Abstract
Background and aims
Diabetes Mellitus (DM) is chronic conditions with devastating multi-systemic complication and may be associated with severe form of Coronavirus Disease 2019 (COVID-19). We conducted a systematic review and meta-analysis in order to investigate the association between DM and poor outcome in patients with COVID-19 pneumonia.
Methods
Systematic literature search was performed from several electronic databases on subjects that assess DM and outcome in COVID-19 pneumonia. The outcome of interest was composite poor outcome, including mortality, severe COVID-19, acute respiratory distress syndrome (ARDS), need for intensive care unit (ICU) care, and disease progression.
Results
There were a total of 6452 patients from 30 studies. Meta-analysis showed that DM was associated with composite poor outcome (RR 2.38 [1.88, 3.03], p < 0.001; I2: 62%) and its subgroup which comprised of mortality (RR 2.12 [1.44, 3.11], p < 0.001; I2: 72%), severe COVID-19 (RR 2.45 [1.79, 3.35], p < 0.001; I2: 45%), ARDS (RR 4.64 [1.86, 11.58], p = 0.001; I2: 9%), and disease progression (RR 3.31 [1.08, 10.14], p = 0.04; I2: 0%). Meta-regression showed that the association with composite poor outcome was influenced by age (p = 0.003) and hypertension (p < 0.001). Subgroup analysis showed that the association was weaker in studies with median age ≥55 years-old (RR 1.92) compared to <55 years-old (RR 3.48), and in prevalence of hypertension ≥25% (RR 1.93) compared to <25% (RR 3.06). Subgroup analysis on median age <55 years-old and prevalence of hypertension <25% showed strong association (RR 3.33)
Conclusion
DM was associated with mortality, severe COVID-19, ARDS, and disease progression in patients with COVID-19.
Keywords: Coronavirus, COVID-19, Diabetes mellitus, Mortality, SARS-CoV-2
1. Introduction
Coronavirus Disease 2019 (COVID-19) has been declared as a public health emergency by the World Health Organization (WHO) on January 30, 2020. At the time this paper is written, COVID-19 has inflicted more than 1.2 million people globally with overall mortality rate of 5.7% [1]. Although the majority of COVID-19 patients present with mild or no symptoms, some patients will develop severe pneumonia, acute respiratory distress syndrome (ARDS), multi-organ failure, and death. Clinical predictors may provide vital clues regarding efficient resource planning and allocation during a pandemic. (see Table 1 )
Table 1.
Characteristics of the included studies.
| Authors | Study Design | Samples | Male (%) | Overall Age (Mean/Median) (years) | Hypertension (%) | CAD/CVD (%) | DM (%) | COPD (%) | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Akbari A 2020 | Observational Retrospective | 440 (13/427) | 56.4 (61.5 vs 56.2) | 48 | 7.9 (15.3 vs 7.7) | 5.7 (15.3 v 5.4) | 7.5 (30.8 vs 6.8) | N/A | Mortality |
| Bai T 2020 | Observational Retrospective | 127 (36/91) | 63 (77.8 vs 57.1) | 55 (67 vs 50) | 28.3 (41.7 vs 23.1) | 2.4 (5.6 vs 1.1) (CVD) | 11.8 (13.9 vs 11.0) | N/A | Mortality |
| Cao J 2020 | Observational Retrospective | 102 (17/85) | 52 (76.5 vs 47.1) | 54 (72 vs 53) | 27.5 (64.7 vs 20) | 4.9 (17.6 vs 2.4) | 10.8 (35.3 vs 5.9) | 9.8 (23.5 vs7.1) | Mortality |
| Chen 2020 | Observational Retrospective | 123 (31/92) | 49 (71 vs 42) | 56 (72 vs 53) | 33.3 (48.4 vs 38.3) | 12.2 (25.8 vs 7.6) | 11.4 (19.4 vs 8.7) | 4.9 (9.7 vs 3.3) | Mortality |
| Chen T 2020 | Observational Retrospective | 274 (113/161) | 62 (73 vs 55) | 62 (68.0 vs 51.0) | 34 (48 vs 24) | 8 (14 vs 4) (CVD) | 17 (21 vs 14) | 7 (10 vs 4) (CLD) | Mortality |
| Fu L 2020 | Observational Retrospective | 200 (34/166) | 49.5 (16.2 vs 67.7) | <49 (5.9 vs 28.3), 50–59 (23.5 vs 27.1), 60–69 (20.6 vs 31.3), >70 (5 vs 13.2) | 50.5 (21.8 vs 12.1) | N/A | N/A | 4 (50.0 vs 15.6) (CLD) | Mortality |
| Li K 2020 | Observational Retrospective | 102 (15/87) | 58 (73 vs 55) | 57 (69 vs 55) | 30 (47 vs 28) | 4 (13 vs 2) | 15 (13 vs 15) | 2 (7 vs 1) | Mortality |
| Luo XM 2020 | Observational Retrospective | 403 (100/303) | 47.9 (57 vs 44.9) | 56 (71 vs 49) | 28 (60 vs 17.5) | 8.9 (16 vs 6.6) | 14.1 (25 vs 10.6) | 6.9 (17 vs 3.6) | Mortality |
| Yuan M 2020 | Observational Retrospective | 27 (10/17) | 45 (47 vs 40) | 60 (68 vs 55) | 19 (50 vs 0) | 11 (30 vs 0) | 22 (60 vs 0) | N/A | Mortality |
