Abstract
Real-world evidence describing the variation in serum creatinine (S-Cre) within 24 hours and its prognostic value is unknown. We enrolled 14 912 adults who received two S-Cre measurements within 24 hours at a tertiary hospital between 2003 and 2016. The study population was divided into four groups according to the hospital service settings where the baseline and second S-Cre were measured: Group 1, Outpatient-to-Outpatient; Group 2, Outpatient-to-ED (emergency department) or Inpatient; Group 3, ED-to-ED or Inpatient; and Group 4, Inpatient-to-Inpatient. The main predictors were the difference between the two S-Cre measurements (ΔS-Cre) and the percent change (ΔS-Cre%). The main outcomes were 30-day, 1-year, or 3-year all-cause mortality. A total of 6753 and 8159 patients with an increase and a decrease within-day ΔS-Cre, respectively. Among 6753 patients who had deteriorating ΔS-Cre or ΔS-Cre%, the adjusted hazard ratio (aHR) for 1-year all-cause mortality for each 0.1 mg/dL or 5% change in S-Cre was 1.09 (95% confidence interval [CI]: 1.07, 1.11) and 1.03 (95% CI: 1.03, 1.04). In 8159 patients with improving ΔS-Cre%, the aHR was 0.97 (95% CI: 0.94, 1.00). Groups 3 and 4 had statistically significant positive linear relationships between deteriorating ΔS-Cre% and 30-day and 3-year mortality. The optimal cut-offs for deteriorating ΔS-Cre% for predicting 30-day mortality were approximately 22% for Group 3 and 20% for Group 4. Inpatient within-day deteriorating ΔS-Cre or ΔS-Cre% above 0.2 mg/dL or 20%, respectively, is associated with all-cause mortality. Monitoring 24-hour S-Cre variation identifies acute kidney injury earlier than the conventional criteria.
Subject terms: Biomarkers, Nephrology
Introduction
The prognostic importance of serum creatinine (S-Cre) within-day variation has not been widely evaluated. In clinical practice, critical variations, defined by an absolute increase of 0.3 mg/dL (26.5 μmol/L) in S-Cre within 48 hours or a 50% increase in S-Cre concentration over a 7-day period, have been used to diagnose in-hospital acute kidney injury (AKI) since 20041,2. A 100% increase in S-Cre concentration is a conventional study endpoint in chronic kidney disease (CKD) research, despite a recent study proposing the less stringent criterion of 30%, with the aim to capture more clinical outcomes while retaining prognostic significance3. However, these cut-offs are arbitrary because of the lack of detailed knowledge, obtained from real-world evidence, regarding the within-day variation in people with a wide range of kidney function.
The diurnal fluctuation in S-Cre, mainly due to alimentary factors, was first observed 50 years ago. Among healthy participants, research has revealed that the maximum mean within-day percent change in S-Cre is almost 30%4. The main sources of within-day S-Cre variation include analytical and biological within-subject variations. Much effort has focused on controlling the analytical variation of inter-assay variability or inherent measurement error by establishing calibration traceability using isotope dilution mass spectrometry (IDMS) reference procedures5. Yet, our understanding of the biological variation in S-Cre remains confined to factors that can influence levels of S-Cre such as the individual’s muscle mass, renal tubular secretion, protein-rich intake, and extra-renal clearance of creatinine through intestinal microbes6–8. More importantly, claiming that S-Cre variation is physiological or pathological without linking an individual’s survival outcome to the within-day variation of S-Cre would be far from evidence-based. To clarify the clinical meaning of within-day variation in S-Cre, we tracked the mortality of patients who had received repeated S-Cre measurements within 24 hours.
Results
A total of 6753 patients with an increase in within-day ΔS-Cre and 8159 patients with a decrease contributed a total of 24 257 and 31 400 person-years of follow-up, respectively (Supplementary Table 1a,b). The median age on the index day was 60.4 (IQR: 46.6, 74.2) years for patients with deteriorating ΔS-Cre and 59.0 (44.8, 73.3) years for patients with improving ΔS-Cre. Patients in the lowest quartile of ΔS-Cre% were older and more likely to be male compared with those in the three highest quartiles (Supplementary Table 1a,b). For comorbidities, the prevalence of impaired kidney function (IKF), acute kidney failure (AKF), diabetes, and hypertension decreased across the quartiles of deteriorating ΔS-Cre% (Supplementary Table 1a), but similar comorbidity trends were also observed among patients with improving ΔS-Cre% (Supplementary Table 1b). The increased use of nonsteroidal anti-inflammatory drugs (NSAIDs) and radiocontrast was observed across the increasing quartiles of improving and deteriorating ΔS-Cre%. The baseline white blood cell counts (WBC) and C-reactive protein (CRP) increased across the increasing quartiles of ΔS-Cre%, and the potassium and serum albumin levels decreased (Supplementary Table 1a,b). Corresponding clinical characteristics of ΔS-Cre, by quartiles, are provided in Supplementary Table 2a,b showing similar results.
