Abstract
A markedly increased demand for vascular ultrasound laboratory and other imaging studies in COVID-19–positive patients has occurred, due to most of these patients having a markedly elevated D-dimer and a presumed prothrombotic state in many of the very ill patients. In the present report, we have summarized a broad institutional consensus focusing on evaluation and recommended empirical therapy for COVID-19–positive patients. We recommend following the algorithms with the idea that as more data becomes available these algorithms may well change.
Keywords: Anticoagulant, Deep venous thrombosis, Duplex ultrasonography, Pulmonary embolism, Venous thromboembolism
Article Highlights.
-
•
Type of Research: Evidence-based algorithm designed for use in times of extreme scarcity
-
•
Key Findings: Standard imaging techniques such as duplex ultrasonography and computed tomography might not be available for venous thromboembolism (VTE) diagnosis owing to the sheer volume of patients, difficulty in disinfecting equipment, and an inadequate number of qualified imaging personnel. Empiric treatment of suspected VTE might be warranted by risk stratification and risk/benefit ratio calculations until imaging studies are available.
-
•
Take Home Message: During the COVID-19 pandemic and other times of extreme scarcity, empiric treatment of VTE without confirmatory imaging might need to be undertaken, balancing the risk/benefit ratios.
The diagnosis of venous thromboembolism (VTE) has traditionally relied on assessment of the patient for history and physical examination findings consistent with the diagnosis, risk stratification, followed by imaging with duplex ultrasonography for deep venous thrombosis (DVT) or with computed tomography (CT) for pulmonary embolism (PE).1 This common diagnosis and its workup are a familiar thread in the fabric of all emergency departments, inpatient wards, and intensive care units across the United States and the world. The COVID-19 pandemic has caused a massive rent in this cloth as hospitals experience a surge in patients. CT for PE has often been delayed or not performed because of comorbid renal failure precluding the use of intravenous contrast and cardiopulmonary instability leading to an unacceptable risk for transfer. Similarly, duplex ultrasonography for the diagnosis of DVT has been difficult to perform owing to the large number of patients, many of whom will be housed in field hospitals without diagnostic vascular unit laboratories, a contracted registered vascular technician workforce, and the length of time associated with, and difficulty in, completely disinfecting the machines between patients.
Beyond the obligation of “do no harm” to our patient population, a global pandemic also shines light on the moral obligations of, and to, the ancillary healthcare staff. Although it has been hotly debated on a national stage whether the physician “duty to treat” moral standard should be upheld without proper personal protective equipment, it is even less clear to what obligation registered vascular technicians (RVTs) should be held to when their services will only indirectly affect morbidity and mortality. It is arguably both within the realm of physician capability and obligation to minimize exposure to RVTs in our diagnostic vascular unit laboratories and minimize harm to patients. Furthermore, with a dedicated skill set requiring the use of expensive equipment, RVTs represent a scare resource during a pandemic, which, if depleted, would inhibit our ability to detect other life- and limb-threatening conditions that require urgent or emergent treatment such as acute limb ischemia, pseudoaneurysms, and carotid disease leading to stroke.
Facing mounting requests for duplex ultrasonography (DVT scans) for VTE diagnosis during the exponential growth phase of the COVID-19 pandemic in our community, we formed an ad hoc committee of venous thrombosis experts, vascular surgeons, hospitalists, critical care physicians, vascular medicine physicians, and RVTs. This committee was charged with rapidly reviewing the evidence for VTE diagnosis and treatment and adapting the clinical algorithms for use during a time when imaging resources, such as ultrasound machines and sonographers with experience and expertise, are expected to be scarce, recognizing both our moral obligation to our patients and our vascular sonographers. Underlying these algorithms is the absolute commitment at the institutional level to ensure appropriate diagnostic imaging, long-term therapy recommendations, and follow-up once the surge has passed. As a frame of reference, we are expected to experience a volume of >3000 patients at our peak in Michigan and to be severalfold in excess of our hospital capacity, requiring the use of field hospitals for ∼8 weeks.
Methods
A committee of vascular thrombosis experts, vascular surgeons, vascular medicine physicians, and vascular technologists was convened. We also sought, via multiple conference calls, input from intensivists, pulmonologists, and hematologists for critique and vetting of the algorithms that resulted. The existing reported protocols for the diagnosis and management of VTE by our faculty practice group were reviewed, in addition to the current American College of Clinical Pharmacy and National Institution for Health and Care Excellence.1 Our institutional experience with VTE events and the utility of empiric low-dose anticoagulation with H1N1 viral pneumonia were reviewed, as well as the emerging evidence regarding VTE risk in those with COVID-19 infection. Feedback from content experts in vascular surgery, hematology, pharmacology, internal medicine, cardiology, anesthesiology, and critical care was solicited. Implementation was rapidly achieved via dissemination in care bundles, computerized physician order entry sets, and best practice alerts.