| Zhou 2020 | Observational Retrospective | 191 (54/137) | 62 (70 vs 59) | 56 (69.0 vs 52.0) | 30.4 (48 vs 23) | 8 (24 vs 1) | 19 (31 vs 14) | 3 (7 vs 1) | Mortality |
| Guan 2020 | Observational Retrospective | 1099 (173/926) | 58.1 (57.8 vs 38.2) | 47 (52.0 vs 45.0) | 15.0 (23.7 vs 13.4) | 2.5 (5.8 vs 1.8) | 7.4 (16.2 vs 5.7) | 1.1 (3.5 vs 0.6) | Severe COVID-19 |
| Hu L 2020 | Observational Retrospective | 323 (172/151) | 51.4 (52.9 vs 49.7) | 61 (65 vs 56) | 32.5 (38.3 vs 25.8) | 12.7 (19.2 vs 5.3) (CVD) | 14.6 (19.2 vs 9.3) | 1.9 (3.5 vs 0) | Severe COVID-19 |
| Li Q 2020 | Observational Retrospective | 325 (26/299) | 51.4 (76.9 vs 49.2) | 51 (65 vs 49) | 24 (46.2 vs 22.1) | 5.5 (19.2 vs 4.3) | 9.2 (19.2 vs 8.4) | 1.2 (7.7 vs 0.6) | Severe COVID-19 |
| Liu J 2020 | Prospective Cohort | 61 (17/44) | 50.8 (58.8 vs 47.7) | 40 (56 vs 41) | 19.7 (35.3 vs 13.6) | 1.6 (5.9 vs 0) (CVD) | 8.2 (1.6 vs 4.5) | 8.2 (1.6 vs 4.5) | Severe COVID-19 |
| Liu Lei 2020 | Observational Retrospective | 51 (7/44) | 62.7 (57.1 vs 63.7) | 45 (52 vs 44) | 7.8 (14.3 vs 6.8) | N/A | 7.8 (57.1 vs 0) | N/A | Severe COVID-19 |
| Ma LK 2020 | Observational Retrospective | 84 (20/64) | 57.1 (60 vs 56.3) | 48 (58 vs 46.5) | 14.3 (20.0 vs 12.5) | 6 (10 vs 4.7) | 11.9 (35 vs 4.7) | 6.0 (10.0 vs 4.7) (CLD) | Severe COVID-19 |
| Qin 2020 | Observational Retrospective | 452 (286/166) | 52.0 (54.2 vs 48.2) | 58 (61 vs 53) | 29.5 (36.7 vs 18.1) | 5.9 (8.4 vs 1.8) (CVD) | 16.4 (18.5 vs 13.3) | 2.6 (3.1 vs 1.8) | Severe COVID-19 |
| Wan 2020 | Observational Retrospective | 135 (40/135) | 53.3 (52.5 vs 54.7) | 47 (56 vs 44) | 9.6 (10 vs 9.4) | 5.2 (15 vs 1) (CVD) | 8.9 (22.5 vs 3.1) | 0.7 (2.5 vs 0) (CLD) | Severe COVID-19 |
| Wang Dan 2020 | Observational Retrospective | 143 (71/72) | 51 (62 vs 40.3) | 58 (65 vs 44) | 25.2 (43.7 vs 6.9) | 11.2 (16.9 vs 5.6) | 9.1 (12.7 vs 5.6) | 7.0 (9.9 vs 4.2) | Severe COVID-19 |
| Wang Y 2020 | Observational Retrospective | 110 (38/72) | 43 (63.2 vs 33.3) | ≤40 (53%), 41–60 (21%), >60 (36%) ≤40 (7.9 vs 69.4), 41–60 (21.0 vs 18.1), >60 (71.0 vs 12.5) |
20.9 (39.5 v 11.1) | N/A | 13.7 (21.0 v 9.7) | 5.4 (10.5 v 2.8) | Severe COVID-19 |
| Yuan B 2020 | Observational Retrospective | 417 (92/325) | 47.5 (53.2 vs 42.8) | 45 (58 vs 41) | 15.1 (28.3 vs 11.4) | N/A | 7.7 (17.4 vs 4.9) | 1.9 (1.1 vs 2.1) | Severe COVID-19 |
| Zhang Guqin 2020 | Observational Retrospective | 221 (55/166) | 48.9 (63.6 vs 44.0) | 55 (62 vs 51) | 24.4 (47.3 vs 16.9) | 10 (23.6 vs 5.4) | 10 (12.7 vs 9.0) | 2.7 (7.3 vs 1.2) | Severe COVID-19 |
| Zhang J 2020 | Observational Retrospective | 140 (58 vs 82) | 50.7 (56.9 vs 46.3) | <30 (1.7 vs 4.9), 30–49 (15.5 vs 34.1), 50–69 (48.3 vs 50), ≥70 (34.5 vs 11.0) | 30 (37.9 vs 24.4) | 5 (6.9 vs 3.7) | 12.1 (13.8 vs 11.0) | 1.4 (3.4 vs 0) | Severe COVID-19 |
| Liu Y 2020 | Observational Retrospective | 109 (53 vs 56) | 59 (52.8 vs 55.4) | 55 (61 vs 49) | 37 (21 vs 26) | 6.4 (5.7 vs 7.1) | 11 (20.8 vs 1.8) | 3.7 (3.8 vs 3.6) | ARDS |
| Wu C 2020 | Observational Retrospective | 201 (84/117) | 63.7 (71.4 vs 58.1) | 51 (58.5 vs 48) | 19.4 (27.4 vs 13.7) | 4 (6 vs 2.6) | 10.9 (19 vs 5.1) | 2.5 (CLD) | ARDS |
| Cao 2020 | Observational Retrospective | 198 (19/176) | 51 (89.5 vs 46.9) | 50.1 (63.7 vs 48.6) | 21.2 (31.6 vs 20.1) | 6.0 (26.3 vs 3.9) (CVD) | 7.6 (10.5 vs 7.3) | N/A | ICU Care |
| Huang 2020 | Observational Retrospective | 41 (13/28) | 73 (85 vs 68) | 49.0 (49.0 vs 49.0) | 14.6 (15 vs 14) | 14.6 (23 vs 11) (CVD) | 19.5 (8 vs 25) | 2.4 (8 vs 0) | ICU Care |
| Wang, Dawei 2020 | Observational Retrospective | 138 (36 vs 102) | 54.3 (61.1 vs 52.0) | 56 (66 vs 51) | 31.2 (58.3 vs 21.6) | 14.5 (25 vs 10.8) | 10.1 (22.2 vs 5.9) | 2.9 (8.3 vs 1.0) | ICU Care |
| Feng 2020 | Observational Retrospective | 141 (15/126) | 51.1 (46.7 vs 51.6) | 44 (58 vs 41) | 14.9 (40.0 vs 11.9) | 2.1 (6.7 vs 1.6) (CVD) | 5.7 (13.3 vs 4.8) | 2.8 (13.3 vs 1.6) | Disease Progression |
| Liu W 2020 | Observational Retrospective | 78 (11/67) | 50 (63.6 vs 47.8) | 38 (55 vs 37) | 40 (18.2 vs 9.0) | N/A | 25 (18.2 vs 4.5) | 10 (9.1 vs 1.5) | Disease Progression |
CAD: Coronary artery disease; COVID-19: Coronavirus disease 2019; CLD: Chronic Lung/Pulmonary Disease; CVD: Cardiovascular Disease; ICU: Intensive Care Unit; N/A: Not available.
