosis of COVID-19
| Epidemiological evidence |
|
| Clinical symptoms |
|
| Laboratory evidence |
|
| Chest imaging evidence |
|
Diagnosis of COVID-19 requires collecting specimens from both the upper respiratory tract (naso- and oropharyngeal samples) and lower respiratory tract, including the expectorated sputum, endotracheal aspirate, or bronchoalveolar lavage. The samples need to be processed quickly and stored at 4°C. The reverse polymerase chain reaction (RT-PCR) assays are a standard procedure for viral genomic sequence identification, which involves the synthesis of a double-stranded DNA molecule from the RNA template. If patients are confirmed COVID-19 positively, their laboratory evaluation should be repeated to evaluate for viral clearance prior to being released from quarantine. Patients with the early stage of COVID-19 may have a normal or decreased total white blood cell count and a decreased lymphocyte count. Subsequently, neutrophils arise wit lymphopenia, and the increased ratio of neutrophils vs. lymphocytes is considered a negative prognostic factor. Patients may have increased values of LDH, muscle enzymes, and C-reactive protein. In critical patients, the thrombogenic biomarker D-dimer may increase, the counts of blood lymphocytes decline persistently, and laboratory alterations in biomarkers of multiorgan injury become prominent.
The clinical profiles and diagnosis for COVID-19 have been well documented to date. However, new evidence is now emerging that this deadly disease is far more mystery than previously thought as many cases appeared atypical and easily misdiagnostic [17]. According to the seventh edition (March 3, 2020) of COVID-19 guideline by the General Office of National Health Committee, and Office of State Administration of Traditional Chinese Medicine, China (The notice on the issuance of program for the diagnosis and treatment of COVID-19, Trial Version 7, 2020), diagnosis of suspected case needs to combine any one item of epidemiological history features with 2 items of clinical manifestations to make a comprehensive analysis, or needs to meet 3 items of clinical manifestations with or without clear epidemiological history (Table 2). Wang et al. [18] suggest that diagnosing the confirmed case should base on suspected case with any 1 item of pathogenic or serological evidence as following: (1) real-time PCR test positive for SARS-CoV-2; (2) viral whole genome sequencing showing high homogeneity to the known novel coronaviruses; (3) positive for the specific IgM antibody and IgG antibody to SARS-CoV-2 in serum test; or a change of the SARS-CoV-2-specific IgG antibody from negative to positive, or titer rising ≥4 times in the recovery phase above that in the acute phase.
4. Pulmonary and cardiac injury caused by COVID-19
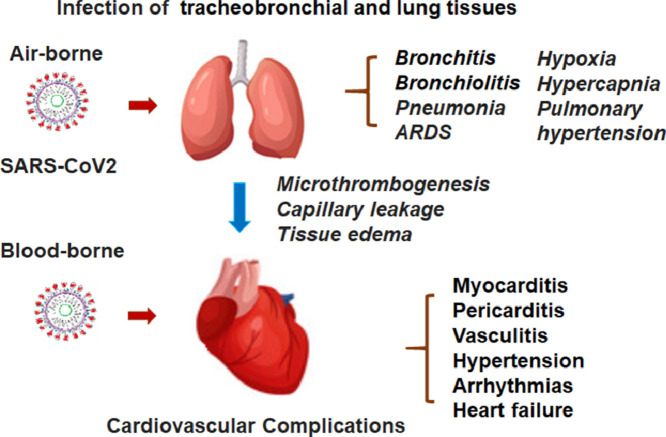
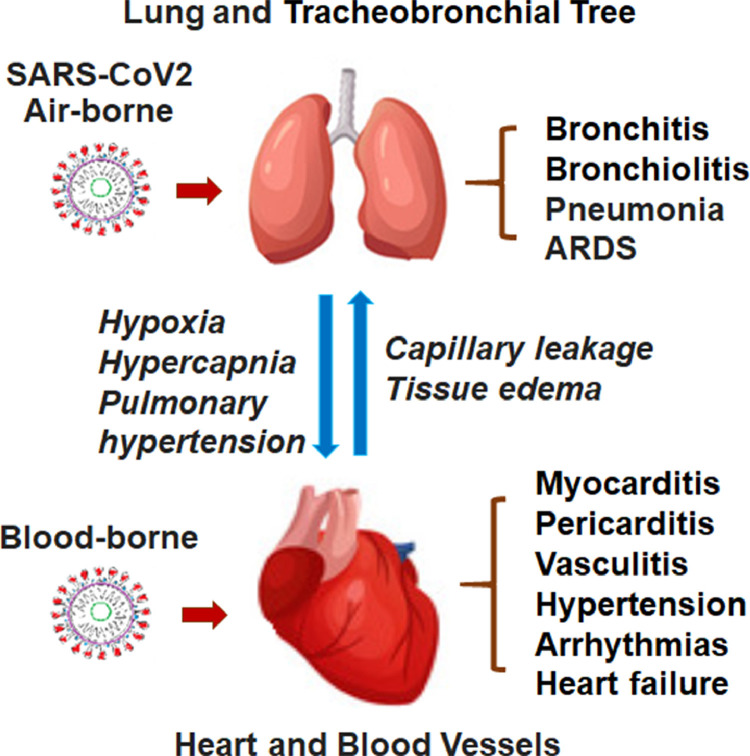
SARS-CoV-2 mainly attacks the respiratory system, clinically characterized by the rapid development of pneumonia, and in severe cases, the acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome [19] . The death rate remains high in those admitted to the intensive care and on ventilator due to complications of respiratory and cardiac failure [16]. Even though the lung is the primary organ damaged by the virus, COVID-19 is now regarded as a systemic disease, involving a broad range of other vital organs, such as heart, liver, and kidney [20]. However, it remains largely unclear whether the organ and tissue injury in patients with COVID-19 is the direct or indirect consequence of the virus infection. ACE2, a known protein binding to SARS-CoV-2, is expressed widely in various organs and tissues, including the cardiovascular, digestive, and urogenital systems beside the respiratory tract [21,22]. Theoretically, the virus may target those organs and tissues with positive expression of ACE2. Cardiovascular cells are known to express ACE2 at high levels, which serves as a key regulator of blood pressure and cardiac contractility [23]. Moreover, the virus infection may trigger a drastic immune response which leads to production of large quantities of cytokines, i.e., “cytokine