Results
Consensus was achieved regarding 9 critical guiding principles (Table I ) developed from best evidence, the available resources, and ethical obligations to patients and staff. High priority was placed on treating suspected VTE without definitive imaging findings available in the context of acceptable bleeding risk, recognizing the potential to decrease morbidity and mortality. Extreme pragmatism was applied, recognizing that the well-being and scarcity of the RVTs was paramount over obtaining a diagnosis when the clinical management would not be altered.
Table I.
Critical guiding principles
| 1. All patients with COVID-19 or suspected COVID-19 should be treated with thromboprophylaxis—this statement places value on avoiding the need to reassess VTE risk when a patient has a change in status and accepts the overall low bleeding risk associated with the use of anticoagulants at thromboprophylactic doses. |
| 2. Elevated D-dimer should be expected with severe COVID infection and should not be a determinant in the decision to obtain imaging studies. Negative D-dimer combined with a low clinical risk score can still safely exclude VTE and might have limited utility for this purpose. |
| 3. Current guidelines recommend empiric treatment of suspected PE if imaging is expected to take >4 hours or for DVT if imaging is expected to take >24 hours. We expect that owing to the stress on the healthcare system, imaging could be delayed for ≥1 month but that patients can be safely empirically treated during this time by determining the risk/benefit ratio. |
| 4. Duplex ultrasonography should be used when the 3 following conditions have been met simultaneously: (1) bleeding risk is high; (2) the results will change management; and (3) clinical suspicion of PE is high and CT PE is unobtainable or clinical suspicion of DVT is high (according to modified Wells and Wells scoring systems). |
| 5. Most patients with confirmed or suspected VTE without a high bleeding risk should receive therapeutic doses of anticoagulation. |
| 6. In patients with ARDS, low-dose non-nomogram heparin infusion might reduce the risk of major bleeding but still protect against thrombotic events. No data are available for this treatment strategy in intubated patients without ARDS. |
| 7. Patients treated with low-dose anticoagulation protocols should be transitioned to full-dose anticoagulation when no longer in the ICU. |
| 8. Referral for CT PE or duplex ultrasonography can be performed once a patient has recovered as an inpatient but might need to be completed in the outpatient setting in a resource-scarce setting. CVC venous clinics (or hematology, if a consulting service as an inpatient) will provide continuity of care in reviewing these outpatient imaging tests and providing long-term anticoagulation recommendations to the patient, thereby expediting discharges without the burden of additional testing and relieving inpatient providers of the burden of follow-up. |
| 9. Upper extremity duplex ultrasonography should be limited to patients with unilateral limb symptoms and meeting the criteria listed in no. 4 and should not be performed routinely. |
ARDS, Acute respiratory distress syndrome; CT, computed tomography; CVC, central venous catheter; ICU, intensive care unit; PE, pulmonary embolism; VTE, venous thromboembolism.
VTE prophylaxis
As an institutional standard, all patients are routinely risk assessed using the Caprini risk assessment model on admission.2 The committee recognized that healthy patients with only a diagnosis might fall into a low-risk category that would not warrant VTE prophylaxis. A paucity of data is available on the presence of VTE in patients with COVID-19. However, 1 study found a mortality benefit to thromboprophylaxis with subcutaneous unfractionated heparin or low-molecular-weight heparin for COVID-19–positive patients with highly elevated (>3 times the upper limit of normal) D-dimer and sepsis-induced coagulopathy score.3 The true incidence of VTE is unknown and likely varies across different patient populations, with the highest case reports of PE in ≤40% of patients with elevated D-dimer undergoing CT for PE evaluation.4, 5, 6 These data suggest that VTE or primary pulmonary thrombi might be an underlying etiology responsible for mortality in those with severe COVID-19 infections. In patients who have initially presented with less severe disease, the risk from failure to reassess and provide timely thromboprophylaxis in an overcapacity healthcare system is much more likely to outweigh the risk of major bleeding from appropriately dosed thromboprophylaxis (∼1%).1 Furthermore, although some might choose to use larger doses of thromboprophylaxis, no clear evidence basis exists for this, and we defer to the front-line practicioner.7 Therefore, the committee has recommended routine thromboprophylaxis of all hospitalized patients with COVID-19, regardless of the risk score (Table II ).
Table II.