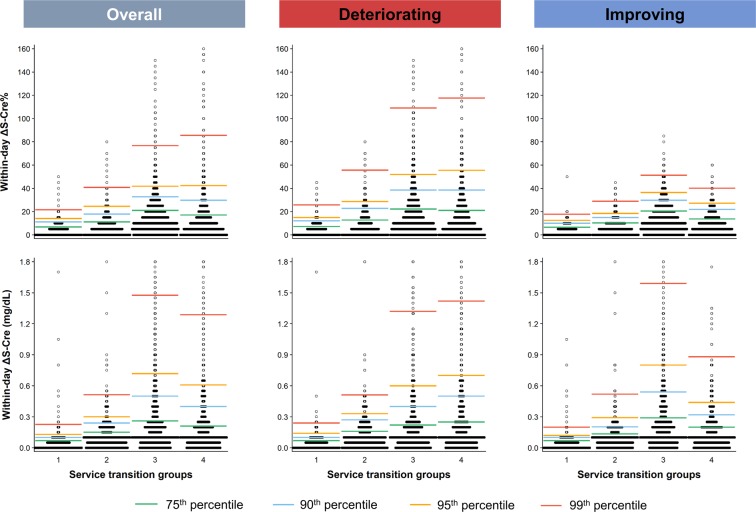
From the perspective of the four service transition groups, patients in Group 4 (INPT-to-INPT) were older; more likely to be male; had the highest prevalence of IKF and noncancerous catastrophic status; were more likely to be exposed to diuretics, NSAIDs, and radiocontrast; and had developed the highest all-cause mortality during the follow-up (Table 1). The proportion of patients who received intravenous fluid therapy on the index day was up to 97.2% for Group 3 and 82.6% for Group 4. Group 4 also had the highest baseline levels of blood urea nitrogen (BUN), S-Cre, serum sodium, CRP, and WBC and had the lowest baseline levels of serum albumin and hemoglobin (Table 1). Figure 1 summarizes the distribution of ΔS-Cre and ΔS-Cre% in the present study population by the four service transition groups.
Table 1.
Baseline demographic and clinical characteristics based on patients’ service transition patterns: ED, emergency department; INPT, inpatient; OPT, outpatient.
| Variables | Group 1 | Group 2 | Group 3 | Group 4 | p-valuea |
|---|---|---|---|---|---|
| OPT to OPT | OPT to ED or INPT | ED to ED or INPT | INPT to INPT | ||
| Demographics, median (IQR) | 4145 (27.8%) | 1761 (11.8%) | 5545 (37.2%) | 3164 (21.2%) | |
| Age, years | 58.7 (47.3, 70.5) | 64.0 (50.2, 75.6) | 56.7 (40.4, 73.2) | 64.3 (49.7, 77.5) | <0.001 |
| Men, n (%) | 2481 (59.9) | 951 (54.0) | 3317 (59.8) | 1894 (59.9) | 0.418 |
| Body mass index (kg/m2) | 24.7 (22.2, 27.6) | 23.8 (21.2, 26.8) | 23.5 (20.8, 26.5) | 23.5 (20.6, 26.6) | <0.001 |
| Comorbidities, n (%) | |||||
| Impaired kidney function | 873 (21.1) | 666 (37.8) | 1641 (29.6) | 1243 (39.3) | <0.001 |
| Acute kidney failure (ICD 584.5–584.9) | 100 (2.41) | 138 (7.84) | 412 (7.43) | 246 (7.77) | <0.001 |
| Diabetes mellitus | 1195 (28.8) | 599 (34.0) | 935 (16.9) | 997 (31.5) | <0.001 |
| Hypertension | 1418 (34.2) | 686 (39.0) | 983 (17.7) | 1017 (32.1) | <0.001 |
| Noncancerous Catastrophic illness status | 356 (8.59) | 229 (13.00) | 1058 (19.08) | 768 (24.27) | <0.001 |
| Therapy, n (%) | |||||
| Fluid therapy between two measurements | 29 (0.70) | 853 (48.44) | 5015 (90.44) | 2060 (65.11) | <0.001 |
| Fluid therapy on the index day | 527 (12.71) | 1649 (93.64) | 5387 (97.15) | 2614 (82.62) | <0.001 |
| Medication, n (%) | |||||
| Angiotensin-converting-enzyme inhibitors | 439 (10.6) | 296 (16.8) | 663 (12.0) | 710 (22.4) | <0.001 |
| Angiotensin II receptor blockers | 1123 (27.1) | 455 (25.8) | 513 (9.3) | 544 (17.2) | <0.001 |
| Diuretics | 957 (23.1) | 751 (42.7) | 1532 (27.6) | 2118 (66.9) | <0.001 |
| Oral hypoglycemic agents | 997 (24.1) | 455 (25.8) | 527 (9.5) | 596 (18.8) | <0.001 |
| Insulin | 550 (13.3) | 490 (27.8) | 983 (17.7) | 1126 (35.6) | <0.001 |
| NSAIDs | 1039 (25.1) | 680 (38.6) | 2514 (45.3) | 1519 (48.0) | <0.001 |
| Radiocontrast | 979 (23.6) | 412 (23.4) | 1911 (34.5) | 1468 (46.4) | <0.001 |
| Lab data, median (IQR) | |||||
| Baseline BUN, mg/dL | 14.0 (11.0, 23.0) | 19.0 (12.0, 42.0) | 15.0 (10.5, 29.0) | 22.0 (12.7, 44.0) | <0.001 |
| Second BUN, mg/dL | 14.0 (11.0, 24.0) | 21.5 (13.0, 48.0) | 14.0 (10.0, 27.0) | 23.0 (13.0, 45.0) | <0.001 |
| Baseline serum creatinine, mg/dL | 0.97 (0.76, 1.32) | 1.15 (0.83, 2.19) | 1.01 (0.78, 1.65) | 1.16 (0.80, 2.01) | <0.001 |
| Second serum creatinine, mg/dL | 0.96 (0.76, 1.33) | 1.15 (0.83, 2.20) | 0.99 (0.75, 1.58) | 1.20 (0.82, 2.10) | <0.001 |
| Baseline eGFR, ml/min/1.73m2 | 81.7 (51.0, 99.4) | 60.1 (25.7, 90.8) | 75.9 (38.9, 100.8) | 58.3 (29.5, 92.1) | <0.001 |
| Serum albumin, g/dL | 4.30 (3.80, 4.60) | 3.60 (3.00, 4.05) | 3.10 (2.50, 3.60) | 2.80 (2.30, 3.30) | <0.001 |