storm,” which may indiscriminately injure vital organs [24]. For instance, SARS-CoV-2 infection injures the myocardium, leading to elevated levels of myocardial biomarkers (e.g., troponin I > 28 pg/mL) and certain abnormalities in electrocardiography and echocardiography [25]. Increasing clinical evidence indicates that virus infection-associated cardiac dysfunction worsen COVID-19 patient's clinical conditions usually in association with the poor prognosis [26,27]. In spite of the observation that myocardial injury occurs frequently in COVID-19 patients [28], the underlying mechanisms remains largely unclear. Several lines of clinical evidence point to the possibility of viral infection directly occurring in the myocardium. Both direct and indirect pathogenic impacts of the virus infection may cause injury of the pulmonary and myocardial tissues (Fig. 2 ).
Fig. 2.
Schematic demonstration of the viral injury to the lung and heart triggering the “Lung-Heart” syndromes with a combination of the respiratory and cardiovascular adverse events and conditions.
In epidemiology, high prevalence of cardiovascular dysfunction has been recently reported in COVID-19 patients, especially those with critical medical conditions [29]. In a study of 138 COVID-19 patients, 10 patients (7.2%) were diagnosed as acute myocardial injury based on the elevation of high-sensitivity cardiac troponin I (hs-cTnI), and 8 of them admitted into the intensive care unit (ICU) [12]. In another retrospective study, cardiac troponin I (cTnI) was significantly elevated in 33.3% of severe and 100.0% of critical patients [30]. Huang et al. also revealed increased numbers of COVID-19 patients with acute myocardial injury and in the critical conditions leading to admission into ICU [19].
5. Pathological characteristics of pulmonary and cardiovascular injury in COVID-19
Recent autopsy reports have demonstrated several anatomical features of COVID-19-induced pulmonary and myocardial injury. The early lung of COVID-19 patients exhibited edema, proteinaceous exudate, focal reactive hyperplasia of pneumocytes with patchy inflammatory cellular infiltration, and multinucleated giant cells [31]. Liu et al. showed that the lung and cardiac tissues contain significant amounts of inflammatory infiltrates, indicating the inflammatory nature of tissue damage by SARS-CoV-2 infection [26]. Patients with a medical history of coronary heart disease are prone to the cardiac injury caused by COVID-19. Although there was no evidence that the acute episode of cardiac injury was caused by coronary events in this patient, one may not rule out the possibility that the underlying medical condition might have made the patient more vulnerable to the hypoxia- and cytokine storm-induced cardiac ischemia [32]. Moreover, postmortem examinations showed moderate pericardial effusion with light yellow and clear liquid and mild epicardial edema indicative of myocardial inflammation, further suggesting that a pericardiac inflammatory response contributes to the myocardial dysfunction in COVID-19 patients. In a pilot study, Xu et al. [33] failed to find evidence of myocardial injury in a histopathologic case by autopsy, in which there were only a few interstitial mononuclear inflammatory infiltrates without other substantial damage in the cardiac tissue. This paradoxical result may be attributed to the small sample size and absent clinical evidence of myocardial injury in this case. Overall, future research is needed to enroll more cases with definite clinical evidence of myocardial injury to further investigate histopathologic characteristics of COVID-19-related myocardial injury. The viral infection induces an excessive immune reaction in the host, which in turn generates a “cytokine storm,” comprising released by pro-inflammatory cytokines known to act on cardiovascular cells [34,35]. One of the protagonists in the cytokine storm is interleukin 6 (IL-6), which is produced and released by activated leukocytes and acts on a large number of cells and tissues. IL-6 promotes the differentiation and activation of B lymphocytes, and stimulates the production of acute phase proteins.