Anticoagulation strategies
| Thromboprophylaxis |
| Low-molecular-weight heparin, 40 mg daily (or 30 mg twice daily) |
| Subcutaneous heparin, 5000 units 3 times daily |
| Full-dose anticoagulation |
| Heparin nomogram for DVT/PE |
| Low-molecular-weight heparin, 1.5 mg/kg daily (or 1 mg/kg twice daily) |
| Direct oral anticoagulant (standard dosing) |
| Low-dose anticoagulation protocol |
| Many patients can receive heparin nomogram for DVT/PE without bolus |
| Heparin nomogram for ACS/AF (Xa target, 0.2-0.5) |
| Non-nomogram heparin at discretion of attending (Xa target 0.2-0.3) |
ACS, acute coronary syndrome; AF, atrial fibrillation; DVT, deep venous thrombosis; PE, pulmonary embolism.
Suspected diagnosis of PE
The existing, reported Michigan Medicine faculty practice guidelines have recommended usage of a modified Wells' score (Table III ) to determine the pretest probability of the presence of PE.8
Table III.
Modified Wells score for assessment of clinical likelihood for pulmonary embolism
| Criteria | Pointsa |
|---|---|
| Clinical signs and symptoms of DVT (objectively measured calf swelling and pain with palpation in deep vein region) | 3 |
| An alternative diagnosis is less likely than a diagnosis of PE | 3 |
| Heart rate >100 beats per minute | 1.5 |
| Immobilization or surgery in previous 4 weeks | 1.5 |
| Previous DVT or PE | 1.5 |
| Hemoptysis | 1 |
| Malignancy (current treatment, treated in previous 6 months, or palliative care) | 1 |
DVT, Deep venous thrombosis; PE, pulmonary embolism.
Total score >4 indicates that PE is likely; and total score ≤4 indicates that PE is unlikely.
Low pretest probability of modified Well PE score (score ≤4; mean probability of PE, 1.7%-2.2% if negative D-dimer; 5.1%-7.8% overall, independent of D-dimer)
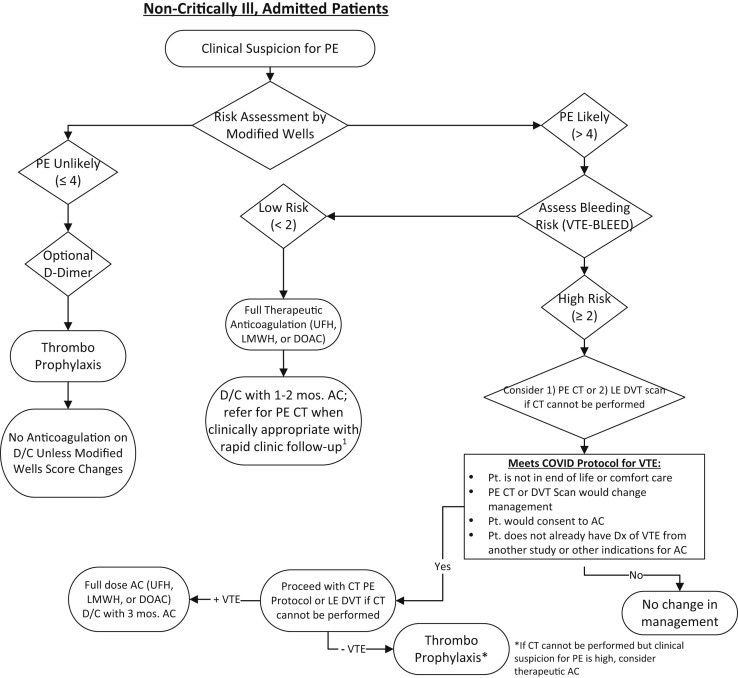
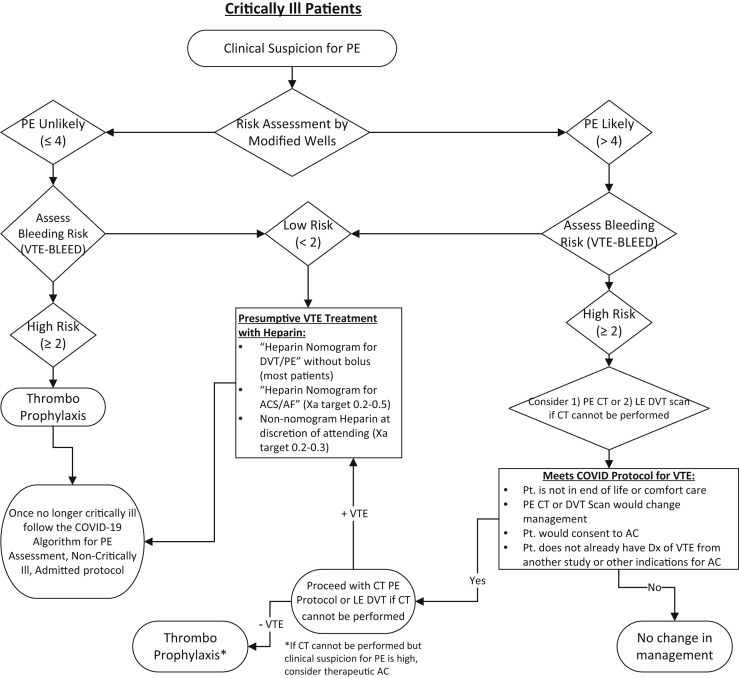
The current recommendation is thromboprophylaxis for the nonintubated, admitted patient and the intubated critically ill patient at high risk of bleeding (Figs 1 and 2 ). Intubated, critically ill patients with a low risk according to the Wells score might qualify for empiric lower dose anticoagulation if their bleeding risk is low. Higher consideration should be given to empiric low-dose anticoagulation if the partial pressure of arterial oxygen/fractional inspired oxygen (P/F) ratio is <200. We do not recommend performance of duplex ultrasonography for patients to exclude the diagnosis of PE. The rationale for this is that the use of DVT imaging in the setting of suspected PE has low accuracy, with a sensitivity of 44%, specificity of 86%, positive predictive value of 58%, and negative predictive value of 77%.9 These data have been substantiated in other studies, with a reported sensitivity of 25% to 38% for the diagnosis of thrombosis when used as a surrogate for PE. Thus, duplex imaging has significant limitations in the diagnosis of PE or in situ pulmonary thrombosis and should not be used as a direct test for PE.