| Hemoglobin, g/dL | 13.50 (11.70, 14.90) | 12.20 (9.90, 14.00) | 12.50 (10.75, 14.15) | 10.70 (9.35, 12.43) | <0.001 |
| Sodium, mEq/L | 138.0 (137.0, 140.0) | 136.0 (133.0, 139.0) | 138.0 (135.0, 140.0) | 138.0 (134.5, 141.5) | <0.001 |
| Potassium, mEq/L | 4.10 (3.80, 4.50) | 3.90 (3.60, 4.40) | 3.70 (3.40, 4.05) | 3.80 (3.35, 4.25) | <0.001 |
| White blood cell count, 103/μL | 6.46 (5.24, 7.95) | 8.19 (6.13, 10.90) | 11.25 (8.27, 14.88) | 10.31 (7.43, 14.58) | <0.001 |
| C-reactive protein, mg/dL | 0.17 (0.06, 0.70) | 0.97 (0.22, 4.85) | 1.60 (0.29, 7.15) | 4.51 (1.17, 12.26) | <0.001 |
| Summary measures of baseline and second S-Cre | |||||
| Time interval (hours) | 0.00 (0.00, 0.43) | 4.63 (2.57, 8.05) | 8.53 (6.15, 12.13) | 8.68 (5.36, 12.85) | <0.001 |
| Difference (ΔS-Cre) | 0.04 (0.02, 0.07) | 0.10 (0.04, 0.15) | 0.13 (0.07, 0.26) | 0.10 (0.06, 0.21) | <0.001 |
| Percent change (ΔS-Cre%) | 3.66 (1.79, 6.82) | 6.00 (2.74, 11.11) | 12.24 (6.20, 21.01) | 9.09 (4.26, 17.12) | <0.001 |
| Outcome | |||||
| Deaths before 2017–12–31 | 736 (17.8) | 677 (38.4) | 2353 (42.4) | 1928 (60.9) | <0.001 |
| 3-year all-cause deaths | 423 (10.2) | 410 (23.3) | 1719 (31.0) | 1563 (49.4) | <0.001 |
| 1-year all-cause deaths | 187 (4.5) | 244 (13.9) | 1219 (22.0) | 1211 (38.3) | <0.001 |
| 30-day all-cause deaths | 14 (0.3) | 41 (2.3) | 604 (10.9) | 634 (20.0) | <0.001 |
ap-values are calculated by Kruskal-Wallis test for continuous variables and chi-square test for categorical variables.
Abbreviations: BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; ICD, Internal Classification of Disease; NSAID, Nonsteroidal anti-inflammatory drugs.
Figure 1.
Box-percentile plot of the difference and percent change in S-Cre levels within 24 hours (within-day ΔS-Cre and ΔS-Cre%) in overall population and in patients with deteriorating or improving kidney function based on patients’ service transition patterns (Four groups: Group 1, OPT-to-OPT; Group 2, OPT-to-ED or INPT; Group 3, ED-to-ED or INPT; and Group 4, INPT–to-INPT). Specific percentiles are highlighted by color lines: median, green; 75th percentile, blue; 90th percentile, yellow; and 95th percentile, red. ED, emergency department; INPT, inpatient; OPT, outpatient; S-Cre, serum creatinine.
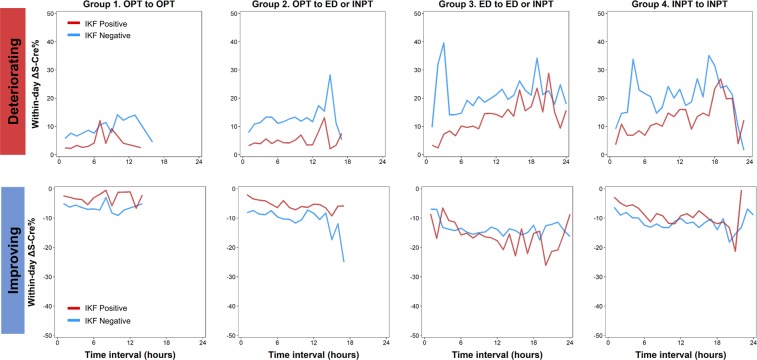
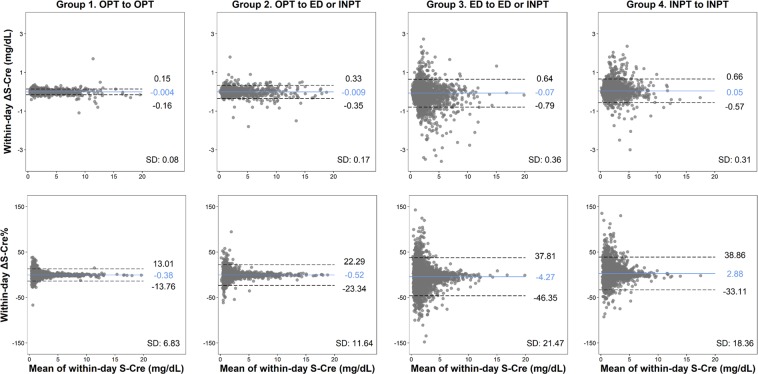
The mean time intervals between the first and second S-Cre measurements were: 0 h, Group 1; 4.63 h, Group 2; 8.53 h, Group 3; and 8.68 h, Group 4. When stratifying by service group and IKF status, the magnitude of ΔS-Cre% and ΔS-Cre increased as the time interval increased, particularly in patients from Groups 3 and 4 who had decreasing change in S-Cre levels (Fig. 2 and Supplementary Fig. 1). The magnitude of within-day ΔS-Cre% was greater among patients without IKF and within-day ΔS-Cre was greater among patients with IKF (Fig. 2 and Supplementary Fig. 1). The spikes in Fig. 2 and Supplementary Fig. 1 represent the most prominent variation in kidney function occurring at a specific time interval. Generally, spikes in ΔS-Cre% occurred early in Groups 3 and 4 but late in Group 2. Spikes were less frequent among patients with improving ΔS-Cre% (Fig. 2). However, the spikes in ΔS-Cre were more likely to occur late (Supplementary Fig. 1). When using the Bland–Altman plot to analyze the concordance of the two S-Cre values by service transition groups, the agreement intervals became wider, from SD 6.83% (Group 1) and 11.64% (Group 2) to 21.47% (Group 3) and 18.36% (Group 4). Additionally, there was a trend for the second S-Cre values to improve when the means of the two S-Cre values increased (Fig. 3).