Diabetes Mellitus (DM) is one of the most prevalent chronic conditions with devastating multi-systemic complication and was estimated to have inflicted 463 million people in 2019 [2]. It is not yet known whether people with DM are more susceptible to COVID-19, but several studies have reported the association between severe COVID-19 infection with DM [3,4]. It was postulated that the angiotensin converting enzyme 2 (ACE2) may be the plausible explanation of this association [5].
In this study, we aimed to perform a systematic review and meta-analysis in order to investigate the association between DM and poor outcome in patients with COVID-19 pneumonia. Our hypothesis is that DM is associated with poor outcome in patients with COVID-19 pneumonia. To the best of the authors knowledge, this is the first systematic review, meta-analysis, and meta-regression that comprehensively describe the association between DM and outcome in COVID-19.
1.1. Subjects
Research articles that evaluate the association between COVID-19 and clinically validated definition of mortality, severe COVID-19, ARDS, intensive care unit (ICU care), and disease progression.
2. Material and methods
2.1. Eligibility criteria
We included all research articles in adult patients diagnosed with COVID-19 with information on DM and clinical grouping or outcome of the clinically validated definition of mortality, severe COVID-19, ARDS, ICU care, and disease progression. The following types of article were excluded: articles other than original research (e.g., review articles, letters, or commentaries); original research with samples below 20 or case reports and series; articles not in the English language; articles on research in pediatric populations (17 years of age or younger).
2.2. Search strategy and study selection
We performed systematic literature search from PubMed and EuropePMC with the search terms (1) “COVID-19″ OR “SARS-CoV-2″ AND “Characteristics”, (2) “COVID-19″ OR “SARS-CoV-2″ AND “Diabetes”, English, MEDLINE. Duplicate results were removed. The remaining articles were independently screened for relevance by its abstracts with two authors (MAL and IH). The full text of residual articles was assessed according to the inclusion and exclusion criteria. The search was finalized on April 8th, 2020 The study was carried out per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.
2.3. Data extraction
Data extraction was performed independently by two authors (IH and RP), we used standardized forms that include author, year, study design, age, gender, cardiovascular diseases, hypertension, DM, need for ICU care, and severe COVID-19.
The outcome of interest was composite poor outcome that comprised of mortality, severe COVID-19, ARDS, need for ICU care, and disease progression. ARDS was defined as per World Health Organization (WHO) interim guidance of Severe Acute Respiratory Infection (SARI) of COVID-19, including the acute onset, chest imaging, and origin of pulmonary infiltrates, and oxygenation impairment [6]. Severe COVID-19 was defined as patients who had any of the following features at the time of, or after, admission: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤93%; (3) ratio of partial pressure of arterial oxygen (PaO2) to fractional concentration of oxygen inspired air (fiO2) ≤300 mmHg; or (4) critical complication (respiratory failure, septic shock, and or multiple organ dysfunction/failure) [7].
2.4. Statistical analysis
The software review Manager 5.3 (Cochrane Collaboration) and Stata version 16 were used for meta-analysis. Dichotomous variables were calculated using Mantel-Haenszel formula with random effects models regardless of heterogeneity. The effect estimate was reported as risk ratios (RRs) along with its 95% confidence intervals (CIs) for dichotomous variables, respectively. P-value was two-tailed, and the statistical significance set at ≤0.05. Random effects meta-regression was performed using restricted-maximum likelihood for pre-specified variables including age, gender, hypertension, cardiovascular disease, and COPD. Subgroup analysis was performed for each component of composite poor outcome. To assess the small-study effect, we performed regression-based Harbord’s test for dichotomous outcome. Begg’s funnel-plot analysis was performed to qualitatively assess the risk of publication bias.