Fig 1.
Algorithm for stable patient with suspected pulmonary embolism (PE). AC, anticoagulation; CT, computed tomography; D/C, discharge; DOAC, direct-acting oral anticoagulant; Dx, diagnosis; LE, lower extremity; LMWH, low-molecular-weight heparin; Pt., patient; UFH, unfractionated heparin; VTE, venous thromboembolism.
Fig 2.
Algorithm for critically ill patient with suspected pulmonary embolism (PE). AC, anticoagulation; Dx, diagnosis; LE, lower extremity; Pt., patient; VTE, venous thromboembolism.
A negative D-dimer combined with a low modified Well score is generally sufficient to exclude PE as a diagnosis. However, in the setting of COVID-19 infection, which is associated with elevated D-dimer, the clinical utility of D dimer is unknown.10 Clinicians might test for D-dimer at their discretion in the setting of a low modified Wells score if a negative result will reassure the patient. However, if positive, which is likely, it could distract from pursuing other, more likely, diagnoses.
High pretest probability of Wells PE score (score >4)
We recommend full-dose anticoagulation for high-risk patients with a low risk of bleeding anticoagulation based on bleeding risk (VTE bleeding score, <2; and no other risk factors, such as thrombocytopenia, cirrhosis, other thrombotic use). Lower dose empiric anticoagulation could be used as clinically appropriate in intubated critically ill patients owing to the bleeding risk associated with hemorrhagic pneumonitis with higher Xa levels. For patients with a high risk of bleeding (Table IV ), clinicians could consider obtaining a CT-PE study, if the findings would alter management. If CT to evaluate for PE is not possible, lower extremity duplex ultrasonography would be an alternative option, recognizing the limitations in sensitivity and specificity. Before obtaining imaging studies, clinicians should screen each patient to ensure that testing will not be futile or redundant.
Table IV.
VTE-BLEED score
| Variable | Score |
|---|---|
| Factor | |
| Active cancera | 2 |
| Male with uncontrolled arterial hypertensionb | 1 |
| Anemiac | 1 |
| History of bleedingd | 1 |
| Age ≥60 years | 1 |
| Renal dysfunctione | 1 |
| Classification | |
| Low bleeding risk | <2 |
| High bleeding risk | ≥2 |
| Other factors that contribute to bleeding | NA |
| Thrombocytopenia | |
| Cirrhosis | |
| Other antithrombotic use | |
| Recent major surgery (eg, neurosurgery, laparotomy) |
NA, Not applicable; VTE, venous thromboembolism.
Cancer diagnosed within 6 months before VTE (excluding basal cell carcinoma or squamous cell carcinoma of the skin), recently recurrent or progressive cancer or any cancer that required anticancer treatment within 6 months before VTE diagnosis.
Men with systolic blood pressure ≥140 mm Hg at baseline.
Hemoglobin <13 g/dL in men or <12 g/dL in women.