Figure 2.
Percent change in S-Cre levels repeated within 24 hours (within-day ΔS-Cre%) over sampling time interval by patients’ service transition patterns. Red line: baseline IKF positive; blue line: baseline IKF negative. IKF, impaired kidney function; S-Cre, serum creatinine.
Figure 3.
Bland-Altman plots of the difference and percent change between the baseline and the second S-Cre level by patient’s service transition patterns. ED, emergency department; INPT, inpatient; OPT, outpatient; S-Cre, serum creatinine.
In the overall population, the adjusted hazard ratio (aHR) for 30-day all-cause mortality for each 5% change (within-day ΔS-Cre%) was 1.06 (95% CI: 1.04, 1.07) and 0.1 mg/dL change (within-day ΔS-Cre) was 1.06 (95% CI: 1.04, 1.08) (Table 2 and Supplementary Table 3). The corresponding aHR for 3-year all-cause mortality was 1.02 (95% CI: 1.01, 1.03) and 1.00 (95% CI: 0.99, 1.01) (Supplementary Table 4 and Supplementary Table 5). Among patients with deteriorating ΔS-Cre%, the aHR of per 5% change in ΔS-Cre% for 30-day all-cause mortality was 1.08 (95% CI: 1.06, 1.10) and 3-year all-cause mortality was 1.03 (95% CI: 1.02, 1.04) (Table 2 and Supplementary Table 3). In Group 3, the effect sizes increased to 1.11 (95% CI: 1.07, 1.15) for 30-day all-cause mortality and 1.03 (95% CI: 1.02, 1.05) for 3-year all-cause mortality (Table 2 and Supplementary Table 3). The significant risk pattern was only observed among patients of service Groups 3 and 4. Moreover, improving ΔS-Cre% was associated with lower risk of 3-year all-cause mortality in Group 3 only (Supplementary Table 4). In Group 1, ΔS-Cre% and mortality was not associated. After further adjusted for time intervals between first and second S-Cre measurements, the main inferences remained robust (Supplementary Table 6 and Supplementary Table 7).
Table 2.
Hazard ratios (95% confidence interval) of 1-year (for Groups 1 and 2) and 30-day (for Groups 3 and 4) all-cause mortality according to every 5% change in S-Cre levels repeated within 24 hours. ED, emergency department; INPT, inpatient; OPT, outpatient; S-Cre, serum creatinine.
| Case/N | Mortality (%) | Crude HR (95% CI) | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Adjusted HR (95% CI) | p-value | Adjusted HR (95% CI) | p -value | Adjusted HR (95% CI) | p -value | ||||
| 1-year mortality | |||||||||
| Overall | 2894/14912 | 19.4% | 1.04 (1.03, 1.04) | 1.05 (1.05, 1.06) | <0.001 | 1.04 (1.03, 1.05) | <0.001 | 1.03 (1.02, 1.04) | <0.001 |
| Deteriorating | 1459/6753 | 21.6% | 1.04 (1.03, 1.05) | 1.06 (1.05, 1.07) | <0.001 | 1.05 (1.04, 1.05) | <0.001 | 1.03 (1.03, 1.04) | <0.001 |
| Improving | 1435/8159 | 17.6% | 1.07 (1.04, 1.09) | 1.03 (1.01, 1.06) | 0.011 | 0.98 (0.95, 1.01) | 0.167 | 0.97 (0.94, 1.00) | 0.041 |
| Group 1 (OPT to OPT) | |||||||||
| Overall | 187/4145 | 4.5% | 0.95 (0.80, 1.13) | 1.06 (0.89, 1.26) | 0.537 | 1.03 (0.86, 1.24) | 0.743 | 0.97 (0.81, 1.17) | 0.768 |
| Deteriorating | 95/1882 | 5.0% | 0.98 (0.80, 1.20) | 1.06 (0.85, 1.31) | 0.616 | 1.01 (0.80, 1.28) | 0.916 | 0.95 (0.75, 1.20) | 0.639 |
| Improving | 92/2263 | 4.1% | 0.89 (0.66, 1.21) | 1.02 (0.73, 1.42) | 0.916 | 1.01 (0.73, 1.41) | 0.936 | 0.98 (0.69, 1.40) | 0.917 |
| Group 2 (OPT to ED or INPT) | |||||||||
| Overall | 244/1761 | 13.9% | 0.99 (0.92, 1.07) | 1.05 (0.99, 1.12) | 0.099 | 1.06 (1.00, 1.13) | 0.063 | 1.05 (0.98, 1.12) | 0.164 |
| Deteriorating | 102/782 | 13.0% | 1.01 (0.93, 1.10) | 1.06 (0.98, 1.15) | 0.117 | 1.07 (0.99, 1.16) | 0.076 | 1.06 (0.97, 1.15) | 0.221 |
| Improving | 142/979 | 14.5% | 0.92 (0.79, 1.08) | 1.05 (0.89, 1.23) | 0.559 | 1.01 (0.86, 1.18) | 0.929 | 1.01 (0.86, 1.20) | 0.882 |