The severity and lethality of COVID-19 may be, at least in part, due to the viral injury to the myocardium and blood vessels. This serves as a major challenge for successful management and care of COVID-19 patients. In an observational study of 52 critical sick adult COVID-19 patients, 19 patients suffered from acute myocardial injury, and 9 of them (75%) died [29], suggesting a higher mortality in COVID-19 patients with cardiovascular complications. Analysis of COVID-19 related death cases has confirmed that myocardial injury was one of the major causes of death. In a review of 25 death cases with COVID-19, Xun et al. found that the serum cTnI were significantly increased in 18 cases, and more COVID-19 cases with heart injury than other organ injuries except for the lung. Zhang et al. reported that 89% of 82 death cases were observed with myocardial injury, which was second only to death cases with pulmonary injury [36]. Moreover, in a study of 416 COVID-19 patients, myocardial injury was independently associated with higher risk of in-hospital mortality [27]. Hence, early myocardial injury in COVID-19 leads to a poor prognosis in COVID-19 patients.
6. Laboratory evidence for inflammation and organ injury
Changes of cardiac-specific biomarkers in the peripheral blood have been reported in patients with COVID-19. As stated above, hs-cTnI acts as one of the specific biomarkers of myocardial injury [37]. Other non- or less-specific myocardial biomarkers may elevate in COVID-19 cardiovascular complications as well, such as creatine kinase (CK), creatine kinase MB isoenzyme (CK-MB) and lactate dehydrogenase (LDH). However, the biomarkers may not always change in the same pattern. For instance, in a study of 99 COVID-19 patients, only 13 patients had elevation of CK while 75 patients showed LDH elevation [38]. The measurements of myocardial biomarkers are believed to possess prognostic values. In the first multicenter study [39] which enrolled 1099 patients with laboratory-confirmed COVID-19 from 552 hospitals in China, 675 patients had measurements of serum LDH, and 277 of them showed elevated LDH levels, accounting for 58.1% of total severe cases. Moreover, increased LDH and CK were also observed in the blood of patients at the composite endpoints (the admission to ICU, mechanical ventilation, and death). The elevation of myocardial biomarkers in COVID-19 patients may offer the prognostic information useful for assessing the disease progression, and development of adverse events. Since some of the biomarkers are not specific to the myocardium, the increased nonspecific biomarkers during the development of adverse events of COVID-19 patients may reflect not only the injury to the myocardium but also other vital organs or tissues.
6.1. Invasion through ACE2
SARS-CoV-2 may directly invade cardiomyocytes and subsequently result in viral myocarditis and corresponding injury. Structurally, the SARS-CoV-2 particle has an ACE2-binding domain [40]. The virus binds to human ACE2 with high affinity, and uses it as an entry receptor to invade target cells. Cryo-electron microscopy reveals the structure of the SARS-CoV-2 spike glycoprotein in 2 distinct conformations, along with inhibition of spike-mediated entry by SARS-CoV polyclonal antibodies, providing a blueprint for the design of vaccines and therapeutics. Although the main target is the epithelium of respiratory system, the virus may use the ACE2 path to invade cardiomyocytes directly as many cardiovascular cells express ACE2 (Fig. 2). SARS-CoV-2 may trigger ACE2 activation and upregulate ACE2 downstream signal transduction, including the activation of Ras-ERK–AP-1 pathway [41]. Consequently, this may induce myocardial inflammation and fibrosis and exacerbate cardiac dysfunction.
As the cellular receptor for the entry of SARS-CoV-2, ACE2 is a monocarboxylase highly expressed in cardiac tissue [42] and exhibits cardio-protective roles as a potent negative regulator of the renin-angiotensin system (RAS) by counterbalancing functions of ACE [43]. As a membrane protein, it can also initiate outside-in signaling [44]. SARS-CoV have been previously demonstrated to induce myocarditis in a ACE2-dependent manner [45]. The trimers of the SARS-CoV spike protein initially extends into a hydrophobic pocket of ACE2 catalytic domain [46], which consequentially leads to the endocytosis of SARS-CoV particles and fusion of virus to cardiomyocytes with the help of type II transmembrane serine proteases (TMPRSS2) [47], finally downregulating ACE2 expression in cardiomyocytes [48,49]. When RAS is over-activated, further myocardial injury is a consequence in SARS patients. Moreover, downregulation of ACE2 may be, at least partly, caused by the shedding of ACE2 ectodomain, mediated by TNF-α-converting enzyme (TACE) in coupling with the production of TNF-α, a well-known pro-fibrotic and myocardial impairing factor [50]. The binding between SARS-CoV spike protein and ACE2 on the cardiomyocyte surface also triggers the Ras-ERK–AP-1 pathway and activates the C-C motif chemokine ligand 2 (CCL2, a pro-fibrosis factor) [41]. The above theories are supported by an autopsy report of heart samples from SARS patients, in which the presence of SARS-CoV in the heart was associated with marked downregulation of ACE2 expression as well as a significant increase in macrophage infiltration and interstitial fibrosis, verifying ACE2 inhibition is involved in SARS-CoV-driven myocardial injury [49].