Including previous major or nonmajor clinically relevant bleeding event, rectal bleeding, frequent nose bleeding, or hematuria.
Glomerular filtration rate <60 mL/min.
Adapted from Klok FA, Barco S, Konstantinides SV. External validation of the VTE-BLEED score for predicting major bleeding in stable anticoagulated patients with venous thromboembolism. Thrombosis Haemost 2017; 117:1164-70.
If the diagnostic study findings are negative, thromboprophylaxis is recommended. If the study findings are positive, consideration should be given to full-dose anticoagulation for nonintubated admitted patients and lower dose empiric anticoagulation (either clinician run without a nomogram or using the acute coronary syndrome nomogram without a bolus; Figs 1 and 2) for intubated critically ill patients because of the bleeding risk or full-dose anticoagulation if thought appropriate by the intensivist.
Rationale for using anticoagulation at lower than full dose but higher than prophylactic dose in patients with severe acute respiratory distress syndrome
Cases of viral pneumonia associated venous thrombotic events resulting in significant mortality were witnessed during the H1N1 2009 pandemic. Patients meeting the following criteria during this period were treated using an empiric low-dose anticoagulation protocol: P/F ratio <200; viral pneumonia suspected or confirmed; and no absolute contraindications to anticoagulation. For patients meeting these criteria, the following protocol reduced the risk of VTE and primary pulmonary thrombi without increasing the incidence of bleeding complications: initiation of non-nomogram heparin infusion with a goal Xa of 0.2 to 0.3 and no bolus dose administered. The lower Xa is necessary, given the risk of bleeding with hemorrhagic pneumonitis. However, this goal Xa does not match an existing heparin nomogram at our institution and requires manual titration by the inpatient team.11 It, therefore, might be a reasonable, although untested, strategy to use the acute coronary syndrome nomogram with lower Xa ranges (0.2-0.5) to reduce the clinician workload if the bleeding risk is acceptable (Table II). For intubated patients without acute respiratory distress syndrome, a paucity of data is available regarding the risk/benefit ratio of empiric anticoagulation strategies. Patients with thrombocytopenia (platelets <30) should not be anticoagulated because of the attendant bleeding risk, based on expert consensus.
Suspected diagnosis of DVT
The existing Michigan Medicine faculty practice guidelines have recommended use of the Wells score to determine the pretest probability of DVT (Table V ).12 We recommend the use of this score during the COVID-19 pandemic, recognizing that the sensitivity and specificity diminishes in the inpatient setting and that its performance in the setting of pandemic pneumonia is untested. However, no reasonable alternative exists.12, 13, 14
Table V.
Wells score for likelihood estimation of lower extremity deep venous thrombosisa
| Clinical characteristic | Score |
|---|---|
| Active cancer (patient receiving treatment for cancer within previous 6 months or currently receiving palliative treatment) | 1 |
| Paralysis, paresis, or recent casting or immobilization of lower extremities | 1 |
| Recently bedridden for ≥3 days or major surgery within previous 12 weeks requiring general or regional anesthesia | 1 |
| Localized tenderness along distribution of deep venous system | 1 |
| Entire leg swollen | 1 |
| Calf swelling ≥3 cm larger than that on asymptomatic side (measured 10 cm below tibial tuberosity) | 1 |
| Pitting edema confined to symptomatic leg | 1 |
| Previously documented DVT | 1 |
| Collateral nonvaricose superficial veins | 1 |
| Alternative diagnosis at least as clinically likely as DVT | −2b |
DVT, Deep venous thrombosis.
Data from Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003; 349:1227-35; and Wells PS, Anderson DR, Bormanis J, Guy F, Mitchell M, Gray L, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet 1997; 350:1795-8.
A score of <2 is considered to indicate a low likelihood of DVT.