| 30-day mortality | |||||||||
| Overall | 1304/14912 | 8.7% | 1.09 (1.07, 1.10) | 1.10 (1.08, 1.11) | <0.001 | 1.07 (1.05, 1.08) | <0.001 | 1.06 (1.04, 1.07) | <0.001 |
| Deteriorating | 745/6753 | 11.0% | 1.10 (1.08, 1.12) | 1.12 (1.10, 1.15) | <0.001 | 1.09 (1.07, 1.11) | <0.001 | 1.08 (1.06, 1.10) | <0.001 |
| Improving | 559/8159 | 6.9% | 1.11 (1.06, 1.16) | 1.07 (1.03, 1.12) | 0.001 | 0.99 (0.95, 1.04) | 0.805 | 0.99 (0.94, 1.04) | 0.633 |
| Group 3 (ED to ED or INPT) | |||||||||
| Overall | 604/5545 | 10.9% | 1.05 (1.03, 1.08) | 1.06 (1.04, 1.08) | <0.001 | 1.06 (1.04, 1.09) | <0.001 | 1.06 (1.04, 1.09) | <0.001 |
| Deteriorating | 270/2184 | 12.4% | 1.08 (1.04, 1.11) | 1.09 (1.06, 1.13) | <0.001 | 1.10 (1.06, 1.15) | <0.001 | 1.11 (1.07, 1.15) | <0.001 |
| Improving | 334/3361 | 9.9% | 0.99 (0.93, 1.05) | 0.97 (0.91, 1.03) | 0.362 | 0.96 (0.9, 1.03) | 0.262 | 0.96 (0.90, 1.03) | 0.261 |
| Group 4 (INPT to INPT) | |||||||||
| Overall | 634/3164 | 20.0% | 1.02 (1.00, 1.04) | 1.04 (1.02, 1.06) | <0.001 | 1.04 (1.02, 1.06) | <0.001 | 1.03 (1.01, 1.06) | 0.002 |
| Deteriorating | 437/1736 | 25.2% | 1.03 (1.01, 1.06) | 1.05 (1.02, 1.08) | <0.001 | 1.05 (1.02, 1.08) | <0.001 | 1.06 (1.03, 1.09) | <0.001 |
| Improving | 197/1428 | 13.8% | 0.93 (0.82, 1.05) | 0.95 (0.83, 1.08) | 0.434 | 0.90 (0.78, 1.04) | 0.150 | 0.95 (0.81, 1.10) | 0.481 |
Model 1: Adjusted for gender, body mass index, diabetes, hypertension, impaired kidney function, noncancerous catastrophic illness, acute kidney failure, baseline eGFR.
Model 2: Further adjusted for medications listed in Table 1 including fluid therapy between two S-Cre measurements.
Model 3: Further adjusted for baseline blood urea nitrogen, C-reactive protein, white blood cell count, serum albumin, hemoglobin.
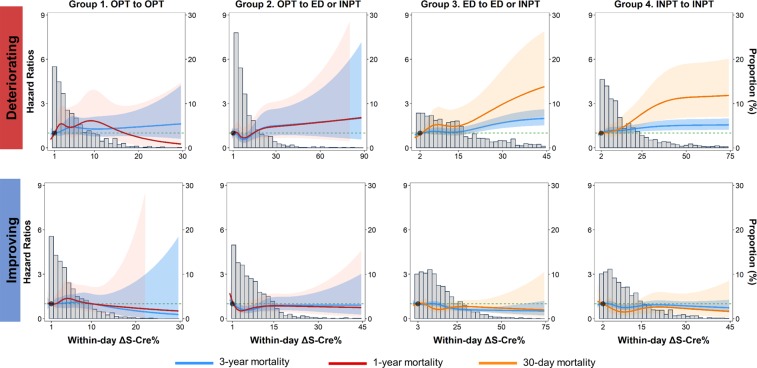
In the dose-response analysis, positive relationships were observed between deteriorating ΔS-Cre and ΔS-Cre% and 30-day and 3-year mortality in Group 3 and 4 patients (Fig. 4 and Supplementary Fig. 2, upper panel). Negative relationships were observed between improving ΔS-Cre and ΔS-Cre% and 30-day and 3-year mortality in Group 3 and 4 patients (Fig. 4 and Supplementary Fig. 2, lower panel). By contrast, the magnitude of improving ΔS-Cre and ΔS-Cre% was not associated with short- or long-term mortality in Group 1 patients (Fig. 4 and Supplementary Fig. 2, lower panel). In Group 3 and 4 patients, the optimal cut-offs for the prediction of 30-day and 3-year mortality were determined to be approximately 0.2 mg/dL increase for ΔS-Cre and 20% increase for ΔS-Cre% (Supplementary Figs. 3 and 4, upper panel). In patients with IKF, the corresponding cut-off for deteriorating ΔS-Cre% dropped to 10–13%; however, among Group 4 patients with IKF, the clinical significance threshold of ΔS-Cre remained consistent at 0.22 (Supplementary Table 8).
Figure 4.
Adjusted hazard ratios (aHRs) for 30-day (red line), 1-year (dark-red line), and 3-year (blue line) all-cause mortality according to the percent change in S-Cre levels repeated within 24 hours (within-day ΔS-Cre%) by patients’ service transition patterns and variation directions (deteriorating vs. improving). Solid lines represent aHRs based on restricted cubic splines for within-day ΔS-Cre%, with knots at the 5th, 25th,, 50th, 75th, and 95th percentiles. Shaded areas represent the upper and lower 95% confidence intervals. Reference was set at 10th percentile of ΔS-Cre% levels. Variables adjusted are the same as that shown in Model 3 of Table 2. S-Cre, serum creatinine.