The specific cellular mechanism for SARS-CoV-2 invading and damaging cardiomyocytes has not been clearly demonstrated yet, but it is probable that SARS-CoV-2 shares the similar mechanism with its relative SARS-CoV. A recent study investigated ultrastructure of full-length human ACE2 with cryo-EM [51], indicating that SARS-CoV-2 also invaded host cells via spike protein trimers binding to an ACE2 homodimer. In addition, the interface between the receptor binding domains of spike proteins on SARS-CoV-2 is quite similar to that between the SARS-CoV and ACE2. Furthermore, the serine protease TMPRSS2 is also indispensable for the entry of SARS-CoV-2 [52]. Given that the high similarity of cellular entry with that of SARS-CoV, it is reasonable to speculate that SARS-CoV-2 interferes in ACE2 expression in the same way. However, this needs to be verified in cardiomyocytes in the further study.
6.2. Hypoxia and ischemic injury
Myocardial oxygen supply is determined by coronary blood flow and its oxygen carrying capacity while myocardial oxygen demand is determined by systolic wall tension, contractility, and heart rate [53]. This physiological mechanism may be involved in SARS-CoV-2-induced myocardial injury. As is well known, pulmonary dysfunction is the primary insult of SARS-CoV-2, which induces hypoxemia, hypotension and, in some cases, shock [20,39]. As a consequence, an insufficient oxygen supply may occur in multiple organs including the heart. Concomitantly, myocardial oxygen demand is increased in virus-infection states, as high metabolic rate induces an augmented burden on the myocardium [54], further causing the imbalance between myocardial oxygen supply and demand. Along with disease progression, this imbalance is increasingly aggravated, which may result in myocardial injury in COVID-2019 patients, especially for those with cardiovascular disorders who have already exhausted myocardial reserve capacity on the supply side. Other systemic contributors may include, in addition to hypoxia, respiratory and/or metabolic acidosis, fluid or electrolyte disorders and an activated neuro-humoral system following severe infection may also lead to myocardial stunning and injury, even inducing malignant arrhythmia and sudden death.
6.3. Cytokine storm and inflammation
Previous studies have confirmed that the immune abnormality is related to the pathogenesis of SARS-CoV infection [45]. Specifically, cytokine storm, an excessive and uncontrollable cytokines production in response to infection, is suggested to be the main contributor, as numerous studies have found that the expression levels of pro-inflammatory cytokines [e.g., Interleukin-1β (IL-1β), IL-6, interferon-γ (IFN-γ), IFNγ inducible protein-10 (IP-10), monocyte chemoattractant protein-1(MCP-1), granulocyte colony-stimulating factor (G-CSF), macrophage inflammatory protein-1α (MIP-1α), tumor necrosis factor-α (TNF-α)] are significantly increased in COVID-19 patients, associated with disease progression [55,56]. Among so many cytokines, IL-6 serves as the core of cytokine storm, given that IL-6 not only amplifies cytokine storm by stimulating production of other pro-inflammatory cytokines, but also results in vascular leakage, interstitial edema [57]. Moreover, IL-6 has also been shown to weaken papillary muscle contraction, which causes myocardial dysfunction [58]. In addition, IFNγ is also regarded as a marker of cytokine storm, causing cell apoptosis through regulating JAK/STAT1 axis and p38-MAPK1 [59]. However, anti-inflammatory cytokines such as IL-4 and IL-10 are also increased in COVID-19 patients, and their levels are also related to disease severity [55,56], demonstrating the close relation between pro- and anti-inflammation. Cytokine storm is a clear contributor to COVID-19-related myocardial injury, demonstrated by a study that revealed that increased levels of IL-6 were significantly associated with high hs-TnI levels [26], a cardiac-selective biomarker of myocardial infarction and injury [37]. Further studies are needed to explore the cytokine expression in cardiomyocytes, which will promote a better understanding for SARS-CoV-2-induced inflammation in cardiac tissue.
Overall, direct infection through ACE2, the imbalance between myocardial oxygen supply and demand, and the abnormal immune response constitute the most plausible explanations for myocardial injury associated with COVID-19. To be noted, however, the above speculation is mostly based on clinical observation of COVID-19. Therefore, more in-depth research is required to understand the pathophysiology of SARS-CoV-2-induced myocardial injury in order to contribute to the future development of effective treatment.
7. Clinical symptoms and profiles
The general clinical symptoms found in COVID-19 patients with cardiac dysfunction are similar to those observed in influenza, such as fever (87.9%), cough (67.7%), fatigue (38.1%), and expectoration (33.4%) [39]. Additionally, confirmed cases of COVID-19 show several more-specific symptoms, such as anosmia, dysgeusia, chest pain [26,38], and palpitation [60]. Electro physiologically, COVID-19 patients are prone to the development of arrhythmia, especially tachycardia, indicative for myocardial injury. In a study of 138 COVID-19 patients, 23 patients (16.7%) presented with arrhythmia, and other evidence for myocardial injury [12]. Indeed, the COVID-19 patients with tachycardia were often in severe or critical situations. For a better understanding of the etiology of cardiac dysfunction in patients with COVID-19, researchers analyzed body temperature in different groups (mild, light, severe, and critical groups), given the fact that high fever caused by the virus infection and inflammation can elevate the heart rate. The result showed that there was no significant difference in the body temperature correlation with arrhythmia between the mild and severe/critical groups. Many COVID-19 patients with tachycardia are severely ill and need critical care, as tachycardia represents a sign of a failing heart [30].