Low pretest probability of modified Wells DVT score (score <2; mean probability of DVT, 3%)
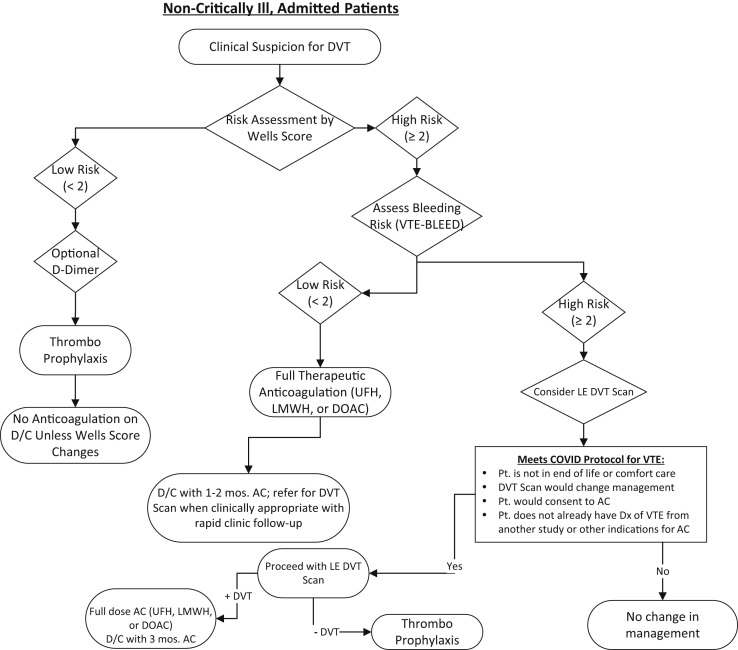
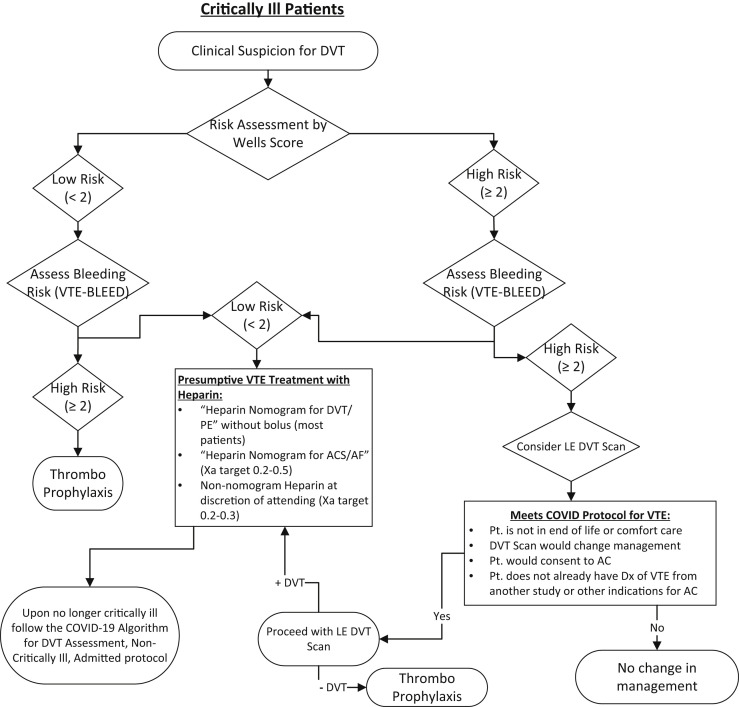
The current recommendation is thromboprophylaxis for nonintubated admitted patients. Intubated, critically ill patients with a low risk according to the Wells score might qualify for empiric lower dose anticoagulation if their bleeding risk is low (Figs 3 and 4 ). Greater consideration should be given to empiric low-dose anticoagulation if the P/F ratio is <200. The use of duplex ultrasound testing for those with a risk of only 3% DVT does not warrant the risk to the technologists to perform the tests in COVID-19–positive patients or those under investigation. Anticoagulation should not be used unless the Wells score has changed. D-dimer can be used only to rule out the presence of DVT. However, the D-dimer is expected to be elevated in those with COVID-19 and should not provide impetus to obtain imaging studies in the absence of any other clinical manifestation of DVT.
Fig 3.
Algorithm for stable patient with suspected deep venous thrombosis (DVT). AC, anticoagulation; D/C, discharge; DOAC, direct-acting oral anticoagulant; Dx, diagnosis; LE, lower extremity; LMWH, low-molecular-weight heparin; Pt., patient; UFH, unfractionated heparin; VTE, venous thromboembolism.
Fig 4.
Algorithm for critically ill patient with suspected deep venous thromboembolism (DVT). AC, anticoagulation; ACS, acute coronary syndrome; AF, atrial fibrillation; Dx, diagnosis; LE, lower extremity; PE, pulmonary embolism; Pt., patient; VTE, venous thromboembolism.