Discussion
This real-world study provides a thorough understanding of the clinical significance of 24-hour ΔS-Cre and ΔS-Cre%, which can be used to inform diagnostic criteria of both outpatient- and inpatient-AKI (AKIOPT and AKIINPT). The clinical significance of within-day ΔS-Cre and ΔS-Cre% is different in inpatient and outpatient settings; the positive linear relationship between all-cause mortality and deteriorating ΔS-Cre or ΔS-Cre% is only observed in the inpatient settings, regardless of whether the all-cause-mortality is short- or long-term or the change in S-Cre is very small. In addition, a 0.2 mg/dL (ΔS-Cre) or 20% (ΔS-Cre%) change is clinically meaningful in predicting the risk of all-cause mortality in inpatient settings. However, for patients with IKF, the clinical threshold of ΔS-Cre% should be reduced to approximately 10%. The physiological daily variation in S-Cre is rarely greater than 10% and the variation can be up to 30% without any prognostic significance. Nevertheless, a 30% change in S-Cre within 24 hours should raise clinical vigilance in the outpatient setting.
Existing evidence separates the variability of S-Cre into within-person, between-person, and analytical variations (CVA). Reinhard et al. found that the analytical variation of S-Cre was stable at less than 2% across a wide range of kidney functions; however, the within-person biological variation (CVI) could vary from 4.7% to 8.9% for individuals with and without impaired renal function9. Similar observations were also made by Carter et al., who found a low CVA of 0.6% and a CVI from 5.1% to above 6% in patients with impaired kidney function (eGFR<60 mL/min/1.73 m2) and proteinuria10. Moreover, previous studies suggested that a low index of individuality (II) for S-Cre supports stable S-Cre levels within an individual over time10,11. However, few studies have associated S-Cre variability, particularly within 24 or 48 hours, with mortality using real-world data. Therefore, the current diagnostic criteria for AKIINPT are empirical and conservatively sensitivity-centered12. Two articles have shown that small differences of 0.3–0.4 mg/dL in S-Cre are associated with in-hospital mortality and 30-day post-surgical mortality; however, neither of them evaluated the effects of within-day S-Cre variation13,14.
The clinical significance of changes in within-day S-Cre variation based on different clinical settings reflects hidden mechanisms underlying the observed inferences. First, in the outpatient setting, a change of up to 30% in S-Cre carries no mortality risk. By contrast, the same magnitude of change in S-Cre is associated with significantly higher risk of death among inpatients. This difference can be explained by the fluid optimization that occurs during the interval between S-Cre sampling in the inpatient setting, which is unusual among outpatients. Second, in the ED setting (Group 3), we found that increasing ΔS-Cre% in improving direction was uniquely associated with protective effects. Whether such acute recovery represents the protective effects of early intervention or the individual’s rapid and efficient compensatory response requires further research. Third, blood samples from patients who had their S-Cre measured twice within 24 hours in the outpatient setting (Group 1) are perfect for quantifying real-world CVA, particularly in paired samples with an examination time difference of less than one hour. In Group 1, the median ΔS-Cre and ΔS-Cre% were 0.04 and 3.41%, respectively, and the 99-percentile of ΔS-Cre and ΔS-Cre% was 0.23 mg/dL and 21.7%, respectively, providing insights into the maximal extent of the physiological variation in S-Cre.
Our results facilitate the in-time diagnosis of AKI by defining actionable cut-offs that accurately differentiate physiological variations from pathological variations of within-day S-Cre. Our findings support the idea that variation in CVI represents the function of biological variation, and an individual’s compensatory capacity to maintain kidney creatinine clearance in common etiologies of AKI associated with renal hypoperfusion or ischemia, such as dehydration, blood loss, or cardiogenic shock. In patients with sepsis, AKI may occur from concomitant systemic hypoperfusion and intrarenal vasodilatation, resulting in acute eGFR change. In the above-mentioned clinical conditions, intravenous hydration and hemodynamic supportive measures remain the treatment of choice15,16. Patients in Groups 3 (ED-to-INPT) and 4 (INPT-to-INPT) are likely to have received fluid replacement between the within-day measurements of S-Cre. Consequently, S-Cre should theoretically decrease in response to the hemodynamic load and dilution effects due to fluid expansion17. In Group 3 and 4, deteriorating rather than improving ΔS-Cre or ΔS-Cre% within 24 hours suggests decompensating renal autoregulation to counterbalance the effects of systemic derangement and predisposes individuals to poor clinical outcomes. By contrast, among patients who had their first S-Cre measured at the ED (Group 3), improving ΔS-Cre or ΔS-Cre% suggests the individual’s preserved kidney compensatory capacity to respond to the initial resuscitation and, therefore, is associated with favorable outcomes. On the other hand, in the ambulatory population, the CVI of S-Cre within 24 hours is small and reflects mainly physiological fluctuation; therefore, in the outpatient setting, a higher cut-off, for example, 30% (99 percentile level plus 10%) (Fig. 1), to alarm pathologic variation is rational in patients with normal kidney function and happened to be consistent with findings reported in 19714. A lower within-day ΔS-Cre% cut-off, for example, 20%, in inpatients who have been admitted or have received empirical hydration is appropriate to identify patients with clinically meaningful kidney decompensation. The within-day ΔS-Cre% cut-off values of 30% and 20% for outpatients and inpatients, respectively, provide the first evidence-based threshold for the identification of clinically significant kidney decompensation. These values imply that for AKIINPT, the existing empirical diagnostic criteria of ΔS-Cre > 0.3 mg/dL within 48 hours or ΔS-Cre% > 50% over a 7-day period may be relatively late for AKIINPT.
This study had several limitations. First,the residual confounding factors could not be completely excluded, particularly, the reasons for the patients receiving two S-Cre measurements on the same day. However, by stratifying the study population according to different service utilization patterns, this bias could be better controlled and understood. This approach further generates novel insights into the clinical meaning of within-day ΔS-Cre or ΔS-Cre% based on different clinical settings. Second, S-Cre in this study was measured by Jaffe method rather than the IDMS (isotope dilution-mass spectrometry) traceable enzymatic method, which may overestimate S-Cre, resulting in misclassification of IKF. Other limitations include the over-adjustment for variables that could be in the causal pathway and the possibility that these results should not be generalized to other ethnic populations.