3. Results
3.1. Study selection and characteristics
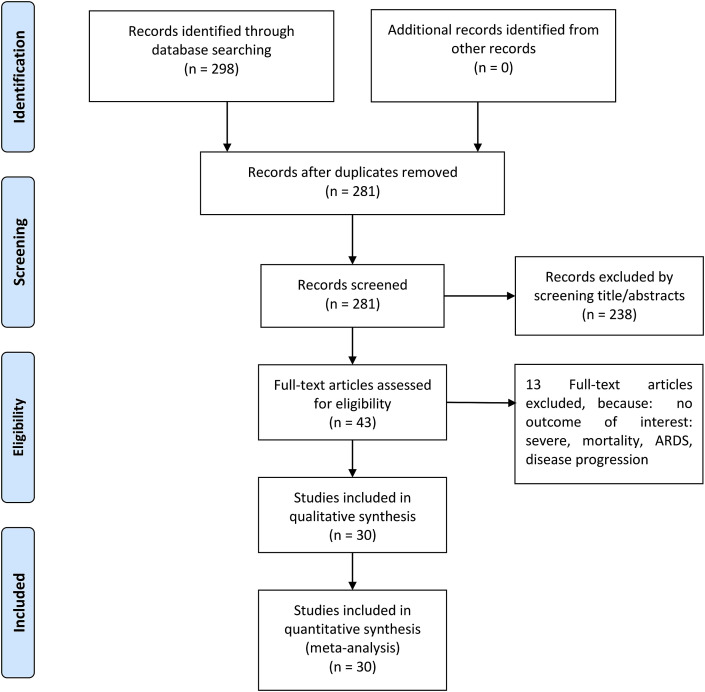
Initial search yields 298 records, and 281 records remained after the removal of duplicates. 238 records were excluded after screening the title/abstracts. After evaluating 43 full-text for eligibility, 13 full-text articles were excluded because: no outcome of interest: severe, mortality, ARDS, disease progression. 30 studies were included in the qualitative synthesis and meta-analysis [Fig. 1 ] [3,[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36]]. There were a total of 6452 patients from 30 studies.
Fig. 1.
Prisma flowchart.
3.2. Diabetes and outcome
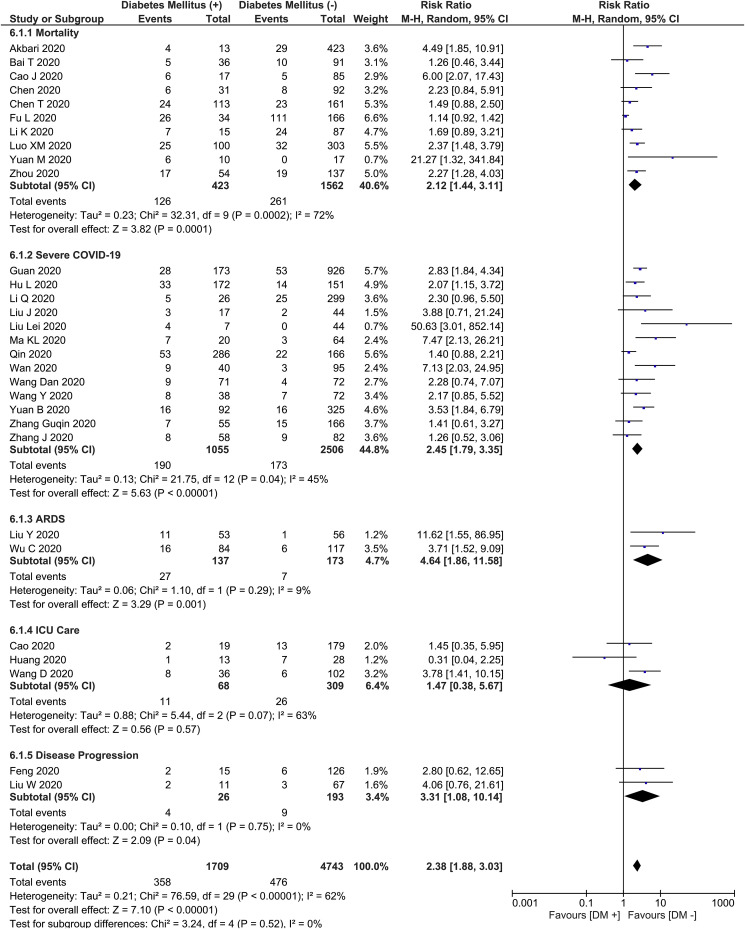
This meta-analysis showed that DM was associated with composite poor outcome (RR 2.38 [1.88, 3.03], p < 0.001; I2: 62%, p < 0.001) [Fig. 2 ]. Subgroup analysis showed that DM was associated with mortality (RR 2.12 [1.44, 3.11], p < 0.001; I2: 72%, p < 0.001), severe COVID-19 (RR 2.45 [1.79, 3.35], p < 0.001; I2: 45%, p = 0.04), ARDS (RR 4.64 [1.86, 11.58], p = 0.001; I2: 9%, p = 0.29), and disease progression (RR 3.31 [1.08, 10.14], p = 0.04; I2: 0%, p = 0.75). DM was not associated with increased need for ICU care (RR 1.47 [0.38, 5.67], p = 0.57; I2: 63%, p = 0.07).
Fig. 2.
Diabetes Mellitus and Poor Outcome. Forest-plot shows that diabetes mellitus was associated with increased composite poor outcome and its subgroup which comprises of mortality, severe COVID-19, ARDS, need for ICU care, and disease progression in patients with COVID-19. ARDS: Acute Respiratory Distress Syndrome, COVID-19: Coronavirus Disease 2019, ICU: Intensive Care Unit.