High pretest probability of Wells DVT score (score >2; mean probability of DVT, 16.6%-74.6%)
With the greater likelihood of DVT in those with a high pretest probability, we would recommend anticoagulation according to the bleeding risk (VTE-BLEED score; Table IV). For patients with a low bleeding risk (VTE-BLEED score <2), we suggest empiric full-dose therapeutic anticoagulation for nonintubated admitted patients. For intubated critically ill patients, we recommend empiric lower dose anticoagulation because of the expected elevated bleeding risk with common comorbid conditions such as renal failure, disseminated intravascular coagulation, and hemorrhagic pneumonitis (Figs 3 and 4). For patients with a high bleeding risk (VTE-BLEED score >2), a lower extremity DVT scan might be indicated if the patients meet the criteria. However, the VTE-BLEED score (Table IV) has been validated in the outpatient population and no similar score exists for use in acutely hospitalized and/or critically ill populations. This specific patient cohort could have additional risk factors that could increase the bleeding risk such as thrombocytopenia, cirrhosis, and other antithrombotic use that should be considered by the clinician at the bedside. Furthermore, the VTE-BLEED score has been validated for the bleeding risk over a longer period than expected for most patients treated using this algorithm. Therefore, physicians might be comfortable with full-dose anticoagulation for patients with multiple risk factors, recognizing the fewer absolute number of days subjected to risk. If the DVT scan shows negative finding, thromboprophylaxis is recommended. If the study shows positive findings, consideration should be given to full-dose anticoagulation or use of an inferior vena cava filter. For patients treated with lower dose empiric anticoagulation in the intensive care unit setting, standard full-dose anticoagulation should be initiated once their condition has stabilized enough for transfer to a regular ward.
Upper extremity DVT
Given the low morbidity of upper extremity line-associated DVT in our critically ill population,15 we do not recommend routine upper extremity duplex ultrasonography. If a patient has unilateral symptoms and a high risk of bleeding, the need for upper extremity imaging studies can be considered on a case by case basis. If the bleeding risk is low but the likelihood of DVT is high, the patient should be empirically treated.
Consideration for long-term therapy
For patients treated with anticoagulants and unable to undergo diagnostic imaging studies during the COVID-19 surge, we recommend that they be discharged with a 1- to 2-month supply of direct oral anticoagulant agents (or vitamin K antagonists) until they are able to undergo diagnostic imaging. For patients deemed at moderate to high risk of PE, a CT-PE protocol within 1 month should be ordered once they are considered negative for COVID-19. For patients deemed at high risk of DVT, lower extremity duplex ultrasonography should be ordered, again, once they are considered negative for COVID-19. All patients should be followed up and provided recommendations for long-term therapy. Current efforts are aimed at developing protocols for high through-put imaging after the pandemic and diligent follow-up via newly expanded video and/or telephone visits. Each institution will likely have guidelines for how the post COVID-19 infected patients will be screened for a negative contagious state.
Discussion
It is said that necessity is the mother of innovation. Just a few short months ago, it was inconceivable that hospitals across the United States and the world would be stretched far beyond capacity, that essential resources would be rationed, and that even the most mundane, everyday clinical diagnosis and treatment algorithms would be altered. This is the current status because only a small aliquot of hours stand between us and the overwhelming tide of patients expected throughout the nation and in many parts of the world. Proactive use of an algorithmic approach to care in this setting has the following advantages: (1) decreases variability in care across the healthcare system; (2) relieves stress on individual providers to determine the optimal treatment without the appropriate and usual diagnostic tests; (3) harnesses existing healthcare infrastructure to ensure conscientious follow-up for patients and restore confidence in the system; (4) protects scare resources; and (5) fulfills our moral obligation to our patients and staff.
Conclusions
As we move forward through the inevitable crush of patients, conserving clinicians' mental energy for critical decisions should be of the highest priority given the expected emotional and mental exhaustion in the pandemic setting. As vascular specialists, using our knowledge of clinical scoring systems and risk/benefit ratios, we can extrapolate the current knowledge and standard of care to provide practical and pragmatic straightforward rules for treatment that alleviates the bedside clinician mired in the purgatory of diagnostic uncertainty. In this treatment paradigm, we emphasize preventing VTE-related morbidity and mortality at the expense of bleeding complications in the short term while imaging studies are delayed and using therapy that has shown benefit for other severe viral states (eg, H1N1). Although direct oral anticoagulant agents are now more readily available and have improved the bleeding profile, we expect that with such a large influx of patients, bleeding complications are a statistical certainty. With such an approach, the commitment to providing follow-up—the DVT scan that would normally be obtained within 24 hours, will now be obtained after 2 to 4 weeks—must be absolute, meticulous and unwavering.
The lack of hospital system beds across the United States, alongside the press reports of temporary morgues and ventilator rationing, could very well serve to undermine the confidence of Americans in the existing healthcare infrastructure. Using content experts and protocolizing treatment plans is 1 method that can allow us to maintain consistency in care delivery and restore faith in the local hospital system. Undoubtedly, areas beyond VTE exist in which vascular surgeons can and are developing protocols that can help to streamline the care of patients during a time of crisis. During the time of crisis, we can further respond by collating data in an organized fashion, adapting protocols as more is learned, and disseminating information rapidly. Vascular surgeons are often the quarterback in a crisis; however, in the current challenge of the COVID-19 pandemic, our role is to use our expertise and tools to protect and aid those serving on the frontlines.