In conclusion, increasing within-day ΔS-Cre or ΔS-Cre% above 0.2 mg/dL or 20%, respectively, were associated with increased all-cause mortality, particularly in inpatient settings. In outpatient settings, increasing within-day ΔS-Cre or ΔS-Cre% above 0.3 mg/dL or 30%, respectively, should raise concerns and trigger vigilance monitoring of kidney function. Moreover, it is the time to reconsider using clinical settings to classify AKI (eg AKIINPT and AKIOPT) rather than location (community acquired vs. hospital acquired).
Methods
Study population
In 2017, China Medical University Hospital (CMUH) established the Clinical Research Data Repository (CRDR) to verify and validate data from a variety of clinical sources to unify the trackable patient information generated during the healthcare process. Between January 1, 2003 and December 31, 2016, CMUH-CRDR had accumulated single unified records of 2 66 0472 patients who had sought medical care at CMUH. The data collected in the CMUH-CRDR includes administrative and demographic information, diagnoses, medical and surgical procedures, prescription drugs, laboratory measurements, physiological data, and status of catastrophic illnesses18. The interoperability of the CMUH-CRDR has expanded access to national population-based health-related databases (e.g., mortality database). The present cohort was composed of patients aged 18–90 years who had ever received two S-Cre measurements on the same day at two different analytical time points. The date of repeated S-Cre measurement was the index date. If patients had multiple events of within-day repeated S-Cre measurements in the CMUH-CRDR, the date of the first event was defined as the index date. We excluded patients who had a history of cancer and those who had undergone dialysis therapy, kidney transplantation, or cardiopulmonary resuscitation within 7 days prior to the index day. The present study included a total of 14 912 patients who were followed up to the date of death or were censored at the corresponding 30-day, 1-year, or 3-year time point after the index date. (Supplementary Fig. 5). The study was approved by the Big Data Center of China Medical University Hospital and the Research Ethical Committee/Institutional Review Board of China Medical University Hospital (CMUH105-REC3–068) and the need to obtain informed consent for the present study was waived by the Research Ethical Committee of China Medical University Hospital.
Quantification of serum creatinine and its within-day variation
S-Cre levels were measured using the Jaffe rate method at CMUH Central Laboratory using a Beckman UniCel DxC 800 immunoassay system (Beckman Coulter Inc., Brea, CA, USA). The estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease-Epidemiology Collaboration equation19. Among patients with multiple S-Cre measurements (>2 times) on the same day, we selected the first two S-Cre measurements for final analysis. The within-day S-Cre difference (ΔS-Cre) was calculated by subtracting the first S-Cre value from the second value. The within-day percentage change (ΔS-Cre%) was calculated by dividing the within-day difference by the first S-Cre value and multiplying by 100. IKF was defined by first S-Cre value greater than 1.5 mg/dL for men or 1.3 mg/dL for women.
Other variables
Sociodemographic variables were retrieved from the CMUH-CRDR. Body mass index (BMI) was calculated using the standard formula: body weight (kg) divided by height squared (m2). Diabetes mellitus and hypertension were defined using physicians’ clinical diagnoses, according to the patients’ ICD-9-CM codes and the use of glucose-lowering or anti-hypertensive agents. Baseline comorbidities and medication use, and any relevant biochemical measures, were determined based on information obtained from the CMUH-CRDR respectively within a 1-year and 3-month window prior to case enrollment. Catastrophic illness status was defined as having a condition classified as catastrophic illness by the Ministry of Health and Welfare in Taiwan. The catastrophic illnesses include 30 disease categories such as: malignancy, hemophilia, inherited hemolytic anemias, chronic kidney disease, systemic autoimmune disease, chronic psychiatric disorders, inherited metabolic disorders, congenital anomalies, severe burn, transplantation, multiple sclerosis, liver cirrhosis with complications, rare diseases, and other conditions that require long-term and systemic medical care (https://ws.nhi.gov.tw/001/Upload/293/RelFile/Ebook/English.pdf). Noncancerous catastrophic status was defined by excluding patients who ever had a diagnosis of malignancy verified by National Catastrophic Illness Registry.
Statistical analyses
The study population was separated into improving (ΔS-Cre < 0) and deteriorating (ΔS-Cre > 0) groups and then assigned into quartile groups based on the quartile cut-offs of the ΔS-Cre or ΔS-Cre%. They were also divided into four groups according to the hospital service where the first and second S-Cre were measured: Group 1, Outpatient (OPT)-to-OPT; Group 2, OPT-to-ED (emergency department) or inpatient (INPT); Group 3, ED-to-ED or INPT; and Group 4, INPT–to-INPT. In the univariable analyses, we separately compared clinical characteristics across quartiles groups of ΔS-Cre% or ΔS-Cre and across four service transition patterns (Group 1–4). Continuous variables were expressed as the median and interquartile range (IQR) and compared using the nonparametric Kruskal–Wallis test due to several biochemical parameters were not normally distributed such as blood urea nitrogen and serum creatinine. Categorical variables were expressed as frequency (percentage) and compared using the chi-square test. The associations between within-day variation (in continuous and quartile categories) and risks of 30-day (Groups 3 and 4), 1-year (Groups 1 and 2), and 3-year (all groups) all-cause mortality were estimated using multivariable Cox regression analysis. The distribution of within-day ΔS-Cre was described according to time and baseline IKF status. Then Bland–Altman analysis was applied to evaluate the agreement between the two S-Cre measurements. To minimize the negative effects of missing data on risk association analysis (e.g., reduced statistical power), we performed multiple imputation through the fully conditional specification method in SAS, an iterative Markov chain Monte Carlo procedure, to replace the missing values for variables in our proposed formula with imputed values. We specified the imputation number as 20 and iteration number as 10020. Multivariable Cox regression models, using age as the time scale, were initially adjusted for sociodemographic variables including age, sex, comorbidities, and baseline eGFR, followed by adjusting for baseline medications, and then by adjusting for baseline nutritional and inflammatory markers. The dose–response relationships were further characterized between all-cause mortality and within-day ΔS-Cre variation using a restricted cubic spline model with five knots located at the 5th, 25th, 50th, 75th, and 95th percentiles of the ΔS-Cre variation distribution of the four transition groups21. To predict 30-day, 1-year, and 3-year mortality, optimal cut-off values were determined for ΔS-Cre and ΔS-Cre% in each service group (Groups 1–4) when the log-rank test statistics was maximal. All statistical analyses were performed in SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R version 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria). The 2-sided statistical significance level was set at α = 0.05.