With regard to the imaging features of cardiac injury, the epicardial adipose tissue (EAT) density, a measurement for inflammation, between the bifurcation of pulmonary artery and diaphragm as determined by lung CT scan may serve as a specific indicator for COVID-19-induced myocardial injury. Hui et al. reported that the mean EAT density of critical/severe groups in chest CT scan were significantly lower than patients in the light/mild groups (−98.77/−96.08 HU vs. −69.37/−84.76 HU), suggesting that myocardial inflammation occurred in COVID-19 patients [30]. As the cytokine storm and consequent inflammatory response are suggested to be the mechanisms contributing to myocardial injury, low EAT density showed in chest CT scan appears to be a high-risk factor for myocardial injury. However, low EAT density is not the optimal imaging parameter of COVID-19-related myocardial injury since it is an indirect sign of inflammation and may be substantively influenced by subjective factors in clinical practice. Other imaging modalities with high sensitivity and specificity such as echocardiography and myocardial magnetic resonance imaging should be preferentially chosen if appropriate.
8. Management and treatment of COVID-19
8.1. Pre-exiting condition management
Treatment of COVID-19 has been mostly restricted to supportive measures as there has been, to date, no specific therapy available to treat this disease. Pre-existing poor-health conditions increase the risk of cardiovascular comorbidity, with poorer prognosis. Patients at age above 60 years old and those with diabetes are more susceptible to COVID-19 induced myocardial injury. They should be prioritized for treatment. In terms of diagnostic criteria, the dynamic change of myocardial biomarkers especially hs-TnI is the main criteria to identify COVID-19 patients with myocardial injury. However, the levels of myocardial biomarkers may be affected by other factors such as the status of infection, hypoxemia, and renal insufficiency, which are commonly observed in COVID-19 patients. Thus comprehensive assessments combining electrocardiography, imaging and histopathology should be performed in clinical practice.
8.2. Antivirus therapy
Since the outbreak of COVID-19 several antivirus agents have been proposed and currently are under intensive clinical investigation. Remdesivir is a broad-spectrum investigational antiviral agent which decreased SARS-Cov-2 RNA transcription with in vitro experiments [61]. The agent was originally developed for treating Ebola virus infection but was not shown to have satisfactory efficacy in clinical trials [62]. A few COVID-19 patients recovered soon after administering remdesivir [63,64], though its safety and efficacy have not been demonstrated in randomized controlled trials (RCTs) [65], [66], [67]. Another antiviral treatment, lopinavir/ritonavir, the protease inhibitor combination used in HIV, was previously given great expectation for treating COVID-19 but failed to show significant benefit in the first RCT. Other antiviral agents are now in RCTs after showing some promise with small numbers of patients during the initial wave of COVID-19 infections in China.
8.3. Anti-inflammatory and immunoregulatory agents
Hydroxychloroquine and chloroquine are traditional antimalarial and autoimmune disease drugs. They have been shown to control the SARS-Cov-2 infection in vitro [61,68]. The underlying mechanism may involve the increase in the endosomal pH required for virus/cell fusion, interference with the glycosylation of ACE2 [69] and inactivation of IL-6 and TNF-α [70]. A small sample size study showed that hydroxychloroquine treatment was significantly associated with viral load reduction/disappearance in COVID-19 patients [71], but this conclusion still has not been verified by ongoing RCTs [72,73]. The cardiotoxicity of hydroxychloroquine and chloroquine should not be neglected. They may prolong QT interval and induce ventricular arrhythmia [74]. Thus, they should be used very cautiously (if at all) to treat COVID-19 patients with cardiovascular comorbidities or uncorrected electrolyte disorder. Given the pivotal role of immunologic overresponse in COVID-19, anti-inflammatory therapy is also promising for treating COVID-19. Corticosteroids were used during the SARV-Cov and MERS-Cov outbreaks and have also been used for patients with COVID-19. However, previous studies on whether corticosteroids exert protective [75,76] or adverse [77] effect in SARV-Cov and MERS-Cov infection came to conflicting conclusions, since it might induce in-hospital secondary infection and long-term complications while inhibiting inflammation-related organ injury. With lack of conclusive evidence, the use of corticosteroids may be prudently considered in critical COVID-19 patients with clear evidence of immune overreaction, but their use is not generally recommended by the WHO or Centers for Disease Control. Some have advocated against nonsteroidal anti-inflammatory agents, notably ibruprofen, which may be associated with worse outcome with COVID-19, based on anecdotal reports. Targeted agents such as tocilizumab are also regarded as potential therapy. Tocilizumab was shown to facilitate chimeric antigen receptor-T cell therapy because it can inhibit IL-6, the core cytokine of cytokine storm syndrome [78]. Several clinical observations demonstrated a predominant increase of IL-6 in COVID-19 patients, which is associated with severe conditions [38,79], so inhibiting IL-6 activation may ease the overreacting immune system. Future well-designed RCTs are expected to clarify tocilizumab's efficiency and its applicable patients.