Author contributions
Conception and design: AO, GB, TW, JE, SB, EA, PH
Analysis and interpretation: Not applicable
Data collection: Not applicable
Writing the article: AO, GB, TW, JE, SB, EA, PH
Critical revision of the article: AO, GB, TW, JE, SB, EA, PH
Final approval of the article: AO, GB, TW, JE, SB, EA, PH
Statistical analysis: Not applicable
Obtained funding: Not applicable
Overall responsibility: PH
Acknowledgments
We greatly appreciate the input of the staff members of our vascular laboratory, the front-line intensivists that participated in reviewing and providing feedback on the algorithms and Dr James Froehlich as a part of the reviewing team.
Footnotes
Dr Obi was supported by a Vascular Cures Wiley Foundation grant.
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Kearon C., Akl E.A., Comerota A.J., Prandoni P., Bounameaux H., Goldhaber S.Z. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e419S–e496S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pannucci C.J., Swistun L., MacDonald J.K., Henke P.K., Brooke B.S. Individualized venous thromboembolism risk stratification using the 2005 Caprini score to identify the benefits and harms of chemoprophylaxis in surgical patients: a meta-analysis. Ann Surg. 2017;265:1094–1103. doi: 10.1097/SLA.0000000000002126. [DOI] [PubMed] [Google Scholar]
- 3.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy [published online ahead of print March 27, 2020]. J Thromb Haemost 10.1111/jth.14817. [DOI] [PMC free article] [PubMed]
- 4.Li X.Y., Du B., Wang Y.S., Kang H.Y.J., Wang F., Sun B. The keypoints in treatment of the critical coronavirus disease 2019 patient. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E026. doi: 10.3760/cma.j.cn112147-20200224-00159. [DOI] [PubMed] [Google Scholar]
- 5.Driggin E., Madhavan M.V., Bikdeli B., Chuich T., Laracy J., Bondi-Zoccai G. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75:2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J., Wang X., Zhang S., Liu B., Wu X., Wang Y. Findings of acute pulmonary embolism in COVID-19 patients. Lancet. 2020 (in press) [Google Scholar]
- 7.Bartlett M.A., Mauck K.F., Daniels P.R. Prevention of venous thromboembolism in patients undergoing bariatric surgery. Vasc Health Risk Manag. 2015;11:461–477. doi: 10.2147/VHRM.S73799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahia A., Albert R.K. The modified Wells score accurately excludes pulmonary embolus in hospitalized patients receiving heparin prophylaxis. J Hosp Med. 2011;6:190–194. doi: 10.1002/jhm.827. [DOI] [PubMed] [Google Scholar]
- 9.Killewich L.A., Nunnelee J.D., Auer A.I. Value of lower extremity venous duplex examination in the diagnosis of pulmonary embolism. J Vasc Surg. 1993;17:934–938. discussion 938-9. [PubMed] [Google Scholar]
- 10.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Obi A.T., Tignanelli C.J., Jacobs B.N., Arya S., Park P.K., Wakefield T.W. Empirical systemic anticoagulation is associated with decreased venous thromboembolism in critically ill influenza A H1N1 acute respiratory distress syndrome patients. J Vasc Surg Venous Lymphat Disord. 2019;7:317–324. doi: 10.1016/j.jvsv.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Wells P.S., Anderson D.R., Bormanis J., Guy F., Mitchell M., Gray L. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997;350:1795–1798. doi: 10.1016/S0140-6736(97)08140-3. [DOI] [PubMed] [Google Scholar]
- 13.Silveira P.C., Ip I.K., Goldhaber S.Z., Piazza G., Benson C.B., Khorasani R. Performance of Wells score for deep vein thrombosis in the inpatient setting. JAMA Intern Med. 2015;175:1112–1117. doi: 10.1001/jamainternmed.2015.1687. [DOI] [PubMed] [Google Scholar]
- 14.Wells P.S., Anderson D.R., Rodger M., Forgie M., Kearon C., Dreyer J. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227–1235. doi: 10.1056/NEJMoa023153. [DOI] [PubMed] [Google Scholar]
- 15.Underhill J., Sherman M.A., Howard R., Hage A., Obi A., Napolitano L. The natural history and outcomes of line-associated upper extremity deep venous thromboses in critically ill patients. J Vasc Surg Venous Lymphat Disord. 2017;5:630–637. doi: 10.1016/j.jvsv.2017.03.018. [DOI] [PubMed] [Google Scholar]