3.3. Meta-regression
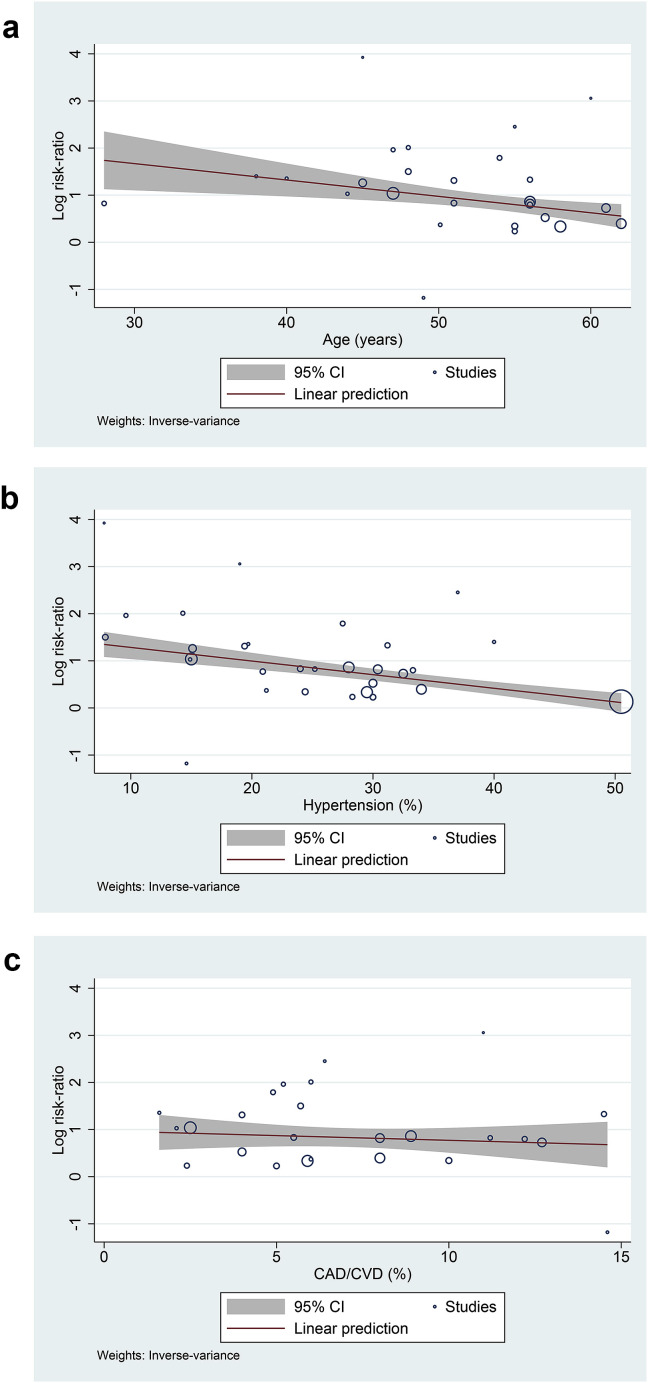
Meta-regression showed that the association between DM and composite poor outcome was affected by age (p = 0.003) [Fig. 3 A] and hypertension (p < 0.001) [Fig. 3B], but not gender (p = 0.895), cardiovascular diseases (p = 0.5) [Fig. 3C], and COPD (p = 0.47). Multivariable meta-regression by including two covariates in single analysis showed age (p = 0.334) and hypertension (p = 0.107) effect is probably dependent on each other.
Fig. 3.
Bubble-plot for Meta-regression. Meta-regression analysis showed that the association between diabetes mellitus and composite poor outcome was affected by age [A] and hypertension [B], but not by cardiovascular diseases [C].
3.4. Subgroup analysis
Subgroup analysis for studies with median age ≥55 years-old (RR 1.92 [1.56, 2.37], p < 0.001; I2: 10%, p = 0.35) showed a lower RR for composite poor outcome compared to <55 years-old (RR 3.48 [2.55, 4.77], p < 0.001; I2: 21%, p = 0.22).
Subgroup analysis for studies with prevalence of hypertension ≥25% (RR 1.93 [1.48, 2.52], p < 0.001; I2: 58%, p < 0.003) showed a lower RR for composite poor outcome compared to prevalence of hypertension <25% (RR 3.06 [2.19, 4.26], p < 0.001; I2: 33%, p = 0.10).
Subgroup analysis for studies with median age <55 years-old and prevalence of hypertension <25% showed association with poor outcome (RR 3.33 [2.35, 4.73], p < 0.001; I2: 28%, p = 0.17).
3.5. Publication bias
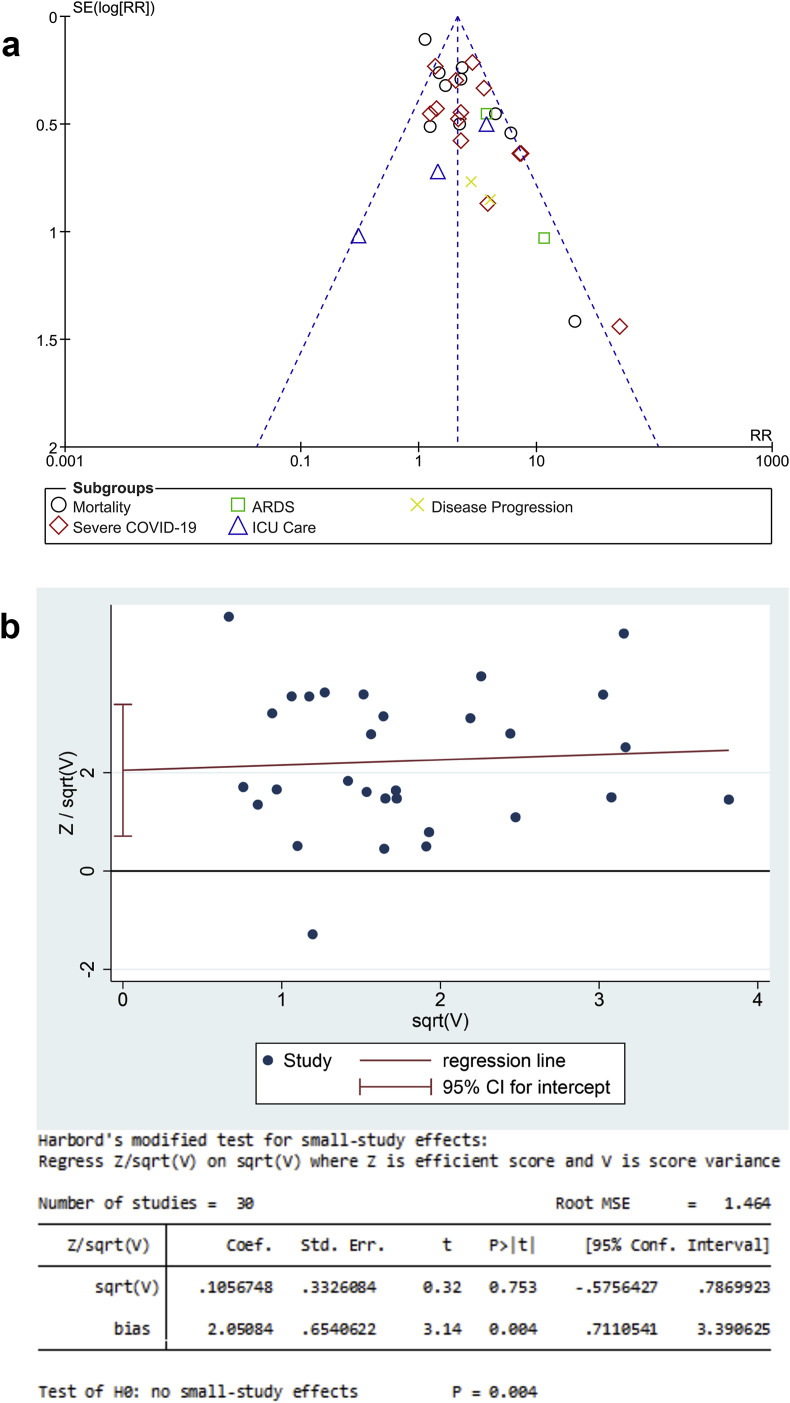
The funnel-plot analysis showed a qualitatively symmetrical inverted funnel-plot for the association between DM and composite poor outcome [Fig. 4 A]. Regression-based Harbord’s test showed indication of small-study effects for DM and composite poor outcome (p = 0.004) [Fig. 4B].
Fig. 4.
Publication Bias Analysis. The Begg’s funnel-plot analysis showed a qualitatively symmetrical inverted funnel-plot for the association between diabetes mellitus and composite poor outcome [A]. Regression-based Harbord’s test showed indication of small-study effects for hypertension and composite poor outcome.
4. Discussion
This comprehensive meta-analysis of 30 studies showed that DM was associated with poor outcome that comprises of mortality, severe COVID-19, ARDS, and disease progression in patients with COVID-19. This association was influenced by age and hypertension. Further analysis based on meta-regression showed that magnitude of risk linked to DM as a single factor was greater in studies with younger and non-hypertensive patients, which is yet to be addressed by the existing literature.