Ethical approval
The study was approved by the Research Ethical Committee/Institutional Review Board of China Medical University Hospital (CMUH105-REC3-068).
Supplementary information
Acknowledgements
This study was supported by the Ministry of Science and Technology of Taiwan (grant number: 106-2314-B-039-041- MY3 and 108-2314-B-039 -038 -MY3) and the China Medical University Hospital (grant number: CRS-106-018; DMR-109-200).
Author contributions
H.C.Y. and C.C.K. designed the study; C.C.K., S.N.C. and Y.C.L. analyzed the data and made the figures; I.W.T., P.L.C., S.N.C. and H.Y.C. drafted and revised the paper; all authors approved the final version of the manuscript.
Data availability
The data that support the findings of this study are available on request from the corresponding author, CCK. The data are not publicly available due to them containing information that could compromise research participant privacy.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-63315-x.
References
- 1.Bellomo R, et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical care. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(KDIGO), K. D. I. G. O. Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury Kidney Int Suppl., 1–138 (2012).
- 3.Coresh J, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. Jama. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasternack A, Kuhlback B. Diurnal variations of serum and urine creatine and creatinine. Scandinavian journal of clinical and laboratory investigation. 1971;27:1–7. doi: 10.3109/00365517109080181. [DOI] [PubMed] [Google Scholar]
- 5.Pieroni L, Bargnoux AS, Cristol JP, Cavalier E, Delanaye P. Did Creatinine Standardization Give Benefits to the Evaluation of Glomerular Filtration Rate? Ejifcc. 2017;28:251–257. [PMC free article] [PubMed] [Google Scholar]
- 6.Mitch WE, Walser M. A proposed mechanism for reduced creatinine excretion in severe chronic renal failure. Nephron. 1978;21:248–254. doi: 10.1159/000181400. [DOI] [PubMed] [Google Scholar]
- 7.Hosten, A. O. In Clinical Methods: The History, Physical, and Laboratory Examinations (eds. rd, Walker, H. K., Hall, W. D. & Hurst, J. W.) (1990). [PubMed]
- 8.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clinical chemistry. 1992;38:1933–1953. doi: 10.1093/clinchem/38.10.1933. [DOI] [PubMed] [Google Scholar]
- 9.Reinhard M, Erlandsen EJ, Randers E. Biological variation of cystatin C and creatinine. Scandinavian journal of clinical and laboratory investigation. 2009;69:831–836. doi: 10.3109/00365510903307947. [DOI] [PubMed] [Google Scholar]
- 10.Carter JL, et al. Biological Variation of Plasma and Urinary Markers of Acute Kidney Injury in Patients with Chronic Kidney Disease. Clinical chemistry. 2016;62:876–883. doi: 10.1373/clinchem.2015.250993. [DOI] [PubMed] [Google Scholar]
- 11.Wu AH. Biological and analytical variation of clinical biomarker testing: implications for biomarker-guided therapy. Current heart failure reports. 2013;10:434–440. doi: 10.1007/s11897-013-0156-6. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter., Suppl., 1–138 (2012).
- 13.Lassnigg A, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. Journal of the American Society of Nephrology: JASN. 2004;15:1597–1605. doi: 10.1097/01.ASN.0000130340.93930.DD. [DOI] [PubMed] [Google Scholar]
- 14.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. Journal of the American Society of Nephrology: JASN. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 15.Endre ZH, Pickering JW, Walker RJ. Clearance and beyond: the complementary roles of GFR measurement and injury biomarkers in acute kidney injury (AKI) Am. J. Physiol. Renal. Physiol. 2011;301:F697–707. doi: 10.1152/ajprenal.00448.2010. [DOI] [PubMed] [Google Scholar]
- 16.Moore PK, Hsu RK, Liu KD. Management of Acute Kidney Injury: Core Curriculum 2018. Am. J. Kidney. Dis. 2018;72:136–148. doi: 10.1053/j.ajkd.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Macedo E, et al. Fluid accumulation, recognition and staging of acute kidney injury in critically-ill patients. Critical care. 2010;14:R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai, C. W. et al. Longitudinal Lipid Trends and Adverse Outcomes in Patients with Chronic Kidney Disease: A 13-Year Observational Cohort Study Using Registry-Based Electronic Medical Records. Journal of lipid research, 10.1194/jlr.P084590 (2019). [DOI] [PMC free article] [PubMed]
- 19.Levey AS, et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeh HC, et al. Variability of red blood cell size predicts all-cause mortality, but not progression to dialysis, in patients with chronic kidney disease: A 13-year pre-ESRD registry-based cohort. Clin. Chim. 2019;497:163–171. doi: 10.1016/j.cca.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 21.Harrel, F. E. Regression Modeling Strategies. 23 (Springer, 2001).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, CCK. The data are not publicly available due to them containing information that could compromise research participant privacy.