8.4. ACE inhibitors and angiotensin receptor blockers
The structural evidence of SARS-Cov-2 invasion of cells via ACE2 has led to the hypothesis that ACE inhibitors (ACEI)/angiotensin receptor blockers (ARB) treatment potentially induces overexpression of ACE2, which subsequently increases the susceptibility of host cells to SARS-Cov-2 invasion and the risk of infection or worsening the severity of disease [80]. For cardiovascular patients under regular antihypertensive treatment, it has been suggested that they should discontinue ACEI or ARB medications. However, ACEI/ARB play a protective role in COVID-19, given that ACE2 shedding caused by SARS-Cov-2 invasion may result in its downregulation, following the activation of RAS axis which is partly responsible for severe organ injury in COVID-19 [48]. Thus far, there have been no clinical data supporting the case for ACEI/ARB discontinuation in COVID-19 patients. According to WHO's guideline, changing the routine antihypertensive therapy under the COVID-19 pandemic to avoid potential harm or pursue potential benefits is not recommended before any compelling evidence published.
8.5. Neutralizing antivirus plasma, stem cell transplantation, traditional herbal remedies, and anti-ischemic therapy
Other therapies with less evidence-based foundation are also worth mentioning. Plasma treatment means transfusing convalescent plasma containing antivirus polyclonal antibodies, and were proved to be effective in treating SARS patients and COVID-19 patients in observational studies [81,82]. Although the number of patients so treated is small, this does make scientific sense. Dilated horse anti-SARS-CoV-2 serum could cross-neutralize SARS-CoV-2 in an in vitro study [8]. Some traditional herbal therapies against coronavirus infection were found to be effective during SARS outbreak and are widely used in current practice in China [83]. Mesenchymal stem cell transplantation has shown both efficiency and safety in treating ARDS [84]. All the above therapies need more verification by RCTs [85], [86], [87], [88], [89]. Due to the widespread cardiac injury in COVID-19 patients, new or worsening heart failure is common especially in those with poor prognosis [79]. After recognizing the onset of heart failure, causes leading to decompensation should be evaluated first, especially those should which could be managed and corrected immediately such as acute coronary syndrome and severe rapid arrhythmias. Hypoxemia following pneumonia and ARDS is common in COVID-19 patients and will be worsened by heart failure. Thus, oxygen therapy and mechanical ventilation should be more rigorously used in COVID-19 patients with heart failure than those without. Diuretics should be considered under the evidence of volume overload, as well as renal replacement therapy which can simultaneously eliminate excessive cytokines. Rehydration should be more prudently prescribed in shock patients with COVID-19 than normal shock counterparts, considering that pulmonary edema following rapid fluid resuscitation will further impair alveolar ventilation. Mechanical assist devices such as intra-aortic balloon pump and extracorporeal membrane oxygenation may help patients get through critical periods and should be considered when necessary.
9. Conclusions
COVID-19 causes not only pulmonary injury but also severe cardiac complications, in particular in those with pre-existing medical conditions. Although the precise mechanism for the development of myocardial injury associated with COVID-19 is not fully understood, a combination of direct and indirect pathogenic factors, such as ACE2-mediated SARS-CoV-2 infection of cardiomyocytes, hypoxia and cytokine storm, may contribute to the development of myocardial injury and other adverse events which aggravate conditions and increase mortality (Table 3 ). Close and careful monitoring of respiratory and cardiac function should be emphasized in order to early identify COVID-19-related pulmonary and myocardial injury.
Table 3.
Major signs and biomarkers for pulmonary and myocardiac injury in COVID-19
| Pulmonary injury |
|
| Myocardiac injury |
|
| Inflammation |
|
Footnotes
Funding: This work was supported in part by grants from the National Institutes of Health (NIH) and Hermann Research Foundation (Y.J. Geng), National Natural Science Foundation of China (H. Qian, 81670337) and from the Clinical and Translational Medicine Research Foundation of Chinese Academy of Medical Sciences (H. Qian, 2019XK320061).
Disclosures: The authors report no competing interests.
References
- 1.World Health Organization. Coronavirus disease (COVID-19) outbreak. April 8, 2020. https://www.who.int/westernpacific/emergencies/covid-19.
- 2.Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID-19 based on current evidence. J Med Virol. 2020 doi: 10.1002/jmv.25722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus disease 2019;(COVID-19) 2020. https://www.cdc.gov/coronavirus/2019-ncov/index.html.
- 4.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;Published online March 27, 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]; doi:10.1001.jamacardio.2020.1286. In press.
- 5.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X., Chen P., Wang J., Feng J., Zhou H., Li X. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forni D., Cagliani R., Clerici M., Sironi M. Molecular evolution of human coronavirus genomes. Trends Microbiol. 2017;25:35–48. doi: 10.1016/j.tim.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization Cumulative number of reported probable cases of severe acute respiratory syndrome (SARS) 2003 https://www.who.int/csr/sars/country/2003_05_01/en/ [Google Scholar]
- 11.World Health Organization. https://www.who.int/csr/don/08-april-2020-mers-saudi-arabia/en/.