Meta-regression showed that the association between DM and poor outcome was influenced by age and hypertension. Age and prevalence of hypertension was inversely proportional with the effect of DM on poor outcome. In other words, the effect estimate of DM was less in older and hypertensive patients. Subgroup analysis further demonstrates the vast difference in RR. Meta-regression also showed that age and prevalence of hypertension seemed to be dependent on one another, this is further demonstrated by subgroup analysis showing that the RR for age <55 years-old, prevalence of hypertension <25%, and both of them combined varies only slightly. The association between DM (as a single risk factor) with composite poor outcome in COVID-19 was greater in younger people and without hypertension. The presence of older age and hypertension may attenuate the association of DM with composite poor outcome. Hence, the total risk is expected to be higher in older patients with HT, but the magnitude of DM as a single risk factor is greater in younger people without hypertension.
It is not yet known whether people with DM are more susceptible to COVID-19, but several studies have reported a greater risk of severe COVID-19 in diabetic patients [3,4]. Diabetic individuals have a greater risk of respiratory infections due to compromised immune system, especially the innate immunity [5,37]. Even transient hyperglycaemia may temporarily affect innate immune responses to infection [38]. It was hypothesized that ACE2 may be the key pathfinder of COVID-19 severity in diabetic individuals [5].
ACE2 is a type 1 integral membrane glycoprotein expressed in the epithelial cells of cardiovascular, pulmonary, renal, brain and intestinal tissue, it acts by breaking down angiotensin II into angiotensin 1–7 [37,39,40]. This enzyme acts by counteracting the inflammatory actions of angiotensin II, lowering the concentration of pro-inflammatory cytokine interleukin (IL)-6, increasing the anti-inflammatory, and increasing the antioxidant action of angiotensin 1-7, escalating the levels of surfactant protein D and promoting vasodilation [41]. The novel coronavirus responsible for COVID-19 is expected to act similarly to Severe Acute Respiratory Syndrome (SARS-CoV). Both utilize ACE2 to bind and gain entry to the host pneumocytes [39]. Viral surface spike (S) protein of COVID-19 binds to ACE2 after spike protein activation by transmembrane protease serine 2 (TMPRSS2) [40]. Routine use of ACEI and ARB as a medication for chronic conditions upregulates ACE2 expression [5,37], thereby facilitating entry of SARS-CoV-2 into the pneumocytes and consequently cause severe and fatal infection [42]. Among other diabetic medications, the use of liraglutide and pioglitazone have also been found to be related with increased ACE2 regulation in animal studies [42,43].