- 12.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan J.F.-W., Yuan S., Kok K.-H., To K.K.-W., Chu H., Yang J. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen Z., Xiao Y., Kang L., Ma W., Shi L., Zhang L. Genomic diversity of SARS-CoV-2 in coronavirus disease 2019 patients. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]; In press. https://doi.org/10.1093/cid/ciaa203.
- 16.Du Y., Tu L., Zhu P., Mu M., Wang R., Yang P. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]; In press. https://doi.org/10.1164/rccm.202003-0543OC.
- 17.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y.Y., Jin Y.H., Ren X.Q., Li Y.R., Zhang X.C., Zeng X.T. Updating the diagnostic criteria of COVID-19 “suspected case” and “confirmed case” is necessary. Mil Med Res. 2020;7:17. doi: 10.1186/s40779-020-00245-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Wang L., Yan S., Yang F., Xiang L., Zhu J. Clinical characteristics of 25 death cases infected with COVID-19 pneumonia: a retrospective review of medical records in a single medical center, Wuhan. China. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053. 2020.02.19.20025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G., Turner A.J. A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J Biol Chem. 2000;275:33238–33243. doi: 10.1074/jbc.M002615200. [DOI] [PubMed] [Google Scholar]
- 22.Hamming I., Timens W., Bulthuis ML, Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oudit G.Y., Crackower M.A., Backx P.H., Penninger J.M. The role of ACE2 in cardiovascular physiology. Trends Cardiovasc Med. 2003;13:93–101. doi: 10.1016/s1050-1738(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 24.Liu J., Zheng X., Tong Q., Li W., Wang B., Sutter K. Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol. 2020;92(5):491–494. doi: 10.1002/jmv.25709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2019;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 26.Wu C, Hu X, Song J, Du C, Xu J, Yang D. Heart injury signs are associated with higher and earlier mortality in coronavirus. MedRxiv. 2020 doi: 10.1101/2020.02.26.20028589. [DOI] [Google Scholar]
- 27.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang, X, Yu Y, Xu, J, Shu, H, Xia, J, Liu, H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. 2020. [DOI] [PMC free article] [PubMed]
- 30.Hui H, Zhang Y, Yang X, Wang X, He B, Li L. Clinical and radiographic features of cardiac injury in patients with 2019 novel coronavirus pneumonia. MedRχiv. 2020 doi: 10.1101/2020.02.24.20027052. [DOI] [Google Scholar]
- 31.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baroldi G. Anatomy and quantification of myocardial cell death. Methods Achiev Exp Pathol. 1988;13:87–113. [PubMed] [Google Scholar]
- 33.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geng Y.J., Wu Q., Muszynski M., Hansson G.K., Libby P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-gamma, tumor necrosis factor-alpha, and interleukin-1 beta. Arterioscler Thromb Vasc Biol. 1996;16:19–27. doi: 10.1161/01.atv.16.1.19. [DOI] [PubMed] [Google Scholar]
- 35.Geng Y.J., Azuma T., Tang J.X., Hartwig J.H., Muszynski M., Wu Q. Caspase-3-induced gelsolin fragmentation contributes to actin cytoskeletal collapse, nucleolysis, and apoptosis of vascular smooth muscle cells exposed to proinflammatory cytokines. Eur J Cell Biol. 1998;77:294–302. doi: 10.1016/S0171-9335(98)80088-5. [DOI] [PubMed] [Google Scholar]
- 36.Zhang B, Zhou X, Qiu Y, Feng F, Feng J, Jia Y, et al. Clinical characteristics of 82 death cases with COVID-19. Medrxiv. 10.1101/2020.02.26.20028191. [DOI] [PMC free article] [PubMed]
- 37.Buja L.M., Zehr B., Lelenwa L., Ogechukwu E., Zhao B., Dasgupta A. Clinicopathological complexity in the application of the universal definition of myocardial infarction. Cardiovasc Pathol. 2020;44 doi: 10.1016/j.carpath.2019.107153. In press. [DOI] [PubMed] [Google Scholar]
- 38.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181(2):281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen I.Y., Chang S.C., Wu H.Y., Yu T.C., Wei W.C., Lin S. Upregulation of the chemokine (C-C motif) ligand 2 via a severe acute respiratory syndrome coronavirus spike-ACE2 signaling pathway. J Virol. 2010;84:7703–7712. doi: 10.1128/JVI.02560-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 43.Kuba K., Imai Y., Ohto-Nakanishi T., Penninger J.M. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohlstedt K., Brandes R.P., Muller-Esterl W., Busse R., Fleming I. Angiotensin-converting enzyme is involved in outside-in signaling in endothelial cells. Circ Res. 2004;94:60–67. doi: 10.1161/01.RES.0000107195.13573.E4. [DOI] [PubMed] [Google Scholar]
- 45.Yu C.M., Wong R.S., Wu E.B., Kong S.L., Wong J., Yip G.W. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82:140–144. doi: 10.1136/pgmj.2005.037515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li F., Li W., Farzan M., Harrison S.C. Structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science. 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- 47.Glowacka I., Bertram S., Muller M.A., Allen P., Soilleux E., Pfefferle S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structural basis for the recognition of the SARS-CoV-2 by full-length human ACE2. Science. 2020;367(6485):1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braunwald E. 50th anniversary historical article. Myocardial oxygen consumption: the quest for its determinants and some clinical fallout. J Am Coll Cardiol. 2000;35 45B–48B. [PubMed] [Google Scholar]
- 54.Heusch G. Myocardial ischemia: lack of coronary blood flow or myocardial oxygen supply/demand imbalance? Circ Res. 2016;119:194–196. doi: 10.1161/CIRCRESAHA.116.308925. [DOI] [PubMed] [Google Scholar]
- 55.Liu J., Li S., Liu J., Liang B., Wang X., Wang H. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020 doi: 10.1016/j.ebiom.2020.102763. 2020.02.16.20023671. [DOI] [PMC free article] [PubMed] [Google Scholar]; [Epub ahead of print].
- 56.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) J Infect Dis. 2020 doi: 10.1093/infdis/jiaa150. [DOI] [Google Scholar]; Online ahead of print.
- 57.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 58.Pathan N., Hemingway C.A., Alizadeh A.A., Stephens A.C., Boldrick J.C., Oragui E.E. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363:203–209. doi: 10.1016/S0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 59.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 60.Liu K., Fang Y.Y., Deng Y., Liu W., Wang M.F., Ma J.P. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020 doi: 10.1097/CM9.0744. [DOI] [PMC free article] [PubMed] [Google Scholar]; Online ahead of print.
- 61.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mulangu S., Dodd L.E., Davey R.T., Tshiani Mbaya O., Proschan M., Mukadi D. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sanville B., Corbett R., Pidcock W., Hardin K., Sebat C., Nguyen M.-V. A community transmitted case of severe acute respiratory distress syndrome due to SARS CoV2 in the United States. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ClinicalTrials.gov. Severe 2019-nCoV Remdesivir RCT. Identifier:NCT04257656. 2020.
- 66.ClinicalTrials.gov. Mild/moderate 2019-nCoV Remdesivir RCT. Identifier: NCT04252664. 2020.
- 67.ClinicalTrials.gov. Study to evaluate the safety and antiviral activity of Remdesivir (GS-5734™) in participants with severe coronavirus disease (COVID-19). Identifier:NCT04292899. 2020.
- 68.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van den Borne B.E., Dijkmans B.A., de Rooij H.H., le Cessie S., Verweij C.L. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24:55–60. [PubMed] [Google Scholar]
- 71.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]; Online ahead of print.
- 72.ClinicalTrials.gov. Efficacy and safety of hydroxychloroquine for treatment of pneumonia caused by 2019-nCoV (HC-nCoV). Identifier:NCT04261517. 2020.
- 73.ClinicalTrials.gov. Chloroquine/hydroxychloroquine prevention of coronavirus disease (COVID-19) in the healthcare setting (COPCOV). Identifier:NCT04303507. 2020.
- 74.White N., J. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7(8):549–558. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 75.Arabi Y.M., Mandourah Y., Al-Hameed F., Sindi A.A., Almekhlafi G.A., Hussein M.A. Corticosteroid therapy for critically ill patients with middle east respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757–767. doi: 10.1164/rccm.201706-1172OC. [DOI] [PubMed] [Google Scholar]
- 76.Stockman L.J., Bellamy R., Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3:e343. doi: 10.1371/journal.pmed.0030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen R.C., Tang X.P., Tan S.Y., Liang B.L., Wan Z.Y., Fang J.Q. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129:1441–1452. doi: 10.1378/chest.129.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kotch C., Barrett D., Teachey D.T. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Rev Clin Immunol. 2019;15:813–822. doi: 10.1080/1744666X.2019.1629904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; Epub 2020 Mar 11.
- 80.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 81.Yeh K.-M., Chiueh T.-S., Siu L.K., Lin J.-C., Chan P.K.S., Peng M.-Y. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020 doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]; Online ahead of print.
- 83.Zhong N. Management and prevention of SARS in China. Philos Trans R Soc Lond B Biol Sci. 2004;359:1115–1116. doi: 10.1098/rstb.2004.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lopes-Pacheco M., Robba C., Rocco P.R.M., Pelosi P. Current understanding of the therapeutic benefits of mesenchymal stem cells in acute respiratory distress syndrome. J Cell Biol Toxicol. 2020;36:83–102. doi: 10.1007/s10565-019-09493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.ClinicalTrials.gov. Anti-SARS-CoV-2 inactivated convalescent plasma in the treatment of COVID-19. Identifier:NCT04292340. 2020.
- 86.ClinicalTrials.gov. Hyperimmune plasma for critical patients with COVID-19 (COV19-PLASMA). Identifier:NCT04321421. 2020.
- 87.ClinicalTrials.gov. NestCell® mesenchymal stem cell to treat patients with severe COVID-19 pneumonia (HOPE). Identifier:NCT04315987. 2020.
- 88.ClinicalTrials.gov. Treatment with mesenchymal stem cells for severe corona virus disease2019(COVID-19). Identifier:NCT04288102. 2020.
- 89.ClinicalTrials.gov. Treatment and prevention of traditional Chinese medicines (TCMs) on 2019-nCoV infection. Identifier:NCT04251871. 2020.