The interconnection between ACE2, renin-angiotensin system (RAS) signalling, aging, DM, hypertension, and severity of COVID-19 may not be as simple as it may seem. As we discussed previously, our meta-regression analysis showed that the association between DM and poor outcome was interdependent with age and hypertension. One of the possible rationale behind this finding is the use of medications, particularly ACEI or ARB in the hypertensive individuals. The risks and benefits associated with ACEI/ARB use in COVID-19 patients remains controversial [44], a specific type of ARB has been shown to ameliorate lung injury related to SARS-CoV infection in animal model [45]. It is unfortunate that all of the included studies in this systematic review did not provide report on diabetic or hypertensive medications. Furthermore, the link between those specific variables could be in line with the hypothesis of AlGhatrif et al. [46] Older hypertensive individuals have lower ACE2 levels but a higher RAS signalling, this difference is further expressed in COVID-19 patients in which ACE2 developed into a critically low levels and RAS signalling is exaggerated even more. Such disturbance result in a potentially decreased susceptibility to the disease, but a greater severity. In contrast, younger people without hypertension have higher ACE2 levels and lower RAS signalling, which transforms into a modestly low ACE2 levels and modestly increased RAS signalling due to COVID-19 infection. This results in a possibly increased susceptibility to the disease, but a lesser severity. Our meta-regression result may support the aforementioned hypothesis. The use of ACEI/ARB is expected in patients with both DM and HTN; and we observe that the age and HTN were in parallel, studies with older subjects having higher prevalence of hypertension. Hence, the clinical significance of DM in the older patients may be attenuated by the risk of hypertension and ACEI/ARB use (which was hypothesized to increase severity in older patients).
Dysfunctional pro-inflammatory cytokine responses in diabetic patients might also be the underlying cause of severe COVID-19 [37,47,48]. Diabetic patients have been shown to have an elevated pro-inflammatory cytokine level, in particular IL-1, IL-6 and tumor necrosis factor (TNF)-α [48]. Different markers, including C-reactive protein, fibrinogen and D-dimer were also found to be elevated in diabetic patients who contracted COVID-19 [47]. Thus, this condition may further exaggerate the cytokine storms in COVID-19 leading to a more severe disease [37,48,49].
4.1. Implications for clinical practice
DM was shown to be associated with poor outcome in patients with COVID-19 and was influenced by age and hypertension. The association was stronger in younger patients and should alert physician even though the patient only presented with one comorbidity. This indicates that DM is a potential prognostic marker that should be explored in triage. We encourage researchers to include DM in studies investigating prognostic model for patients with COVID-19. Moreover, this finding adds the needs of further studies concerning the use of ACEI/ARB in COVID-19.
4.2. Limitations
Data on diabetic/hypertensive medications were lacking in the included studies, hence, cannot be analysed. Since ACEI/ARB is often used in DM, it may have influence on prognosis. Most of the articles included in this meta-analysis were preprints; nevertheless the authors have made exhaustive efforts to ensure that only sound studies were included. Most of the reports were from China, hence, the samples might overlap across the reports. The included studies were retrospective in design.
5. Conclusion
DM was associated with mortality, severe COVID-19, ARDS, and disease progression in patients with COVID-19. The association was weaker in the older and hypertensive patients.
Funding
None.
Data availability
The data used to support the findings of this study are included within the article.
Funding statement
None.
Declaration of competing interest
None.
Acknowledgement
IH and RP conceived and designed the study. IH, RP, and MAL acquired the data. IH and MAL drafted the manuscript. IH and RP performed data extraction and interpreted the data. IH and MAL performed extensive research on the topic. RP reviewed and performed extensive editing of the manuscript. All authors contributed to the writing of the manuscript. IH and RP performed the statistical analysis.
Footnotes
The authors agreed on transferring the copyright of the manuscript to Diabetes and Metabolic Syndrome: Clinical Research and Reviews.
References
- 1.World Health Organization . 2020. Coronavirus disease 2019 (COVID-19) situation report – 78. [Google Scholar]
- 2.Saeedi P., Petersohn I., Salpea P., Malanda B., Karuranga S., Unwin N. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;6736:1–9. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma R.C.W., Holt R.I.G. COVID-19 and diabetes. Diabet Med. 2020 doi: 10.1111/dme.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected; pp. 1–21. [Google Scholar]
- 7.World Health Organization. Report of the WHO-China joint mission on coronavirus disease 2019 COVID-19 report. vol. vol. 2019. n.d.
- 8.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;2600:1–7. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang G., Hu C., Luo L., Fang F., Chen Y., Li J. Clinical features and outcomes of 221 patients with COVID-19 in Wuhan, China. MedRxiv. 2020 doi: 10.1101/2020.03.02.20030452. 2020.03.02.20030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J jin, Dong X., Cao Y yuan, Yuan Y dong, Yang Y bin, qin Yan Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy Eur J Allergy Clin Immunol. 2020:1–12. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 12.Wan S., Xiang Y., Fang W., Zheng Y., Li B., Hu Y. Clinical features and treatment of COVID-19 patients in Northeast Chongqing. J Med Virol. 2020:1. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu R., Ling Y., Zhang Y.-H., Wei L.-Y., Chen X., Li X. Platelet-to-lymphocyte ratio is associated with prognosis in patients with Corona Virus Disease-19. J Med Virol. 2020:3. doi: 10.1002/jmv.25767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;53:1689–1699. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y., Zhou Y., Yang Z., Xia D., Geng S. Clinical characteristics of patients with severe pneumonia caused by the 2019 novel coronavirus in Wuhan, China. MedRxiv. 2020:1–15. doi: 10.1101/2020.03.02.20029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Z., Yu Q., Yao S., Luo L., Duan J., Yan Z. Early prediction of disease progression in 2019 novel coronavirus pneumonia patients outside Wuhan with CT and clinical characteristics. MedRxiv. 2020 doi: 10.1101/2020.02.19.20025296. 2020.02.19.20025296. [DOI] [Google Scholar]
- 17.Lei L., Jian-ya G., Hu W., Zhang X., Gua L., Liu C. Clinical characteristics of 51 patients discharged from hospital with COVID-19 in Chongqing,China. MedRxiv. 2020 doi: 10.1101/2020.02.20.20025536. 2020.02.20.20025536. [DOI] [Google Scholar]
- 18.Liu J., Li S., Liu J., Liang B., Wang X., Wang H. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. MedRxiv. 2020:2020. doi: 10.1101/2020.02.16.20023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai Q., Huang D., Ou P., Yu H., Zhu Z., Xia Z. COVID-19 in a designated infectious diseases Hospital Outside Hubei Province,China. MedRxiv. 2020 doi: 10.1101/2020.02.17.20024018. 2020.02.17.20024018. [DOI] [PubMed] [Google Scholar]
- 20.Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C. Neutrophil-to-Lymphocyte ratio Predicts severe illness patients with 2019 novel coronavirus in the early stage. MedRxiv. 2020 doi: 10.1101/2020.02.10.20021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabata S., Imai K., Kawano S., Ikeda M., Kodama T., Miyoshi K. Non-severe vs severe symptomatic COVID-19: 104 cases from the outbreak on the cruise ship “Diamond Princess” in Japan. MedRxiv. 2020 doi: 10.1101/2020.03.18.20038125. [DOI] [Google Scholar]
- 22.Chen M., Fan Y., Wu X., Zhang L., Guo T., Deng K. Clinical characteristics and risk factors for fatal outcome in patients with 2019-coronavirus infected disease (COVID-19) in Wuhan, China. SSRN Electron J. 2020 doi: 10.2139/ssrn.3546069. [DOI] [Google Scholar]
- 23.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA, J Am Med Assoc. 2020:1–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao M., Zhang D., Wang Y., Lu Y., Zhu X., Li Y. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. MedRxiv. 2020:2020. doi: 10.1101/2020.03.04.20030395. [DOI] [Google Scholar]
- 25.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020:1–10. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanli L., Wenwu S., Jia L., Liangkai C., Yujun W., Lijuan Z. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. MedRxiv. 2020:1–9. doi: 10.1101/2020.02.17.20024166. [DOI] [Google Scholar]
- 27.Guan W.-J., Ni Z.-Y., Hu Y., Liang W.-H., Ou C.-Q., He J.-X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020:1–13. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu W., Tao Z.-W., Lei W., Ming-Li Y., Kui L., Ling Z. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl) 2020:1. doi: 10.1097/cm9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan M., Yin W., Tao Z., Tan W., Hu Y. Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PloS One. 2020;15:1–10. doi: 10.1371/journal.pone.0230548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li K., Chen D., Chen S., Feng Y., Chang C. Radiographic findings and other predictors in adults with Covid-19. MedRxiv. 2020;2 doi: 10.1101/2020.03.23.20041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu L., Fei J., Xiang H.-X., Xiang Y., Tan Z.-X., Li M.-D. Analysis of death risk factors among 200 COVID-19 patients in Wuhan, China: a hospital-based case-cohort study. SSRN Electron J. 2020;86 doi: 10.2139/ssrn.3551430. [DOI] [Google Scholar]
- 33.Luo X., Xia H., Yang W., Wang B., Guo T., Xiong J. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan , China. MedRxiv. 2020 doi: 10.1101/2020.03.19.20033175. [DOI] [Google Scholar]
- 34.Li Q., Ling Y., Zhang J., Li W., Zhang X., Jin Y. Clinical characteristics of SARS-CoV-2 infections involving 325 hospitalized patients outside Wuhan. Res Sq. 2020:1–15. doi: 10.21203/rs.3.rs-18699/v1. [DOI] [Google Scholar]
- 35.Yuan B., Chen Y., Wang J., Wang C., Song S., Liu H.-Q. Epidemiological Characteristics of 417 patients infected with COVID-19 and 368 discharged cases among them in Shenzhen City , China CURRENT STATUS : under review. Res Sq. 2020:1–14. doi: 10.21203/rs.3.rs-19554/v1. [DOI] [Google Scholar]
- 36.Wang D, Li R, Wang J, Jiang Q, Gao C, Yang J, et al. Correlation analysis between disease severity and clinical and biochemical characteristics of 143 cases of COVID-19 in Wuhan, China: a descriptive study. Res Sq n.d.:1–17. doi:10.21203/rs.3.rs-19680/v1. [DOI] [PMC free article] [PubMed]
- 37.Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020;162:108132. doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jafar N., Edriss H., Nugent K. The effect of Short-term hyperglycemia on the innate immune system. Am J Med Sci. 2016;351:201–211. doi: 10.1016/j.amjms.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long Structural studies of SARS coronavirus. J Virol. 2020;94:1–9. doi: 10.1128/jvi.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is Blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossi G.P., Sanga V., Barton M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. Elife. 2020;9 doi: 10.7554/eLife.57278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romaní-Pérez M., Outeiriño-Iglesias V., Moya C.M., Santisteban P., González-Matías L.C., Vigo E. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156:3559–3569. doi: 10.1210/en.2014-1685. [DOI] [PubMed] [Google Scholar]
- 44.Gupta R., Misra A. Contentious issues and evolving concepts in the clinical presentation and management of patients with COVID-19 infection with reference to use of therapeutic and other drugs used in Co-morbid diseases (Hypertension, diabetes etc) Diabetes Metab Syndr Clin Res Rev. 2020;14:251–254. doi: 10.1016/j.dsx.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.AlGhatrif M., Cingolani O., Lakatta E.G. The dilemma of coronavirus disease 2019, aging, and cardiovascular disease: Insights from cardiovascular aging science. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maddaloni E., Buzzetti R. Covid-19 and diabetes mellitus: unveiling the interaction of two pandemics. Diabetes Metab Res Rev. 2020 doi: 10.1002/dmrr.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geerlings S.E., Hoepelman A.I.M. Immune dysfunction in patients with diabetes mellitus (DM) FEMS Immunol Med Microbiol. 1999;26:259–265. doi: 10.1016/S0928-8244(99)00142-X. [DOI] [PubMed] [Google Scholar]
- 49.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.